Abstract
The behavior of the entire medial gastrocnemius (MG) superficial and deep aponeurosis structure was investigated with velocity-encoded phase-contrast, spin-tag, and three-dimensional morphometric magnetic resonance imaging. The displacements and strain of both these aponeuroses, muscle length, and the cross-sectional segment length of the deep aponeurosis were measured during isometric plantarflexion at 20% and 40% of maximal voluntary contraction (MVC). The length of the entire MG shortened during 20% and 40% MVC. All regions of interest in both aponeuroses moved proximally. Positive strain (lengthening) occurred in both ends of the deep aponeurosis and in the proximal region of the superficial aponeurosis. In contrast, negative strain (shortening) was observed in the middle region of the deep aponeurosis and in the distal region of the superficial aponeurosis. Consistent with this shortening of the deep aponeurosis length along the proximal-distal axis was expansion of the aponeuroses in the medial-lateral and anterior-posterior directions in the cross-sectional plane. It is concluded that at low to moderate force levels of isometric contraction, regional differences in strain occur along the proximal-distal axis of both aponeuroses, and some regions of both aponeuroses shorten.
Keywords: strain, skeletal muscle, morphology, velocity-encoded phase-contrast magnetic resonance imaging, aponeurosis
during human movement, force produced by muscle fibers is transmitted through tendinous tissue, that is, tendons and aponeuroses, to bones since skeletal muscle is attached to the bones via tendinous tissues at both proximal and distal ends of the muscle. These tendinous tissues play an important role as series elastic components and can store elastic energy during the movement (1, 4). Ultrasonographic techniques have suggested that during an isometric contraction the superficial and deep aponeuroses of medial gastrocnemius (MG) are homogeneously stretched along their lengths in opposite directions; the superficial aponeurosis is stretched distally, whereas the deep aponeurosis is stretched proximally (13, 14). A rather different conclusion was drawn in other references in the literature (16, 24, 25), which pointed out that the strain properties of aponeurosis may be more complex than the ultrasound data suggest. Direct measurements of aponeurosis strain in contracting rat gastrocnemius muscle indicate inhomogeneous strain (25), and several models of contracting unipennate muscle also indicate inhomogeneous strain in the aponeuroses (16, 24).
Phase-contrast (PC) magnetic resonance imaging (MRI) has been used to investigate the mechanical behavior of the muscle and tendinous tissue during muscle contraction (5–7, 20). The PC MRI technique has the advantage of superior soft tissue contrast and provides a large field of view (FOV), enabling measurements over a large length of multiple inner tissue structures with a high image resolution during muscle contraction, compared with the technique of ultrasonography. To test the hypothesis that both of the MG aponeuroses would exhibit inhomogeneous strain along the proximal-distal axis, we have therefore revisited the question of the mechanical behavior along the MG aponeuroses with PC MRI techniques. In view of the potential differences in aponeurosis behavior reported in the literature, we also verified our observations with the alternative MRI method of spin tagging and three-dimensional (3D) morphological imaging. Spin tagging is considered to be the gold standard for tracking the magnitude and direction of movement of tissue with in vivo MRI (23). With this technique, protons (or tissue points) in predetermined locations can be followed with either straight lines or grids (as in this case) in the targeted tissue during the contraction cycle, ensuring a high level of confidence that the observed movements represent the actual movements of the tissues. The present study was therefore directed toward a better understanding of the relationships between the nature and extent of the deformations of a muscle and mechanical properties of the aponeurosis and subsequently the dynamics of passive and active tissues within a unipennate muscle-tendon complex.
MATERIALS AND METHODS
Subjects.
Eight men [age 29.6 ± 3.8 yr, height 171.3 ± 6.3 cm, weight 70.4 ± 13.9 kg (means ± SD)] were recruited to participate in this study after the requisite Institutional Review Board (IRB) approval was obtained. All the subjects were in good health, with no orthopedic or neuromuscular abnormalities. IRB-approved written informed consent was obtained from all subjects. The studies were conducted according to the Declaration of Helsinki.
Experimental protocol.
Subjects were positioned in the foot-first supine position on the bed of a 1.5-T Signa HDx (GE Medical Systems, Milwaukee, WI) MR scanner (shown in Fig. 1 of Ref. 10). The right lower leg was fastened to a custom-built MR-compatible foot-pedal device with a Velcro tape at an ankle angle of 90° and a knee angle of 180°. Before the experiment we ensured that the axis of rotation of the ankle joint was parallel to the axis of the exercise apparatus and that both axes were aligned as closely as possible. The upper portion of the lower leg was encased in a receive-only eight-channel cardiac coil, so that the entire MG was covered. While several coils, such as the transmit-receive head coil and the eight-channel knee coil, were investigated during the initial stages, this cardiac coil was found to be optimal in terms of its signal-to-noise ratio (SNR), the FOV covered, and the ability to accommodate the knee. The device was specially designed to accommodate isometric plantarflexion during the MR scanning (18). During the isometric plantarflexion, the force was measured with an optical strain gauge (Fiberscan 2000, Luna Innovations, Blacksburg, VA) on the footplate. The force signal was digitally sampled at the rate of 250 Hz (DAQ-6024E, National Instruments, Austin, TX) and stored for later analysis.
Fig. 1.
