Abstract
How different regimens of nicotine administration and withdrawal affect systemic inflammation is largely unknown. We studied the effects of chronic and acute nicotine administration and of nicotine withdrawal on the outcome of aseptic and septic systemic inflammation. Male C57BL/6 mice were implanted with subcutaneous osmotic pumps (to deliver nicotine) and intrabrain telemetry probes (to measure temperature). Aseptic inflammation was induced by lipopolysaccharide (40 mg/kg ip); sepsis was induced by cecal ligation and puncture. The chronic nicotine administration group received nicotine (28 mg·kg−1·day−1) for 2 wk before the induction of inflammation and continued receiving nicotine until the end of the experiment; the acute nicotine administration group received saline for 2 wk and nicotine thereafter; the nicotine withdrawal group received nicotine for 2 wk and saline thereafter; and the no-nicotine group was infused with saline throughout the experiment. Compared with no nicotine, the chronic nicotine administration did not affect survival in either model of inflammation, possibly due to the development of nicotine tolerance. The acute nicotine administration increased the survival rate in aseptic inflammation from 11 to 33% (possibly by suppressing inflammation) but worsened the outcome of sepsis (possibly because the suppression of inflammation promoted microbial proliferation). Oppositely to acute nicotine, nicotine withdrawal increased the survival rate in sepsis from 18 to 40%. The effects on survival were not due to changes in body temperature. We conclude that acute nicotine administration and nicotine withdrawal affect survival in systemic inflammation and that these effects strongly depend on whether inflammation is aseptic or septic.
Keywords: sepsis, acetylcholine, mortality, body temperature
the systemic inflammatory response syndrome (SIRS) is a leading cause of death in hospitalized patients. SIRS can be associated with infection (in which case it is called sepsis), or it can be triggered by noninfectious insults such as blunt trauma (6, 27). In the laboratory, a procedure called cecal ligation and puncture (CLP) is often used to induce peritonitis and sepsis in laboratory animals, whereas systemic administration of lipopolysaccharide (LPS, a constituent of the cell wall of Gram-negative bacteria) is often used to cause SIRS aseptically. In either case, shock, multiple organ failure, and eventually death can occur, largely as the result of an overt production of proinflammatory mediators (8). Activation of nicotinic acetylcholine receptors, possibly on macrophages, either by the electrical stimulation of the efferent vagus nerve (which releases acetylcholine) or by the acute administration of nicotine has been shown to suppress the production of several proinflammatory cytokines and inhibit several symptoms of experimental SIRS (5, 28, 50). On the other hand, transection of the vagus nerve has been shown to result in exaggerated responses to shock-inducing doses of LPS (5, 16, 39). However, whether and how nicotine influences the outcome of SIRS remains unclear. In studies by Wang et al. (49), Pavlov et al. (31), and Hofer et al. (13), acute administration of nicotine diminished mortality in mice with SIRS, regardless of whether SIRS was induced aseptically (by LPS) or was associated with infection (caused by CLP), whereas a study by van Westerloo et al. (48) showed that nicotine increased mortality in a mouse model of Escherichia coli-induced sepsis.
Perhaps more important than clarifying how SIRS is affected by the acute administration of nicotine is to determine how it is affected by the chronic administration of this substance (as in smoking tobacco) and by nicotine withdrawal (as with smoking cessation). Humans and experimental animals that receive nicotine chronically become tolerant to it, a phenomenon that is thought to result from the desensitization of nicotinic acetylcholine receptors (26, 32). Tolerant subjects depend on the regular consumption of nicotine to maintain its concentration in the body high enough to prevent the precipitation of withdrawal symptoms such as anxiety, depression, irritability, restlessness, bradycardia, hyperphagia, and others (22). These withdrawal symptoms are manifested both in healthy people trying to quit smoking (10, 14) and in patients with various conditions who abruptly stop smoking on admission to an intensive care unit (20). The latter group deserves special attention, because critical care patients are at a high risk of developing SIRS (6, 7, 47).
The purpose of the present study was to determine the effects of nicotine administered acutely and chronically, as well as the effects of nicotine withdrawal, on the mortality of mice with experimentally induced SIRS. Two established models of SIRS were employed: LPS administration (to induce SIRS in the absence of an infection) and CLP (to induce SIRS associated with a polymicrobial infection).
