Abstract
We investigate how the biarticular long head and monoarticular lateral head of the triceps brachii function in goats (Capra hircus) during jumping and landing. Elbow moment and work were measured from high-speed video and ground reaction force (GRF) recordings. Muscle activation and strain were measured via electromyography and sonomicrometry, and muscle stress was estimated from elbow moment and by partitioning stress based on its relative strain rate. Elbow joint and muscle function were compared among three types of limb usage: jump take-off (lead limb), the step prior to jump take-off (lag limb), and landing. We predicted that the strain and work patterns in the monoarticular lateral head would follow the kinematics and work of the elbow more closely than would those of the biarticular long head. In general this prediction was supported. For instance, the lateral head stretched (5 ± 2%; mean ± SE) in the lead and lag limbs to absorb work during elbow flexion and joint work absorption, while the long head shortened (−7 ± 1%) to produce work. During elbow extension, both muscles shortened by similar amounts (−10 ± 2% long; −13 ± 4% lateral) in the lead limb to produce work. Both triceps heads functioned similarly in landing, stretching (13 ± 3% in the long head and 19 ± 5% in the lateral) to absorb energy. In general, the long head functioned to produce power at the shoulder and elbow, while the lateral head functioned to resist elbow flexion and absorb work, demonstrating that functional diversification can arise between mono- and biarticular muscle agonists operating at the same joint.
Keywords: biarticular, fascicle strain, muscle work
in vertebrate limbs, multiple muscles may power rotation at a given joint. One way to make sense of this redundancy is to investigate how anatomical differences among synergistic muscles lead to different patterns of muscular strain, force, and work (e.g., Ref. 35). Understanding how these differences may lead to functional differences is critical to interpreting neural control strategies (47) and to analyzing the biomechanical ramifications of musculoskeletal physiology and form (21). One way synergist muscles may differ is in the number of joints they cross (8). Here we investigate in vivo muscle strain and inverse dynamic estimates of muscle work and stress to understand how biarticular and monoarticular synergists of the goat (Capra hircus) triceps brachii might differ in their function across differing locomotor tasks.
The functional repertoires available to biarticular muscle have been extensively discussed and modeled (reviewed in Refs. 25, 44). A biarticular muscle may shorten and produce positive work—simultaneously at each of the joints it crosses (13). Alternatively, a biarticular muscle may lengthen at one joint while shortening at the common joint, reducing strain rates relative to its monoarticular synergist, thus allowing it to generate greater force (44, 36). If the relative joint rotations are sufficient, the biarticular muscle may also contract isometrically, transmitting energy from one joint to another, without the muscle itself doing any work. More generally, it has been proposed that biarticular muscles are responsible for controlling joint moments while monoarticular muscles are responsible for powering joint rotation (44). Finally, if no rotation occurs at the second joint, a biarticular muscle may not differ in its function from its monoarticular synergist. This might be especially true when the moment arm across one of the joints is small (10).
The wealth of prediction about how biarticular muscles may function relative to their monoarticular agonists has motivated several empirical comparisons of function in mono- and biarticular muscles of the hindlimb triceps surae (e.g., Refs. 25, 35). However, to our knowledge, only two comparisons of directly measured muscle fascicle strain in monoarticular/biarticular synergists have been published (23, 43), which were based on ultrasound studies of the human monoarticular soleus compared with the biarticular medial gastrocnemius (MG). Both studies found differing functional patterns between the two muscles. The soleus was observed to follow the kinematics of the ankle, stretching and shortening with ankle movement in both walking (23) and landing prior to a drop jump (43), in a pattern similar to other monoarticular muscles (15, 22). In contrast, the MG showed isometric strain patterns during walking (23) and fascicle shortening in low-intensity drop jumps, but stretching in high-intensity drop jumps (43). Reduced strain and strain rates during walking in the human MG are consistent with the predictions of isometric function that may arise from a biarticular muscle that transfers energy between joints but does little or no work itself. Such behavior has also been observed in vivo for the biarticular lateral gastrocnemius in various animals during running and hopping (e.g., Refs. 2, 39).
The paucity of empirical comparisons of biarticular and monoarticular muscle strain and work production, however, may limit or bias our understanding of the range of functional differences between biarticular and monoarticular synergists. A main goal of this study, therefore, is to augment these studies of the hindlimb triceps surae with an analogous forelimb muscle synergist pair, by comparing the biarticular long head of the triceps brachii (TrLONG) and monoarticular lateral head (TrLAT). Muscle activation, strain, stress, and work patterns are compared between the muscle heads during upward and downward jumps in goats (Capra hircus). The forelimb was also chosen because it is involved in landing from downward jumps as well as powering upward jumps and would therefore be expected to display a broad range of joint and muscle function. We hypothesize that strain and work patterns of the monoarticular TrLAT, like those of the soleus in humans (23), will follow the kinematics of the elbow joint more closely than the biarticular TrLONG. Specifically, we predict that shoulder flexion during upward jumps (3) might allow the long head to shorten despite elbow flexion in the early periods of stance. However, similar to the human soleus and MG during high-intensity landings (43), we predict that the TrLONG and TrLAT would both function to absorb energy when goats land from a downward jump.
METHODS
Animals.
Four goats (25, 28, 42, and 45 kg) were used in the study as a minimum but sufficient sample size for statistical significance (based on previous studies, e.g., Ref. 15). Animals were housed outdoors in fields at the Concord Field Station, Bedford, MA, and experiments were conducted in accordance with Harvard Institutional Animal Use and Care Committee guidelines and U. S. Department of Agriculture regulations. Over a period of 2–3 wk goats were trained to jump onto and off of a wooden platform that measured 1.0 m high for the three larger goats and 0.82 m for the 25-kg goat (1.0 × 0.3 m top surface) until jumps were made smoothly and without hesitation.
Anatomy.
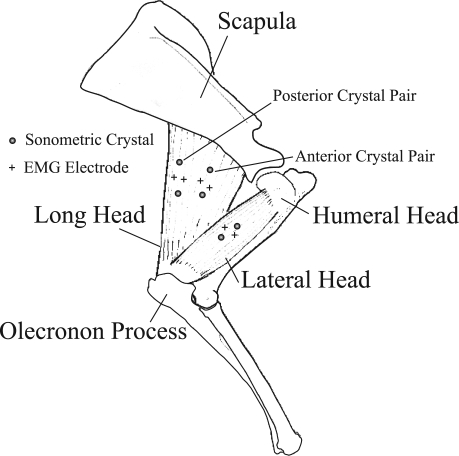
The long head (TrLONG) and the lateral head (TrLAT) of the triceps both insert on the olecranon process of the ulna (Fig. 1; Ref. 9). A much smaller monoarticular medial head is also present; its mass and cross-sectional area were grouped with that of the lateral head for the purposes of this study. The lateral and medial triceps heads originate from the humerus, while the long head originates from the distal-most third of the ventrocaudal margin of the scapula. A much smaller tensor fascia antebrachii (9) also runs from the scapula to the olecranon, and its mass and cross-sectional area were grouped with that of the long head. The TrLAT was found to be smaller than the TrLONG, averaging 53% of the mass and 39% of the physiological cross-sectional area (PCSA) of the TrLONG. Architecturally, the TrLAT contains parallel fascicles that run the extent of its length (ranging from an average of 6.0 cm in a 25-kg goat to 8.0 cm in a 45-kg goat). The TrLONG also contains long fascicles of similar length (average: 6.5 cm in a 25-kg goat to 8 cm in a 45-kg goat), but is slightly unipinnate (15 ± 4°).
Fig. 1.
Anatomy of the goat (Capra hircus) triceps brachii. The biarticular long head (TrLONG) and monoarticular lateral head (TrLAT) both insert on the olecranon process of the ulna. The TrLONG originates on the scapula, whereas the TrLAT originates on the humerus. Symbols indicate the location of sonometric crystals and electromyographic (EMG) electrodes for 3 of 4 goats used in this study (Table 3). In the fourth goat, 1 set of crystals was used to record from the midbelly of the long head, rather than 2 sets (anterior and posterior) as in the other goats.