Aponeuroses displacement calculated from phase-contrast (PC) images superimposed on corresponding magnitude images. A: representative axial magnetic resonance (MR) image at rest, showing the graphical prescription of a sagittal-oblique slice, aligned with the longitudinal axis of the medial gastrocnemius (MG), along which subsequent PC MR images were acquired. B: regions of interests (ROIs) are prescribed graphically along the superficial aponeurosis at rest (left). Their displaced positions during voluntary isometric plantarflexion [40% maximum voluntary contraction (MVC)] are shown on right in the superficial aponeurosis. Dashed lines are added for visualization of the displacement of the ROIs during isometric contraction. Fascicle length was used as a reference distance (12). C: ROIs are prescribed graphically along the deep aponeurosis. All conventions are the same as in B. D: typical example of the displacement distribution along the superficial and deep aponeuroses during 20% (top) and 40% (bottom) MVC from 1 subject. ROI 1 corresponded to the most proximal region, and ROI 12 corresponded to the most distal region of the MG.
After the subjects were familiarized with the experimental apparatus and procedure by performing plantarflexion exercises at submaximal force, they performed maximal voluntary isometric contraction (MVC) of plantarflexion for 3–5 s. This was repeated four times, and the highest value for MVC was determined. In addition, the measurements of MVC were repeated after the completion of the MRI measurements to confirm the absence of fatigue.
During the PC and morphological MRI measurements, the isometric plantarflexion was performed at two different force levels of 20% and 40% MVC. From our prior experience in using this technique, 40% MVC is about the maximum resistance at which the subject can sustain the ∼64 repeated contractions that are required to acquire a PC image without significant fatigue. The target forces were projected and displayed on the scanner face in real time, allowing the subject to achieve the target forces accurately and consistently. The two force levels were performed in a randomized order with a 5-min rest interval. The PC MR images were acquired first, followed by the morphological MRI.
For PC MRI measurements, the subject performed ∼64 isometric contractions at a rate of 35 cycles/min with the guidance of a computer-generated audio metronome cue fed to the subject via headphones. All subjects were retested on the same day to ensure reproducibility of the PC MRI measurements. For morphological MRI measurements, the subject performed sustained submaximal isometric contractions at 20% and 40% MVC throughout the entire duration of the scan. In a separate set of experiments, spin tag and PC measurements were carried out on one subject for the validation study. The spin tag method of imaging was first optimized on the scanner, with respect to coils, number of views per segment, averages, repetition time (TR), and contraction cycles per minute. The spin-tag acquisition was followed by the PC acquisition with a 10-min rest interval. The contraction rate was increased to 50 cycles/min in order to increase the portion of the contraction cycle available for analysis since the spin-tag label decayed to undetectable levels within ∼0.8 s.
Image acquisition.
Oblique sagittal PC MR images were acquired with a two-dimensional gradient echo PC sequence [15.9-ms TR, 6.3-ms echo time (TE), 20° flip angle, 300 × 150-mm FOV, 128 × 256 image matrix, 5-mm slice thickness, bandwidth 488 Hz/pixel, 2 views/segment, 1 average, 1 slice, and ∼1:53 scan time]. The slice location was prescribed from the axial images and chosen to visualize the entire length of the MG (Fig. 1). Encoding velocity was set at 10 cm/s in the proximal-distal direction. A total of 22 phases were acquired in each contraction cycle. The temporal resolution of each phase was ∼78 ms. A comparison between the 22 images of the PC sequence showed no systematic change in image distortion, indicating that the measurement errors remained constant over the duration of the image acquisition. Various parameters of the scanning protocol, such as slice thickness, FOV, time resolution and number of phases per contraction cycle, and number of averages were determined after an extensive optimization process in the initial stages of the experiment. Factors such as SNR and time resolution required to draw meaningful physiological conclusions as well as a practical total scan time were taken into consideration. Detailed descriptions are provided in Ref. 20.
Axial morphological images were acquired during a sustained isometric contraction with a fast gradient echo sequence to minimize fatigue (150-ms TR, 3.7-ms TE, 45° flip angle, 180 × 135-mm FOV, 256 × 160 image matrix, 5-mm slice thickness, 0-mm slice interval, bandwidth 244 Hz/pixel, 2 averages, 52–59 slices, and 1:38 scan time). Sagittal images were used to prescribe the beginning and end locations of the stack of axial morphological images, so that they covered the entire length of the MG.
For the spin-tag acquisition, a standard, vendor-supplied, gradient-recalled acquisition in the steady state (GRASS)-based sequence was used with grid tagging (8.6-ms TR, 4.1-ms TE, 20° flip angle, 300 × 300-mm FOV, 160 × 256 image matrix, 5-mm slice thickness, 7-mm tag spacing, bandwidth 244 Hz/pixel, 8 views/segment, 3 averages, 1 slice, 22 phases, and ∼0:49 scan time). The tag lines faded after ∼550 ms from longitudinal relaxation time (T1). For this reason, the contraction cycle length was adjusted to ∼1.2 s−1 (corresponding to 50 beats/min) so that the tag lines were visible over a greater fraction of the contraction cycle. An oblique sagittal slice was prescribed similar to the above scans in one subject, and a spin-tag scan was acquired with an eight-channel cardiac coil. This acquisition could be completed in 20 contraction cycles at 40% MVC isometric plantarflexion. This was followed with a comparable PC scan using the same slice prescription and contraction cycle duration as the spin-tag acquisition. MVCs before and after the tests indicated no fatigue during data collection.
Image analysis.
