Abstract
The locus ceruleus (LC) contains neurons that increase their firing rate (FR) in vitro when exposed to elevated CO2/H+ and have been proposed to influence the respiratory network to make compensatory adjustments in ventilation. Prp57 transgenic mice express green fluorescent protein (GFP) in the LC and were used to isolate, culture, and target LC neurons for electrophysiological recording. We hypothesized that GFP-LC neurons would exhibit CO2/H+ chemosensitivity under primary culture conditions, evidenced as a change in FR. This is the first study to quantify CO2/H+ responses in LC neuron FR in cell culture. Neurons were continuously bathed with solutions containing antagonists of glutamate and GABA receptors, and the acid-base status was changed from control (5% CO2; pH ∼7.4) to hypercapnic acidosis (9% CO2; pH ∼7.2) and hypocapnic alkalosis (3% CO2; pH ∼7.6). FR was quantified during perforated patch current clamp recordings. Approximately 86% of GFP-LC neurons were stimulated, and ∼14% were insensitive to changes in CO2/H+. The magnitude of the response of these neurons depended on the baseline FR, ranging from 155.9 ± 6% when FR started at 2.95 ± 0.49 Hz to 381 ± 55.6% when FR started at 1.32 ± 0.31 Hz. These results demonstrate that cultured LC neurons from Prp57 transgenic mice retain functional sensing molecules necessary for CO2/H+ responses. Prp57 transgenic mice will serve as a valuable model to delineate mechanisms involved in CO2/H+ responsiveness in catecholaminergic neurons.
Keywords: CO2/H+ chemosensitivity, central respiratory chemoreceptors, carbon dioxide, primary cell culture, pH response
central respiratory chemoreceptors detect fluctuations in CO2 and/or pH, alter their neuronal activity, and subsequently influence the respiratory network to appropriately adjust ventilation. Chemoreceptors were first proposed to be located in distinct cellular aggregations at or near the ventrolateral medullary surface (37, 57). However, it is now well established that there are many neurons widely distributed along the neuraxis that increase their firing rate (FR) in response to acidosis, including cells within the ventrolateral medullary surface (36, 37, 57), medullary raphé (6, 49, 51, 70, 71, 74), pre-Bötzinger complex (34, 52, 61), retrotrapezoid nucleus (RTN) (20, 27, 56), hypothalamus (15, 16, 75), nucleus tractus solitarius (NTS), and locus ceruleus (LC) (12, 18, 22, 23, 28, 33, 40, 42, 53, 55). It has been proposed that all of these groups of pH-sensitive neurons are central respiratory chemoreceptors, each one contributing to the increased respiratory motor output to ensure that the pH of the blood and cerebrospinal fluid is restricted to normal physiological limits (9, 10, 41). The relative importance of each one to the overall response, and the conditions under which each one contributes, is not known.
Considerable evidence suggests that the LC contains chemosensitive neurons that participate in the ventilatory response to CO2/H+ (22, 23, 33, 35, 44–46, 54, 56, 63). This dorsal pontine region bilaterally situated at the floor of the fourth ventricle, contains the greatest concentration of catecholaminergic neurons in the central nervous system and is known to exhibit arousal state-dependent activity and play a modulatory role in the processing of sensory information, arousal, feeding behaviors, nociception, and cardiovascular control (3, 32, 45). The relative importance of LC neurons in central chemoreception is unclear. However, retrograde labeling studies have provided evidence for direct connections from the LC to phrenic motoneurons (17). Focal acidosis within the LC causes an increase in respiratory frequency and phrenic nerve discharge in cats (9). Furthermore, LC neurons have been suggested to play an essential role in the proper development of the respiratory network (68) and exhibit a modulatory action on respiratory rhythm (2, 8, 26, 31, 43, 65). Targeted elimination of the A6/A5 noradrenergic cell groups with antidopamine beta hydroxylase conjugated to saporin administered into the fourth ventricle (35) or focal deletion of LC cells with 6-hydroxydopamine (7) results in a 28 and 64% reduction of the hypercapnic ventilatory response, respectively. Haxhiu and coworkers have shown that prolonged exposure to elevated CO2 induces c-fos expression in the LC in vivo (29). Hypercapnic acidosis (HA) leads to an increase in FR in >80% of neurons in the LC in vitro (4, 5, 45, 46). This response is due in part to a decrease in intracellular pH (22) but can also occur in response to a decrease in extracellular pH alone (28).
Most of the aforementioned studies were conducted in whole animals, brain slices, or en bloc brain stem spinal cord preparations. However, the chemosensory properties of neurons from other regions have been studied in dissociated cell cultures. Studies were conducted on respiratory neurons cultured from the upper medulla (24), and respiratory pacemaker-like cells exhibited responses to hypercapnia (52). Richerson and coworkers quantified CO2/H+ chemosensitive responses of medullary raphé respiratory chemoreceptors in primary culture (71, 72, 74) and found that the FR of 73% of serotonergic neurons was increased by small elevations in CO2/H+ (74). More recently, Su et al. (64) studied CO2 chemosensitivity in cultured neurons (from the medulla and pons) using microelectrode array recordings and found that 20% of the neurons were stimulated by CO2. Although culture conditions provide much better recording stability than is possible in slices, lead to better isolation of intrinsic responses, and allow optimal access and control over the external environment of the cells, it was necessary in these studies to use immunohistochemistry after recording to confirm the phenotype of each neuron.
Additional studies are needed to define the intrinsic properties of putative central chemoreceptive neurons to determine the mechanisms that induce changes in neuronal FR during CO2/pH challenges. We have employed primary cell culture and patch-clamp recordings to study catecholaminergic neurons of the LC phenotypically identified by endogenous expression of green fluorescent protein (GFP). The present investigation focuses on characterization of the CO2/H+ responsiveness of cultured GFP-LC neurons obtained from the Prp57 transgenic mouse (67) and assesses the feasibility of the transgenic model for future studies. A portion of the data presented in this paper was previously published in abstract form (33).
MATERIALS AND METHODS
Prp57 transgenic mice expressing GFP in the LC.