Surgery.
Prior to surgery, animals were starved for 1 day. Goats were induced for intubation with 8 mg/kg ketamine and 0.05 mg/kg xylazine administered into the jugular vein. Following intubation, goats were maintained in a surgical plane of anesthesia with 2–4% isoflurane by volume, administered via inspired oxygen. The skin of the lateral, proximal forelimb was sterilized. Under aseptic conditions, an incision was made superficial to the long and lateral triceps muscle bodies, and their fascicles were exposed by separation of overlying fascia. 2-mm sonometric crystals (Sonometrics, London, ON, Canada) and bipolar electromyography (EMG) electrodes, fashioned from twisted strands of 0.102 mm Teflon-coated silver wire (California Fine Wire, Grover Beach, CA) with a 3–4 mm dipole distance (28) were soaked overnight in a sterilizing solution (Nolvasan Solution, Aveco, Fort Dodge, IA). A 2-cm incision was made in the neck of the animal and a subcutaneous channel was hollowed between this incision and the incision over the triceps. Sonometric crystals and electrodes were passed from the neck to the forelimb incision, leaving their connectors outside the smaller neck incision. Sonometric crystals were inserted into the muscle, and fascicles were gently sutured over the crystal with 4–0 silk. Care was taken to insert each pair of sonometric crystals along the fascicle axis 10–14 mm apart. In the long head, this meant implanting the distal crystal slightly deeper than the proximal due to the muscle's slight fascicle pinnation. EMG electrodes were inserted immediately lateral to those fascicles containing crystals via an 18.5-gauge hypodermic needle and were secured to the fascicles with 4–0 silk suture. The connectors were then sutured to the skin of the neck with sterile 0 surgical wire. Both incisions were closed with 3–0 vicryl suture, and the goats were allowed to recover overnight. Every 12 h between surgery and death animals were given a nonsteroidal anti-inflammation agent (Flunixin 1 mg/kg) and a broad spectrum antibiotic (Polyflex, Wyeth, Madison, NJ). Data collection took place within 1–2 days following surgery. All animals were killed following data collection with 120 mg/kg of commercial pentobarbital sodium solution injected into the jugular vein. No animal showed signs of infection or lameness prior to death.
Joint kinematics.
The hoof and the skin overlying the metacarpophalangeal, wrist, and shoulder joints and the scapular spine (at its most proximal palpable extent) were marked with nontoxic white paint. Jumps and landings were digitally recorded at 250 Hz using a Redlake PCI-500 video system (Redlake, Morgan Hill, CA). Joint locations were digitized using a custom auto-tracking digitization program in MatLab (v. 6.5; The MathWorks, Natick, MA) written by T. L. Hedrick, UNC, Chapel Hill, NC (http://www.unc.edu/∼thedrick/software1.html). Digitized data were filtered at 35 Hz with a fourth-order recursive (zero lag) Butterworth filter and used to determine hoof position, limb segment position, and elbow and shoulder angles for each sequence. The camera was controlled by an analog trigger, and the voltage signal from this trigger was used to synchronize camera recordings with EMG, sonomicrometry, and forceplate recordings. As opposed to the definition for humans (Moore and Agar, 34a), shoulder flexion is this study is defined as closing of the caudal (posterior) angle between the humerus and scapula in the sagittal plane.
EMG.
EMG signals were filtered (60-Hz notch and 30- to 3,000-Hz band pass) and amplified at 1,000× with Grass P511 amplifiers (Grass-Telefactor, West Warwick, RI). Outputs from the Grass amplifiers were digitized at 2,000 Hz through a 12-bit analog-to-digital converter (Digidata 1200B, Axon Instruments, Union City, CA). EMG signals were later digitally filtered with a 100- to 1,000-Hz band pass fourth-order zero-lag Butterworth filter before analysis. Onset and offset times were measured relative to timing of hoof contact. Rectified EMG intensity (mV) was normalized by the largest recorded value for each electrode over all trials.
Muscle strain.
Sonometric crystals measure latency of ultrasound to determine instantaneous muscle length. The speed of sound in muscle was estimated to be 1,550 m/s (34). Latency of crystal response was transduced and amplified with a Triton sonometrics system (model 120–1001; Triton Technology, San Diego, CA) and digitized with the EMG signals at 2,000 Hz as described above. Due to its filtering circuitry, there is a 5-ms delay inherent in the Triton system that was accounted for in subsequent data analysis. Crystal signals manually cleaned to eliminate “drop-outs,” were digitally filtered at 30 Hz with a fourth-order recursive (zero lag) Butterworth filter and a custom MatLab script. Crystal distances were normalized to resting length at stance.
Elbow joint moment analysis.
Elbow moments were calculated using inverse dynamics (29). Goats jumped onto or off of one of two forceplates (0.4 m by 0.6 m, model AMTI BP400600HF, Watertown, MA), covered with grip tape (3M Safety-Walk Medium Duty Resilient Tread 7741), flush with ground level, and resting in a bed of sand. Plate forces and moments were recorded using a custom LabView program (written by D. V. Lee). The center of pressure of each plate was calculated from these data and transformed into the camera frame of reference using a custom MatLab script. Elbow joint moments were estimated from ground reaction force, segment mass, translational and rotational segment inertia, and moment of the more distal joint using a MatLab script modified from that developed by Craig McGowan (31). Segmental properties were determined from two goats (20 and 55 kg; Lee et al., 2008 27a) and scaled to the size of the goats used in the current study.
Elbow work was measured as the numerical integration of elbow moment and elbow angle change over the period of interest. Elbow work was normalized by the combined mass of the TrLONG and TrLAT heads measured post mortem. Muscle work was measured as the numerical integration of muscle fascicle shortening and estimated force (calculated as described below) and was normalized by the mass of each muscle.
Muscle stress.
Muscle stress was estimated from extensor moments acting across the elbow joint by assuming that the triceps are the only muscles capable of extending the elbow:
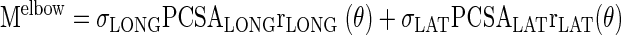 |
(1) |
where Melbow is the moment at the elbow, σMUS is the stress produced by each muscle as a function of time, PCSAMUS is the physiological cross-sectional area of each muscle (measured post mortem), and rMUS(θ) is the moment arm of each muscle as a function of elbow angle (θ).
Because this equation cannot be solved for muscle stress, it was necessary to make further assumptions to estimate the stress acting in each of the muscles. Therefore, muscle stress was assigned based on the estimated capacity of each muscle to generate force as a function of its shortening velocity (18). This was done by calculating a single common stress σ̂, which was distributed to each muscle according to a weighting factor, kMUS, based on the relative estimated force for each muscle. For example the weighting factor for the TrLAT was:
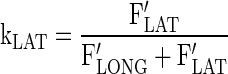 |
(2) |
where F′MUS is the relative force estimated for each muscle based on instantaneous measurement of fascicle velocity (as described below). Thus the following equation was used to solve for stress in each individual muscle.
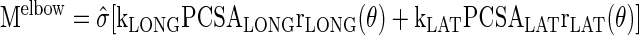 |
(3) |
with estimated individual muscle stress equal to the product of σ̂(t) and kMUS(t), and muscle force equal to the product of stress and physiological cross sectional area of each muscle (PCSAMUS).
Relative force for each muscle was calculated to depend on muscle velocity (strain rate) by an inverse hyperbolic relationship (19). During shortening this relationship was described by the following equation (32):
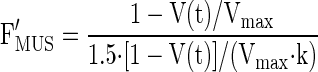 |
(4) |
where F′MUS is normalized to a maximum eccentric force ∼1.5 times isometric force (see below). The normalized hill parameter, k (=b/Vmax and a/Fo), was estimated as 0.20 (5, 41). Vmax was estimated as −7 fiber lengths (FL/s) based on the assumption that the triceps is composed of both type I and type II fibers with a Vmax of −5 and −9 FL/s, respectively [based on temperature normalized values for humans (46), horses (40), and sheep (42)].