The algorithms used for image analysis were developed indigenously in a MATLAB software platform (The Mathworks, Natick, MA). Displacements of both superficial and deep aponeuroses were determined by tracking the position of regions of interest (ROIs) over all the phases of the contraction cycle, as described in Refs. 6, 7, and 20. The images were smoothed with a 3-by-3 lower-pass filter, after which the systematic phase errors (from phase shading) were quantified and subtracted from each image. The 1 × 1 pixel ROIs were positioned at every 12 points from the most proximal end to the most distal end of each aponeurosis (Fig. 1). ROI 3 from the top of each aponeurosis was defined as an anatomic landmark, which corresponded to the same level as the most distal end of the femur. All ROIs were prescribed graphically with the first magnitude image of the cine sequence. The mean velocity of the ROI was obtained from the first PC image. The positions in the subsequent PC images were then estimated by the product of the velocity in the first phase image and the time difference between the consecutive images (∼78 ms), thereby tracking the position of the ROI throughout the contraction cycle. This procedure allowed us to calculate the displacement (velocity × time) of the aponeurosis. Care was taken to ensure that the projected ROI in the subsequent images did not fall into the aponeurosis region. For each ROI, the resting position value was subtracted from the calculated trajectory positions to calculate the displacement. To compensate for bulk leg movement during the isometric contraction, the displacement of the most proximal end of the femur (×, Fig. 1) was determined at all 22 phases with ImageJ (version 1.37, National Institutes of Health, Bethesda, MD) and was subtracted from the displacement. Strain [(l − lo)/lo] was calculated by using the distance between two adjacent ROIs (l) at a given temporal phase and the resting length (lo). The resting length was determined just before the initiation of contraction.
For the purpose of estimating the reproducibility of muscle displacement measurements between scan sessions in these PC experiments, a large rectangular ROI located in the middle portion of the MG for each subject was used. The size of the ROI, ∼71.3 mm × 12.1 mm, was varied to best fit each subject's anatomy. The larger side of the ROI was aligned with the superior-inferior direction of the leg. The pixels within the ROI were averaged to generate a mean velocity, which was used to calculate bulk displacement of the ROI. This method was used to minimize discrepancy in pixel locations that would have occurred if one pixel was used as the ROI.
The length of the entire MG was measured from the oblique-sagittal magnitude image, and the length of the cross-sectional segment of the deep aponeurosis was measured from the axial morphological image with ImageJ at rest, 20% MVC, and 40% MVC. The SNR, which was calculated as the ratio of the mean signal intensity within the center of muscle to the SD of background signal intensity, and overall image quality was sufficient (SNR: 42.2 ± 10.3 for 20% MVC, 41.3 ± 6.7 for 40% MVC) for this analysis. The muscle length was measured as the distance between the most proximal and distal ends of MG in the oblique-sagittal image in which MG was identifiable. The cross-sectional segment length of deep aponeurosis was measured as the distance from the start point to the end point of the deep aponeurosis in the axial image in which the deep aponeurosis was identifiable. The cross-sectional segment length was calculated from the axial morphological images at 30%, 50%, and 90% proximal-distal length, where 0% represented the most proximal end and 100% the most distal end of MG. The rationale for the choice of these points was that 30% was the anatomic limit for calculating aponeurosis length at the most proximal region, 50% represented the center region of whole MG, and 90% was the anatomic limit for calculating aponeurosis length at the most distal region.
3D reconstruction.
The axial morphological images were used for 3D reconstruction and visualization of the imaged leg with Amira 4.1.1 software (Visage Imaging, Carlsbad, CA). The cross-sectional contours of the MG and deep aponeurosis, where identifiable, were traced in each slice, and standard volume-rendering options were used to display its 3D structure. The accuracy of segmentation depends to some extent on the slice thickness producing the images and the partial volume effects involved. Given a slice thickness, the errors from partial volume have tended to increase if the structure being segmented is very convoluted or irregular within that thickness, such as the white or gray matter in the brain (22). However, the leg is a comparatively regular structure along the superior-inferior direction, and the error from partial volume from a thicker slice was not likely to be as significant as in the brain.
Comparison between PC and spin tag.
Three points at the intersections of the grid lines in the spin-tag image series along the superficial and deep aponeuroses were chosen for comparison to determine the validity of the displacement measurements made from the corresponding PC images. Location of these points at the intersection of the grid lines in the spin-tag images allowed tracking of the tissue points from one phase to another. Subsequent measurements of displacement throughout the contraction cycle were made manually for the spin-tag images with ImageJ. Only the superior-inferior components of displacement were measured for both methods. Identical phases within the contraction cycle of both methods were chosen for analysis and comparison.
Statistics.
Values are presented as means and SD. Differences in the displacement and strain between two aponeuroses (superficial vs. deep aponeurosis) and two force levels (20% vs. 40%) among ROIs were examined with one-way analysis of variance. Differences in the muscle length and segmented length of deep aponeurosis among three force levels (rest vs. 20% vs. 40%) were also tested by one-way analysis of variance. Post hoc comparison (Fisher) was performed when significance was found. Reproducibility of displacement measurement was assessed by intraclass correlation coefficient (ICC) and Student's paired t-test. ICC has been commonly used in reproducibility tests (2, 19). This correlation test is based on variance components analysis and measures the homogeneity within groups relative to the total variation. The comparison between the displacement determined by PC and spin-tag imaging was assessed by linear regression analyses, and a Pearson product-moment correlation (r) was calculated. The level of statistical significance was set at P < 0.05.