Previous reports have described the methods used to generate the Prp57 transgenic mouse strain, which exhibits strong expression of GFP in the LC (66, 67). In brief, a mouse prion promoter was used to drive a cDNA construct of an enhanced, red-shifted, mammalian codon-corrected GFP. Greater than 98% of GFP-expressing LC neurons found in the Prp57 mouse also express the enzymatic marker, tyrosine hydroxylase (TH), which is used to identify noradrenergic neurons of the LC (67). There were no significant differences found in basic electrophysiological properties between GFP-expressing LC neurons from slices of transgenic mice to non-GFP-expressing neurons from nontransgenic mice of the same strain (67). All animal procedures were conducted in accordance with experimental protocols approved by the Yale University Animal Care and Use Committee.
Primary cell culture.
Using aseptic technique, GFP-LC primary cell cultures were prepared using protocols previously described for raphé neurons by Richerson and coworkers (71–74). Briefly, Prp57 neonatal mouse pups (P0–P3) were rapidly decapitated, and a craniotomy was performed to expose the dorsal surface of the brain and spinal cord. Transverse cuts were made rostral to the midbrain-pontine border and at the level of the cervical spinal cord (C1–C3). The brain stem-spinal cord was sliced rostrocaudally with a McIlwain tissue chopper (Frederick Haer, Bowdoin, ME) in ∼500-μm increments or manually with a microknife until the level was reached containing the LC. GFP expression was initially verified in pontine slices using a fluorescence microscope (Fig. 1), and tissue samples including the region of the LC were bilaterally excised from three to four neonatal mice. There was a limited possibility that we obtained GFP-expressing cells from an area outside of the LC, but slices were cut in a manner that minimized the possibility of contamination from the closest area that also expressed GFP, the A5 medullary region. The LC tissue samples were initially stored in HEPES-buffered solution containing (in mM) 130 NaCl, 4 KCl, 1 MgCl2, 1.5 CaCl2, 10 HEPES, 10 dextrose, and 3 NaOH, pH 7.3. Samples were then transferred to HEPES digestion solution containing papain (100 units/10 ml) (Worthington-Biochem, Lakewood, NJ) and placed on a rotator within an incubator with 5% CO2 and 95% air at 37°C. Subsequently, tissue was triturated in modified Eagle's medium and plated onto poly-l-ornithine-coated glass coverslips at a density of ∼50,000 cells/ml. Approximately 1 h after plating, a single coverslip was examined with a fluorescence microscope equipped with GFP filters to verify GFP expression. Cultures were maintained in glial-conditioned medium (10% fetal bovine serum in 70% modified Eagle's medium + 30% Neurobasal medium with B27 supplement + penicillin-streptomycin). Basic fibroblast growth factor (0.1–1 ng/ml) and fibroblast growth factor 5 (1–10 ng/ml) were also added to enhance neuronal survival. On days 4–7, a one-half volume change was made with Neurobasal/B27 supplemented with cytosine α-d-arabinofuranoside hydrochloride (0.3–3 μM) to reduce glial growth. Subsequent maintenance feedings were made every 1–2 wk with a one-half volume change of Neurobasal/B27. Cell cultures established under these conditions were characterized by a glial bed with neurons dispersed on its surface and were maintained for periods ≥8 wk.
Fig. 1.
Expression of green fluorescent protein (GFP) was used to target locus ceruleus (LC) neurons. A: fluorescence photomicrograph of a pontine slice from a P2 Prp57 transgenic mouse pup that expressed GFP in the LC. Fluorescently labeled neurons appear as a densely packed elongated nucleus at the level of the fourth ventricle (4V; arrowheads) lateral to the midline (scale bar, 200 μm). Inset: the region bilaterally excised for primary cell culture. B–C: the dendritic processes and cell bodies of individual neurons (arrows) are seen at high magnification (scale bars, 100 μm and 20 μm, B and C, respectively). D: neurons in culture were targeted for patch-clamp recordings using visible light Nomarski differential interference contrast (DIC) and fluorescence (scale bar, 50 μm).
Perforated patch electrophysiological recordings.
Coverslips were transferred to a recording chamber (E. W. Wright, Guilford, CT). The chamber was mounted on a fixed-stage upright microscope (Axioskop 2 FS, Zeiss) equipped with visible light Nomarski differential interference contrast and fluorescence capabilities. Candidate cells for chemosensitivity tests were identified by expression of GFP and subsequently targeted for patch-clamp recordings. Our goal was to obtain a homogeneous data set from GFP-LC neurons. The cultures did include some cells from areas adjacent to the LC that were likely to be heterogeneous; however, we specifically focused on GFP-LC neurons and did not study cells that did not express GFP. However, earlier experiments from cultured rat neurons indicated that the majority of neurons cultured from this region (most of which are not catecholaminergic) were not chemosensitive. All experiments were conducted at room temperature while extracellular pH was continuously monitored with an electrode inserted into an inflow port to the recording chamber (MI-414; Microelectrodes, Londonderry, NH).
Under control conditions, neurons were bathed with Ringer solution containing (in mM) 124 NaCl, 26 NaHCO3, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, and 10 dextrose equilibrated with medical-grade-certified 95% O2 and 5% CO2 (Airgas Northeast, Cheshire, CT). To eliminate fast glutamatergic and GABAergic synaptic transmission, all bath solutions included kynurenic acid (1 mM) and picrotoxin (100 μM) during recordings (71). Electrodes (3–10 MΩ) were fabricated from borosilicate glass using a micropipette puller (Sutter P-97, Sutter Instruments) and filled with an intracellular solution containing (in mM) 135 KOH, 135 methanesulphonic acid, 10 KCl, 5 HEPES, and 1 EGTA; pH 7.2; osmolarity 275 ± 10 mosM. Perforated patch recordings were made using gramicidin (50–100 ng/ml) (69). All recordings were made in current clamp mode. Neurons were considered healthy when the resting membrane potential was less than or equal to −40 mV and action potential height was ≥60 mV. Cells were also considered healthy with maintenance of continuous spiking, either spontaneous or with depolarizing current injection under control conditions (5% CO2, pH ∼7.4). Signals were amplified (Axopatch 1D, Axon Instruments), filtered (10-kHz low pass), and acquired at 10 kilosamples/s with a computerized data acquisition board (PCI-MIO-16E-1, National Instruments, Austin, TX) using custom-written software.
