Abstract
In this work, we investigate 3He magnetic resonance imaging as a noninvasive morphometric tool to assess emphysematous disease state on a local level. Emphysema was induced intratracheally in rats with 25 U/100 g body wt of porcine pancreatic elastase dissolved in 200 μl saline. Rats were then paired with saline-dosed controls. Nine three-dimensional (3D) 3He diffusion-weighted images were acquired at 1, 2, or 3 wk postdose, after which the lungs were harvested and prepared for histological analysis. Recently introduced indexes sensitive to the heterogeneity of the air space size distribution were calculated. These indexes, D1 and D2, were derived from the moments of the mean equivalent airway diameters. Averaged over the entire lung, it is shown that the average 3He diffusivity (Dave) correlates well with histology (R = 0.85, P < 0.0001). By matching small (0.046 cm2) regions in 3He images with corresponding regions in histological slices, Dave correlates significantly with both D1 and D2 (R = 0.88 and R = 0.90, respectively, with P < 0.0001). It is concluded that 3He MRI is a viable noninvasive morphometric tool for localized in vivo emphysema assessment.
Keywords: hyperpolarized gas, elastase, lung histology, diffusion anisotropy
emphysema is a chronic obstructive pulmonary disease (COPD) typified by tissue destruction and airway expansion (40). Emphysema, particularly the early stages, may be characterized by a heterogeneous distribution of air space sizes (13). Indeed, McLaughlin and Tueller (23) showed that in apparently normal lungs of smokers harvested at autopsy (none of the deaths were attributed to COPD), numerous localized regions of tissue destruction were surrounded by normal tissue. Because early detection of COPD can play a significant role in managing the disease and improving patient outcomes (2, 20), a locally specific and sensitive method of detection may prove beneficial. Hyperpolarized (HP) 3He and 129Xe gas have been exploited in clinical and preclinical magnetic resonance imaging (MRI) studies to evaluate lung tissue structure (5, 6, 8, 18, 22, 33), although perfluorinated gases have also been used (14). In several studies, 3He MRI has been demonstrated to be sensitive to mild or early emphysema (10, 28, 34, 38). Gas-phase MRI is well suited for detecting emphysema because the diffusivity (Brownian motion) of gas is strongly affected by restrictive barriers, such as alveolar walls. Additionally, the gas contained in larger air spaces contributes proportionally more to the MRI signal in each voxel, making the signal within each voxel a volume-weighted average sensitive to the presence of enlarged air spaces or blebs. Therefore, MRI provides noninvasive, localized information about lung structure while allowing for frequent assessment of disease state without repeated exposure to ionizing radiation.
Despite its potential utility, validation of 3He diffusion MRI with histology at the local, or near-pixel, level is still needed before the technique can be generally accepted as a reliable localized measure of tissue destruction. Only a few studies demonstrating a correlation between histology and MRI exist (22, 28, 41), and they generally exhibit a correlation between MRI and regions of the lung of a much larger scale than the image resolution. Woods et al. (41) studied cylindrical cork-bore samples (1.3-cm diameter × 2-cm thick) from explanted emphysematous human lungs. These lungs were either healthy but rejected for transplant, or severely emphysematous and removed at transplant. Their results found a fair correlation between morphometry and 3He diffusivity (R = 0.611) using the lung samples and corresponding regions from the diffusion images, although their whole lung results correlated much more strongly (R = 0.964). Unfortunately, no mildly emphysematous lungs were available for their study. Peces-Barba et al. (28) studied mild elastase-induced emphysema in rats using a dose three times larger than used herein: 75 U/100 g body wt vs. 25 U/100 g body wt. Forty-five days after the dose, 3He diffusion MRI was performed and was compared with morphometry on a lobar level (with R = 0.71). Mata et al. (22) studied mild elastase-induced emphysema in rabbits and found that 3He diffusivity correlated well with regional morphometry that was performed by dividing lungs into four parts (R = 0.78).
Previous work has established that 3He MRI is sensitive to tissue destruction and that measured gas diffusion correlates well with regional histology. To correlate MRI results with tissue characteristics, previous studies used various methods of histological analysis. For example, Mata et al. (22) measured an average chord length within the air spaces of rabbit lungs, Woods et al. (41) measured the mean linear intercept (Lm) in excised human lungs using small cork-bore samples of lung sections, and Peces-Barba et al. (28) measured the number average of alveolar air space area and Lm in rats. Although Lm measurements have become generally accepted as the standard stereological measure of air space size, it is actually a measure of volume-to-surface area ratio. Using point-counting methods, Lm is essentially the ratio of the number of test points within air spaces to the number of grid intercepts with air space walls (39). It is therefore expected to be rather insensitive to a few enlarged air spaces in the presence of many small ones. Indeed, it has been shown that Lm is not a sensitive method for identifying early or mild emphysema in humans (36) nor in a rat elastase model, even after 5 wk (9). Furthermore, Lm is not intended to provide localized information in a heterogeneous structure since its rigorous calculation requires exhaustive, unbiased sampling of the entire lung or large lung regions. Consequently, a weighted index sensitive to distributions of air space size is argued to be more appropriate for this study, in which the aim is to compare MRI and local histology.