RESULTS
Force.
The peak force over 64 contractions across subjects was an average of 77.8 ± 20.5 N and SD of 6.3 ± 1.8 N for 20% MVC and an average of 145.3 ± 51.5 N and SD of 11.6 ± 3.3 N for 40% MVC. The duration of the contraction cycles over 64 contractions across subjects was an average of 1.74 ± 0.06 s and SD of 0.11 ± 0.05 s for 20% MVC and an average of 1.72 ± 0.02 s and SD of 0.14 ± 0.10 s for 40% MVC. Mean peak force levels were 26 ± 8% and 46 ± 8% of the target force levels, and cycle time variation had a SD of <10% of the target cycle time. MVC was not significantly different between before (296.7 ± 116.8 N) and after (326.1 ± 152.5 N) the PC measurements.
ICC.
The ICC value of the displacement was 0.97 for 20% MVC and 0.91 for 40% MVC. There were no significant differences in the displacement between mean values of the two measurements. Therefore, reproducibility of displacement measurement in each subject was found to be excellent.
PC vs. spin tagging.
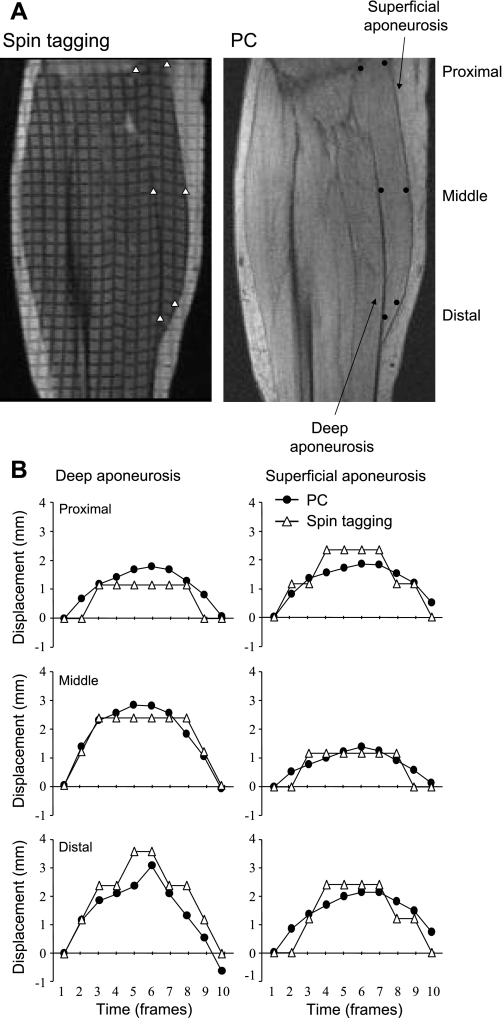
Figure 2B shows a comparison between the displacement measured from the spin-tag experiments with that from the PC experiment, at three different points along the superficial and deep aponeuroses, during the first 10 phases. Phase 6 corresponded to the peak torque. The displacement from these two methods showed excellent agreement for the superficial (r = 0.89, P < 0.05) and deep (r = 0.89, P < 0.05) aponeuroses within the limits of the pixel resolution.
Fig. 2.
Validation of displacement as determined from PC MRI by comparison with spin-tag experiment. A: representative oblique-sagittal spin-tagging and PC images during phase 6, which correspond to the peak torque. ROIs are prescribed graphically along both the superficial and deep aponeuroses. B: displacement at 3 different points along the superficial and deep aponeuroses during the first 10 phases is compared between the PC and spin-tagged data. The single-pixel resolution of the spin-tag data results in steps in the displacement curves, whereas the subpixel resolution of the PC imaging provides smoother curves. The displacement measurements show good correlation (r = 0.89 for superficial aponeurosis and r = 0.89 for deep aponeurosis).
Displacement.
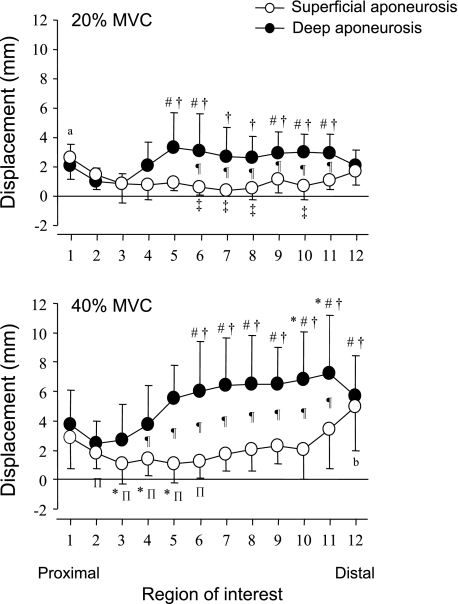
Figure 3 shows the displacement of the superficial and deep aponeuroses along their lengths during 20% and 40% MVC of isometric plantarflexions. All displacement values were corrected by subtraction of the femur displacement (28 ± 15 mm for 20% and 41 ± 14 mm for 40% MVC). Locations above the zero line indicate movement of the ROI in the proximal direction, and points below the line indicate a distal movement. Thus all ROIs in the superficial and deep aponeuroses moved proximally. For 20% MVC, in the superficial aponeurosis, the displacement of ROI 1 was significantly larger than that of ROIs 2-11. The displacement of ROI 12 was significantly larger than ROIs 6-8 and 10. In the deep aponeurosis, the displacements of ROIs 5, 6, and 9-11 were significantly larger than those of ROIs 2 and 3. There were significant differences in the displacement from ROIs 6-11 between the superficial and deep aponeuroses. For 40% MVC, in the superficial aponeurosis, the displacement of ROI 1 was significantly larger than those of ROIs 3-5. The displacement of ROI 12 was significantly larger than those of ROIs 1-10. In the deep aponeurosis, the displacements of ROIs 6-12 were significantly larger than that of ROI 1. There were significant differences in the displacements of ROIs 4-11 between the superficial and deep aponeuroses. ROI 2 of the deep aponeurosis, which attaches to the femur, had the almost lowest displacement among the ROIs. This point was almost static during muscle contraction. Together, the superficial aponeurosis (open circles in Fig. 3) in the proximal and distal regions moved the most, while the aponeurosis in the middle region remained almost static or moved only slightly. The deep aponeurosis (filled circles in Fig. 3) in the middle and distal regions moved more than in the proximal region.
