Abstract
The objective of this study was to measure and monitor changes in Achilles tendon mechanical properties and force production capability of triceps surae muscles after 4 wk of limb suspension and 6 wk of physical rehabilitation. Five healthy volunteers underwent unilateral lower limb suspension followed by weekly physiotherapy. A velocity-encoded, phase-contrast magnetic resonance imaging (VE-PC-MRI) technique was used to estimate the tendon strain as a function of force produced during the submaximal isometric contractions. After limb suspension, triceps surae muscle strength decreased to 53.2 ± 15.6% (mean ± SD) of the presuspension level (P < 0.05). Young's modulus, estimated from the slope of the tendon stress-strain relationship, decreased by 17.1% (from 140.50 ± 29.33 to 119.95 ± 36.07 MPa, P < 0.05), while the tendon transition point, reflecting the “toe region,” increased by 55.7% (from 2.2 ± 1.0% to 3.4 ± 1.24%). Muscle strength, tendon stiffness, and transition point recovered to presuspension levels by the end of 6 wk of rehabilitation. Calcaneus movement was significant during the “isometric” contraction, accounting for 52.13 ± 7.63% of the tendon displacement. Tendon cross-sectional area determined from anatomic magnetic resonance axial images remained unchanged, suggesting that the altered tendon elastic modulus and transition point were largely due to material deterioration. The increase in the transition point following chronic unloading as measured by the VE-PC-MRI technique has not been previously reported and offers new insights into the biomechanical changes that may occur in the tendon crimp structure.
Keywords: Young's modulus, toe region, tendon stress-strain, triceps surae strength, atrophy
the effects of chronic unloading on skeletal muscle have been widely studied in light of its clinical importance in an expanding population of geriatric patients and in the rarer incidence of astronauts exposed to microgravity. Well-documented alterations include myofibril protein loss (35), muscle fiber size reduction (11, 32, 36), altered neuronal recruitment pattern, and fiber type conversion (10). These cellular changes lead to macroscopically observable degradation of muscle structure and function, which include a marked decline in muscle volume (9, 17, 23), a reduced load production capability (7, 13, 17), and an increased fatigability (6), all of which underscore the importance of countermeasures for preserving the well-being and safety of such individuals.
Since the interaction between muscular and tendinous tissues is the result of skeletal muscle kinetics, both need to be studied in tandem to elucidate the overall effect on function. Aside from their well-known role as a structural link between muscle and bone, tendons are known to exhibit a viscoelastic response under tension, allowing efficient use and recycling of stored energy during stretch, modulating joint position control, and providing protection from muscle injuries. Consequently, changes in mechanical properties of tendons will significantly affect musculoskeletal performance. Similar to muscles, tendons have been reported to be adaptable to loading level (5, 31, 37), although much less is known about their responses to chronic unloading. B-mode ultrasonography has become popular for in vivo measurement of several tendons of the leg (e.g., gastrocnemius and patella tendons), but there is no adequate in vivo method for quantifying the mechanical behavior of the Achilles tendon, defined as the thick or external tendon running from the calcaneus insertion to the distal part of the soleus muscle. The mechanical behavior of this segment is of particular clinical importance, since it is known to be the most likely site of tendon rupture and tear in humans (1).
Given this background, we used a technique of velocity-encoded phase-contrast magnetic resonance imaging (VE-PC-MRI) to noninvasively measure Achilles tendon strain and changes in its force-displacement relationship concomitant with chronic unloading and subsequent recuperation. We quantified the Achilles tendon Young's modulus (MPa) from a stress-strain curve after adjusting for bulk movement of the leg, which is a common and significant issue in “isometric” experiments using the ultrasound technique (28). Higher spatial resolution and large field of view (FOV) afforded by MRI also allowed us to clearly define and segment the two ends of the Achilles tendon; such capabilities are important for elimination of undesirable strain contributed by exogenous tissues and for consistent monitoring of the same anatomic landmarks over the duration of several months. Thus, in this report, Achilles tendon stress-strain data are adjusted for calcaneus movement and tendon cross-sectional area (CSA), both of which have been shown to reduce the intersubject variability in the data (28, 33).
Our laboratory previously reported the effects of unilateral lower limb suspension (ULLS) on Achilles tendon and soleus aponeurosis strain distribution at a specific contraction level, i.e., 20% of the maximal plantarflexion (17). The present study was designed to monitor, in a larger cohort, changes in the Achilles tendon stress-strain relationship by measuring its elastic modulus and transition point, thus providing a new and independent insight with regard to the mechanical properties of the tendon. The term “transition point” was introduced to identify a characteristic region in the tendon stress-strain curve where it transitions from a nonlinear, low-stiffness phase to a high and almost constant-stiffness phase.