Once a stable membrane potential was achieved, non-spiking neurons were depolarized with current until a consistent baseline FR was obtained. In most cases, the level of depolarizing current was then maintained constant throughout the rest of the experiment to enable monitoring of CO2/H+ induced changes in neuronal firing patterns. Neurons requiring a hyperpolarizing current to stabilize FR and membrane potential were not included in the analysis of chemosensitivity since this can be an indication of an unhealthy recording.
Solutions used to alter pH and CO2.
FR was measured under three conditions; control (5% CO2, pH ∼7.4), HA (9% CO2, pH ∼7.2), and hypocapnic alkalosis (3% CO2, pH ∼7.6). Each analyzed neuron was subjected to a minimum of four CO2-induced pH transitions to establish the consistency of its response. Prior to any acid-base perturbation, a baseline FR was established at 5% CO2, pH ∼7.4. In most neurons, this was followed by a change to 9% CO2 with washout back to control, followed subsequently by exposure to 3% CO2 and return to 5% CO2. For stable recordings, multiple changes were then made between 5 and 9% CO2. In other cases, the baseline FR was established at 5%, and then cells were exposed to 3% CO2 followed by return to 5% CO2, and then multiple changes between 5 and 9% CO2. Based on these protocols, neurons were classified either as CO2/H+ stimulated or insensitive. We did not encounter any neurons inhibited by CO2/H+.
Data analysis and quantification of response to CO2/H+.
GFP-LC neurons were evaluated to assess their chemosensitive properties based on the criteria previously used to define responsiveness of medullary raphé neurons in culture (49, 71–74). We defined GFP-LC neurons as chemosensitive if they met the following criteria: 1) reproducible responses to a minimum of four pH transitions; 2) consistency in time course for each pH response; 3) opposite responses to acidosis and alkalosis if exposed to both; and 4) a statistically significant increase or decrease in mean FR of at least 20% for a 0.2-unit change in pH (P ≤ 0.05, Student's t-test). Cells that exhibited consistent increases in FR during acidosis and reductions in FR during alkalosis were classified as acidosis-stimulated neurons. GFP-LC cells that did not consistently respond to CO2/H+ challenges were considered CO2/H+ insensitive. Our approach was to establish whether a neuron had a reproducible response and to avoid false classification due to random changes in neuronal FR that might be mistaken for chemosensitivity. However, in general, if a neuron responded once it would respond every time it was exposed to acidosis.
Neuronal responses were quantified from data obtained with a software-based spike threshold detection algorithm that generated FR plots using a moving average (bin size, 1–2 s, 20 bins per point). The steady-state mean FR and pH were calculated from the last 60 s of each epoch at each pH level as previously described (74). The mean FR during steady state was determined for each neuron under a given test condition and compared with the control period immediately before and after the pH change to reduce the effect of FR variability. Values for percent change in FR were calculated by dividing the steady-state FR during challenge by the control FR. For example, a neuron's FR would be 150% of control when the FR was 3 Hz during acidosis and 2 Hz during the control period. We also calculated the chemosensitivity index (CI) for each neuron, as previously described (71). Neurons exhibited FRs between 1 and 3 Hz under control conditions. We did, in some cases, encounter neurons that had a baseline FR that was <0.2 Hz. This would lead to a deceptively large percent change in FR. For those neurons, we substituted a baseline FR of 0.2 Hz to produce a more conservative estimate of the percentage change in FR, as was previously done for neurons of the medullary raphé (71). Data are presented means ± SE, unless otherwise noted. A Student's t-test (two-tailed with unequal variance; Microsoft Excel) was used to determine whether means were significantly different (P < 0.05, unless otherwise noted). NS indicates that results were not statistically different.
TH immunohistochemistry.
We performed immunofluorescence experiments to verify that GFP-LC neurons produced TH and to characterize their morphological features. Coverslips were fixed with 4% paraformaldehyde for 1 h, rinsed with phosphate-buffered saline (PBS; pH ∼7.35), and stored in cryoprotectant solution (1% polyvinylpyrrolidone, 30% sucrose, 30% ethylene glycol in 0.05 M phosphate buffer) until processed. Coverslips were washed with 0.1 M PBS three times for 10 min each. Nonspecific binding was reduced by incubating cultures in blocking solution containing 5% normal goat serum (NGS) in 0.1 M PBS + 0.3% Triton-X for 30 min. Coverslips were incubated (18–24 h at 4°C) with a rabbit polyclonal antiserum against TH (1:2,000, Chemicon) diluted in PBS with 0.3% Triton-X and 2% NGS and then washed with 0.1 M PBS for 30 min. Next, coverslips were exposed to goat anti-rabbit rhodamine conjugated secondary antibody (1:200) at room temperature for 2 h to produce red fluorescent labeling of TH-positive neurons. For negative controls, primary antibody was excluded from the protocol.
Coverslips were mounted onto glass slides with Vectashield mounting medium (Vector Laboratories) and observed with a fluorescence microscope using filters for GFP and rhodamine (Olympus Provis). We identified and counted GFP-LC neurons from two litters of Prp57 mice cultured on different dates and determined how many of these GFP-LC neurons also expressed TH.
RESULTS
Endogenous GFP and TH immunofluorescence in cultured LC neurons.
Unlike previous studies that required the use of immunostaining to determine the phenotype of neurons after recording the response to changes in CO2/H+, the expression of GFP by catecholaminergic LC neurons allowed us to target a specific cell type before electrophysiological recording. To validate this approach, we examined the expression of GFP in neurons cultured from the LC of Prp57 mouse pups and determined that GFP-LC neurons coexpressed TH (Fig. 2, A–C). Total cell counts of 337 GFP-expressing neurons from two independent culture dates revealed that 98.89% (333 of 337) were immunoreactive for TH (Fig. 2D).
Fig. 2.
Morphology of LC neurons in culture. A: GFP-LC neurons were immunoreactive (IR) for TH (scale bar, 20 μm). B: the cell bodies, nuclei, and dendritic processes of GFP-LC neurons were clearly discernable (scale bars, 20 μm). Left two images are TH immunofluorescence, and right two images are GFP fluorescence. C: this neuron was identified by visualizing expression of GFP (inset; scale bar, 20 μm), and TH immunohistochemistry allowed visualization of the dendritic field (scale bar, 100 μm). Although multiple processes were TH-IR, it is possible that some of the dendrites emanated from nearby overlapping cells. D: most LC neurons were found to coexpress both GFP and TH. Bar graph shows the number of cells that expressed GFP (green), were TH immunofluorescent (red), and were both GFP+ and TH-IR (yellow).