As part of previous efforts to characterize local airway structure from histological sections, Parameswaran et al. (27) demonstrated that variability in air space sizes may not be well reflected by Lm measurements. The alternatives they proposed were weighted indexes of air space size distributions D1 and D2. Mathematically, these describe the first (D1) and second (D2) moments of the distribution of airway sizes determined from histological samples. To implement this approach, airway sizes are first quantified in terms of an equivalent diameter (Deq = 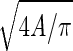 ) that is calculated from measurements of individual air space areas (A). The mean μ, the variance σ2, and the skewness γ of Deq are then determined to calculate D1 and D2:
) that is calculated from measurements of individual air space areas (A). The mean μ, the variance σ2, and the skewness γ of Deq are then determined to calculate D1 and D2:
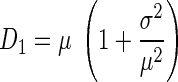 |
(1a) |
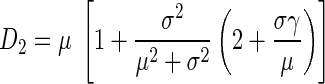 |
(2b) |
It is seen that a normal distribution, but with a large variance, will weight D1 to higher values. A nonnormal, skewed distribution of air space size will further weight D2. Hence, the presence of a few large air spaces causes the indexes to increase rapidly, unlike with Lm. Nevertheless, it must be emphasized that D1 and D2 provide information about the air space size distribution, not actual air space sizes. Therefore they may be used to describe a disease state but cannot strictly be used to quantify air space geometry parameters.
In this paper we examine an emphysematous disease state in an animal model on a local level. This is done by applying a model of air space geometry introduced by Yablonskiy et al. (42) to diffusion-weighted, three-dimensional (3D) 3He MRI (15). The results are then compared with weighted indexes of the alveolar size distribution from histological samples. In brief, the Yablonskiy model depicts the lung parenchyma as a network of cylindrical bronchioles sleeved by alveoli. Diffusion longitudinal to a bronchiole, DL, and diffusion transverse to a bronchiole, DT, are separated out as key parameters that can be used to describe lung microstructure. The Yablonskiy model then allows for direct measurement of the diffusion anisotropy Dan = DL − DT, and the voxel-average diffusivity Dave = (DL + 2DT)/3 through acquisition of multiple diffusion-weighted images. We note that Dave is equivalent to what is commonly referred to as the apparent diffusion coefficient (ADC) (42).
METHODS
Animal dosing.
Humane animal handling procedures approved by the Institutional Animal Care and Use Committee at Pacific Northwest National Laboratory were followed. A total of 18 male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study; half were dosed intratracheally with porcine pancreatic elastase (Calbiochem, La Jolla, CA), and the other half with sterile saline. The average body weight of the rats at the time of dosing was 209 ± 9 g. About 10–15 min before dosing, the rats were administered 0.02 ml/kg body wt glycopyrrolate subcutaneously to inhibit oral secretions and facilitate insertion of an endotracheal tube. The animals were then anesthetized with 2.5–3.5% isoflurane in oxygen and placed supine on a tilting intubation table. Approximately 0.02 ml of lidocaine was applied directly to the larynx; then a 14-gauge catheter tube was orally inserted into the trachea. The tube was precut to a length to ensure that it would pass through the larynx but not come near the carina. A high-pressure aerosolizing syringe (PennCentury, Philadelphia, PA) was sterilized with ethanol, then thoroughly rinsed with sterile saline before dosing. The elastase solution (or saline) was drawn into the syringe, the end of the aerosolizer was inserted through the length of the trachea tube, and the dose was administered. Rats were dosed intratracheally with either 25 U/100 g body wt of elastase in 200 μl of sterile physiological saline or with 200 μl saline only as a control, with nine rats in each group. After the dose was administered, rats were then tilted upward for several breaths after which the trachea tube was removed. When a rat began to revive from the anesthetic, it was returned to its cage and provided food and water ad libitum. The dosing was conducted over three consecutive days with six weight-matched animals dosed per day (3 each of elastase and saline). At each 1-, 2-, and 3-wk time point, elastase-dosed animals were imaged with a control. The different time delays were intended to generate a range of responses to the elastase dose. Animal mortality was zero.
HP 3He generation and delivery.
HP 3He was generated via hybrid spin-exchange optical pumping (1, 37) using a home-built, spectrally-narrowed (3), dual-laser apparatus. Two ≈110-ml Pyrex 3He vessels, each filled to ≈7 atm (for a total of ≈800 ml of HP gas per vessel), were used to generate a 3He polarization of ≈50% overnight (16). For imaging, a bolus of 3He gas was released into a 2-liter Tedlar bag (Jensen Inert Products, Coral Springs, FL) that resided in the fringe field of the imaging magnet. The HP 3He was mixed with ≈0.4 liter of N2 to ensure an adequate volume of gas for an entire imaging experiment. The gas mixture was then delivered to the rat via a positive-pressure mechanical ventilator, as described below.