MATERIALS AND METHODS
Study design.
The effect of chronic unloading and physiotherapy on the force production capability of the triceps surae muscles was assessed by comparing the baseline (Pre) with four time intervals: immediately after 4 wk of limb suspension (P0) and after 1, 3, and 6 wk of a physical rehabilitation program (P1, P3, and P6). The Achilles tendon mechanical behavior (stress-strain relationship) was monitored at Pre, P0, and P6 only. During the suspension period, subject compliance to the protocol was closely monitored through weekly telephone interviews. A physician was on standby to carefully screen for any symptoms of deep vein thrombosis. Immediately after completion of the protocol (P0), thorough examinations were performed by a professional physical therapist to assess the progression of muscle atrophy. Twice each week the subjects were required to attend a 15-min physical rehabilitation session, which was supervised by the same therapist.
Rehabilitation.
The rehabilitation program was custom designed specifically for this study at the Rehabilitation Services of UCLA Healthcare. Each subject received one-on-one supervision. Each session consisted of six exercise routines: 1) warm-up (prepain rating, treadmill, and stationary bike), 2) strength (ankle weights and calf raise), 3) balance (Dyna-disk exercises), 4) stretching of gastrocnemius and soleus (both on a tilt board), hamstring, quadriceps, hip flexor, and piriformis, 5) cool-down (stationary bike), and 6) postevaluation (pain rating). The program was adjusted each week according to the subject's tolerance.
Subjects.
Five healthy subjects (4 men and 1 woman) with no history of neurological and musculoskeletal disorders were recruited. Before their participation, the experimental protocols were explained to the subjects, who then signed a research participation consent form approved by the Institutional Medical Review Board. Age, body mass, and height were as follows (means ± SD): 24.2 ± 5.4 yr, 76.4 ± 9.7 kg, and 178.8 ± 18.5 cm.
ULLS.
Muscle atrophy was induced on the (self-reported) dominant leg with 4 wk of chronic unloading using the ULLS model (4), which was designed to simulate the microgravity condition commonly experienced by astronauts in space. The model was shown to be effective, yet economical to implement, with only moderate encroachment on a subject's daily physical activities.
A Velcro strap was looped around the foot of the dominant limb and tied to the waist to flex the knee, preventing the foot from touching the ground. The nondominant foot was supplemented with a 5-cm raised heel on the shoe to further minimize accidental dragging (loading) of the foot. The subjects relied on crutches for mobility at all times.
Measurement of muscle force.
The leg was placed in the posterior half of a fiberglass cast with the ankle joint at 90° between the foot and the tibia. Isometric plantarflexion of the triceps surae muscles was achieved by immobilization of the leg within the cast: the leg was tightly wrapped with layers of elastic bandage that were secured with adhesive tape. A magnetic resonance (MR)-compatible fiber-optic sensor (Fiberscan 2000, Luna Innovations) was glued onto the sole of the cast and used to monitor subtle changes (strain resolution = 1 μɛ) of cast deformation. The voltage output from the measurement system, a measure of the force exerted on the sole of the foot, was sampled at a rate of 200 Hz and stored in a personal computer using an indigenously developed data acquisition program (LabView, National Instruments, Austin, TX). For each cast used in the study, the strains were calibrated with four known weights to obtain a calibration factor relating cast strain with the force on the ball of the foot. For measurement of maximum voluntary contraction (MVC), the subject was asked to maximally plantarflex the ankle joint three times, and the highest reading was taken as the MVC; 40% of the MVC was calculated and used as the target during dynamic VE-PC-MRI acquisition. The peak contraction force was matched to the target force level (39.3 ± 3% MVC), thereby achieving the consistency of muscle contractions needed for good-quality motion-artifact-free phase-contrast MRI (PC-MRI) data.
Estimation of Achilles tendon force.
Achilles tendon force was estimated by multiplying the force at the sole of the foot by 2.67 following the method of Haxton (14). Haxton calculated the ratio of the tension in the Achilles tendon to the resistance at the ball of the foot to be 2.67 ± 0.038 (SE) in cadaveric limbs and noted that this ratio was remarkably constant over five samples. The system was modeled as a first-class lever in which the ankle joint acted as the fulcrum while the tendon force and foot resistance acted as loads at the opposite ends of the lever.
VE-PC-MRI.