Relative force during stretch was estimated using the following equation (30):
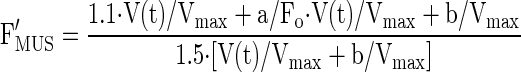 |
(5) |
a′/Fo was estimated as 0.4 (30) while b′/Vmax was estimated as 0.15 (27, 30).
The asymptotic force in our model is set at 1.5 times that of isometric force. While values as high as 1.8 are often cited in in vitro and in situ literature (e.g., Ref. 24), values are frequently lower during voluntary contractions (e.g., 1.2; Ref. 12). Asymptotic force was assumed to be achieved at approximately −1/2 Vmax as observed in numerous studies (e.g., Refs. 4, 12, 30).
The active force-length properties of each muscle head were also estimated based on the following equation and parameters from Brown et al. (4):
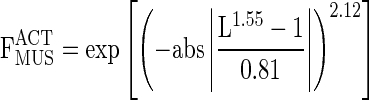 |
(6) |
where L is the normalized length (i.e., resting length = 1 and F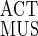 equals a maximum of 1 at resting length). FMUS was multiplied by F
equals a maximum of 1 at resting length). FMUS was multiplied by F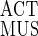 to take into account reductions in muscle force at lengths other than resting length. Passive force was not estimated because it accounts for <5% of total force up to 1.2 times muscle resting length (4), which is near the maximum strains measured in this study.
to take into account reductions in muscle force at lengths other than resting length. Passive force was not estimated because it accounts for <5% of total force up to 1.2 times muscle resting length (4), which is near the maximum strains measured in this study.
The slight pennation angle of the TrLONG was not taken into account. On the basis of an emperical study of the turkey lateral gastrocnemious (1), a maximum fiber angle of ∼20° was estimated, which would lead to a 7% reduction in muscle force output. However, because it is impossible to estimate actual fiber rotation in vivo it was not incorporated into the model. EMG timing and amplitude were also not used to estimate muscle force because of the intrinsic difficulties in comparing EMG preparations (28) and because of the difficulty of accurately estimating activation and deactivation force kinetics due to lack of published data for these muscles. Instead, we assumed that both muscles produced force when an extensor muscle moment acted at the elbow.
Data analysis.
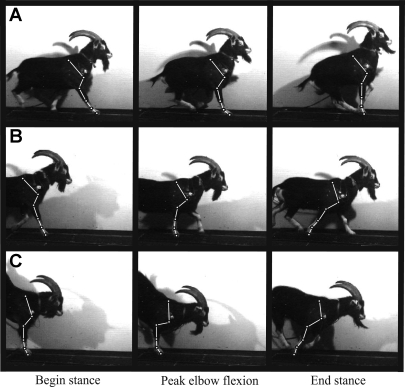
Goats used both forelimbs to land from downward jumps, but only one forelimb was used during take-off in upward jumps (Fig. 2). If the marked limb was used in take-off (being the last forelimb to leave the ground), it was described as the “lead limb” of an upward jump (Fig. 2). When it was used in penultimate step prior to take-off, the marked limb was described as the “lag limb” of an upward jump (Fig. 2). Thus we defined three distinct categories of limb usage in this study: the lead limb of an upward jump (lead limb); the lag limb of an upward jump (lag limb); and the marked limb of a downward jump (landing limb). Kinematic and ground reaction force differences appeared to justify this categorization (Figs. 3 and 4). Consequently, all muscle and joint parameters were compared among these categories of limb usage.
Fig. 2.
Video frames from a goat (45 kg) showing kinematics of the lead limb (A) and lag limb (B) of an upward jump and the landing limb (C) of a down jump. Goats jumped off or onto a force plate. Frames show time points of foot-on, peak elbow flexion, and foot-off. These kinematic events defined periods used to analyze muscle and joint function in this study.
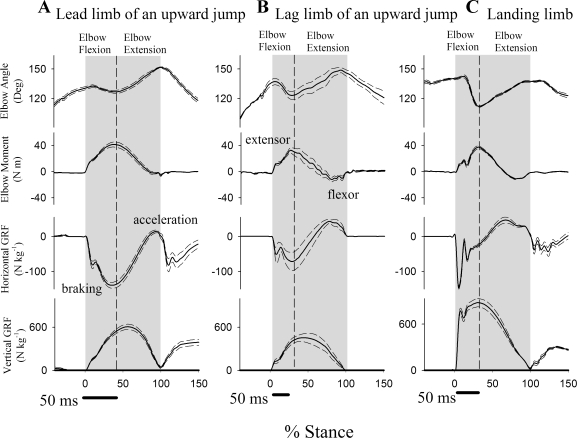
Fig. 3.
Mean (solid line) and standard error (dashed line) for elbow angle, elbow moment, horizontal ground reaction force (GRF), and vertical GRF for a 45-kg goat as percent of stance. The gray area indicates stance (foot-on to foot-off) and the dashed line indicates peak elbow flexion. Forelimb use resulted in differences in joint kinematic, force, and moment profiles. Note different scales for horizontal vs. vertical GRF.
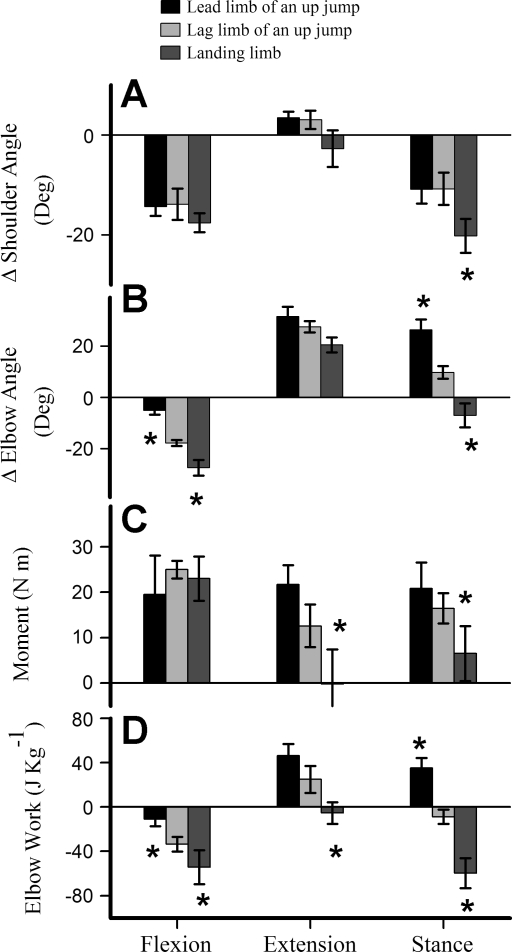
Fig. 4.
Histogram showing means (±SE) across the 4 individual goats for changes in shoulder angle (A), elbow angle (B), elbow moment (C), and elbow joint work (D). Values for the 3 patterns of limb use (lead and lag limb of up jumps and landing from down jumps) are shown for phases of elbow flexion and extension, and net values over the duration of stance. *Significant difference among jump types in Tukey's Honestly Significant Difference (HSD) post hoc tests with a starting α of 0.05. Differences in limb function resulted in significant differences among kinematic and dynamic parameters, particularly elbow work, which differed significantly over all phases for the 3 categories of forelimb usage.
EMG, forceplate, joint, and muscle parameters were grouped within each individual and statistics were run on the individual means for each parameter. Differences among jump types were tested using a two-factor ANOVA with limb usage and individual as factors (Table 1). All data was tested for normality prior to ANOVA using a Shapiro-Wilk test. Tukey's Honestly Significant Difference post hoc tests with a starting α of 0.05 were run to determine which parameters differed over periods of stance or muscle cycle. Differences in muscle parameters between the long and lateral heads were tested with a two-factor ANOVA with muscle head and individual as factors (Table 2). ANOVA, post hoc tests, and normality tests were run in .Jmp (SAS institute, Cary, NC). Correlations among muscle strain and work were tested using SigmaPlot (Systat Software, San Jose, CA), and were performed on pooled data from all individuals.