Imaging.
All of the animal preparation procedures, specific ventilation parameters, and details of imaging parameters used are described in detail elsewhere (15) and will be explained only briefly here. Animals were prepared for imaging by anesthetizing with isoflurane, following which an endotracheal tube was inserted, as was done for the dosing. With the tube inserted, the anesthetized rat was secured supine to a wooden tray and attached to a home-built, computer-controlled mechanical ventilator based on those described by others (8, 12). A rectal temperature probe was then inserted and ECG leads attached to the forepaws for physiological monitoring. A breathing rate of 50 breaths/min was used to maintain the animals under gas anesthesia while allowing sufficient time for breathing maneuvers and imaging. Each breathing cycle consisted of ≈1 ml of the HP 3He-N2 gas mixture followed by ≈4 ml of air to a maximum pressure of ≈15 cmH2O (an oxygen-enhanced mixture of 30% O2-70% N2 was used in place of normal breathing air). Following a passive exhalation period, imaging data were acquired during a breath hold. Based on 3He ventilation imaging studies by others (4) and on 3D 3He diffusion imaging on healthy rats in our laboratory using this same gas delivery technique (15), we expect that this stacked method of ventilation results in a homogeneous distribution of 3He gas in the lungs. The air pressure at the trachea after exhale, or end-expiratory pressure (EEP), was measured by a solid-state pressure transducer and was recorded for each rat. Although EEP was not rigorously controlled, its value was documented so that diffusion data could be corrected for rat-to-rat differences in lung inflation at the same point in each rat's breathing cycle.
The wooden tray with the animal and ECG/temperature-monitoring unit (SA Instruments, Stony Brook, NY, model 1025) slid into the MRI coil on a mating rail to enable animal positioning. Warm air, typically ≈50°C, was circulated within the magnet bore to maintain body temperature. The rectal temperature was maintained at 35 ± 2°C, and the heart rate was generally 250–350 beats/min, depending on body weight and level of anesthesia.
All imaging was performed in a 2.0-T horizontal-bore magnet (Oxford, UK) with 150 mT/m gradients (Resonance Research, Billerica, MA) and a Varian UnityPlus spectrometer (Palo Alto, CA). A home-built, birdcage-style MRI coil tuned to both 3He and 1H frequencies (64.4 MHz and 84.9 MHz, respectively) was used. A trigger signal from the ventilator ensured that image data were acquired at the same point of each breathing cycle; cardiac motion was ignored. A standard 2D multislice spin-echo sequence was used for proton imaging for animal positioning. A hybrid 3D imaging sequence combining radial acquisition (in the xy-plane) with conventional phase encoding (along the z-axis, parallel to the magnetic field) was used for 3D 3He diffusion imaging. This pulse sequence is referred to as ZIPR in Ref. 29 and is described in detail there and in Ref. 15. Diffusion encoding was applied along the phase-encode axis with a gradient pulse duration of 600 μs and a gradient separation of 1.8 ms. The 9 b-values used were 0, 1, 3, 6, 10, 15, 20, 30, and 40 s/cm2. Each 3D 3He data set consisted of nine interleaved images, each with a different diffusion sensitization, and required 990 breathing cycles to complete, consuming ∼1 liter of the 3He-N2 mixture. Images were reconstructed to 3D Cartesian grids, which were then divided into 2-mm-thick transverse slices for analysis. Final reconstructed 3He image planar resolution was 0.5 mm × 0.5 mm. We refer the reader to Ref. 15 for additional details on image acquisition and reconstruction.
After reconstruction, images were thresholded to eliminate background noise from the diffusion maps. A model-based approach of acquiring quantitative information about air space size was then implemented; we refer the reader to Yablonskiy et al. (42) for full details of the model. The voxel-average diffusivity, Dave, diffusion anisotropy Dan, and apparent airway radius Rmean were calculated (using equations 5 and 8 in Ref. 42) on a pixel-by-pixel basis using the Non LinearRegress function of Mathematica (Wolfgram Research, Champaign, IL). Pixels with a standard error (i.e., fit uncertainty) exceeding 50% were discarded and assigned a value of zero. Global median values for the whole lung diffusivities were then calculated for each rat. Global means were not used because the distributions of diffusivities were nonnormal (typical skewness = 7.1). In such cases, medians are not distorted by extreme values, such as high diffusivities in the trachea and major airways, but generally provide a useful measure of the typical value (24). The 3He free diffusivity was also calculated for each animal by measuring the apparent diffusion coefficient from the first two b-values in several slices that included only the trachea, which was aligned generally parallel with the z-axis of the magnet.