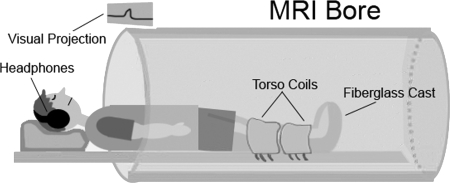
The leg under observation remained within the fiberglass cast, and both legs were placed in a multichannel, phased-array torso coil for MR signal detection. The supine subject was moved, feet first, into the bore of a 3.0-T scanner (Trio, Siemens, Malvern, PA; Fig. 1). As part of the warm-up protocol, each subject underwent ∼3 min of repeated submaximal muscle contractions before data acquisition.
Fig. 1.
Experimental set-up. Imaged leg is fixated inside the fiberglass cast, which is placed inside the torso coil. Consistency of generated forces between contractions is controlled by the audiovisual feedback system, consisting of a magnetic resonance (MR)-compatible headphone feeding the audio cue and the projection of the trace of force produced on the magnet face from a digital projector located outside the magnet room.
The details of the VE-PC-MRI technique are described elsewhere (12, 17, 34). Briefly, the technique produces a series of time-resolved frames throughout the contraction-relaxation cycle. Corresponding to each frame are two image types: one containing anatomic (magnitude) information and the other containing velocity (phase) information. In the phase dataset, the intensity of a given voxel reflects the average spin velocity at that specific spatial location. With knowledge of the time interval between frames, the trajectory of an anatomic region of interest (ROI) can be determined. For the present study, an oblique sagittal imaging plane was prescribed, such that it bisected the centerline of the Achilles tendon (Fig. 2B).
Fig. 2.
Oblique sagittal (A) and axial (B) images of the lower leg. Sagittal slice location is marked with a white dashed line in the axial image. White dots represent anatomic locations used for measurement of tendon displacement and calcaneus excursion. Initial tendon length (∼5 cm) was defined as the distance between the two regions of interest. C: magnified axial image showing automatically segmented tendon cross-sectional area.
A segmented, two-dimensional spoiled gradient echo velocity-encoded phase-contrast (VE-PC) sequence, with velocity encoding of 10 cm/s in the superior-inferior direction, three views per segment (VPS), 13.3 ms/7.5 ms/20° TR/TE/flip angle, 3-mm slice thickness, 290-Hz receiver bandwidth/pixel, 128 × 256 matrix size, 160 × 320 mm FOV, and two averages, was used in the retrospective gated mode for acquisition of time-resolved frames during 86 isometric contractions. The temporal resolution of each frame was ∼79.8 ms (TR*VPS*2). To maintain the repeatability of the muscle contractions, a computer-generated audio cue at the rate of 35 beats/min was provided to the subject through the scanner audio system, and real-time force signals were projected onto the face of the magnet, giving continuous audiovisual feedback to the subject (Fig. 1). The force signals were filtered through a custom-made electronic trigger box, which was interfaced to the scanner ECG port. The initial rise in each signal generated a voltage impulse that served as a trigger input to the scanner. Since the duration of each contraction cycle was ∼1.71 s (60 s per 35 beats/min) and the image temporal resolution was ∼79.8 ms, the completion of a PC-MRI acquisition generated 22 uninterpolated time-resolved frames.
Axial morphological images were acquired throughout the length of the lower leg using a spoiled gradient echo sequence (371 ms/2.42 ms/45° TR/TE/flip angle, 5-mm slice thickness, 320 Hz receiver bandwidth/pixel, 256 × 240 imaging matrix, 180 × 168.75 mm FOV, 2 averages, 30 slices). Special care was taken to ensure that one of the axial slices corresponded to the level on the PC-MRI oblique sagittal plane where the tendon strain was calculated (Fig. 2). This level, located ∼5 cm (48.25 ± 0.68 mm) from the calcaneal insertion, was specifically chosen, since this region is the most common site of tendon rupture and tear in humans (1). The tendon CSA at this slice was estimated with an indigenously developed region-growing algorithm (Fig. 2C). The reference lines corresponding to the oblique plane displayed on the axial images were saved on a laptop computer to be referenced in subsequent imaging sessions (P0 and P6), thereby preserving the exact plane orientation prescribed in Pre.
Image processing and analysis.
Image processing and analysis algorithms were indigenously developed in MATLAB (Mathworks, Natick, MA). The systematic phase shading errors were quantified and subtracted from the individual phase image of the cine sequence using the technique of Sinha et al. (34). The error-corrected phase data were converted to velocity data using velocity encoding, such that the gray-scale intensity at each pixel represented its tissue velocity.
For tendon displacement, a voxel on the soleus muscle adjacent to the Achilles tendon was used as an initial seed location (Fig. 2A, top) in the first frame. The muscle tissue just adjacent to the tendon was assumed to represent the tendon tissue movement, as in previous studies (12, 17), since it is not possible with this imaging technique to track the tendon itself. The tracking algorithm calculated the mean velocity of its 3 × 3 pixel centered window and multiplied the mean by the temporal resolution of its frame to calculate the displaced location in the next frame. This process was repeated iteratively until the tendon displacement was fully tracked throughout the contraction-relaxation cycle. The algorithm allowed for linearly interpolated subpixel velocity estimation, thereby achieving submillimeter displacement resolution.