Fig. 3.
Displacement distribution along the superficial and deep aponeuroses. Average displacement distribution along the superficial and deep aponeuroses during 20% (top) and 40% (bottom) MVC from 8 subjects is shown. All conventions are the same as in Fig 2D. Values are means ± SD. *P < 0.05 vs. ROI 1; #P < 0.05 vs. ROI 2; †P < 0.05 vs. ROI 3; ΠP < 0.05 vs. ROI 11; ‡P < 0.05 vs. ROI 12; aP < 0.05 vs. from ROI 2 to ROI 8; bP < 0.05 vs. from ROI 1 to ROI 10; ¶P < 0.05, 20% vs. 40% MVC.
Strain.
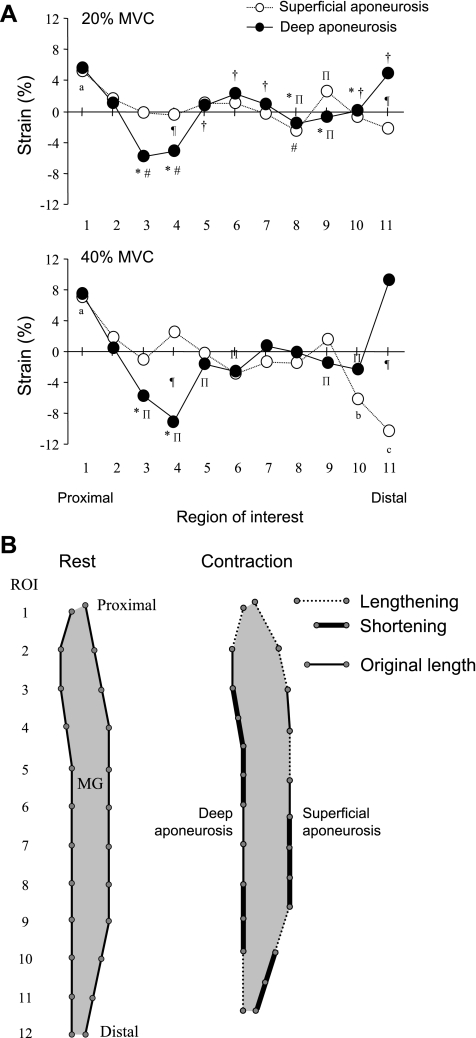
With regard to Fig. 4, if the ROIs did not move relative to the neighboring ROI, the strain value would remain at 0%. Values above 0% indicate a lengthening and points below a shortening. Thus, in general, the strain distributions of both of the aponeuroses were not uniform along the length of the MG for both force levels. The superficial aponeurosis lengthened in the most proximal region and shortened in the most distal region. The deep aponeurosis lengthened in the most proximal and the distal region but shortened in some regions, which were just below the attachment point of the deep aponeurosis and femur. In both aponeuroses, the strain varied in the middle regions along the length of the MG. There were significant differences in the strains of ROIs 4 and 11 between the superficial and deep aponeuroses.
Fig. 4.
Strain distribution along the superficial and deep aponeuroses. A: average strain distribution along the superficial and deep aponeuroses during 20% (top) and 40% (bottom) MVC from 8 subjects. Positive and negative strain indicate lengthening and shortening, respectively. Values are means. *P < 0.05 vs. ROI 1; #P < 0.05 vs. ROI 2; †P < 0.05 vs. ROI 3; ΠP < 0.05 vs. ROI 11; aP < 0.05 vs. from ROI 3 to ROIs 8, 10, and 11; bP < 0.05 vs. ROIs 2, 4, and 10; cP < 0.05 vs. ROIs 2-9; ¶P < 0.05, 20% vs. 40% MVC. B: model of strain distribution along the proximal-distal axis of both aponeuroses. This model resulted from the strain during 40% MVC. Dashed lines indicate location of aponeurosis lengthening, and thick solid lines indicate location of aponeurosis shortening. Thin solid lines are the original length of the aponeurosis, i.e., where strain is undetectable.
Muscle length and segment length of deep aponeurosis.