After calculation of the diffusion coefficients, 8–10 regions of interest (ROI), each ∼5 mm in diameter and generally excluding major airways, were selected from the 2D slices of each 3D image set. These regions were then used to guide histological sampling of the lung tissue with the intention of facilitating localized correlation between MRI and histology.
Morphometric analysis.
Following imaging, animals were humanely killed using CO2 asphyxiation. The lungs were exsanguinated, harvested, trimmed, and filled with 10% buffered formalin to a pressure of 25 cmH2O (19) using a Mariotte bottle system. After filling, the trachea was tied off and the lung placed in a formalin bath for >48 h. We estimate tissue shrinkage due to fixation to be approximately 10–15%. The lungs were then sectioned into 2-mm-thick transverse slices using the MR images as a guide. Lung sections that corresponded to the image slices that had a circled ROI were retained, resulting in a total of 101 sections. After imbedding in paraffin, a representative 5-μm-thick slice of each section was placed on a microscope slide for histological analysis.
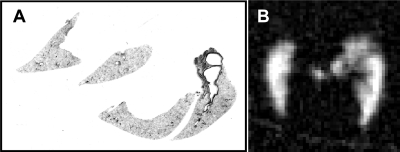
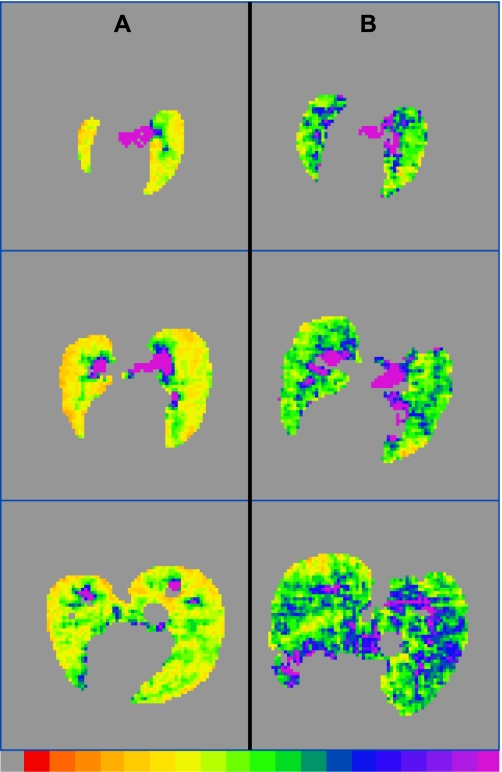
For a global (i.e., averaged over the whole lung) comparison between MRI and histology, slides were examined at 100× magnification (10× objective, 10× ocular), and 1,600 × 1,200 pixel digital images (covering ≈1.0 mm × 0.7 mm) were acquired for analysis. Global characterization was performed by taking 8–10 digital images of tissue sections for each rat; images were taken from all histological sections to span the entire lung. Generally, large airways and blood vessels were avoided. By design, histological sampling was guided by the ROIs initially identified in 3He images. In many cases, however, histological sections bore little resemblance to the 3He images they were intended to represent. Primarily, this discrepancy occurred because lung shape was generally distorted during sectioning. This is illustrated in Fig. 1, where the histological slide (Fig. 1A) and the MRI slice (Fig. 1B) it was supposed to represent are shown.
Fig. 1.
Histological slice (A) and 3He image slice (B) that were intended to represent the same transverse lung section. The distortion of the lung tissue in A due to sectioning illustrates the difficulties encountered in attempting localized correlations between histology and MRI.
Because of difficulty matching 3He ROIs to histological sections, a different approach was adopted for performing local correlations. To prevent bias, this was done without knowledge of the dose history, animal identity, or the 3He diffusivity results within each slice. Each histology slide and a printout of the corresponding 3He spin-density image (such as that shown in Fig. 1B) were examined together and evaluated qualitatively for regions that could be matched with a high degree of confidence, as judged by the blinded reviewer. Of the 101 slides from all the rats, a total of only 12 regions were deemed to match sufficiently well for analysis. Once a region was selected, its location was marked on the 3He image, and six slightly overlapping 1,600 × 1,200 pixel digital microscope images were acquired within the corresponding region on the slide covering an approximately rectangular region of ≈2.0 mm × ≈2.3 mm.