Table 1.
ANOVA results for limb function (lead, lag, or down jumps) effects on muscle parameters
| DF | TrLAT Stretch |
TrLAT Shortening | Stance | ||||
|---|---|---|---|---|---|---|---|
| F Ratio | P | F Ratio | P | F Ratio | P | ||
| Long head | |||||||
| Strain | 2 | 19.4 | 0.002 | 1.5 | 0.299 | 33.5 | <0.001 |
| Strain rate | 2 | 26.5 | 0.001 | 6.1 | 0.036 | 45.0 | <0.001 |
| Stress | 2 | 0.2 | 0.797 | 29.9 | <0.001 | 2.7 | 0.1423 |
| Work | 2 | 9.4 | 0.014 | 7.1 | 0.027 | 10.3 | 0.0114 |
| Lateral head | |||||||
| Strain | 2 | 11.5 | 0.009 | 5.8 | 0.081 | 11.3 | 0.009 |
| Strain rate | 2 | 4.5 | 0.064 | 2.3 | 0.177 | 8.0 | 0.020 |
| Stress | 2 | 17.8 | 0.003 | 6.9 | 0.027 | 30.6 | <0.001 |
| Work | 2 | 10.1 | 0.012 | 2.3 | 0.173 | 12.0 | 0.008 |
Biarticular long head of the triceps brachii (TrLONG) and monoarticular lateral head (TrLAT). Bold values indicate significance (P < 0.05). Differences among types of limb function were determined by post hoc tests and are shown in Fig. 7. DF, degrees of freedom.
Table 2.
ANOVA results for muscle head (lateral or long head) effects on muscle parameters
| DF | TrLAT Stretch |
TrLAT Shortening | Stance | ||||
|---|---|---|---|---|---|---|---|
| F Ratio | P | F Ratio | P | F Ratio | P | ||
| Up Jumps | |||||||
| Lead limb | |||||||
| Strain | 1 | 141 | 0.001 | 0.4 | 0.565 | 6.1 | 0.091 |
| Strain rate | 1 | 27.6 | 0.013 | 3.1 | 0.176 | 0.6 | 0.507 |
| Stress | 1 | 46.4 | 0.007 | 0.9 | 0.407 | 14.2 | 0.033 |
| Work | 1 | 14.2 | 0.032 | 4.44 | 0.125 | 17.3 | 0.025 |
| Lag limb | |||||||
| Strain | 1 | 127.8 | 0.002 | 0.1 | 0.782 | 73.1 | 0.003 |
| Strain rate | 1 | 43.6 | 0.007 | 2.0 | 0.256 | 19.0 | 0.022 |
| Stress | 1 | 6.53 | 0.043 | 1.7 | 0.284 | 11.0 | 0.045 |
| Work | 1 | 38.5 | 0.009 | 11.8 | 0.041 | 31.5 | 0.011 |
| Down Jumps | |||||||
| Landing limb | |||||||
| Strain | 1 | 2.1 | 0.202 | 0.9 | 0.418 | 1.4 | 0.326 |
| Strain rate | 1 | 3.77 | 0.147 | 7.5 | 0.070 | 4.4 | 0.126 |
| Stress | 1 | 3.3 | 0.164 | 2.6 | 0.208 | 5.8 | 0.094 |
| Work | 1 | 4.8 | 0.116 | 1.6 | 0.297 | 5.7 | 0.090 |
Bold values indicate significant difference between muscles (P < 0.05).
RESULTS
Goats did not appear to favor their noninstrumented forelimb, as all but one individual used the instrumented forelimb as the lead limb to jump up in most cases (Table 3), suggesting that instrumentation did not alter limb usage. Regardless of forelimb usage, all jumps began with a period of elbow flexion (duration: lead 0.047s ± 0.002 s; lag 0.078+0.01 s; landing 0.083 ± 0.008 s, mean ± SE) followed by a period of elbow extension (duration: lead 0.067 ± 0.009 s; lag 0.092+0.01 s; landing 0.074 ± 0.01 s; Figs. 3 and 4B). Periods of elbow flexion and elbow extension, as well as the total period of stance, were used to test for differences among joint parameters in all subsequent analyses (Fig. 4).
Table 3.
Numbers of trials recorded for each type of jump used in this study
| Up Jumps |
Down Jumps | ||
|---|---|---|---|
| Lead Limb | Lag Limb | ||
| Goat 1 (45 kg) | |||
| Long* | 18 | 6 | 22 |
| Lateral | 8 | 3 | 12 |
| Goat 2 (42 kg) | |||
| Long | 2 | 6 | 9 |
| Lateral | 3 | 6 | 8 |
| Goat 3 (28 kg) | |||
| Long* | 12 | 4 | 11 |
| Lateral | 6 | 2 | 6 |
| Goat 4 (24 kg) | |||
| Long* | 5 | 5 | 5 |
| Lateral | 4 | 5 | 3 |
Two samples were simultaneously recorded from the long head as in Fig. 1.
Elbow moments.
During elbow flexion, no significant differences in mean elbow moment were observed among types of forelimb usage. During elbow extension, elbow moments differed significantly among all jump types (Fig. 4C), reflecting differences in relative limb retraction angle (Fig. 2) and GRF magnitudes (Fig. 3).
Elbow work.
Regardless of forelimb usage, the elbow experienced similar extensor moments during the flexion period and, thus, absorbed energy (Fig. 4D). The amount of energy absorbed during flexion, however, varied significantly with limb usage (Fig. 4D), reflecting differences in elbow joint angular deflection (compare Fig. 4, B and C). During elbow extension, significant net joint work was done only during upward jumps (46 ± 9 J/kg in the lead limb and 25 ± 9 J/kg in the lag limb; Fig. 4D). No work was done during elbow extension in downward jumps because the extensor elbow moment fell to zero during this period (Fig. 4D). Over the duration of stance, the elbow did net positive work in the lead limb of an upward jump (35 ± 10 J/kg) but the work done in the in the lag limb was not significantly different from zero (P > 0.10). In contrast, the elbow joint absorbed a great deal of energy (−58 ± 10 J/kg) during landing (Fig. 4D).
Muscle activity.
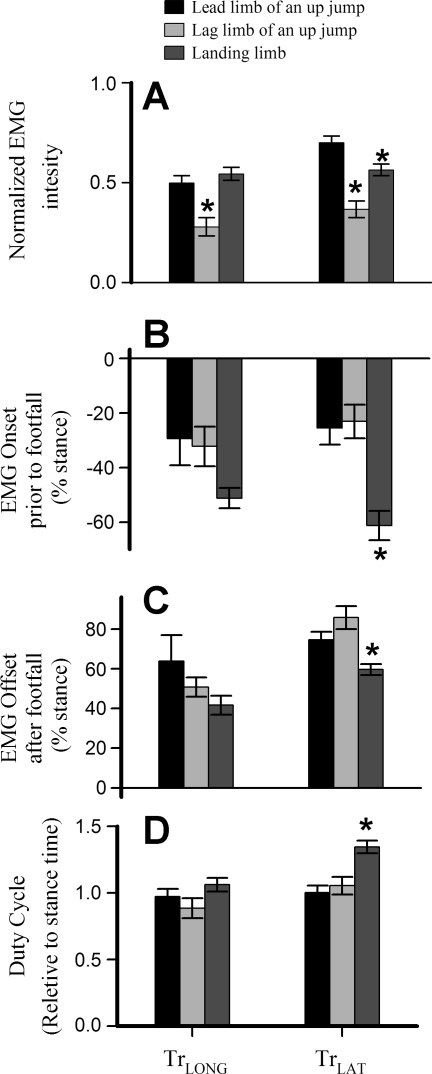
Timing parameters did not vary with limb usage in the TrLONG but, in the TrLAT, EMG onset was significantly earlier when animals landed from a downward jump. This resulted in a longer duty cycle (Fig. 5D), despite significantly earlier offset of the TrLAT (Fig. 5C). In both muscles, normalized EMG intensity was higher in the lead forelimb than in the lag forelimb of an upward jump (Fig. 5A). TrLAT EMG intensity in the lead forelimb of an upward jump was also significantly higher than during landing from a downward jump (P < 0.05; Fig. 5A).