METHODS
Animals
Male C57BL/6 mice weighing 25–35 g (Charles River Laboratories, Wilmington, MA) were initially housed four per cage in cages (“shoe boxes”) kept in a Maxi-Miser ventilated rack (Thoren Caging Systems, Hazleton, PA) at room temperature (24–27°C). From the day before they were subjected to LPS administration, CLP, or the respective control procedures to the end of the experiments, the mice were housed singly in cages kept inside an environmental chamber (model 3940; Forma Scientific, Marietta, OH) at tightly controlled ambient temperature (27.8–28.2°C) and air humidity (45–55%). Inside the chamber, the cages were kept on top of telemetry receivers (series 3000; MiniMitter, Bend, OR) to allow for continuous monitoring of brain temperature and gross locomotor activity (for those mice implanted with telemetry probes). Whether during the initial housing in the ventilated rack or during the subsequent housing in the environmental chamber, the mice were on a 12:12-h light-dark cycle (lights on at 7:00 AM) and had free access to standard chow and tap water. The study was approved by the St. Joseph's Hospital Animal Care and Use Committee.
Study Design
Experiment 1: characterizing the models of LPS- and CLP-induced SIRS.
To induce SIRS, one group of mice was injected intraperitoneally with LPS (40 mg/kg), and the other group was subjected, under anesthesia, to the CLP procedure. Control groups were injected with saline or subjected to a sham procedure, respectively. LPS (or saline) was injected at 5:00 PM; CLP (or sham surgery) was performed between 12:00 PM and 4:00 PM. To monitor mortality, all mice were periodically examined for the presence of cardiac and respiratory activities and spontaneous movements. Brain temperature and gross locomotor activity were measured in approximately one-half of the mice in each group by telemetry (MiniMitter). If a mouse was designated for injection of LPS (or saline), a telemetry transmitter was implanted into the brain 2 days before the injection. If a mouse was designated for CLP (or the sham procedure), a transmitter was implanted immediately before the procedure, thus avoiding a second anesthesia.
Experiment 2: determining the effects of acute and chronic nicotine administration and nicotine withdrawal on the outcomes of LPS- and CLP-induced SIRS.
The mice were first implanted subcutaneously with Alzet osmotic minipumps (Durect, Cupertino, CA) to deliver nicotine (28 mg·kg−1·day−1) or saline continuously for 13 days (Table 1). The rate of nicotine infusion was selected based on the fact that it elevates the level of nicotine in the blood plasma of mice to ∼50 ng/ml (25), a level frequently observed during unrestricted smoking in humans (43). On day 13, each implanted pump was surgically removed and replaced with a new, second pump to continue delivering nicotine or saline (at the same rate as the first pumps) or to change the treatment (from nicotine to saline and vice versa). Mice designated to telemetry measurements were implanted with brain transmitters immediately before the pump replacement. CLP (which induces a slowly developing SIRS) was performed immediately after the pump replacement, whereas LPS (which induces a rapidly developing SIRS) was injected on the second day after pump replacement (day 15 overall). This timeline was chosen to ensure that the majority of deaths in either model of SIRS occur on the second day after pump replacement. During the first 3 days after pump replacement (days 14–16), the group that received the saline infusion via the first pump followed by nicotine infusion via the second pump was not expected to have developed significant nicotine tolerance (26) and hence was suitable for studying the effects of acute nicotine administration on the outcomes of SIRS; in the text below and in the figures, we refer to this group as the “acute nicotine” group. During the same time window, the group that received nicotine through the first pump followed by nicotine through the second pump was expected to be fully tolerant to the drug (26); we refer to this group as the “chronic nicotine” group. At the same time, the group that received nicotine through the first pump followed by saline through the second pump was expected to display a full-blown withdrawal syndrome (12, 46); we refer to this group as the “nicotine withdrawal” group. The fourth group received saline via the first pump followed by saline via the second pump; this was the “no-nicotine” group. In all groups, physical examination and telemetry recordings were performed as in experiment 1. On day 18 all monitoring was ended, and all survivors were euthanized.
Table 1.
Timeline of experiment 2
| Time | Model |
|
|---|---|---|
| LPS-induced SIRS (develops faster) | CLP-induced sepsis (develops slower) | |
| Day 0 | 1st pump implanted (delivers nicotine or saline during days 0–13) | 1st pump implanted (delivers nicotine or saline during days 0–13) |
| Day 13 | Telemetry probe implanted | Telemetry probe implanted |
| 1st pump removed | 1st pump removed | |
| 2nd pump implanted (delivers nicotine or saline during days 13–18) | 2nd pump implanted (delivers nicotine or saline during days 13–18) | |
| Temperature and activity recording started | CLP or sham surgery performed | |
| Temperature and activity recording started | ||
| Mortality recording started* | ||
| Day 15 | LPS or saline injected | |
| Mortality recording started* | ||
| Day 18 | Experiment ended | Experiment ended |
SIRS, systemic inflammatory response syndrome; CLP, cecal ligation and puncture.