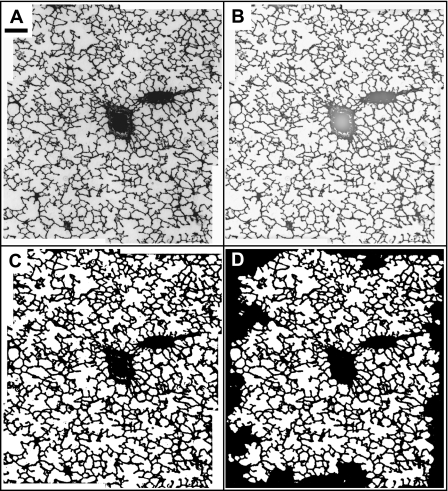
Analysis of all digital histology images was done using ImageJ (32). Image sets for local correlation (those consisting of 6 images) were first assembled into a mosaic using MosiacJ (35), an ImageJ plugin. All images were filtered with a 2.0-pixel radius Gaussian blur to remove speckle and a 200-pixel radius rolling ball background subtract filter to remove background intensity variations. Next, the optical images were threshholded and examined. Particles were removed, major airways, and blood vessels were manually filled in, and unthresholded tissue regions were repaired. Figure 2 shows an example of the image processing sequence for an image mosaic. Finally, the areas of the individual air spaces were measured by selecting the air spaces that were entirely bounded by tissue, measuring the areas, and reporting all results in a spreadsheet. The equivalent diameter Deq of each air space was then calculated and converted to micrometers using a microscope calibration. The average equivalent diameter μ for the entire image mosaic was found, and D1 and D2 were then calculated to represent the air space size distribution of each mosaic. We note that partial airways truncated by the edge of the image mosaics were excluded (see Fig. 2). For the smaller single images used in the global comparison, all air spaces were included in the analysis. Airways bordering the edge were therefore defined by the edge of optical data. In the emphysematous samples most of the largest air spaces were truncated by at least one edge of the 1,600 × 1,200 image frame, thereby skewing the results toward somewhat smaller values. Although this method is imperfect, excluding the edge-bordering airways would serve to further skew the results toward lower values by eliminating the largest air spaces altogether. Several of the images were randomly selected and reanalyzed with all edge-bordering airways excluded; this test confirmed the hypothesis. The exclusion of edge air spaces in the saline controls had little effect.
Fig. 2.
An example of the sequence for processing of image mosaics. A: raw image of the assembled mosaic. B: after filtering and background subtraction. C: after threshholding. D: after manual repair. Scale bar represents ∼200 μm.
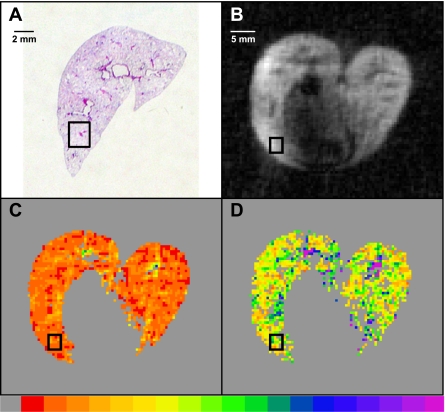
To calculate the diffusion results in each 3He image region corresponding to a mosaic, the 3He spin-density image and Dave, Dan, and Rmean maps were opened in ImageJ. An image stack was created with the spin-density image on top. Looking only at the spin-density image, a 2.0 × 2.5 mm rectangular region (i.e., 4 pixels × 5 pixels) was selected at the location chosen by the blinded reviewer. Without moving the selected region, the average values of Dave, Dan, and Rmean were then measured. Pixels within the region with a value of zero were excluded from the average. Figure 3 shows an example of this process. Figure 3A shows a digital photograph of the histological slide with the targeted region outlined in black. The blinded observer deemed this region to correlate with the region in the 3He image (Fig. 3B) with high confidence. Figure 3, C and D, shows the Dave and Dan maps, respectively, with the same region outlined (the Rmean map is not shown). Hence, the histology results from the mosaic images were correlated with the average MRI results from the corresponding region. We note that the mosaic in Fig. 2 is from the selected region in Fig. 3A.
Fig. 3.
Illustration of one of the 12 regions, this from rat E9, used for the localized correlation between histology and MRI. A: photograph of the histology slide (only the right lung is shown) with a selected region outlined in black. B: the corresponding 3He spin-density image with the region outlined. C: the average diffusivity (Dave) map. D: the diffusion anisotropy (Dan) map. The bottom color scale for both diffusion images ranges from 0.0 (gray, left) to ≥0.8 cm2/s (purple, right).