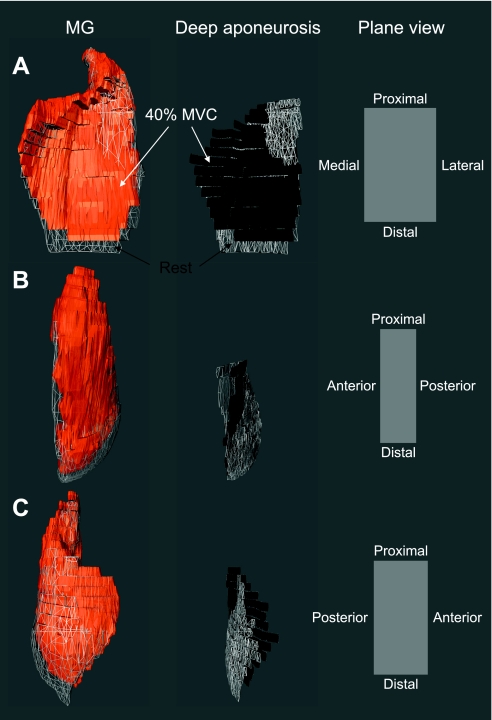
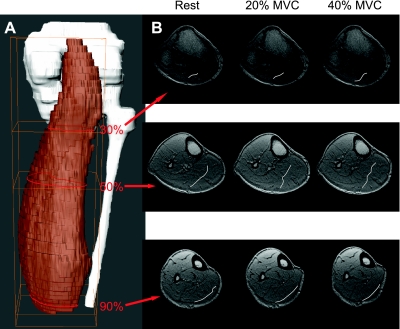
The entire length of MG was significantly shorter for 20% (244.0 ± 25.5 mm) and 40% (242.2 ± 25.6 mm) MVC than at rest (248.8 ± 26.1 mm), although the differences were small (Table 1). This is shown in Fig. 5 and Supplemental Video Clips S1–S3.1 During a sustained isometric contraction the volume in the distal region of the muscle was smaller than at rest, while the proximal region was increased (Figs. 5 and 6). The entire length of the deep aponeurosis also shortened proximally during 40% MVC. The deep aponeurosis appears as a sinuous curve in axial sections, as seen in Figs. 5 and 6. Figure 6 shows a 3D reconstructed image of the entire MG and axial morphological MR images at 30%, 50%, and 90% locations along the proximal-distal direction under rest, 20% MVC, and 40% MVC from one subject. At the 50% location, the deep aponeurosis exhibited greater sinuosity in the cross section as force levels increased, which resulted in a significantly greater segment length for 40% MVC (Table 1). In contrast, the cross-sectional segment length of the deep aponeurosis at the 90% location decreased significantly with increasing force levels.
Table 1.
Muscle length and segment length of deep aponeurosis at rest, 20% MVC, and 40% MVC
| Rest | 20% MVC | 40% MVC | |
|---|---|---|---|
| Muscle length | 249.3±24.2 | 244.9±23.7* | 242.4±25.6* |
| Segment length of deep aponeurosis | |||
| 30% Location | 16.6±13.9 | 18.8±17.1 | 23.1±19.2 |
| 50% Location | 39.0±3.7 | 47.0±4.3 | 54.6±5.5* |
| 90% Location | 46.7±5.0 | 41.9±8.0 | 38.7±8.5* |
Values (in mm) are means ± SD. MVC; maximum voluntary contraction.
P < 0.05 vs. rest.
Fig. 5.
Three-dimensional (3D) reconstructed images showing changes in the MG and deep aponeurosis during force production. 3D reconstructed MG (left) and deep aponeurosis (center) from 1 subject in which the aponeurosis was identifiable at rest and at 40% MVC are shown. The added 3D reconstruction of the deep aponeurosis enables one to identify the locations from one-third to three-thirds of the distance from the proximal end to the distal end of the MG. Plane views on right indicate the anatomic orientation of the 3D MG and deep aponeurosis from 3 different frontal perspectives (A–C). Orange and black colors indicate the MG and deep aponeurosis during 40% MVC, respectively. Gray mesh represents the MG and deep aponeurosis in the rest condition. See Supplemental Video Clips S1–S3.
Fig. 6.
Changes in cross-sectional segment shape and length of the deep aponeurosis. A: 3D reconstructed images of the MG, tibia, and fibula from a stack of axial MR images in 1 representative subject. Red lines correspond to positions at 30%, 50%, and 90% locations along the proximal-distal axis. B: axial morphological images at 30%, 50%, and 90% locations as indicated in the 3D image at rest, 20% MVC, and 40% MVC from 1 subject. The deep aponeurosis is indicated by white lines in these axial images and reveals changes in shape and cross-sectional segmental length after force production.
DISCUSSION
The present results showed that during a low- to moderate-level isometric contraction of the plantar flexors of the ankle 1) both the superficial and deep aponeuroses of the MG moved in the proximal direction, 2) the deep aponeurosis shortened in the middle regions and lengthened in the proximal and distal regions, and 3) the superficial aponeurosis lengthened in the proximal region and shortened in the distal region. These results revealed that the behavior of the two aponeuroses varied and that there were regional differences in strain along the proximal-distal axis of both aponeuroses. Given that some of the present observations were counter to existing experimental evidence and intuition, we carefully reexamined our experimental and mathematical approaches. We collected and analyzed data with two methodologically different approaches (PC and spin tag) and found close agreement between the two techniques. Some of the issues that may be pertinent in the apparent differences in observations are discussed below.