In either model, deaths occurred on the 2nd and 3rd days after pump replacement (days 15 and 16, respectively), when the effects of acute nicotine and nicotine withdrawal are known to be maximal.
Surgical Procedures
Anesthesia.
All surgeries were performed under ketamine-xylazine-acepromazine (42.0, 4.8, and 0.6 mg/kg ip) anesthesia. A mouse was kept on a heating pad and periodically ventilated with oxygen through a custom-made mask.
Telemetry probe implantation.
The head of a mouse was fixed to a stereotaxic apparatus (David Kopf, Tujunga, CA), the skin over the sagittal suture was incised, the periosteum was excised, and two supporting miniature screws were driven into the skull. The incisor bar of the apparatus was adjusted to place the intersections of the sagittal suture with bregma and lambda in the same horizontal plane. A hole was drilled in the skull surface (1.0 mm caudal to bregma; 1.0 mm right of the midline), and the 26-gauge thermistor extension of an XM-FH transmitter (MiniMitter) was stereotaxically lowered through the hole so that its tip was located 4.0 mm ventral to the skull surface. The capsule of the transmitter was attached to the supporting screws with acrylic cement. Postmortem histology revealed that the thermistor tip was located in the lateral hypothalamus. Hypothalamic temperature is commonly used as an index of core body temperature (34, 35, 38). The lateral location of the thermistor within the hypothalamus was chosen to avoid damaging the medially located hypothalamic structures such as the median preoptic nucleus and medial preoptic area; lesioning these medial structures results in severe hyperthermia and other unwanted physiological effects (1, 40).
Osmotic pump implantation.
A 1-cm skin incision was made over the lumbar spine, the skin was bluntly separated from the underlying tissues to form a pocket on the right side, an Alzet osmotic minipump (model 2002; delivers its content for 14 days) was inserted into the pocket, and the surgical wound was sutured. At the time of pump replacement, the skin was incised at the same site, the previously implanted pump was removed, a new pocket was made on the left side, a new pump (model 1007D; delivers its content for 7 days) was inserted into the new pocket, and the skin was re-sutured.
CLP.
Following a midline laparotomy, the cecum was pulled out of the abdominal cavity and placed on a saline-soaked gauze. The cecum was filled with the intestinal content by gently squeezing the content from the ascending colon. The cecum was then ligated with 3-0 silk just distal to the ileocecal junction (intestinal transit was not interrupted). The cecal wall was punctured through at the antimesenteric side with a 26-gauge needle, the cecum was placed back into the abdominal cavity, and the abdominal wall was sutured in layers.
Drugs
LPS.
A 2.4-mg/ml suspension of LPS from E. coli 0111:B4 (Sigma-Aldrich, St. Louis, MO) in saline was prepared, aliquoted, and stored at −20°C. On the day of the injection, the suspension was thawed, sonicated for 5 min, and then injected in bolus (40 mg/kg ip).
Nicotine.
A 200-mg/ml (70 mg/ml, free base) solution of (−)nicotine bitartrate (Sigma-Aldrich) in saline was prepared and loaded into Alzet minipumps. The pumps were then submerged in saline at room temperature for 24 h to ensure that they would be releasing their contents at the time of implantation (immediately after the incubation in saline). Both pump models used (2002 and 1007D) release their content at a rate of 12 μl/day, thus delivering nicotine at a rate of 0.84 mg/day (∼28 mg·kg−1·day−1).
Statistical Analyses
The survival rate was calculated as a percentage of survivors at a certain point in time relative to the total number of mice that received a given treatment. The survival rate data were analyzed by using the logrank test (4) and by performing a point-by-point χ2 test (Statistica AX'99, StatSoft, Tulsa, OK). The results of the logrank test were used to reveal those intergroup differences in the survival rate that persisted throughout the entire duration of the experiment (4). Student's t-test was used to evaluate changes in the time to death. The brain temperature and locomotor activity responses were compared across treatments and points in time by two-way ANOVA. For all tests used, the level of significance was set at P < 0.05.