RESULTS
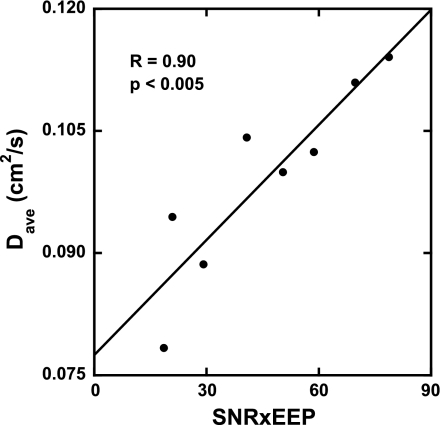
It was observed that each animal exhibited a different EEP, despite each being ventilated in exactly the same way. The signal-to-noise ratio (SNR) for 3He images also varied from rat to rat, presumably, due to differences in gas polarization and/or mixing with N2 in the Tedlar bag. Figure 4 shows that the global median value of Dave for the saline controls varied linearly with the product of EEP and SNR over the range of measured diffusion coefficients (R = 0.90, P < 0.005). In principle, this is not surprising since differences in EEP indicate that diffusion data were likely collected at slightly different levels of lung inflation, and because measured diffusion is affected by 3He SNR (25). To facilitate comparisons between different animals and treatment groups, an empirical, linear correction was made to all Dave values to EEP = 0 cmH20. Linear variations with the product of EEP and SNR were also found for Dan (R = 0.85, P < 0.01) and Rmean (R = 0.91, P < 0.002), with respective corrections made to those data. We note that Dave error bars are not shown in Fig. 4 due to the lack of a conventional measure of uncertainty of a median value. However, the average fractional error determined from the numerical fitting for Dave ranged from 0.10 to 0.27 for the highest and lowest SNR saline rats, respectively.
Fig. 4.
Plot showing the empirical relationship between the global median of Dave vs. the product of 3He signal-to-noise ratio (SNR) and end-expiratory pressure (EEP) for the saline-treated control rats. Data for all rats were then corrected to the y-intercept (EEP = 0 cmH2O). Similar relationships were found for, and corrections made to, Dan and mean airway radius (Rmean).
Table 1 shows physiological and MRI data collected from each rat. The rat label refers to the type of dose given (S for saline, E for elastase) and the rat number. Saline and elastase rats of the same number were imaged on the same day. Rats S1 and E1 were eliminated from the analysis due to technical problems encountered during imaging.
Table 1.
Data from 16 of the 18 rats in the study
| Rat | BW, g | LW, g | LV, ml | EEP, cmH2O | 3He S/N | D0, cm2/s | Dave*, cm2/s | Dan*, cm2/s | Rmean*, mm |
|---|---|---|---|---|---|---|---|---|---|
| S2 | 271 | 1.700 | 5.148 | 1.49 | 12.46 | 1.08 | 0.0696 | 0.219 | 0.148 |
| S3 | 240 | 1.308 | 4.918 | 2.98 | 16.95 | 0.94 | 0.0762 | 0.222 | 0.157 |
| S4 | 323 | 1.631 | 6.770 | 2.24 | 13.06 | 1.03 | 0.0749 | 0.218 | 0.150 |
| S5 | 309 | 1.443 | 4.827 | 3.59 | 21.92 | 0.97 | 0.0770 | 0.228 | 0.153 |
| S6 | 261 | 1.347 | 3.540 | 2.73 | 21.52 | 0.99 | 0.0747 | 0.222 | 0.147 |
| S7 | 344 | 1.616 | 5.854 | 4.33 | 16.10 | 0.97 | 0.0781 | 0.235 | 0.153 |
| S8 | 347 | 1.759 | 7.596 | 2.24 | 18.24 | 1.03 | 0.0850 | 0.247 | 0.158 |
| S9 | 337 | 1.542 | 6.973 | 1.37 | 15.25 | 1.02 | 0.0846 | 0.243 | 0.157 |
| E2 | 292 | 1.881 | 6.857 | 2.24 | 14.15 | 0.95 | 0.0728 | 0.221 | 0.145 |
| E3 | 240 | 1.877 | 7.188 | 1.74 | 15.90 | 0.97 | 0.0977 | 0.269 | 0.174 |
| E4 | 267 | 1.461 | 7.405 | 3.22 | 14.08 | 1.07 | 0.1005 | 0.282 | 0.173 |
| E5 | 321 | 1.482 | 3.511 | 2.11 | 21.65 | 1.01 | 0.0858 | 0.246 | 0.159 |
| E6 | 298 | 1.920 | 10.040 | 3.59 | 19.24 | † | 0.1097 | 0.308 | 0.170 |
| E7 | 381 | 1.823 | 5.796 | 2.36 | 18.33 | 1.01 | 0.0967 | 0.272 | 0.166 |
| E8 | 386 | 2.017 | 9.323 | 2.48 | 18.84 | 1.02 | 0.0870 | 0.255 | 0.160 |
| E9 | 356 | 1.908 | 8.089 | 3.47 | 11.77 | 1.01 | 0.0892 | 0.277 | 0.161 |
S rats were given saline dose; E rats were given elastase dose. Two rats, S1 and E1, were excluded due to technical problems with the imaging data. BW, body weight at time of imaging; LW, lung weight at time of necropsy; LV, lung volume after fixation determined by fluid displacement; EEP, end- expiratory pressure; 3He S/N, average global 3He signal-to-noise ratio for the b = 0 s/cm2 image, excluding major airways; D0, 3He free diffusivity measured in the trachea.
Data for average diffusivity (Dave), diffusion anisotropy (Dan), and mean airway radius (Rmean) are corrected global medians from MRI.
Insufficient data.