Neurons that coexpressed GFP and TH immunoreactivity were most commonly characterized by large cell bodies with multiple dendrites. The somata of these neurons ranged from ∼20 to 30 μm in diameter with a prominent nucleus. TH immunoreactivity revealed elaborate labeling of neuronal processes that was better visualized than with GFP fluorescence (Fig. 2B). Some of the processes had projections with en passant swellings that were detectable.
Effect of pH changes on GFP-LC neuron FR.
We quantified responses to changes in CO2/H+ from LC neurons cultured for 12–56 days (32.9 ± 12 days; mean ± SD). This corresponds to previous studies conducted on raphé neurons where chemosensory responses were shown to develop in culture and in brain slices at ∼12 days (72), and other neurons studied in dissociated cultures (24, 52, 64). We did not attempt to make patch recordings before this time.
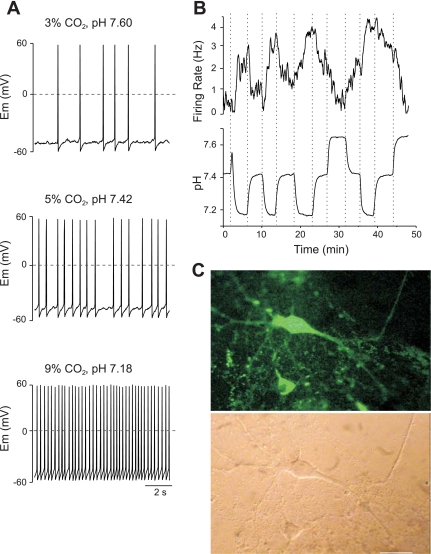
We targeted 36 GFP-LC neurons, exposed them to 9% CO2, and determined that 31 of 36 GFP-LC cells (86.1%) were stimulated by HA and 5 of 36 GFP-LC cells (13.9%) were insensitive to HA. HA stimulated neurons exhibited a reproducible increase in FR during exposure to 9% CO2/91% O2 and a bath pH of ∼7.2. As the bath pH decreased, FR increased within the first 30–60 s of the pH change reaching steady-state FR within 2–4 min (Figs. 3–5). The FR was sustained above baseline throughout exposure to HA and decreased toward baseline on switching back to control solution. The FR during HA ranged from 122 to 927% of the baseline FR with a mean of 250.6 ± 30.2% (n = 31). The confidence internal (CI) ranged from 116 to 535%, with a mean of 220 ± 19.5% (n = 31). The morphology and CO2/H+ response for a pH-sensitive GFP-LC neuron is shown in Fig. 3, where the mean FR increased from 1.82 Hz at baseline to 3.02 Hz during HA exposure or an increase to 165.9% of control. Subsequent pH tests revealed that hypocapnic alkalosis exposure caused the FR of this cell to decrease to 80.2% of the baseline FR.
Fig. 3.
GFP-LC neuron stimulated by hypercapnic acidosis (HA). A: membrane potential (Em) of a GFP- LC neuron stimulated by HA. Top: hypocapnic alkalosis (3% CO2, pH ∼7.6). Middle: control (5% CO2, pH ∼7.4). Bottom: HA (9% CO2, pH 7.2). The mean FRs (in Hz) were 1.01 during alkalosis, 1.82 in control solution, and 3.02 during acidosis. B: simultaneous recordings of FR (top) and extracellular bath pH (bottom) induced by alterations in CO2. C: this LC neuron was visualized with both fluorescence microscopy (top) and Normaski DIC optics (bottom). Scale bar, 50 μm.
Fig. 5.
Changes in FR of high response GFP-LC neurons during HA. Shown are two examples of high response neurons, one with a baseline FR that was >2 Hz (A) and the other with a baseline FR that averaged <2 Hz(B). Both cells exhibited a large increase in FR during HA.
Some GFP-LC neurons were more sensitive to small fluctuations in pH than others (Figs. 4 and 5). Based on their CO2 response, we established two categories of HA-stimulated neurons: low responders (<200% of baseline FR during HA; n = 18; 58%) and high responders (>200% baseline FR during HA; n = 13; 42%) with group means of 155.9 ± 6% and 381 ± 55.6%, respectively. The CI for low- and high-response neurons was 146 ± 5.8 and 332 ± 26.4%, respectively. Examples of recordings of high responder GFP-LC neurons are shown in Figs. 4 and 5. The cell in Fig. 4 exhibited FRs (in Hz) of 0.39 (alkalosis), 1.37 (baseline), and 4.86 (acidosis) and increased its FR to a mean of 354.7% of baseline during HA exposure. The CI was 273%. The magnitude of the response was not dependent on the order of presentation of pH perturbation. In this case, the cell shown in Fig. 4 was exposed to hypocapnic alkalosis after establishing a baseline FR. Here, we observed a reduction in FR during exposure to alkalosis followed by a progressive increase in FR as the CO2 increased from 3 to 5 to 9%. Subsequently, the neuron was exposed to control solution followed by multiple transitions between 5 and 9% CO2. The FRs during HA and hypocapnic alkalosis were statistically different from the baseline (P < 0.001). High response neurons shown in Fig. 5 consistently increased and decreased FR as expected during shifts between acidosis and alkalosis.
Fig. 4.
Membrane potential of a high response GFP-LC acidosis-stimulated neuron. A: membrane potential of a cultured LC neuron recorded during hypocapnic alkalosis (3% CO2, pH ∼7.6); control conditions (5% CO2, pH ∼7.4); and HA (9% CO2, pH ∼7.2). B: changes in FR and pH induced by changes in CO2. Exposure to 3 or 9% CO2 caused a reproducible inhibition or stimulation of spontaneous FR, respectively. Traces in A were taken from the first three episodes shown in B.
A low response HA-stimulated neuron is shown in Fig. 6. This neuron received a depolarizing current injection of 130 pA to establish a baseline FR and this was maintained during acid challenges. The neuron exhibited a reproducible increase in FR in response to acidosis during the first three trials. At the end of the third trial, the FR was lower than the baseline. Subsequently, the depolarizing current was increased from 130 to 140 pA to reestablish the baseline FR at 5% CO2, and the CO2/H+ challenge was repeated. The cell continued to respond to acid challenge with a similar responsiveness. This GFP-LC HA-stimulated neuron also responded to alkalosis with a reduction in FR during transition to 3% CO2.