RESULTS AND DISCUSSION
LPS and CLP Models of SIRS
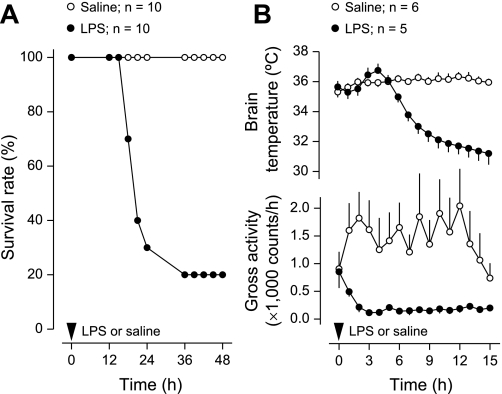
All mice injected with saline survived, whereas the survival rate among the mice that received LPS was 20% (P < 0.001, logrank test; Fig. 1A). All LPS-related deaths occurred between 15 and 36 h after LPS administration; the mean time to death among nonsurvivors was 21 ± 1 h. Such a rapid progression is typical for LPS-induced SIRS (16). The fast progression of SIRS was also revealed by changes in brain temperature (Fig. 1B): a small but significant (P < 0.05, 2-way ANOVA) rise in brain temperature (fever) peaked at 4 h after LPS administration and was followed by pronounced and long-lasting hypothermia (P < 0.001, 2-way ANOVA). This bimodal pattern is typical for the body temperature response of mice to high doses of LPS (42). Both the febrile and hypothermic phases of the response were associated with locomotor depression (P < 0.001, 2-way ANOVA), a nonspecific “sickness symptom” (11, 23, 33) that also occurs in LPS-treated animals (36).
Fig. 1.
Outcome of mice injected with LPS (40 mg/kg ip) or saline. A: survival rate of the mice. B: deep body (brain) temperature and gross locomotor activity responses (mean ± SE) before any casualties; n = no. of animals in each group. Compared with saline, LPS significantly reduced survival rate (P < 0.001 for the duration of the experiment, logrank test), altered body temperature (fever at 4 h postinjection, P < 0.05, 2-way ANOVA; hypothermia at 6–15 h, P < 0.001, 2-way ANOVA), and suppressed locomotor activity (1–15 h, P < 0.001, 2-way ANOVA).
In the CLP experiment, all mice survived the sham procedure, whereas CLP-induced sepsis had a survival rate of 14% (P < 0.001, logrank test; Fig. 2A). Although LPS and CLP resulted in similar overall survival rates, CLP-induced sepsis was characterized by a slower dynamics. All CLP-related deaths occurred between 24 and 78 h after the CLP procedure, and the mean time to death among nonsurvivors was 42 ± 5 h. As in the LPS-treated mice, marked hypothermia and locomotor depression occurred in the mice subjected to CLP (P < 0.001 for both, 2-way ANOVA; Fig. 2B). These symptoms became obvious after the mice recovered from anesthesia (∼6 h after CLP). At this point, the body temperature and activity of the sham-operated mice started to increase, whereas hypothermia and malaise persisted in the CLP-subjected mice.
Fig. 2.
Outcome of mice subjected to cecal ligation and puncture (CLP) or sham surgery. A: survival rate of the mice. B: deep body (brain) temperature and gross locomotor activity responses (mean ± SE) before any casualties; n = no. of animals in each group. Compared with sham surgery, CLP significantly reduced survival rate (P < 0.001 for the duration of the experiment, logrank test), decreased body temperature (6–18 h, P < 0.001, 2-way ANOVA), and suppressed locomotor activity (6–18 h, P < 0.001, 2-way ANOVA).