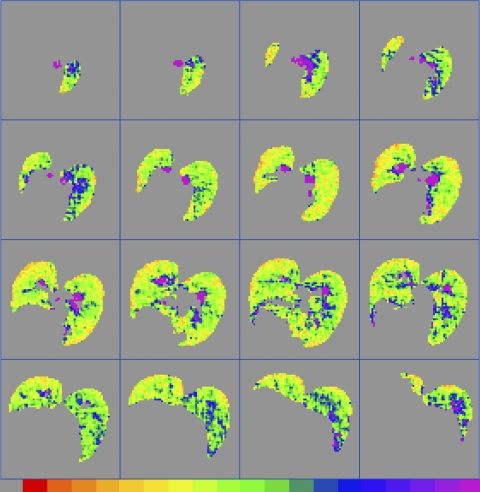
Figure 5 shows an example of a 3D data set from rat E4 for which the Dave values have been calculated for each pixel using the Yablonskiy model (42). Each slice is 2-mm thick with a planar resolution of 0.5 mm × 0.5 mm. The color scale (in units of cm2/s; see Fig. 5 legend) shows the range of values of Dave; major airways are generally visible as regions of the highest Dave values.
Fig. 5.
An example of a full 3D data set, taken from rat E4, showing Dave calculated on a pixel-by-pixel basis from 3He MRI data and corrected to EEP = 0 cmH2O. The left lung is on the observer's left. Planar resolution is 0.5 × 0.5 mm, and the slice thickness is 2 mm. The bottom color scale ranges from 0.0 (gray, left) to ≥2.5 cm2/s (purple, right).
Figure 6 shows a comparison between two rats, rats S6 and E6, both imaged on the same day 2 wk after dosing. Figure 6 demonstrates differences in 3He diffusivity between a saline-dosed and an elastase-dosed animal. Three approximately corresponding representative slices are shown for each animal; the color scale is the same for each. The saline-dosed animal (Fig. 6, column A) has significantly lower Dave (P < 0.0001) and less apparent heterogeneity than the elastase-dosed animal (Fig. 6, column B).
Fig. 6.
Comparison between calculated Dave values in approximately corresponding slices from a saline-dosed rat (A) and an elastase-dosed rat (B), rats S6 and E6, respectively. The bottom color scale ranges from 0.0 (gray, left) to ≥2.5 cm2/s (purple, right). Data were corrected to EEP = 0 cmH2O.
For the global histological analysis, the overall mean Deq (i.e., μ), its variance (σ2), and skewness (γ) were calculated by combining the results from the 8–10 microscope images from each rat. D1 and D2 were then calculated using Eqs. 1a and 1b (see Table 2). The fact that D2 > D1 is largely due to the skewness of the Deq distributions. For the saline-dosed rats, the average value of μ was 35.5 ± 3.4 μm and the average value of D2 was 102 ± 13 μm. These uncertainties are comparable to those reported for Lm in the literature (for example, Ref. 9 reported an Lm of 37 ± 10 μm in 7 control rats, and Ref. 28 reported an Lm of 85 ± 14 μm in 6 control rats). Therefore, we assume that any consequences of tissue processing did not have an unusual effect on our data analysis.
Table 2.
Global histological results
| Rat | μ, μm | σ2 | γ | D1, μm | D2, μm |
|---|---|---|---|---|---|
| S2 | 30.77 | 648.03 | 2.2856 | 51.835 | 79.416 |
| S3 | 40.73 | 1324.78 | 2.1908 | 73.256 | 112.30 |
| S4 | 35.55 | 996.07 | 2.8170 | 63.568 | 106.08 |
| S5 | 35.23 | 1175.83 | 3.0967 | 68.606 | 121.17 |
| S6 | 30.86 | 739.37 | 2.5834 | 54.818 | 88.537 |
| S7 | 36.60 | 1033.43 | 2.4723 | 64.835 | 103.09 |
| S8 | 36.01 | 1082.87 | 2.7781 | 66.081 | 110.39 |
| S9 | 38.01 | 981.16 | 2.4394 | 63.822 | 99.661 |
| E2 | 37.73 | 1270.06 | 2.5269 | 71.392 | 115.77 |
| E3 | 52.55 | 2315.44 | 2.1770 | 96.611 | 148.26 |
| E4 | 53.15 | 2594.00 | 2.7272 | 101.95 | 170.53 |
| E5* | 30.34 | 881.67 | 3.4295 | 59.400 | 109.84 |
| E6 | 56.52 | 3854.04 | 2.6469 | 124.71 | 208.18 |
| E7 | 38.07 | 1272.48 | 2.6047 | 71.495 | 117.11 |
| E8 | 43.53 | 1509.03 | 2.6031 | 78.197 | 126.95 |
| E9 | 45.80 | 1933.69 | 3.1648 | 88.020 | 156.49 |
μ, σ2, and γ are the mean, variance, and skewness of equivalent diameter Deq; D1 and D2 are weighted indexes of air space size distribution.