Fig. 6.
Changes in FR of a low-response GFP-LC neuron during HA. Simultaneous recordings of FR and pH from a low-response GFP-LC acidosis-stimulated neuron. The vertical line marks a point at which the level of depolarizing current was increased to adjust the baseline FR. The magnitude of the response was similar before and after the increase in depolarizing current.
All of the HA-stimulated neurons tested for responses to alkalosis exhibited a reproducible reduction in FR with a mean reduction of 30.2 ± 5.11% so that cells fired at 69.8 ± 5.36% of baseline FR.
The magnitude of CO2/H+ responsiveness was dependent on baseline FR.
We found that the likelihood of a neuron being classified as a low or high response cell was dependent on the baseline FR (Fig. 7; Tables 1 and 2). The majority of high response cells (11 of 13, 85%) had a baseline FR of <2 Hz (mean 1.04 ± 0.17 Hz). During HA, these neurons increased their FR on average to 392.7 ± 82.1% of the baseline FR. In contrast, high response cells that started out with a baseline FR of >2 Hz only increased their FR to 243 ± 38.2% of control during HA exposure (P = 0.056).
Fig. 7.
The response of GFP-LC neurons was dependent on baseline FR. Neurons that had a lower baseline FR (<2 Hz) had a greater increase in FR than neurons with higher baseline FRs (>2 Hz).
Table 1.
CO2/H+ responsiveness for GFP-LC neurons during hypercapnic acidosis and hypocapnic alkalosis
| Condition | n | pH | Firing Rate, % of Control |
|---|---|---|---|
| Acidosis stimulated | |||
| Control (5% CO2) | 31 | 7.43±0.03 | 100.0±0.00 |
| Acidosis (9% CO2) | 31 | 7.19±0.04 | 250.6±30.2* |
| Alkalosis (3% CO2) | 14 | 7.64±0.03 | 69.8±5.11* |
| Acidosis insensitive | |||
| Control (5% CO2) | 5 | 7.42±0.01 | 100.0±0.00 |
| Acidosis (9% CO2) | 5 | 7.21±0.01 | 105.9±1.34 |
Values are means ± SE.
Values are statistically different from control (P < 0.001).
Table 2.
Effect of baseline firing rate on the response to hypercapnic acidosis
| Baseline Firing Rate | <2 Hz | >2 Hz | All Neurons |
|---|---|---|---|
| High response | 392.7±82.1 (n=11) | 243±38.2 (n=2) | 381.6±55.6*(n=13) |
| Mean baseline firing rate | 1.04±0.17 | 2.85±0.05 | 1.32±0.31† |
| % of neurons | 85% | 15% | |
| Low response | 166±7.9 (n=9) | 149.6±12.7 (n=9) | 155.9±6.0*(n=18) |
| Mean baseline firing rate | 1.14±0.17 | 4.04±0.76 | 2.95±0.49† |
| % of neurons | 50% | 50% |
Values are means ± SE. The firing rate during hypercapnic acidosis (expressed as a % of control) shown for high-response and low-response neurons, was statistically different
(P < 0.001) as were the baseline firing rates
(P = 0.006).
As a group, low-response neurons had a higher baseline FR than high-response neurons. The mean baseline FR was 2.95 ± 0.49 Hz (n = 18) for low-response neurons compared with 1.32 ± 0.31 Hz (n = 13) for high-response neurons. This difference in baseline FR was statistically significant (P = 0.006). However, among low-response neurons, there was no correlation between baseline FR and CO2/H+ responsiveness; r2 = 0.014. Thus 50% of low-response cells had a baseline FR of <2 Hz and 50% had a baseline FR of >2 Hz. The mean increase in FR during HA for these two groups was 166 ± 7.9% (<2 Hz) and 149.6 ± 12.7% (>2 Hz), respectively (NS, P = 0.31).
Acidosis-insensitive GFP-LC neurons.
Of GFP-LC neurons, 13.9% (5 of 36) did not change their FR by >20% in response to HA. The action potentials of insensitive neurons were stable and repetitive with membrane potential trajectories that were similar to stimulated neurons. There were no distinguishing morphological or electrophysiological features of these neurons. The average baseline FR for HA-stimulated neurons was 2.26 ± 0.32 Hz (n = 31) and 2.16 ± 0.73 for insensitive neurons (P = 0.89). The average membrane potential obtained after formation of a perforated patch was −58.75 ± 1.21 and −55.6 ± 3.2 mV for HA-stimulated and -insensitive neurons, respectively (P = 0.40). The mean FR of insensitive neurons during HA was 105.9 ± 1.34% of baseline FR. An example is shown in Fig. 8, where the FR remained ∼1 Hz throughout the CO2/H+ protocol.
Fig. 8.
Membrane potential and firing rate of a CO2-insensitive GFP-LC neuron. A: membrane potential of a CO2/H+-insensitive GFP-LC neuron during exposure to hypocapnic alkalosis (top), control conditions (middle), and HA (bottom). B: the FR of the same neuron did not change in response to acidosis or alkalosis.
Effect of age in culture on responsiveness to hypercapnia.
The number of days that a neuron was maintained in culture did not have a significant effect on CO2/H+ responsiveness. We compared the responses to HA of cells maintained in culture for 12–28, 29–34, and 35–56 days in vitro (DIV) (Fig. 9). There was no significant difference in the response to acidosis of cells 12–28 DIV (P = 0.85) or 35–56 DIV (P = 0.31) compared with 29–34 DIV. There was also no difference in the response of cells 12–28 DIV compared with those 35–56 DIV (P = 0.18).
Fig. 9.
The number of days in culture did not influence CO2/H+ responsiveness. Cells in culture <29 days and >35 days were compared with neurons in culture 29–34 days. There was no statistical difference in CO2/H+ responsiveness of neurons over this age range.
DISCUSSION
We have used perforated patch recordings from the Prp57 transgenic mouse that expresses GFP in catecholaminergic neurons of the LC (66, 67) to quantify changes in the FR of LC neurons in primary cell culture during CO2/H+ challenge. Consistent with previous studies from slices, we found that cells from the LC exhibited chemosensitivity in culture after pharmacological block of fast excitatory and inhibitory synaptic transmission. Our results show that >85% of GFP-positive LC neurons are stimulated by elevated CO2/H+.