Nicotine and the Outcome of SIRS
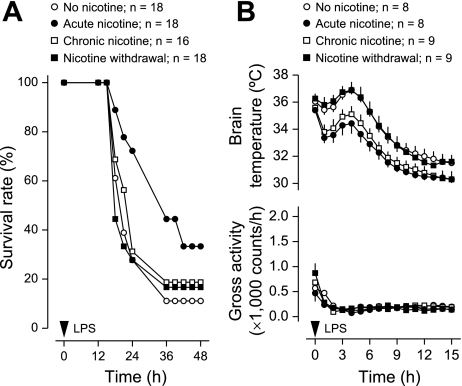
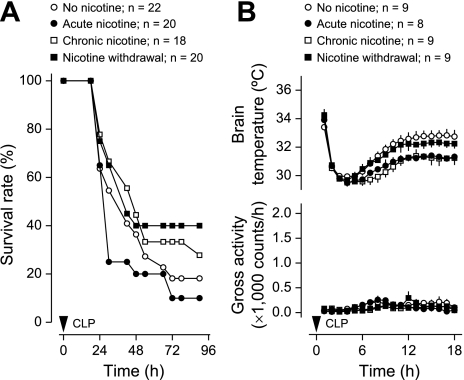
In the no-nicotine group of experiment 2, the overall survival rate of the LPS-treated mice implanted with osmotic pumps was 11% (Fig. 3A). This rate was not statistically different from the survival rate (20%) found in experiment 1 for the LPS-treated mice not implanted with osmotic pumps (Fig. 1A). Relative to no nicotine, the acute treatment with nicotine increased survival of mice with LPS-induced SIRS threefold: from 11 to 33% (P < 0.001, logrank test; Fig. 3A). The same treatment delayed the mean time to death for animals that succumbed: from 22 ± 1 h in the no-nicotine group to 27 ± 2 h in the acute nicotine group (P < 0.05; t-test). In CLP-induced sepsis (Fig. 4A), the effects of acute nicotine were opposite to its effects in LPS-induced SIRS. The survival rate at 30 h post-CLP was reduced more than twofold (from 55% to 25%; P < 0.05, χ2 test), and the average time to death for nonsurvivors tended to be shorter (37 ± 5 vs. 41 ± 5 h in the no-nicotine group).
Fig. 3.
Effects of nicotine treatments or withdrawal on the outcome of mice injected with LPS (40 mg/kg ip). For details on the regimens of nicotine infusion, see methods. A: survival rate of the mice. B: deep body (brain) temperature and gross locomotor activity responses (mean ± SE) before any casualties; n = no. of animals in each group. Relative to no nicotine, the acute treatment with nicotine increased survival rate (P < 0.001 for the duration of the experiment, logrank test). LPS-induced hypothermia was enhanced by acute and chronic nicotine (1–15 h, P < 0.001, 2-way ANOVA). None of the treatments affected LPS-induced locomotor depression.
Fig. 4.
Effects of nicotine treatments or withdrawal on the outcome of mice subjected to CLP. For details on the regimens of nicotine infusion, see methods. A: survival rate of the mice. B: deep body (brain) temperature and gross locomotor activity responses (mean ± SE) before any casualties; n = no. of animals in each group. Relative to no nicotine, acute nicotine reduced survival rate (P < 0.05 at 30 h, χ2 test), whereas nicotine withdrawal increased it (P < 0.05 for the duration of the experiment, logrank test). CLP-induced hypothermia was enhanced by acute and chronic nicotine (6–18 h, P < 0.001, 2-way ANOVA). None of the treatments affected LPS-induced locomotor depression.
Interestingly, both the beneficial effects in the LPS model and the deleterious effects in the CLP model may be related to the same, anti-inflammatory action of nicotine (5, 28, 50). Suppression of inflammation can be expected to aid recovery in a situation when the inflammatory response is a major damaging factor (such as in LPS-induced SIRS), whereas the situation with infection-associated SIRS (as in the case of CLP) is more complex. When SIRS and an infection occur in parallel, inhibiting inflammation may facilitate microorganism spreading, and thus promote infection (2). In support of the proposed scenario, nicotine has been shown to impair the ability of macrophages to eliminate live bacteria in vitro (28). Furthermore, van Westerloo et al. (48) have reported an association between the impairment of bacterial clearance and the decreased survival rate in nicotine-treated mice infected with E. coli. Factors other than nicotine have also been shown to affect septic and aseptic SIRS differently. For example, increasing core temperature did not improve survival of LPS-challenged mice in a study by Jiang et al. (18), whereas it improved survival and reduced the bacterial load in mice with Klebsiella pneumoniae peritonitis in another study by the same authors (17).