The ROIs in the present study surrounded the entire MG muscle and allowed us to sample the behavior in all regions of the muscle. These included the ends of the muscle between the aponeuroses where the epimysium covered muscle fibers, which presumably shorten during a contraction. On the basis of reported muscle fiber lengths (12) and presumed anatomy of the MG, we would expect ROIs 10-12 of the superficial aponeurosis as we have defined them to lie over the muscle fibers at the distal end of the muscle. The negative slope connecting ROIs 10-12 is consistent with shortening of fibers in this region. If the remaining (force transmitting) regions of the aponeurosis (beginning at ROI 9) stretched, the remainder of the data points would lie along a line with a positive slope since stretch would reduce the proximal distance traveled by progressively more distal points. The points on deep and superficial surfaces at the ends of the muscle should roughly coincide because these points lie close together. Contrary to the reports of several studies that used ultrasound to evaluate aponeurosis strain (13, 14), we found that the strain in both aponeuroses was slight or variable in 20% MVC and predominantly negative in 40% MVC. Unlike the distal region of the soleus muscle, where the aponeurosis also shortens (6, 7), there is no clear internal structure to explain this unexpected finding. Close inspection of the strain patterns computed in finite-element models of muscle indicate theoretical considerations that suggest that negative strains may occur in some regions of the muscle and aponeurosis (16, 24). Furthermore, some reports of in situ contractions of rat muscles indicate shortened aponeuroses in active relative to passive loading (25). Therefore, it is possible that aponeurosis shortening may be a normal property of skeletal muscle under some contractile conditions. The two-dimensional structure of the aponeurosis, combined with the 3D muscle, may be one possible reason for the observed strains. Shortening muscle fibers must expand in at least one plane orthogonal to the long axis of the fiber in order to maintain a constant volume. The axial in-plane length of the superficial aponeurosis may increase during a contraction, corresponding to a significant positive (lengthening) strain in the axial plane. This was evident in our measures of aponeurosis segment length in axial sections. The simple mechanical consequence of this positive strain, orthogonal to the strain vector normally measured in contracting muscle, may be a negative strain along some regions of the proximal-distal axis of the aponeurosis. Figures 5 and 6 illustrate that the contracted muscle is shorter with greater thicknesses in the proximal and middle regions. Presumably, the deep aponeurosis expanded in the medial-lateral axis. The distal region of the contracted muscle was thinner and the axial segment length of the aponeurosis decreased, accompanied by aponeurosis stretch along the proximal-distal axis.
The strain distribution observed in our experiments may be linked to the distribution and orientation of the forces generated by the muscle fibers (3). In terms of the MG, longer fibers are found in the distal region, while shorter fibers are found in the proximal region (12). Such inhomogeneity of fiber length can alter the length-tension characteristic of the MG (8). In addition, the orientation of the muscle fibers with respect to the aponeurosis is different along the length of the aponeurosis. It has been shown that the pennation angle of MG is smaller in the distal region than in the proximal region (11, 15, 21). Therefore, fiber architecture may cause nonuniformity of fiber shortening, with corresponding changes in aponeurosis strain.
The different compliance of the deep aponeurosis may also be a contributor to its nonuniform shortening. Higher compliance of the distal region compared with that of the proximal region of the aponeurosis was found in mammalian (25) and human (9) MG studies. The high compliance of the distal region may be related to the fact that it is a junctional area, where two different types of material are connected (17). Thus the mechanical properties of the MG are not entirely the same throughout the muscle. An additional mechanical issue would be increasing the stress transmitted by the more distal regions of the aponeurosis, which would result in higher strains. A greater aponeurosis cross-sectional area in the more distal regions would be required to compensate for this.
There is a clear discrepancy between our data from MRI studies presented here and others' reports in the literature. Fukunaga and colleagues (13, 14) have reported that both the superficial and deep aponeuroses are stretched uniformly along their lengths in opposite directions: the superficial aponeurosis is stretched distally, whereas the deep aponeurosis is stretched proximally. The differences in the aponeuroses' behavior between our present study and their studies are unsettling. In reference to Fig. 2B, as indicated in our previous papers (e.g., Ref. 20), PC MRI measures pixel velocity and therefore allows subpixel resolution in estimates of tissue movements. Spin tagging relies on the actual displacement of tagged pixels in an image, meaning that resolution is largely limited to the size of the pixel. In the case of our images, this was 1.17 mm (for both techniques), which corresponds to the steps in Fig. 2. Within these limitations of image resolution and inherent measurement uncertainty, the spin-tagged data correspond very closely to our findings from PC MRI data (as noted in results, r = 0.89, P < 0.05 for both superficial and deep aponeuroses). This suggests that the difference between our observations and those from ultrasound are not due to errors in the PC MRI method or in our computations. Such conflicting observations will have to be resolved before a proper understanding of muscle function can be realized. It is possible that the contractile conditions themselves may contribute to the observed differences. Previous studies based on ultrasound used 100% MVC, whereas the present study used 20% and 40% MVC. Our results suggested that under some contractile conditions, even those involving significant force levels, the distribution of strain along muscle aponeuroses was not homogeneous and even involved shortening of the aponeurosis in some regions. Another criticism of our observations may be that approximately two-thirds of the entire length of the MG was in contact with the leg device, which might have restricted its shape. It could be argued that displacements in these regions corresponding to the middle region of the aponeurosis were disrupted by the presence of the apparatus. However, such criticism does not invalidate the observation that a stressed aponeurosis may shorten rather than elongate under certain conditions.