Fig. 5.
Histogram showing means (±SE) across the 4 individual goats for electromyogram (EMG; A) intensity, EMG onset (B), and offset times (as percent of stance and relative to the time of foot on; C), and EMG duty cycle (EMG duration/stance duration; D). *Significant difference among jump types in Tukey's HSD post hoc tests with a starting α of 0.05. In both TrLAT and TrLONG, EMG intensity was greater in lead jumps than in lag jumps, but intensity during landing was also greater than in the lag limb, despite a lower elbow moment (Fig. 4B).
Muscle strain and strain rate.
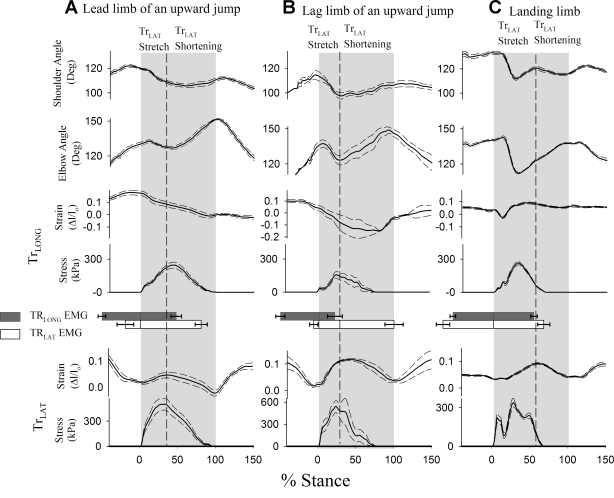
Muscle function parameters were measured over stance and within stretch and shortening periods of the TrLAT to allow comparisons between the muscle heads (Fig. 6). In upward jumps, periods of stretch and shortening in the TrLAT were simultaneous with those defined for the elbow joint (elbow flexion = TrLAT stretch; elbow extension = TrLAT shortening, Fig. 6; t-test: P > 0.25). The TrLONG sometimes displayed a slight lengthening at the end of stance (Fig. 6B), so the end of shortening in this was measured at the nadir of its strain profile. In downward jumps, however, peak elbow flexion occurred before peak TrLAT stretch (P < 0.05; Fig. 6C), apparently due to the stretch of in-series compliance in the muscle-tendon unit.
Fig. 6.
Mean profiles (solid lines) ± standard error (dashed lines) across trials for lead limb up jump (A), lag limb up jump (B); and a forelimb landing from a down jump (C), showing temporal patterns of shoulder angle, elbow angle, and fascicle strain and stress in the TrLONG and TrLAT muscle heads, as a percentage of stance in a 45-kg goat. The number of trials included in each graph is given in Table 3. Gray areas indicate stance time, and dashed lines indicate peak stretch in the TrLAT, which was concurrent with peak elbow flexion, except when the forelimb was used to land from a downward jump.
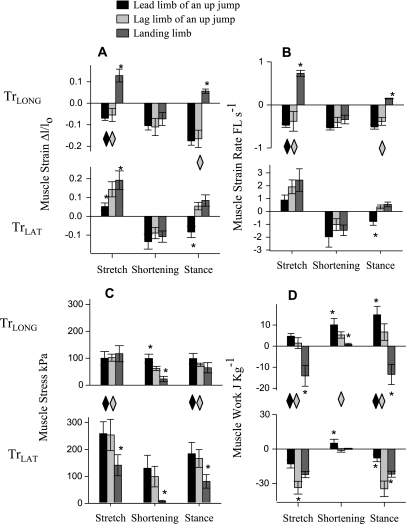
The TrLAT stretched (during elbow flexion) in upward jumps, with significantly less stretch in lead limb than in the lag limb (net fascicle strain: 5 ± 2 vs. 14 ± 4% post hoc test with starting value of P < 0.05). Over the same period, the TrLONG shortened by −6 ± 2% with no significant difference found between the lead and lag limbs of an upward jump in post hoc tests (Fig. 7A). In landing, both muscles stretched simultaneously at the onset of stance (net strain: 19 ± 5 and 13 ± 3%; Table 2; Fig. 7A). Subsequently, during elbow extension, the muscles shortened by a similar amount in both the lead and lag limb of an upward jump (net strain: −11 ± 4% pooled mean; P > 0.25). Over the full stance period these patterns resulted in net TrLAT shortening in the lead limb of an upward jump (−8 ± 3%) but resulted in net stretching during landing (8 ± 3%). Surprisingly, the TrLAT also displayed net stretch over the period of stance in the lag limb during jump takeoff (5 ± 2%), although the elbow underwent net extension (comparing Fig. 4 with Fig. 7A) In contrast to the TrLAT, the TrLONG showed net shortening over forelimb stance in upward jumps regardless of limb usage (net strain: −17 ± 2% pooled mean; P > 0.25) and, similar to TrLAT, underwent net stretch when landing from a downward jump (net strain: 6 ± 1%).
Fig. 7.
Histogram showing means (±SE) across the 4 individual goats and 3 jump conditions for muscle strain (A), muscle strain rate (B), mean muscle stress (C), and muscle work (D) during TrLAT stretch, TrLAT shortening, and over net stance. *Significant difference among jump types in Tukey's HSD post hoc tests with a starting α of 0.05. ◊Significant differences between muscles with gray indicating a difference in the lag limb, and black indicating a difference in the lead limb (statistics given in Table 2). Parameter values shown in the figure are also presented in Table 4. The TrLAT absorbed substantial work as it stretched concurrent with elbow flexion and joint work absorption. In contrast the TrLONG generated work in both the lead and lag limb of upward jumps, but absorbed energy in downward jumps. This shift appeared to be mediated by changes in the relative kinematics of the elbow and shoulder.
Muscle strain rate followed patterns of muscle strain (Fig. 7B), with the exception that strain rates during shortening were significantly higher in the TrLONG of the lead limb during upward jumps than during downward jumps (Fig. 7B; −0.5 ± 0.05 vs. −0.3 ± 0.01 FL/s). The differences in strain rate between muscle heads during shortening were not significant (Table 2). When the data for lead and lag forelimbs of upward jumps were grouped; however, the TrLONG was found to shorten significantly more slowly than the TrLAT (−0.5 ± 0.1 vs. −1.5 ± 0.2 FL/s; P < 0.05).
Muscle stress.
Stresses calculated for both muscles followed the general pattern of elbow moment (compare Figs. 4C and 7C). During the period of muscle stretch, stress in the TrLAT did not differ significantly between the lead and lag limbs of an upward jump (pooled mean: 258 ± 27 kPa; P = 0.10), but in downward jumps the TrLAT generated significantly less stress in post hoc tests (Fig. 7C; 142 ± 39 kPa). Over the same period, no significant difference in muscle stress was found in the TrLONG among the lead limb of an upward jump, lag limb of an upward jump, or in landing from a downward jump (pooled mean: 99 ± 13 kPa). Consequently, during the period of TrLAT stretch, stress was significantly higher in the TrLAT than the TrLONG in the lead and lag limbs of an upward jump (P < 0.01), but not in down jumps (P > 0.1; Fig. 7C; Table 2).