Seemingly in contradiction with this scenario are studies by Wang et al. (49), Pavlov et al. (31), and Hofer et al. (13), in which nicotine (or nicotine agonists) increased the survival rate in both LPS-induced SIRS and CLP-induced sepsis. It should be considered, however, that the CLP procedure was followed by administration of antibiotics in the studies by Wang et al. (49) and Pavlov et al. (31); the antibiotic therapy should have limited the underlying infection, thus making the model employed different from that of an untreated sepsis and more similar to that of an aseptic SIRS. The study by Hofer et al. (13) did not use antibiotics to treat CLP-induced sepsis, but it differed from ours in that it involved repeated intraperitoneal injections (and not a constant-rate infusion) of nicotine. Because high concentrations of nicotine can promote neutrophil activation (15), marked surges in nicotine levels following the intraperitoneal bolus injections might have aided protection against infection. Although Hofer et al. (13) found no neutrophil activation in the lungs, neutrophils still could have become activated in the peritoneal cavity, the compartment in which nicotine was injected directly and, therefore, was expected to reach the highest concentration.
When nicotine was infused chronically, it did not affect the survival rate significantly in either the LPS or the CLP model (Figs. 3A and 4A). This finding suggests that the anti-inflammatory action of nicotine is subject to tolerance development (desensitization). Among the nicotinic acetylcholine receptor subtypes desensitized by chronic administration of nicotine are the homomeric α7-receptors and the heteromeric α4β2-receptors (30), both of which have been implicated in the anti-inflammatory action of nicotine (9, 28, 50).
Since the physiological effects produced by nicotine withdrawal are usually opposite to those produced by acute nicotine administration, one could expect that nicotine withdrawal would reduce the survival rate in the LPS model but would increase it in the CLP model (relative to the survival rate in the respective no-nicotine group). Nicotine withdrawal did increase the survival rate more than twofold (from 18 to 40%; P < 0.05, logrank test) in mice with CLP-induced sepsis (Fig. 4A). However, it failed to cause any effect in the LPS-induced SIRS (Fig. 3A). This lack of a measurable effect may be linked to the fact that LPS-induced SIRS causes death so rapidly (see above) that any further decrease in the survival rate may be hard to detect in this model. In neither model of SIRS was the time to death in nonsurvivors significantly altered by nicotine withdrawal.
The effects of acute nicotine administration and withdrawal on the survival rate in experimental SIRS are pronounced: 2.2- to 3.0-fold according to the present study. It should be noted, however, that effects found in animal studies of SIRS are usually much stronger than those revealed in clinical trials. Indeed, several drugs have been reported to drastically (sometimes from 0 to 75%) increase the survival rate in LPS-induced SIRS (19, 29) and in CLP-induced sepsis (21) in mice and rats, whereas the single most successful clinical trial (with protein C) reported only a marginal (from 69 to 75%) increase in the survival rate of patients with severe sepsis (3).
Both fever (due to its antimicrobial and immunostimulating effects) and hypothermia (due to its anti-hypoxic and possibly anti-inflammatory effects) may be adaptive in systemic inflammation (41). Because body temperature is likely to affect the outcome of both aseptic and septic SIRS (17, 24, 37), we asked whether the effects of nicotine treatment and withdrawal revealed in this study were due to effects on body temperature. Although acute nicotine treatment affected the survival rate in LPS-induced SIRS and CLP-induced sepsis in the opposite directions (increased and decreased, respectively, compared with the corresponding no-nicotine group; Figs. 3A and 4A), it exaggerated both LPS- and CLP-induced hypothermia (P < 0.001 for both effects, 2-way ANOVA; Figs. 3B and 4B). Moreover, the effect of acute nicotine treatment on brain temperature was similar to that of chronic nicotine treatment in either LPS-induced SIRS (Fig. 3B) or CLP-induced SIRS (Fig. 4B), whereas the two treatment regimens differently affected survival rate in either model of SIRS (Figs. 3A and 4A). Hence, we conclude that the effects of nicotine on the survival rate and deep body temperature are dissociated. Based on the fact that chronic nicotine treatment affected LPS hypothermia similarly to acute nicotine (Figs. 3B and 4B), we also conclude that the hypothermic effect of nicotine is not subject to tolerance development. Further supporting this conclusion is the fact that the withdrawal of nicotine increased body temperature relative to chronic nicotine treatment in either LPS SIRS (P < 0.001, 2-way ANOVA; Fig. 3B) or CLP SIRS (P < 0.001, 2-way ANOVA; Fig. 4B) but did not change the temperature response to SIRS relative to no-nicotine treatment in either model of inflammation (Figs. 3B and 4B). It is possible that the hypothermic effect of nicotine involves a nicotinic acetylcholine receptor that is resistant to desensitization, such as the α4β4-receptor (51). Indeed, the hypothermic response to nicotine has been proposed to be mediated by a receptor containing the β4 subunit (44).