To compensate for bulk leg movement induced by isometric contractions and to accurately define the scope of the tendon tissue under study, the calcaneus displacement at the distal end of the Achilles tendon (Fig. 2A, bottom) was manually tracked from each magnitude image frame using ImageJ (National Institutes of Health, Bethesda, MD) and was subtracted from the tendon displacement.
Measurement of tendon stress and strain.
The Achilles tendon strain [(l − lo)/lo, where l is segment length] was calculated as the distance between the two ROIs (distance between the 2 ends of the tendon) at each temporal frame, and the initial length (lo) was defined in the frame corresponding to the resting muscle.
Force signals recorded during the PC-MRI (see Measurement of muscle force) were averaged to generate one representative force curve. The average tendon force was divided by the estimated tendon CSA to obtain tendon stress.
Estimation of Young's modulus and transition point.
The relationship between tendon strain and stress was least-squares fitted to a cubic polynomial. From the polynomial curve, we estimated the Achilles tendon stiffness by finding a line extending down from the peak force (40% MVC), such that it achieved the correlation coefficient of 0.98 with the stress-strain curve. In other words, the “linear” portion of the curve had to conform to a correlation coefficient of 0.98. This approach was taken to ensure that, despite the intra- and intersubject variations in the stress-strain curves, the stiffness was objectively measured without user intervention or subjectivity. To reflect the adaptations in the toe region of the stress-strain curve, the transition point was defined as the x-intercept of the stiffness line to be used as an objective measure (Fig. 3B).
Fig. 3.
A: time history of tendon stress and strain from a representative subject (subject in Fig. 4A) immediately after 4 wk of limb suspension (P0). B: stress-strain relationship fitted to a cubic polynomial and a stiffness line used to calculate the Young's modulus and transition point. Quality of cubic fit and stiffness line is represented by their respective R value.
Statistics.
Values are means ± SD. Differences between Pre, P0, and P6 groups in tendon Young's modulus, transition point, and muscle strength were examined using repeated-measures ANOVA with Fisher's (least significant difference) adjustment for multiple comparisons. Significance level was set at P < 0.05.
RESULTS
Figure 3A represents a time history of stress and strain of the Achilles tendon during a contraction-relaxation cycle obtained after postprocessing of tendon force (normalized to CSA) and displacement (ankle joint motion-adjusted) data. The subtracted calcaneus movement accounted for 52.1 ± 7.6% of the total tendon displacement.
The data in Fig. 3A demonstrate that the maximum plantarflexion and tendon elongation occurred ∼0.48 s after the signal trigger. Figure 3B shows the stress vs. strain data pairs of the same subject fitted to the least-squares polynomial cubic function. Overall, the cubic fit demonstrated an excellent match to the data, with a correlation coefficient of 0.997 ± 0.003%. The linear fit (R = 0.98) of the stress-strain relationship was used to assess the Young's modulus as the slope of the curve and the transition point at the intercept of the abscissa.
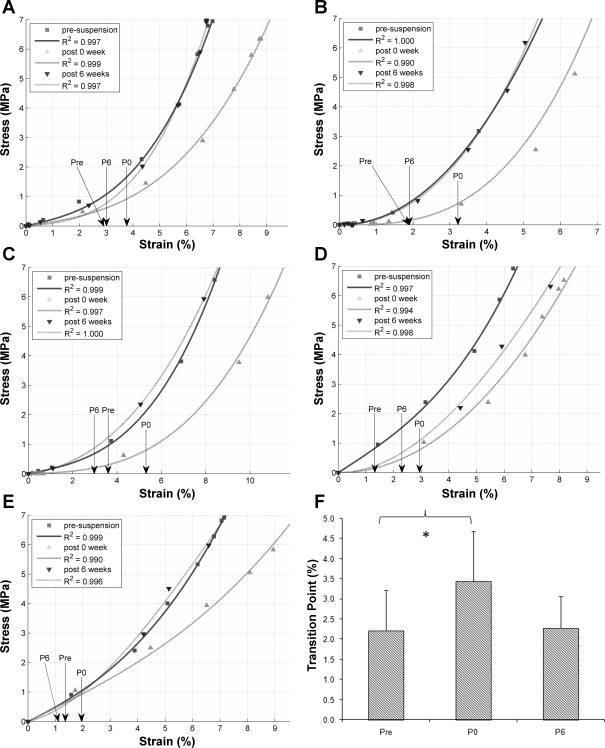
The subject-specific and time-dependent (Pre, P0, and P6) stress-strain relationships are shown for all five subjects in Fig. 4, A–E. The tendon strain at the transition point was significantly different (P = 0.013) between Pre and P0 (Fig. 4F), but not between Pre and P6: 2.2 ± 1.0% for Pre, 3.4 ± 1.2% for P0, and 2.3 ± 0.8% for P6.