During the period in which both muscles shortened, stress in the TrLAT was not significantly different among the lead and lag limbs of upward jumps (pooled mean: 115 ± 30 kPa; P > 0.10), but was much lower in landing from a downward jump in post hoc tests (8 ± 4 kPa). Over the same period, stress in the TrLONG was similar in the lead limb and lag limbs of an upward jump (P > 0.25; Table 4) and again, similar to TrLAT, much lower in landing (17 ± 4 kPa; Fig. 7C). No significant difference in TrLAT and TrLONG stress was observed during shortening, even when data from both categories of upward jumps were pooled (P > 0.10).
Table 4.
Muscle parameters for the long and lateral heads of the triceps brachii of jumping goats
| Strain (Δl/lo) |
Strain Rate (FL s−1) | Stress (kPA) | Work (J kg−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Long | Lateral | Long | Lateral | Long | Lateral | Long | Lateral | |
| Up Jumps | ||||||||
| Lead limb | ||||||||
| TrLAT stretch | −0.07±0.01 | 0.05±0.02 | −0.47±0.05 | 0.89±0.39 | 100±25 | 259±44 | 4.7±1.3 | −13.0±3.5 |
| TrLAT shortening | −0.10±0.02 | −0.13±0.04 | −0.53±0.05 | −1.97±0.80 | 99±16 | 130±48 | 10.1±3.0 | 5.1±3.4 |
| Stance | −0.17±0.02 | −0.08±0.03 | −0.51±0.05 | −0.78±0.30 | 99±19 | 185±42 | 14.8±4.0 | −7.8±3.2 |
| Lag limb | ||||||||
| TrLAT stretch | −0.05±0.03 | 0.14±0.04 | −0.38±0.23 | 1.92±0.54 | 101±13 | 253±58 | 1.4±2.6 | −33.7±5.6 |
| TrLAT shortening | −0.11±0.04 | −0.09±0.03 | −0.41±0.12 | −1.02±0.46 | 62±8 | 99±38 | 5.3±1.5 | −1.5±1.5 |
| Stance | −0.17±0.04 | 0.05±0.02 | −0.38±0.09 | 0.35±0.15 | 76±6 | 166±33 | 6.7±3.9 | −34.7±6.8 |
| Down Jumps | ||||||||
| Landing limb | ||||||||
| TrLAT stretch | 0.13±0.03 | 0.19±0.05 | 0.73±0.07 | 2.44±0.88 | 117±30 | 142±39 | −14.1±4.9 | −24±2.79 |
| TrLAT shortening | −0.07±0.03 | −0.11±0.03 | −0.34±0.10 | −1.47±0.41 | 17±10 | 8±4 | 0.8±0.5 | 0.2±0.5 |
| Stance | 0.06±0.01 | 0.08±0.03 | 0.15±0.01 | 0.54±0.18 | 65±19 | 82±25 | −13.4±4.8 | −21.9±2.6 |
Means ± SE of 4 individual means for muscle parameters for the long and lateral heads of the triceps brachii of jumping goats.
Muscle work.
While being stretched, the TrLAT absorbed more work in the lag limb (−34 ± 5.6 J/kg) than in either the lead limb (−13.0+3.5 J/kg) or in landing (−24 ± 2.8 J/kg; Fig. 7D; Table 4). Over the same period, the TrLONG shortened to produce work in both the lead and lag limbs of an upward jump, without significant differences among limb function (pooled mean: 3 ± 1 J/kg). In landing, however, the TrLONG stretched and absorbed energy from a downward jump (−13 ± 4.9 J/kg; Fig. 7D; Table 4).
During the period of TrLAT shortening both muscles shortened to produce work in the lead limb (TrLONG: 10 ± 3 J/kg and TrLAT: 5 ± 3 J/kg; P > 0.05). In the lag limb, positive work was produced only by the TrLONG (5 ± 1.5 J/kg), as net work in the TrLAT was not significantly different from zero (−1.5 ± 1.5 J/kg; P > 0.25; Table 4). When landing from a downward jump neither muscle produced significant work during the period of TrLAT shortening (P > 0.1; Fig. 7D, Table 4) due to low stress calculated from the reduced elbow moment (Fig. 4).
Despite the fact that the elbow produced positive net joint work over stance in the lead limb, net work done by the TrLAT was negative (−8 ± 3.2 J/kg). TrLAT net work reflected even greater energy absorption in the lag leg of an upward jump (−35 ± 7 J/kg) and in landing from a downward jump (−22 ± 3 J/kg), which were consistent in the sign, but not in the magnitude, of elbow joint work (Fig. 4). In contrast, the TrLONG did positive net work over stance in both the lead and lag limbs of an upward jump (15 ± 4 and 7 ± 4 J/kg; Fig. 7D) but, similar to TrLAT, absorbed energy during landing from a downward jump (−13 ± 5 J/kg). Hence, over the duration of stance, net work of the TrLONG and TrLAT are opposite in sign and significantly different during upward jumping, whereas these muscles absorb statistically similar, negative net work during downward jumping.
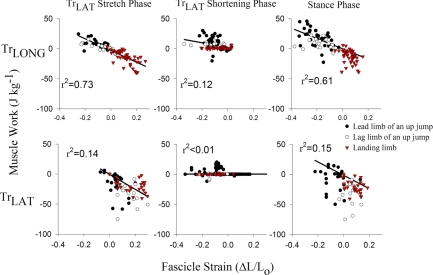
Muscle work during shortening was regressed against fascicle strain for each period of TrLAT stretching and shortening, and over net stance, to assess the dependence of muscle work on fascicle strain patterns over all categories of limb usage (Fig. 8). A strong correlation indicates that fascicle strain influences muscle energy absorption or production. During the period of TrLAT stretch and over stance, strong correlations are found between muscle work and muscle strain in the TrLONG but were lower in the TrLAT (Fig. 8). During the period defined by TrLAT shortening, there was a weak but significant correlation in the TrLONG (r = 0.35) and no correlation in the TrLAT (r < 0.01; P > 0.25), indicating that its work was determined almost entirely by muscle stress.
Fig. 8.
Least-square regression correlations between muscle work (y-axis) and fascicle strain (x-axis) for the TrLONG and TrLAT during the phases determined by TrLAT stretch, TrLAT shortening and net over stance for the lead limb (•) and lag limb (○) of an up jump, and for down jumps (red ▵). Correlations were higher in the TrLONG overall. Correlations for both muscle heads were lowest during elbow extension, indicating that muscle stress most strongly determined work output during this period. Correlations were assessed on the basis of pooled data from all individuals. A weak correlation between muscle work and fascicle strain indicates that work was determined by muscle stress. Two samples were simultaneously recorded from the long head as in Fig. 1.
DISCUSSION
The principal aim of this study is to understand how muscle function in a monoarticular vs. a biarticular synergist compares across varying locomotor tasks. This is assessed by examining the relative relationship between muscle strain and elbow joint kinematics and between muscle work and elbow joint work for the monoarticular TrLAT and biarticular TrLONG of the goat triceps brachii during jumping and landing tasks.
Fascicle strain and elbow kinematics.
Our prediction that strain patterns in the monoarticular TrLAT would follow elbow kinematics more closely than those in the biarticular TrLONG is generally supported. The TrLAT always stretched during elbow flexion and shortened during elbow extension. In contrast, the TrLONG shortened throughout stance in upward jumps (Fig. 6, A and B). In landing from a downward jump both muscles stretched with elbow flexion and shortened with elbow extension (Fig. 6C). While it is possible that these differences in functional pattern may be due to differences in muscle-tendon architecture between the muscle heads, we believe the most likely explanation is that the TrLONG is biarticular and the TrLAT is monoarticular.
Earlier studies of muscle function in the biarticular MG and monoarticular soleus muscles of cats found very little difference in strain patterns among the muscles (e.g., Refs. 17, 35). However, in these studies fascicle strain was estimated from joint kinematics, which can be unreliable (2, 20). When fascicle strain in human MG and soleus fascicles was measured directly with ultrasound during walking (23) and drop jumps (43), strain patterns in the soleus were found to follow the kinematics of the ankle more closely than those of the MG. Similarly, strain in the goat TrLAT follows rotation of the elbow more closely than that of the TrLONG.