It should also be noted that in the present study the most proximal extent of superficial aponeurosis might not be the most proximal extremity of the muscle. The most proximal extent of the observed superficial aponeurosis was displaced ∼2 mm for 20% and 3 mm for 40% MVCs during isometric contractions, in contrast to our expectations that the most proximal end of the superficial aponeurosis would be fixed during muscle contraction. Empirical definition of this point from the oblique-sagittal slices prescribed for imaging in this study might not be the most optimal approach to defining the most proximal end of superficial aponeurosis. Nevertheless, this should not alter the basic conclusions presented here. Missing the extreme origin of the muscle is equivalent to ignoring the most proximal ROI in our graphs.
In conclusion, the present study indicates that at low to moderate contractile force levels, even in an unipennate muscle, strain distributions along the proximal-distal axis are nonuniform in both deep and superficial MG aponeuroses. A novel finding is that some regions of both aponeuroses shortened along the proximal-distal axis under loading. This behavior was correlated with the cross-sectional changes in shape of the deep aponeuroses as revealed by 3D morphological imaging. The present data suggest the importance of a comprehensive assessment of multiple and interactive factors that contribute to the dynamics of shortening and lengthening of passive and active tissues within a muscle-tendon complex, in order to understand the net result of the forces and displacements that occur in vivo at the tendon-bone interface.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1-AR-53343.
Supplementary Material
Acknowledgments
The authors thank all the participating subjects in the study. The authors especially acknowledge Dr. T. Finni (University of Jyväskylä, Jyväskylä, Finland) for comments in preparing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Alexander RM Elastic energy stores in running vertebrates. Am Zool 24: 85–94, 1984. [Google Scholar]
- 2.Bartko JJ The intraclass correlation coefficient as a measure of reliability. Psychol Rep 19: 3–11, 1966. [DOI] [PubMed] [Google Scholar]
- 3.Blemker SS, Pinsky PM, Delp SL. A 3D model of muscle reveals the causes of nonuniform strains in the biceps brachii. J Biomech 38: 657–665, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cavagna GA Storage and utilization of elastic energy in skeletal muscle. Exerc Sport Sci Rev 5: 89–129, 1977. [PubMed] [Google Scholar]
- 5.Drace JE, Pelc NJ. Elastic deformation in tendons and myotendinous tissue: measurement by phase contrast MR imaging. Radiology 191: 835–839, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol 95: 829–837, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson JA, Finni T, Lai AM, Edgerton VR, Sinha S. Influence of structure on the tissue dynamics of the human soleus muscle observed in MRI studies during isometric contractions. J Morphol 267: 584–601, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol 85: 398–404, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Kubo K, Kawakami Y, Kanehisa H, Fukunaga T. Measurement of viscoelastic properties of tendon structures in vivo. Scand J Med Sci Sports 12: 3–8, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Lee HD, Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Soleus aponeurosis strain distribution following chronic unloading in humans: an in vivo MR phase-contrast study. J Appl Physiol 100: 2004–2011, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Lichtwark GA, Bougoulias K, Wilson AM. Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech 40: 157–164, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Maganaris CN, Baltzopoulos V, Sargeant AJ. In vivo measurements of the triceps surae complex architecture in man: implications for muscle function. J Physiol 512: 603–614, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muramatsu T, Muraoka T, Kawakami Y, Fukunaga T. Superficial aponeurosis of human gastrocnemius is elongated during contraction: implications for modeling muscle-tendon unit. J Biomech 35: 217–223, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Muramatsu T, Muraoka T, Takeshita D, Kawakami Y, Hirano Y, Fukunaga T. Mechanical properties of tendon and aponeurosis of human gastrocnemius muscle in vivo. J Appl Physiol 90: 1671–1678, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol 496: 287–297, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oomens CW, Maenhout M, van Oijen CH, Drost MR, Baaijens FP. Finite element modelling of contracting skeletal muscle. Philos Trans R Soc Lond B Biol Sci 358: 1453–1460, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proske U, Morgan DL. Tendon stiffness: methods of measurement and significance for the control of movement. J Biomech 20: 75–82, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Shin D, Kinugasa R, Mishra C, Hodgson JA, Edgerton VR, Sinha S. Mapping of movements in the dynamically contracting human triceps surae muscles using a computer controlled hydraulic foot-pedal device with velocity encoded phase contrast MRI (Abstract). Proceedings of the International Society for Magnetic Resonance in Medicine 16th Scientific Meeting and Exhibition, Toronto, 2008; pg 3671.
- 19.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86: 420–428, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Hodgson JA, Finni T, Lai AM, Grinstead J, Edgerton VR. Muscle kinematics during isometric contraction: development of phase contrast and spin tag techniques to study healthy and atrophied muscles. J Magn Reson Imaging 20: 1008–1019, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle. J Magn Reson Imaging 24: 182–190, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Sisodiya S, Free S, Fish D, Shorvon S. MRI-based surface area estimates in the normal adult human brain: evidence for structural organisation. J Anat 188: 425–438, 1996. [PMC free article] [PubMed] [Google Scholar]
- 23.Young AA, Axel L, Dougherty L, Bogen DK, Parenteau CS. Validation of tagging with MR imaging to estimate material deformation. Radiology 188: 101–108, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Yucesoy CA, Koopman BH, Huijing PA, Grootenboer HJ. Three-dimensional finite element modeling of skeletal muscle using a two-domain approach: linked fiber-matrix mesh model. J Biomech 35: 1253–1262, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Zuurbier CJ, Everard AJ, van der Wees P, Huijing PA. Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J Biomech 27: 445–453, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.