Fig. 4.
A–E: stress-strain relationship of Achilles tendon at presuspension (Pre), at P0, and after 6 wk of physical rehabilitation (P6) for each subject. Arrow, transition point at each period. Quality of each cubic fit with respect to experimental data is denoted with the correlation coefficient value. F: average tendon transition point at Pre, P0, and P6. *P < 0.05, Pre vs. P0.
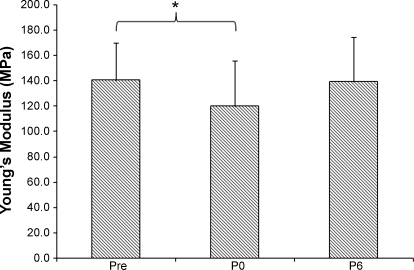
Repeated-measures ANOVA showed that unloading and rehabilitation had an effect on the Achilles tendon Young's modulus (P = 0.021). The pairwise comparisons with Fisher's (least significant difference) correction showed a significant difference between Pre and P0 (P = 0.042), but not between Pre and P6: 140.5 ± 29.3 MPa for Pre, 120.0 ± 36.1 MPa for P0, and 139.1 ± 35.1 for P6 (Fig. 5). In summary, the Young's modulus was decreased by 17.1% following ULLS and returned to the normal level after 6 wk of recovery.
Fig. 5.
Achilles tendon Young's modulus at Pre, P0, and P6. Values are means ± SD. Repeated-measures ANOVA with Fisher's (least significant difference) pairwise comparison showed 17.1% reduction in the modulus after unilateral lower limb suspension: *P < 0.05.
Figure 6 shows that the average tendon CSA was unaltered between the three time points: 52.0 ± 3.8 mm2 at Pre, 54.2 ± 3.6 mm2 at P0, and 54.8 ± 3.4 mm2 at P6. However, the tendon CSA showed substantial intersubject variability, with a range of 41.3–62.8 mm2.
Fig. 6.
Tendon cross-sectional area (CSA) at Pre, P0, and P6. Tendon CSA was not changed over the duration of the study. Values are means ± SD.
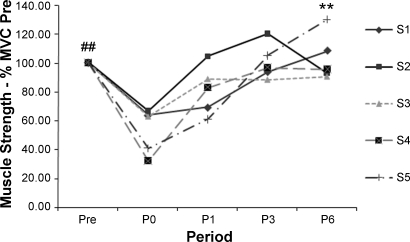
After limb suspension, maximum muscle strength of the triceps surae decreased (P < 0.05) to 53.2 ± 15.6% of the presuspension level and incrementally increased during the week of physical rehabilitation (Fig. 7). By P6, the strength level recovered to its original level: 103.4 ± 6.2% of initial strength.
Fig. 7.
Subject-specific maximum plantarflexion force of triceps surae at P0, P1, P3, and P6 relative to Pre. ##P < 0.05, Pre vs. P0. **P < 0.05, P0 vs. P6.
DISCUSSION
The aim of the present study was to elucidate the effects of chronic unlading on the mechanical properties of the human Achilles tendon in vivo. The main findings were that the tendon stress-strain curve shifted to the right and Young's modulus decreased moderately (17%) after unloading. These changes recovered to baseline values within 6 wk of rehabilitation, suggesting that tendons are able to adapt within weeks.
The Achilles tendon mechanical properties were measured with the stress-strain relationship established from the PC-MRI data and MR-compatible dynamometry. To the best of our knowledge, this technique has not been used as an in vivo method in humans or animals to investigate the effect of chronic unloading on the stress-strain relationship. Ultrasonography is an established in vivo technique utilized by various investigators (15, 30) to address this question but localized at a higher anatomic location (e.g., gastrocnemius-myotendinous junction) because of difficulties associated with the measurement technique. To the best of our knowledge, only one ultrasound study (21) has measured the stress-strain relationship of the free Achilles tendon as defined in the present study (Fig. 2). Magnusson et al. (21) used a thin needle insertion as a marker to overcome the technical challenge, although their overall tendon length was ∼4 cm longer than that used in the present study. Given that the thick portion of the Achilles tendon is highly active and the most common region of tendon tear and rupture and that mechanical properties of proximal and distal regions may differ under loading (18, 21, 39), we believe that the present study is unique and may offer added insights.