Interestingly, this strain pattern differs from that measured in the TrLAT of horses during trotting, which showed minimal (∼2%) lengthening despite elbow flexion (20). This difference may reflect a general trend toward reduced stretch in monoarticular muscles as animal body size increases. For instance, the magnitude of stretch in the VL is also smaller in the horse than in rats (14), dogs (6), or goats (15).
In downward jumps, the two goat forelimb muscles function similarly because the TrLONG shifts from the monophasic shortening pattern required for upward jumps to a stretch-shortening pattern resembling that observed in the TrLAT. Similarly, the human MG exhibited net shortening over stance when subjects landed from low-intensity drop jumps but shifted to a stretch-shorten pattern when subjects landed from greater drop heights (43). The stretch-shorten pattern exhibited by the monoarticular goat TrLAT during landing from downward jumps also parallels the pattern observed for the monoarticular human soleus in both low- and high-intensity drop jumps (43).
Muscle work and elbow work.
Consistent with our predictions, patterns of energy absorption and production of the goat TrLAT also generally followed patterns of elbow joint work more closely than those of the TrLONG. For instance, in the lead limb the monoarticular TrLAT absorbed energy during elbow flexion and produced energy during elbow extension, whereas the biarticular TrLONG produced energy throughout stance (Figs. 4D and 7D). A similar contrast in muscle work production was found between the two muscles in the lag limb (Table 4). However, in downward jumps, both muscles followed the pattern found in the elbow: stretching to absorb energy early in stance as the elbow flexed (Fig. 7D), with neither muscle contributing significant positive work during elbow extension.
A major result of this study that might be predicted from force-velocity effects is that net shortening by a monoarticular muscle may not indicate a net contribution of work across a joint: on average, the TrLAT did not produce net work over stance, even when there was net fascicle shortening under load, as in the lead limb of an upward jump. Because muscles can produce greater forces when actively stretched than when shortening (19), the TrLAT was estimated to have absorbed more energy while stretching than it produced during shortening in the lead limb due to this force-velocity effect (Fig. 7D). In contrast to the TrLAT, the biarticular TrLONG shortened to produce work throughout stance in both the lead and lag limb of upward jumps. Thus, when work production is required of the elbow, the biarticular TrLONG appears to contribute most of the positive work, whereas the TrLAT predominately absorbs energy while resisting elbow flexion.
Alternate stretch-shorten cycles, similar to that found in the TrLAT have been interpreted as periods of work absorption and production in other muscles, such as the vastus lateralis of rats (14) and the iliotibialis lateralis pars postacetabularis (ILPO) of turkeys (38). Additionally, the rat VL (14) and turkey ILPO (38) were found to meet demands of increased joint work production or absorption by decreasing or increasing fascicle stretch. Likewise, the TrLAT underwent less stretch when joint work was required in the lead limb of an upward jump and greater stretch when more energy was absorbed across the elbow joint as the animals landed from downward jumps. Unlike the rat VL and turkey ILPO, however, the goat TrLAT did not increase the magnitude of fascicle shortening to produce more work during elbow extension. Instead, work production during fascicle shortening appears to be modulated mainly by the level of activation and stress developed in the muscle (Fig. 8B).
Energy transfer via the biarticular TrLONG.
One of the proposed functions of biarticular muscles is to transfer power between joints (43). The lower strain rates measured in the TrLONG relative to the TrLAT in upward jumps, may indicate that shoulder extension reduces the effect of elbow extension on TrLONG fascicle strain. Thus TrLONG fascicles would need to perform less work for a given elbow extension, because the whole muscle-tendon unit is displaced due to shoulder extension. Reduced shortening rates in the TrLONG may, therefore, indicate power transfer from the shoulder to the elbow through this muscle head. However, architectural differences between the two muscles may also affect their fascicle strain behavior, which could influence our interpretation of energy transfer via the TrLONG.
Another way to test for energy transfer is to compare the work done by the elbow during extension with that done by the two muscles. In fact, In the lead leg of an upward jump the combined work of the TrLONG and TrLAT during elbow extension was ∼15 J/kg (Table 4), significantly less than the work done at the elbow (∼ 46 J/kg; P < 0.05). This strongly indicates that power transfer occurs from shoulder extensors, operating as antagonistics to the TrLONG, although it is also possible that energy storage and return from elastic structures may explain some of this difference (16). Consistent with our interpretation, proximal to distal joint energy transfer has been hypothesized to occur in human and animal movement (36, 37, 45). However, to our knowledge, this is the first study to use direct measurements of in vivo fascicle strain to quantitatively estimate the amount of energy transferred by a biarticular muscle.
Limitations of the stress-partition model.
Because joint moments alone cannot determine muscle stress when more than one muscle crosses a joint, we used differences in relative fascicle velocity and fascicle strain to partition stress based on the inherent force-velocity and force-length characteristics modeled for the two muscles (18, 19). Muscle force is also affected by differential recruitment within a muscle. However, this was not included in our models because EMG amplitude provides only a rough estimation of muscle recruitment (28) and is strongly dependent on variable contractile conditions of the muscle, as we observed here.
Nevertheless, we observed a correspondence between muscle stress and normalized EMG intensity that provides an independent validation of our stress-partitioning model. During upward jumps, the goat TrLONG and TrLAT displayed a correlated increase in mean stress with increased EMG intensity in the lead limb compared with the lag limb (Fig. 5A vs. Fig. 7C). However, when animals landed from downward jumps, EMG intensity of the two muscles was greater than that predicted based on estimates of muscle stress alone. One explanation for this discrepancy is antagonist cocontraction of elbow flexors, which would lead to a underestimation of muscle stress in the triceps. Antagonist cocontraction across the knee and hip has been found within the biceps femoris and rectus femoris in humans prior to and during landing from a downward jump (26, 33). Similarly, antagonist cocontraction during landing in goats might well occur to stabilize the elbow during the rapid switch from an extensor to a flexor moment at the elbow after landing (Fig. 3). Therefore, our estimates of energy absorption during landing may underestimate actual energy absorption by the triceps. Antagonist cocontraction may have also been present during upward jumps, but it is unclear why goats would employ such a motor pattern, as this would reduce the work produced at the elbow and limit overall jumping performance.
Finally, our model for calculating muscle stress did not include history-dependent effects, such as stretch-induced force enhancement (7), which may have increased work production in the TrLAT. On the basis of the results of (11), however, only a 12% increase in torque due to the force enhancement following prestretch would be expected, which would still have been insufficient for the TrLAT to produce positive work over stance in the lead limb (values from Table 4), although it may have resulted in positive work production in the TrLAT during extension in the lag limb.
Conclusions.
The anatomical differences between the biarticular TrLONG and the monoarticular TrLAT of goats resulted in differences in muscle function with respect to elbow joint kinematics and work. In general, consistent with our hypothesis and as suggested previously (44), the shortening pattern and work of the monoarticular TrLAT followed the kinematics and work of the elbow joint more closely than the biarticular TrLONG during the flexion and extension periods of stance. Over the duration of stance, however, the TrLAT did not produce significant positive net work, despite net fascicle shortening. As a result, the TrLONG appeared to be mainly responsible for powering upward jumps, while the TrLAT generated force and absorbed the work of elbow flexion. Both muscles functioned similarly to absorb work during landing. As this study demonstrates, empirical measurement of muscle strain in relation to muscle and joint work output is key to analyzing how synergist muscles function to modulate work and force across a common joint.
GRANTS
This research was supported by National Institutes of Health Grant AR-047679 to A. A. Biewener.