We also found that the effects of nicotine treatment (whether acute or chronic) and withdrawal on the survival rate were dissociated from the locomotor manifestations of SIRS. Specifically, none of the regimens of nicotine administration affected LPS- or CLP-induced locomotor depression (Figs. 3B and 4B) despite affecting the survival rate (Figs. 3A and 4A).
Conclusions
We conclude that while chronic administration of nicotine does not affect the survival rate in SIRS (possibly due to the development of nicotine tolerance), acute nicotine administration and nicotine withdrawal both have pronounced effects on the outcome of experimental SIRS. As for the acute administration of nicotine, it is beneficial in an aseptic situation (LPS model), possibly due to suppression of inflammation. The same acute nicotine treatment, however, is detrimental in sepsis (CLP model), possibly because the suppression of inflammation renders the host defenseless against microbial proliferation. As for the effect of nicotine withdrawal, it can be seen in sepsis (CLP model), and it is opposite to the effect of acute nicotine administration. We further conclude that none of the reported effects of nicotine administration or withdrawal on survival in SIRS are due to changes in body temperature.
Perspectives
The present study has discovered a robust experimental phenomenon: differential effects of nicotine administration and withdrawal on the overall outcome of LPS- and CLP-induced systemic inflammation. However, the mechanisms of this phenomenon were not studied in the present work and are open for exploration. As the next step, it will be particularly important to determine whether the differential effects described here are indeed due to the aseptic vs. septic nature of systemic inflammation in the two models studied. To this end, studies of the effects of different regimens of nicotine administration on the levels of inflammatory mediators and the extent microbial proliferation are warranted.
Also of interest are the clinical implications of the phenomenon discovered. A large number of SIRS patients may experience nicotine withdrawal, e.g., smokers who undergo a traumatic injury and abruptly stop smoking due to the severity of trauma. There may also be a small cohort of patients who receive nicotine acutely, e.g., those patients who develop SIRS within a few days of being placed on transdermal nicotine therapy for unrelated conditions such as ulcerative colitis (45) or within a few days after starting cigarette smoking or using other tobacco products. The present work suggests that future clinical studies on this subject need to account for the fact that the effects of nicotine on outcomes of SIRS may depend on whether SIRS occurs aseptically or is associated with an infection (sepsis), and on how successfully the underlying infection is treated.
GRANTS
The study was funded by grants from the Arizona Biomedical Research Commission (Category II, No. 8016), the National Institute of Neurological Disorders and Stroke (R01-NS-41233), and St. Joseph's Foundation to A. A. Romanovsky.
Acknowledgments
We thank Dr. Ronald J. Lukas for critical comments and Julie M. Turko for editing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Almeida MC, Steiner AA, Branco LGS, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE 1: e1, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol 288: L633–L640, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344: 699–709, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bland JM, Altman DG. The logrank test. BMJ 328: 1073, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson C The epidemiology of the systemic inflammatory response. Intensive Care Med 26: S64–74, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Regnier B. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units French ICU Group for Severe Sepsis. JAMA 274: 968–974, 1995. [PubMed] [Google Scholar]
- 8.Cohen J The immunopathogenesis of sepsis. Nature 420: 885–891, 2002. [DOI] [PubMed] [Google Scholar]
- 9.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immun 6: 844–851, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Gross J, Stitzer ML. Nicotine replacement: ten-week effects on tobacco withdrawal symptoms. Psychopharmacology (Berl) 98: 334–341, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Hart BL Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12: 123–137, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Helton DR, Modlin DL, Tizzano JP, Rasmussen K. Nicotine withdrawal: a behavioral assessment using schedule controlled responding, locomotor activity, and sensorimotor reactivity. Psychopharmacology (Berl) 113: 205–210, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36: 404–408, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 48: 52–59, 1991. [DOI] [PubMed] [Google Scholar]