First, the PC-MRI technique uses a much faster rate of loading than the ramp conditions used in the ultrasonography studies. This can be physiologically very relevant, since the stiffness of a tendon is rate dependent because of its viscoelastic nature, and the present rapid contractions are similar to those that occur in normal activities such as walking and running.
Second, a large FOV (32 cm along the length of the leg) is afforded by MRI. Since the bulk leg motion combined with calcaneus rotation during the isometric contraction can introduce significant error in measured tendon displacement (20, 21, 33), we were able to quantify and subtract this error from the strain calculation, which was relatively easy to achieve with the large FOV used in the present method. However, because of signal void in bony structures in MRI, manual tracking (instead of PC-MRI) of an anatomic landmark was employed using the magnitude data of the cine sequence. In the present study, 52% of the measured tendon displacement was caused by the calcaneus excursion, underscoring the difficulty of achieving the ideal isometric muscle experiments. In addition, we normalized the tendon force to each individual's tendon CSA to quantify stress. Both parameters (calcaneus error correction and CSA normalization) were shown in our previous study to reduce the scatter in the intersubject force-length relationship of the tendon (33).
The cubic polynomial function used to describe the stress-strain relationship showed excellent fit to the experimental data, and the fitted curve exhibited the classical viscoelastic response of the tendon (Fig. 3B and Fig. 4, A–E) with two distinct regions: the initial “toe region” followed by the more linear region. The toe region can be characterized with the curve showing low, but variable, slope, whereas the linear region can be characterized with increased, but more constant, slope.
The Young's modulus (MPa) defined in the linear region showed a 17% reduction after ULLS and returned to baseline by the end of the study (Fig. 5). Previously, the decrease in stiffness was reported mainly in ultrasonographic studies in humans (15, 19, 30) but also in some animal studies (2, 37). The extent of decline found in the present study is similar to that reported by Kubo et al. (15), who studied humans subjected to 20 days of bed rest (−28%), but is much more moderate than the percent decline reported in studies of 90 days of simulated microgravity (−58%) (30) and paralysis (−59%) (19). This suggests that the extent of stiffness decrement may have a direct correlation to the duration of unloading, although an objective comparison with these studies is difficult, because different sections of the tendon were investigated. For this study, a P value (0.042) close to the critical level (0.05) associated with stiffness reduction should be noted; this may warrant a larger subject sample in future studies. However, the trend toward dynamic tendon adaptations to unloading and physiotherapy-induced recovery is an important and clear finding. The modulus values reported in the present study are relatively low compared with those reported in ultrasound studies. However, the values are in good agreement with the studies conducted by Reeves et al. (30) and Kubo et al. (16). Since the PC-MRI and ultrasound techniques yield similar tendon displacement values, we believe that a large variation of absolute modulus values in the literature is largely attributable to the difference in the way the tendon force is estimated. It is also possible that the modulus measurement from a stress-strain relationship at a submaximal contraction level may have contributed to the lower estimates.
The exact mechanisms behind the tendon stiffness reduction remain unelucidated, although collagen fibers are generally understood to be directly responsible. Some of the relevant findings include a decline in tendon fibril diameter (27), fewer medium- and larger-sized fibrils (22), and a reduction in the covalent intramolecular cross links (3) as the direct result of chronic unloading. The notion that the stiffness is affected mainly by the deterioration of the material properties is also supported by the finding that the tendon CSA remained unchanged over the duration of the present study (Fig. 6). Therefore, it can be inferred that the geometrical properties (CSA) were not the major determinants of the extensibility of the Achilles tendon (24). The longer-term or permanent effect of unloading on the tendon thickness may be different, however. Maganaris et al. (19) reported that the CSA of the patella tendon was 77% lower in spinal cord-injured individuals.
There are clear functional ramifications of a decrease in Achilles tendon stiffness. First, decreased stiffness results in less efficient transfer of contractile force produced by the muscle to the skeleton, leading to delayed execution of motor tasks (29). Furthermore, the precision of the motor movement can be compromised, presenting challenges in the coordination of fine movement and balancing. Since tendon and muscle are connected in series, this also implies that muscle fibers effectively would have to shorten to a greater extent to generate a given level of force, particularly in efforts requiring relatively low force. All other factors being constant, the compliant tendon results in a leftward shift of the length-tension relationship curve of the muscle fiber, resulting in a decline in force production for a given amount of fiber shortening (38).