Comparison to previous work on chemosensitivity of LC neurons.
In vitro investigations have provided an extensive body of information regarding LC responsiveness to changes in CO2. The first report of LC chemosensitivity (46) revealed that 38 of 40 cells reduced their FR to ∼64% of baseline when CO2 was reduced from 5% to 0%. When CO2 was increased from 2.5% CO2 to 10% CO2 or 15% CO2, LC neurons increased their FR by 53 and 54%, respectively (46). Since then, additional studies have reported similar results, with LC neurons having relatively low sensitivity to pH fluctuations during recordings from brain slices (22, 28, 48, 54, 56) and the in vitro brain stem spinal cord preparation (45).
The responses we observed here from LC neurons in cell culture (a mean increase in FR to 251% of baseline in response to 9% CO2) were considerably larger than those previously reported in brain slices (up to ∼193% of baseline in response to 15% CO2) (22, 45, 46, 54, 56). The reasons for the larger response are not clear. One possibility is that LC neurons studied in cell culture are older than those studied in slices, since this leads to larger responses in raphé neurons (see below). However, others have not found a significant effect of age on chemosensitivity of LC neurons (47, 63), and we did not find a significant effect of age on the response of LC neurons in culture after P12 (Fig. 9). Another possibility is that, in culture, the entire cell body and all dendritic arborizations are exposed to changes in bath pH, and/or there is less pH buffering by surrounding cells, as opposed to slices where many dendritic processes are cut or are beneath the surface of the slice where they are protected from bath pH changes. However, previous work demonstrated that a full increase in LC neuron FR induced with HA was only seen with somatic stimulation, and that stimulation of dendrites alone evoked no FR response (54). An additional possibility is that the recordings in brain slices were from LC neurons with a higher baseline FR (1.95 ± 0.12 Hz) (22) than our recordings from cultured neurons (1.04 ± 0.17 Hz). Indeed, the responses of LC neurons reported in brain slices are consistent with our low response group in culture. It will be important to consider this possibility in the future and will be useful to present data in more ways than just the percent change from baseline, as done here and in recent work from raphé neurons (70).
Possible mechanisms for enhanced HA responsiveness in a subpopulation of chemosensitive GFP-LC neurons.
More than 90% of LC neurons in slices or brain stem spinal cord preparations are CO2 responsive, representing the largest concentration of pH-sensitive neurons for any identified putative central chemoreceptive site (46). Similarly, we observed that 86.1% of cultured GFP-LC neurons were stimulated by HA during recordings where we blocked glutamate and GABA receptors. These conditions eliminate the possibility of fast glutamatergic or GABAergic synaptic influences of chemosensitive neurons on non-chemosensitive cells (25, 49, 71, 72, 74). Thus our data are consistent with studies from slice and en bloc brain stem spinal cord preparations with regard to the percentage of neurons exhibiting responses to CO2/H+.
There is evidence that LC neuron chemosensitivity is highly dependent on gap junctions (28). There may be differences in gap junction expression in our culture system compared with brain slices. Gap junctions may play a role in the regulation of ventilation during development before the maturation of synaptic connections (11, 44, 58–60, 62). Gap junctions have been shown to enhance or amplify the CO2 response observed in younger animals in the RTN and NTS (30). In a recent study, Hartzler et al. (28) provided evidence that gap junctions influence the percentage of LC neurons with intrinsic chemosensitivity at different ages during development from P0 up to P18. They found that 100% of LC neurons at P0–P4, 80% at P5–P9, and 42% at P10–P18 were stimulated by HA with intact gap junctions. However, when gap junctions were blocked, the percentage of LC neurons activated by HA fell to 50% at P5–P9 and 25% at P10–P18; the P0–P4 group was unaffected. These data suggest that LC chemosensitivity and CO2 responsiveness changes during development and is dependent on gap junction coupling within the region. We did not explore the possible role of gap junctions in this study, nor did we attempt to block other forms of chemical synaptic transmission, which may have occurred between neurons within our cultures.
There are other aspects of our experimental approach that could lead to differences in the degree of chemosensitivity of LC neurons compared with previous work. For example, there may be differences in glial influences in culture compared with brain slices (20). Our use of 95% O2 could also alter excitability of LC neurons. Recent studies by Dean and coworkers (13, 14, 38, 39) indicate that some central chemoreceptive neurons in the solitary complex are stimulated by high levels of O2 and reactive oxygen species. It is not known whether increased O2 levels can lead to an increase in the response of chemosensitive neurons to CO2/H+, although this is a possibility. Since most in vitro studies and all of the previous studies on raphé neurons were conducted using 95% O2, we followed the same protocol for studies of cultured LC neurons. This enabled a direct comparison of LC responses to previous data from cultured raphé neurons.
Another important consideration is that we performed our experiments at room temperature. This limits comparison to data from studies that were conducted on LC neurons at higher temperatures, since it is possible that temperature modulates responsiveness to CO2/H+.
Comparison to previous studies of chemosensitive 5-HT neurons in culture.
Dissociated cultures have previously been used to characterize the electrophysiological properties of chemosensitive neurons in the NTS and nucleus ambiguus (24, 52), medulla and pons (64), and medullary raphé (71). These studies employed different approaches to test for and quantify CO2 responsiveness. To allow direct comparisons, we used the same data acquisition and analysis protocols as used previously to study medullary raphé cells in culture (71, 73). Those previous studies were done on unidentified raphé neurons that were then subsequently verified to be serotonergic using immunohistochemistry for tryptophan hydroxylase. In those experiments, only 15–30% of recorded cells were HA stimulated. However, 73% (22 of 30) of those neurons later identified to be serotonergic neurons were HA stimulated (74). Similarly, we initially began by recording from cultured LC neurons from rats, in which we randomly recorded from neurons and identified LC neurons using TH immunohistochemistry after recording. In that case, most of the cells we recorded were TH negative and unresponsive to changes in pH.
A greater percentage (86.1%) of GFP-LC neurons responded to CO2 with at least a 20% increase in FR, but LC neurons were slightly less chemosensitive on average than medullary raphé neurons. However, there is overlap between these two cell types, with some chemosensitive LC neurons increasing their FR to >392% of baseline and having an average CI of 332%, a response that is equivalent to some medullary raphé neurons.