Acknowledgments
Pedro Ramirez maintained and helped to train the goats used in this study. Carlos Moreno, Russell Main, Craig McGowan, Edwin Yoo, Ivo Ros, Kieth Egan, and Trevor Higgins aided in data collection. Craig McGowan also donated his inverse dynamics script. The comments and attention of three anonymous reviewers were indispensable in improving this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Azizi E, Brainerd EL, Roberts TJ. Variable gearing in pennate muscles. Proc Natl Acad Sci USA 105: 1745–1750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biewener AA, Konieczynski DD, Baudinette RV. In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J Exp Biol 201: 1681–1694, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Biewener AA, Thomason J, Lanyon LE. Mechanics of locomotion and jumping in the forelimb of the horse (Equus): in vivo stress developed in the radius and metacarpus. J Zool Lond 201: 67–82, 1983. [Google Scholar]
- 4.Brown IE, Cheng EJ, Loeb GE. Measured and modeled properties of mammalian skeletal muscle. II The effects of stimulus frequency on force-length and force-velocity relationships. J Muscle Res Cell Motil 20: 627–643, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri MJ, Hull ML. Are the maximum shortening velocity and the shape parameter in a Hill-type model of whole muscle related to activation? J Biomech 38: 2172–2180, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Carrier DR, Gregersen CS, Silverton NA. Dynamic gearing in running dogs. J Exp Biol 201: 3185–3195, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Cavagna GA, Dusman B, Margaria R. Positive work done by a previously stretched muscle. J Appl Physiol 24: 21–32, 1968. [DOI] [PubMed] [Google Scholar]
- 8.Cleland J On the actions of muscles passing over more than one joint. J Anat Physiol 1: 85–93, 1867. [PMC free article] [PubMed] [Google Scholar]
- 9.Constantinescu GM Guide to Regional Ruminant Anatomy Based on the Dissection of the Goat. Ames, IA: Iowa State University Press, 2001.
- 10.Dul J, Johnson GE, Shiavi R, Townsend MA. Muscular synergism-II, A minimum-fatigue criterion for load sharing between synergistic muscles. J Biomech 17: 675–684, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Finni T, Ikegawa S, Komi PV. Concentric force enhancement during human movement. Acta Physiol Scand 173: 369–377, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Finni T, Ikegawa S, Lepola V, Komi PV. Comparison of force-velocity relationships of vastus lateralis muscle in isokinetic and in stretch-shortening cycle exercises. Acta Physiol Scand 177: 483–491, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara M, Basmajian JV. Electromyographic study of two-joint muscles. Am J Phys Med 54: 234–242, 1975. [PubMed] [Google Scholar]
- 14.Gillis GB, Biewener AA. Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J Appl Physiol 93: 1731–1743, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Gillis GB, Flynn JP, McGuigan P, Biewener AA. Patterns of strain and activation in the thigh muscles of goats across gaits during level locomotion. J Exp Biol 208: 4599–4611, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gregersen CS, Silverton NA, Carrier DR. External work and potential for elastic storage at the limb joints of running dogs. J Exp Biol 201: 3197–3210, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change, and ankle extensor EMG patterns. J Neurophysiol 95: 1397–1409, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Happee R Inverse dynamic optimization including muscular dynamics, a new simulation method applied to goal directed movements. J Biomech 27: 953–960, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Hill AV The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci 126: 136–195, 1938. [DOI] [PubMed] [Google Scholar]
- 20.Hoyt DF, Wickler SJ, Biewener AA, Cogger EA, De La Paz KL. In vivo muscle function vs speed. I Muscle strain in relation to length change of the muscle-tendon unit. J Exp Biol 208: 1175–1190, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson JR Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morphol 262: 421–440, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa M, Finni T, Komi PV. Behaviour of vastus lateralis muscle-tendon during high intensity SSC exercises in vivo. Acta Physiol Scand 178: 205–213, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP. Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol 99: 603–608, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Katz B The relation between force and speeding muscular contraction. J Physiol 96: 45–64, 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaya M, Jinha A, Leonard TR, Herzog W. Multi-functionality of the cat medical gastrocnemius during locomotion. J Biomech 38: 1291–1301, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kellis E, Arabatzi F, Papadopoulos C. Muscle co-activation around the knee in drop jumping using the co-contraction index. J Electromyogr Kinesiol 13: 229–238, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Krylow AM, Sandercock TG. Dynamic force responses of muscle involving eccentric contraction. J Biomech 30: 27–33, 1997. [DOI] [PubMed] [Google Scholar]
- 27a.Lee DV, McGuigan MP, Yoo EH, Biewener AA. Compliance, actuation, and work characteristics of the goat foreleg and hindleg during level, uphill, and downhill running. J Appl Physiol 104: 130–141, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeb GE, Gans C. Electromyography for Experimentalists. University of Chicago Press, 1986.
- 29.Manter JT The dynamics of quadrupedal walking. J Exp Biol 15: 1938. [DOI] [PubMed]
- 30.Mashima H, Akazawa K, Kushima H, Fujii K. The force-load-velocity relation and the viscous-like force in the frog skeletal muscle. Jpn J Physiol 22: 103–120, 1972. [DOI] [PubMed] [Google Scholar]
- 31.McGowan CP, Baudinette RV, Biewener AA. Modulation of proximal muscle function during level versus incline hopping in tammar wallabies (Macropus eugenii). J Exp Biol 210: 1255–1265, 2007. [DOI] [PubMed] [Google Scholar]
- 32.McMahon TA Muscles, reflexes, locomotion. Princeton, NJ:. Princeton University Press, 1984.
- 33.McNitt-Gray JL, Hester DM, Mathiyakom W, Munkasy BA. Mechanical demand and multijoint control during landing depend on orientation of the body segments relative to the reaction force. J Biomech 34: 1471–1482, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Mol CR, Breddels PA. Ultrasound velocity in muscle. J Acoust Soc Am 71: 455–461, 1982. [DOI] [PubMed] [Google Scholar]
- 34a.Moore KL, Agur AM. Essential Clinical Anatomy. Baltimore, MD Williams and Wilkins, 2002.
- 35.Prilutsky BI, Herzog W, Allinger TL. Mechanical power and work of cat soleus, gastrocnemius and plantaris muscles during locomotion: possible functional significance of muscle design and force patterns. J Exp Biol 199: 801–814, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Prilutsky BI, Zatsiorsky VM. Tendon action of two-joint muscles: transfer of mechanical energy between joints during jumping, landing, and running. J Biomech 27: 25–34, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Roberts TJ, Belliveau RA. Sources of mechanical power for uphill running in humans. J Exp Biol 208: 1963–1970, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Roberts TJ, Higginson BK, Nelson FE, Gabaldon AM. Muscle strain is modulated more with running slope than speed in wild turkey knee and hip extensors. J Exp Biol 210: 2510–2517, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science 275: 1113–1115, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Rome LC, Sosnicki AA, Goble DO. Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J Physiol 431: 173–185, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scovil CY, Ronsky JL. Sensitivity of a Hill-based muscle model to perturbations in model parameters. J Biomech 39: 2055–2063, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Seow CY, Ford LE. Shortening velocity and power output of skinned muscle fibers from mammals having a 25,000-fold range of body mass. J Gen Physiol 97: 541–560, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sousa F, Ishikawa M, Vilas-Boas JP, Komi PV. Intensity- and muscle-specific fascicle behavior during human drop jumps. J Appl Physiol 102: 382–389, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Van Ingen Schenau GJ On the action of bi-articular muscles, a review. Neth J Zool 40, 1990.
- 45.Van Soest AJ, Schwab AL, Bobbert MF, van Ingen Schenau GJ. The influence of the biarticularity of the gastrocnemius muscle on vertical-jumping achievement. J Biomech 26: 1–8, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Widrick JJ, Romatowski JG, Karhanek M, Fitts RH. Contractile properties of rat, rhesus monkey, and human type I muscle fibers. Am J Physiol Regul Integr Comp Physiol 272: R34–R42, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Zajac FE Muscle coordination of movement: a perspective. J Biomech 26, Suppl 1: 109–124, 1993. [DOI] [PubMed] [Google Scholar]