In addition to the reduction in the Young's modulus of the Achilles tendon, we observed a consistent shift in the stress-strain curve concomitant with unloading. Specifically, there appeared to be an extended range of tendon strain at low stresses (<2 MPa), implying that the tendon remained in the toe region longer before it entered the linear region. Physiologically, this is an interesting phenomenon, because the toe region is associated with straightening of crimps in collagen fibrils. This sinusoidal crimp structure surrounding them is made up of a woven mesh of connective tissue containing elastic fibers (29). The linear region is believed to become dominant only after the crimps have been fully straightened during stretching of the tendon fibrils (26). To quantify this aspect of the stress-strain relationship, we coined the term transition point, which is an arbitrary point that separates the stress-strain curve into these two regions. This point was defined as the x-intercept of the stiffness line (Fig. 3B) and was shown to accurately reflect this dynamic shift in the stress-strain curve over the course of the study (Fig. 4). The strain at the transition point increased significantly between Pre and P0 but was restored at P6. This unique finding seems to indicate that the material properties and/or the structure in the crimp region may be responsive to changes in loading level. Another physiological explanation for this shift may be changes in muscle architecture following muscle atrophy. The atrophied muscle is known to have lower pennation angles, which may make the tendon operate at shorter lengths (25). This would have the effect of moving the tendon resting position to the left of the force-length curve and, apparently, moving the limit of the toe region to the right.
In a previous study, we were unable to detect any changes in the Achilles tendon peak strain at 20% MVC after ULLS (16). The more detailed analysis reported in the present study demonstrates a more sensitive technique for investigating strain changes. In the present study, we employed a repeated-measures model, rather than a test for differences between group means, to account for variability between subjects. This approach offers much more statistical power, particularly in a study where the difference between groups is small relative to the variation within groups. In addition, we have corrected for calcaneus movement during isometric contraction. Although perhaps not directly influencing the sensitivity of our method, Lee et al. (17) measured a single displacement at the peak of a 20% MVC, whereas the present study constructed force-length curves for all data points measured during the rising phase of a 40% MVC. The viscoelastic nature of the tendon must be recognized when such dynamic contractions are evaluated, since the force-length curve during the rising phase of the contraction-relaxation exhibits much greater apparent strain than the falling phase or the peak of an intermediate-level contraction (Fig. 7) (12).
The extent of the effects of ULLS was demonstrated by reduction of the muscle force to 53% of the preloading level. The recovery of force was gradual, and it returned to the normal level at P6. Although muscle volume and strength reduction are well-documented findings (4, 8, 17), the highly responsive nature of the triceps surae muscles should be noted as reflected by the quick degradation of force production capacity and equally resilient full recovery toward the normal level. In two of the five subjects, the maximal plantarflexion force exceeded the baseline level (Fig. 7), possibly indicating the efficacy of physical therapy, although a control group is needed to substantiate this claim.
Methodological considerations.
Because of the inherent physics of cine PC-MRI, the complete dataset is acquired after 128–256 repetitions or phase-encoding levels, where each level corresponds to one muscle contraction. For this study, the subject was required to repeat the contractions ∼86 times (256 levels/3 VPS). This presents two challenges: 1) the magnitude of muscle contraction is limited to submaximal (40% MVC in this study) because of fatigue, which also limits the range of the stress-strain relationship at which the Young's modulus is determined, and 2) these repeated contractions must be uniform for optimal PC-MRI data collection. This challenge could be surmounted with adequate pretraining and a continuous audiovisual feedback system during the data acquisition. With pretraining and a feedback system in place, the study subjects were able to maintain the high repeatability of the target contraction level (39.3 ± 3% MVC). Our previous study also showed that, under normal setting, the force-length relationship determined by our method had sufficiently high reproducibility (33). The need for more contractions can be reduced to some extent by increasing the VPS, which, in turn, would yield poorer temporal resolution of the image frame, giving rise to fewer stress-strain data pairs. Given these limitations, the development of a faster velocity-encoding pulse sequence is desirable: it can drastically reduce the number of muscle contractions needed without sacrificing the image temporal resolution.
In conclusion, 4 wk of chronic unloading decreased the Young's modulus by 17% due to changes in the material properties of the tendon. Importantly, the transition point of the Achilles tendon shifted to longer length, indicating increased compliance in the toe region. These changes in tendon properties were accompanied by a 47% decrease in force production capacity of the triceps surae muscles. The Young's modulus, transition point, and muscle strength returned to the normal level at the end of 6 wk of physical therapy, emphasizing the dynamic and adaptive nature of muscle and tendon to external loading.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1 AR-53343.