Unlike our recordings from cultured LC neurons, raphé neurons increase their chemosensitivity with age in culture and in brain slices (72). Since it is difficult to make stable recordings from raphé neurons in brain slices older than P21, all the published data on chemosensitivity of raphé neurons in slices are from these younger animals, and as such the responses are not as large as many published examples of chemosensitive raphé neurons in culture. However, when raphé neurons of the same age are compared between P5 and P21, the degree of chemosensitivity is approximately the same in culture and slices. Thus there is no evidence that raphé neurons are more chemosensitive in culture than in slices.
In prior experiments, it was found that the morphological features of HA-activated raphé neurons distinguished them from HA inhibited cells from that region (71). Our TH immunofluorescence allowed detailed morphological analysis of LC neurons. Insensitive cells as well as low and high response HA activated cells all expressed GFP, and showed no obvious differences in appearance. Therefore, there was no means to predict based on morphological traits whether a cell would respond, and if it did whether it would be classified as a low or high response neuron or insensitive.
Why are some LC neurons more sensitive than others?
All cultured GFP-LC neurons did not respond with the same robustness. Forty-two percent of HA-stimulated neurons were highly stimulated (Figs. 4 and 5), whereas other GFP-LC neurons responded with a less robust response to the same pH challenge. There are several possible explanations for this. One possibility is that GFP-LC neurons have a heterogeneous composition of pH-sensitive mechanisms. This hypothesis is supported by the recent work by Su et al. (64), which suggests that chemosensitive cells cultured from the medulla and pons may express a broad spectrum of pH-sensitive molecules. Another possibility is simply that all CO2-sensitive neurons have more or less a similar capability for chemosensitivity, but their response depends on the baseline conditions such as the baseline FR, as shown here. A third possibility is that LC neurons may have non-glutamatergic/non-GABAergic modulatory inputs that lead to differences in the responsive to pH. For example, the LC has intracoerulear inhibitory connections mediated by α2-adrenoceptors that contribute to the overall level of activity, possibly in response to HA (1, 19, 45). It is also presently unclear whether there are other cell types within our culture system that could lead to release of neuromodulators that influence the response of LC neurons to pH/CO2.
Functional significance of chemosensitivity of LC neurons.
There are numerous groups of central nervous system neurons that have been proposed to be central respiratory chemoreceptors (21, 48, 50). Currently, it remains controversial which of these pH-sensitive neuronal groups are involved in modulation of respiratory output in response to hypercapnia in vivo, and if so under what conditions this occurs. The presence of pH sensitivity in vitro does not prove that a neuron is a central respiratory chemoreceptor. However, support for this conclusion also comes from in vivo studies, which have shown that cells in the LC increase their spike frequency in response to elevated levels of CO2, before and after peripheral chemoreceptor deafferentation (18). In other reports, focal acidification of the A6 region caused an increase in phrenic nerve activity (9). In addition, lesions of the LC lead to a decrease in the ventilatory response to hypercapnia in vivo (7, 35). Despite this evidence, it remains unclear how important LC neurons are for control of breathing, and the possibility should be considered that they may play their most important role in cortical arousal by hypercapnia.
Primary cultures of GFP-LC neurons as a model for studying intrinsic pH mechanisms.
The approach we use here offers advantages in the study of LC neuron chemosensitivity. First, GFP expression allows targeting of catecholaminergic neurons specifically. Second, primary culture provides optimal stability for patch-clamp recordings. Third, culture allows optimal access of solutions and pharmacological agents of neurons, since they exist in a monolayer. We conclude that the Prp57 transgenic mouse will serve as a useful experimental model for delineating CO2/H+ chemosensory mechanisms of phenotypically identified catecholaminergic neurons.
GRANTS
Research supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant NS-047422 to S. M. Johnson, by National Institute of Child Health and Human Development and Veterans Affairs Medical Center to G. B. Richerson, and in part by NINDS Grant NS-039407.
Acknowledgments
The authors are grateful for the generous donation of Prp57 mice provided by Dr. Anthony van den Pol of Yale University Department of Neurosurgery and the technical support of Dr. Vitaliy Rogulin.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aghajanian GK, Cedarbaum JM, Wang RY. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res 136: 570–577, 1977. [DOI] [PubMed] [Google Scholar]
- 2.Arata A, Onimaru H, Homma I. The adrenergic modulation of firings of respiratory rhythm-generating neurons in medulla-spinal cord preparation from newborn rat. Exp Brain Res 119: 399–408, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Foote SL, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience 15: 765–777, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne D, Scheid P. Central chemosensitivity of respiration: a brief overview. Respir Physiol 129: 5–12, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Ballantyne D, Scheid P. Central respiratory chemosensitivity: cellular and network mechanisms. Adv Exp Med Biol 17–26, 2001. [DOI] [PubMed]
- 6.Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol 80: 108–115, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch 455: 1119–1128, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bou-Flores C, Lajard AM, Monteau R, De ME, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci 20: 4646–4656, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Coates EL, Li AH, Nattie EE. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol 441: 433–451, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean JB, Ballantyne D, Cardone DL, Erlichman JS, Solomon IC. Role of gap junctions in CO2 chemoreception and respiratory control. Am J Physiol Lung Cell Mol Physiol 283: L665–L670, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dean JB, Bayliss PA, Eriksson LI, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Dean JB, Mulkey DK, Garcia AJ, III, Putnam RW, Henderson RA, III. Neuronal sensitivity to hyperoxia, hypercapnia, and inert gases at hyperbaric pressures. J Appl Physiol 95: 883–909, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Dean JB, Mulkey DK, Henderson RA III, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol 96: 784–791, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Dillon GH, Waldrop TG. In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience 51: 941–950, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Dillon GH, Waldrop TG. Responses of feline caudal hypothalamic cardiorespiratory neurons to hypoxia and hypercapnia. Exp Brain Res 96: 260–272, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–68, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res 222: 373–381, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Ennis M, ston-Jones G. Evidence for self- and neighbor-mediated postactivation inhibition of locus coeruleus neurons. Brain Res 374: 299–305, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Erlichman JS, Putnam RW, Leiter JC. Glial modulation of CO2 chemosensory excitability in the retrotrapezoid nucleus of rodents. Adv Exp Med Biol 605: 317–321, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol 541: 493–509, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald SC, Willis MA, Yu C, Rigatto H. In search of the central respiratory neurons: I. Dissociated cell cultures of respiratory areas from the upper medulla. J Neurosci Res 33: 579–589, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda Y, See WR, Honda Y. H+-sensitivity and pattern of discharge of neurons in the chemosensitive areas of the ventral medulla oblongata of rats in vitro. Pflügers Arch 388: 53–61, 1980. [DOI] [PubMed] [Google Scholar]