- 15.Iho S, Tanaka Y, Takauji R, Kobayashi C, Muramatsu I, Iwasaki H, Nakamura K, Sasaki Y, Nakao K, Takahashi T. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-κB. J Leukoc Biol 74: 942–951, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI, Kulchitsky VA, Sugimoto N, Simons CT, Romanovsky AA. Does the formation of lipopolysaccharide tolerance require intact vagal innervation of the liver? Auton Neurosci 85: 111–118, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 68: 1265–1270, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, DeTolla L, van Rooijen N, Singh IS, Fitzgerald B, Lipsky MM, Kane AS, Cross AS, Hasday JD. Febrile-range temperature modifies early systemic tumor necrosis factor alpha expression in mice challenged with bacterial endotoxin. Infect Immun 67: 1539–1546, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Yang R, Marsters SA, Bunting SA, Wurm FM, Chamow SM, Ashkenazi A. Protection against rat endotoxic shock by p55 tumor necrosis factor (TNF) receptor immunoadhesin: comparison with anti-TNF monoclonal antibody. J Infect Dis 170: 1323–1326, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Jones C, Griffiths RD, Skirrow P, Humphris G. Smoking cessation through comprehensive critical care. Intensive Care Med 27: 1547–1549, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Murata A, Ishida H, Toda H, Tanaka N, Hayashida H, Monden M, Matsuura N. Interleukin 10 reduces mortality from severe peritonitis in mice. Antimicrob Agents Chemother 39: 1336–1340, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 70: 531–549, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci 13: 24–28, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science 188: 166–168, 1975. [PubMed] [Google Scholar]
- 25.Marks MJ, Rowell PP, Cao JZ, Grady SR, McCallum SE, Collins AC. Subsets of acetylcholine-stimulated 86Rb+ efflux and [125I]-epibatidine binding sites in C57BL/6 mouse brain are differentially affected by chronic nicotine treatment. Neuropharmacology 46: 1141–1157, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther 235: 619–628, 1985. [PubMed] [Google Scholar]
- 27.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 167: 6518–6524, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ogata M, Matsumoto T, Kamochi M, Yoshida SI, Mizuguchi Y, Shigematsu A. Protective effects of a leukotriene inhibitor and a leukotriene antagonist on endotoxin-induced mortality in carrageenan-pretreated mice. Infect Immun 60: 2432–2437, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human α3, α4, and α7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther 283: 675–683, 1997. [PubMed] [Google Scholar]
- 31.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35: 1139–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Robinson SE, Vann RE, Britton AF, O'Connell MM, James JR, Rosecrans JA. Cellular nicotinic receptor desensitization correlates with nicotine-induced acute behavioral tolerance in rats. Psychopharmacology (Berl) 192: 71–78, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Romanovsky AA Signaling the brain in the early sickness syndrome: are sensory nerves involved? Front Biosci 9: 494–504, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Romanovsky AA, Blatteis CM. Biphasic fever: what triggers the second temperature rise? Am J Physiol Regul Integr Comp Physiol 269: R280–R286, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Romanovsky AA, Blatteis CM. Heat stroke: opioid-mediated mechanisms. J Appl Physiol 81: 2565–2570, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. The two phases of biphasic fever—two different strategies for fighting infection? Ann NY Acad Sci 813: 485–490, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock-associated hypothermia. How and why does it occur? Ann NY Acad Sci 813: 733–737, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol 270: R693–R703, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol Regul Integr Comp Physiol 273: R407–R413, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol 285: R420–R428, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses 50: 219–226, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289: R1244–R1252, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Russell MA, Wilson C, Patel UA, Feyerabend C, Cole PV. Plasma nicotine levels after smoking cigarettes with high, medium, and low nicotine yields. Br Med J 2: 414–416, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sack R, Gochberg-Sarver A, Rozovsky U, Kedmi M, Rosner S, Orr-Urtreger A. Lower core body temperature and attenuated nicotine-induced hypothermic response in mice lacking the β4 neuronal nicotinic acetylcholine receptor subunit. Brain Res Bull 66: 30–36, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Sandborn WJ Nicotine therapy for ulcerative colitis: a review of rationale, mechanisms, pharmacology, and clinical results. Am J Gastroenterol 94: 1161–1171, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology (Berl) 168: 280–292, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Smail N, Messiah A, Edouard A, Descorps-Declere A, Duranteau J, Vigue B, Mimoz O, Samii K. Role of systemic inflammatory response syndrome and infection in the occurrence of early multiple organ dysfunction syndrome following severe trauma. Intensive Care Med 21: 813–816, 1995. [DOI] [PubMed] [Google Scholar]
- 48.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis 191: 2138–2148, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, Tracey KJ, Ulloa L. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10: 1216–1221, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Liu Q, Yu K, Hu J, Kuo YP, Segerberg M, St John PA, Lukas RJ. Roles of nicotinic acetylcholine receptor β subunits in function of human α4-containing nicotinic receptors. J Physiol 576: 103–118, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]