Acknowledgments
We thank Ziya Altug, Anthony Hines, and Janet J. Tucay (UCLA Rehabilitation Services) for planning and conducting physical therapy.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J Orthop Res 16: 591–596, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Ameida-Silveira MI, Lambertz D, Perot C, Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol 81: 252–257, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bailey AJ Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol 70: 1882–1885, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan CI, Marsh RL. Effects of long-term exercise on the biomechanical properties of the Achilles tendon of guinea fowl. J Appl Physiol 90: 164–171, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol 76: 1764–1773, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Convertino VA Physiological adaptations to weightlessness: effects on exercise and work performance. Exerc Sport Sci Rev 18: 119–166, 1990. [PubMed] [Google Scholar]
- 8.Convertino VA, Doerr DF, Mathes KL, Stein SL, Buchanan P. Changes in volume, muscle compartment, and compliance of the lower extremities in man following 30 days of exposure to simulated microgravity. Aviat Space Environ Med 60: 653–658, 1989. [PubMed] [Google Scholar]
- 9.Desplanches D, Mayet MH, Ilyina-Kakueva EI, Sempore B, Flandrois R. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol 68: 48–52, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Edgerton VR, Roy RR. Neuromuscular adaptations to actual and simulated spaceflight. In: Handbook of Physiology. Environmental Phsyiology. Bethesda, MD: Am. Physiol. Soc., 1996, p. 721–763.
- 11.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol 78: 1733–1739, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol 95: 829–837, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Greenleaf JE, Bulbulian R, Bernauer EM, Haskell WL, Moore T. Exercise-training protocols for astronauts in microgravity. J Appl Physiol 67: 2191–2204, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Haxton HA Absolute muscle force in the ankle flexors of man. J Physiol 103: 267–273, 1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of 20 days of bed rest on the viscoelastic properties of tendon structures in lower limb muscles. Br J Sports Med 38: 324–330, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubo K, Kawakami Y, Kanehisa H, Fukunaga T. Measurement of viscoelastic properties of tendon structures in vivo. Scand J Med Sci Sports 12: 3–8, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Lee HD, Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Soleus aponeurosis strain distribution following chronic unloading in humans: an in vivo MR phase-contrast study. J Appl Physiol 100: 2004–2011, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. J Biomech 33: 1453–1459, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve 33: 85–92, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531: 277–288, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M, Kjaer M. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol 21: 369–377, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Martin TP, Edgerton VR, Grindeland RE. Influence of spaceflight on rat skeletal muscle. J Appl Physiol 65: 2318–2325, 1988. [DOI] [PubMed] [Google Scholar]
- 24.Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Geometric and elastic properties of in vivo human Achilles tendon in young adults. Cells Tissues Organs 178: 197–203, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Narici M, Cerretelli P. Changes in human muscle architecture in disuse-atrophy evaluated by ultrasound imaging. J Gravit Physiol 5: 73–74, 1998. [PubMed] [Google Scholar]
- 26.Nemetschek T, Jonak R, Nemetschek-Gansler H, Riedl H, Rosenbaum G [Translated by Nemetschek T]. On the determination of changes in the large periodic structure of collagen. Z Naturforsch [C] 33: 928–936, 1978. [PubMed] [Google Scholar]
- 27.Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci 203: 305–321, 1978. [DOI] [PubMed] [Google Scholar]
- 28.Paxton JZ, Baar K. Tendon mechanics: the argument heats up. J Appl Physiol 103: 423–424, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Proske U, Morgan DL. Tendon stiffness: methods of measurement and significance for the control of movement. J Biomech 20: 75–82, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98: 2278–2286, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol 88: 567–572, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Shin D, Finni T, Ahn S, Hodgson JA, Lee HD, Edgerton VR, Sinha S. In vivo estimation and repeatability of force-length relationship and stiffness of the human Achilles tendon using phase contrast MRI. J Magn Reson Imaging. In press. [DOI] [PMC free article] [PubMed]
- 34.Sinha S, Hodgson JA, Finni T, Lai AM, Grinstead J, Edgerton VR. Muscle kinematics during isometric contraction: development of phase contrast and spin tag techniques to study healthy and atrophied muscles. J Magn Reson Imaging 20: 1008–1019, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Steffen JM, Musacchia XJ. Spaceflight effects on adult rat muscle protein, nucleic acids, and amino acids. Am J Physiol Regul Integr Comp Physiol 251: R1059–R1063, 1986. [DOI] [PubMed] [Google Scholar]
- 36.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol 516: 915–930, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology 19: 397–408, 1982. [DOI] [PubMed] [Google Scholar]
- 38.Zajac FE Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17: 359–411, 1989. [PubMed] [Google Scholar]
- 39.Zuurbier CJ, Everard AJ, van der Wees P, Huijing PA. Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J Biomech 27: 445–453, 1994. [DOI] [PubMed] [Google Scholar]