- 26.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of cental chemoreceptors. Exp Physiol 90: 247–253, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv Exp Med Biol 605: 333–337, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Haxhiu MA, Yung K, Erokwu B, Cherniack NS. CO2-induced c-fos expression in the CNS catecholaminergic neurons. Respir Physiol 105: 35–45, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt A, Barrie R, Graham M, Bogus K, Leiter JC, Erlichman JS. Ventilatory effects of gap junction blockade in the RTN in awake rats. Am J Physiol Regul Integr Comp Physiol 287: R1407–R1418, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev 79: 325–360, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Hobson JA, McCarley R, Pivik RT, Freedman R. Selective firing by cat pontine brain stem neurons in desynchronized sleep. J Neurophysiol 37: 497–511, 1974. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SM, Haxhiu MA, Richerson GB. GFP expressing locus coeruleus neurons obtained from Prp57 transgenic mice exhibit intrinsic CO2/H+ responses in primary cell culture. FASEB J 21: lb571, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson SM, Trouth CO, Smith JC. Chemosensitivity of respiratory pacemaker neurons in the pre-Botzinger complex in vitro. Neuroscience 24: 875, 1998. [Google Scholar]
- 35.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles R Does low pH stimulate central chemoreceptors located near the ventral medullary surface? Brain Res 271: 349–353, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW. Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol 18: 523–533, 1963. [DOI] [PubMed] [Google Scholar]
- 38.Mulkey DK, Henderson RA III, Putnam RW, Dean JB. Hyperbaric oxygen and chemical oxidants stimulate CO2/H+-sensitive neurons in rat brain stem slices. J Appl Physiol 95: 910–921, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Mulkey DK, Henderson RA III, Ritucci NA, Putnam RW, Dean JB. Oxidative stress decreases pHi and Na+/H+ exchange and increases excitability of solitary complex neurons from rat brain slices. Am J Physiol Cell Physiol 286: C940–C951, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Mulkey DK, Henderson RA III, Ritucci NA, Putnam RW, Dean JB. Chemical oxidants acidify solitary complex (SC) neurons in the rat. Undersea Hyperb Med 31: 107–111, 2004. [PubMed] [Google Scholar]
- 41.Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol 81: 1987–1995, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol 47: 385–403, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Oyamada Y, Andrzejewski M, Muckenhoff K, Scheid P, Ballantyne D. Locus coeruleus neurones in vitro: pH-sensitive oscillations of membrane potential in an electrically coupled network. Respir Physiol 118: 131–147, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P. Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol 513: 381–398, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Putnam RW, Conrad SC, Gdovin MJ, Erlichman JS, Leiter JC. Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir Physiol Neurobiol 149: 165–179, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Richerson GB Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Richerson GB, Wang W, Hodges MR, Dohle CI, ez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Richerson GB, Wang W, Tiwari J, Bradley SR. Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir Physiol 129: 175–189, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Rigatto H, Rehan V, Lemke RP, Idiong N, Hussain A, Cates D. Respiratory pacemaker cells responsive to CO2 in the upper medulla: dose response and effects of mediators. Pediatr Pulmonol 30: 359–367, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Ritucci NA, Chambers-Kersh L, Dean JB, Putnam RW. Intracellular pH regulation in neurons from chemosensitive and nonchemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 275: R1152–R1163, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am J Physiol Cell Physiol 289: C1094–C1104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ritucci NA, Dean JB, Putnam RW. Intracellular pH response to hypercapnia in neurons from chemosensitive areas of the medulla. Am J Physiol Regul Integr Comp Physiol 273: R433–R441, 1997. [DOI] [PubMed] [Google Scholar]
- 56.Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol Regul Integr Comp Physiol 289: R851–R861, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlaefke ME, See WR, Loeschcke HH. Ventilatory response to alterations of H+ ion concentration in small areas of the ventral medullary surface. Respir Physiol 10: 198–212, 1970. [DOI] [PubMed] [Google Scholar]
- 58.Solomon IC Connexin36 distribution in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol Neurobiol 139: 1–20, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Solomon IC, Chon KH, Rodriguez MN. Blockade of brain stem gap junctions increases phrenic burst frequency and reduces phrenic burst synchronization in adult rat. J Neurophysiol 89: 135–149, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Solomon IC, Dean JB. Gap junctions in CO2-chemoreception and respiratory control. Respir Physiol Neurobiol 131: 155–173, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Solomon IC, Edelman NH, O'Neal MH III. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol 88: 1996–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Solomon IC, Halat TJ, El-Maghrabi MR, O'Neal MH III. Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir Physiol 129: 101–121, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Stunden CE, Filosa JA, Garcia AJ, Dean JB, Putnam RW. Development of in vivo ventilatory and single chemosensitive neuron responses to hypercapnia in rats. Respir Physiol 127: 135–155, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Su J, Yang L, Zhang X, Rojas A, Shi Y, Jiang C. High CO2 chemosensitivity versus wide sensing spectrum: a paradoxical problem and its solutions in cultured brainstem neurons. J Physiol 578: 831–841, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thoby-Brisson M, Ramirez JM. Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J Neurosci 20: 5858–5866, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Pol AN, Ghosh PK. Selective neuronal expression of green fluorescent protein with cytomegalovirus promoter reveals entire neuronal arbor in transgenic mice. J Neurosci 18: 10640–10651, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol 541: 169–185, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J Neurosci 24: 928–937, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang F, Xiao C, Ye JH. Taurine activates excitatory non-synaptic glycine receptors on dopamine neurones in ventral tegmental area of young rats. J Physiol 565: 503–516, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pH(o) and Pco2. J Physiol 540: 951–970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, Richerson GB. Chemosensitivity of non-respiratory rat CNS neurons in tissue culture. Brain Res 860: 119–129, 2000. [DOI] [PubMed] [Google Scholar]
- 74.Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA 104: 10685–10690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]