All sections of lung E5 appeared underinflated on the histological slides.
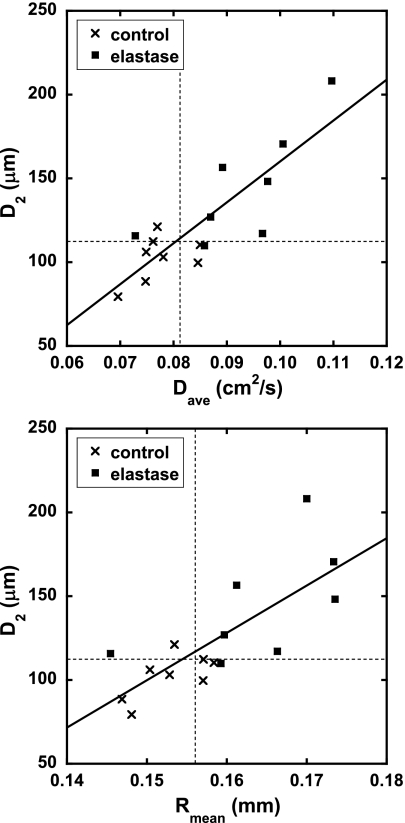
Figure 7 is a plot of the MRI parameters Dave and Rmean vs. D2; correlations between the global 3He diffusivity results (Table 1) and D2 (Table 2) for each rat are shown in Table 3. We note that Dave and Dan correlate strongly with each other in both the global and local measurements (R = 0.99 and R = 0.97, respectively), implying that uniform scaling of the airway structures is occurring. Thus the plot of Dan vs. D2 is not shown. The dashed lines in Fig. 7 represent the 95% confidence intervals from the mean of the saline control group, as calculated from the Student's t-distribution. That is, points lying to the right or above the dashed lines have high confidence of representing emphysematous rats according to either MRI or histology, respectively. For Dave, two saline controls were outside the confidence interval, while three were outside for Rmean. Only a single elastase-dosed animal, rat E2, was within the confidence interval for Dave and Rmean. For D2, two saline controls were outside the confidence interval while one elastase animal, rat E5, was within. We note that rat E5 appeared quite underinflated on the histological slides, thus resulting in low histological measurements. Because the elastase-treated rats were imaged at various time points and therefore presented a range of disease response, we examined the confidence intervals based on the mean of the saline group, as opposed to attempting direct comparisons between the elastase and saline groups.
Fig. 7.
Correlations between global results of weighted index of air space size distribution D2, and Dave and Rmean. The dashed lines indicate the 95% confidence interval from the mean values of the saline controls. Fit quality parameters are shown in Table 3.
Table 3.
Correlation parameters for global and local measurements of histology and MRI
| Dave | Dan | Rmean | |
|---|---|---|---|
| Global | |||
| μ | R = 0.86 | R = 0.87 | R = 0.80 |
| P < 0.0001 | P < 0.0001 | P < 0.0005 | |
| D1 | R = 0.85 | R = 0.86 | R = 0.79 |
| P < 0.0001 | P < 0.0001 | P < 0.0005 | |
| D2 | R = 0.85 | R = 0.88 | R = 0.77 |
| P < 0.0001 | P < 0.0001 | P < 0.001 | |
| Local | |||
| μ | R = 0.81 | R = 0.85 | R = 0.42 |
| P < 0.002 | P < 0.0002 | P = 0.175 | |
| D1 | R = 0.88 | R = 0.93 | R = 0.50 |
| P < 0.0002 | P < 0.0001 | P = 0.196 | |
| D2 | R = 0.90 | R = 0.93 | R = 0.56 |
| P < 0.0001 | P < 0.0001 | P = 0.0593 |
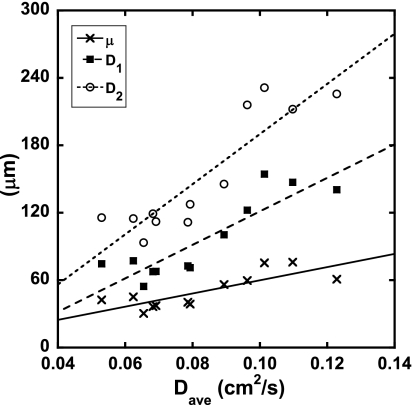
The local correlations between the MRI results and the mosaic histological results are shown in Fig. 8. The quantities μ, D1, and D2 are plotted against Dave. Significant correlations between the 3He diffusivities and histological measurements were found, as shown in Table 3. Data for the mean airway radius Rmean were not found to correlate significantly.
Fig. 8.
Plots showing the correlations between the local MRI 3He diffusion, Dave, and the local histology measurements, μ, D1, and D2. Fit quality parameters are shown in Table 3.
DISCUSSION
We found a significant correlation between 3He diffusion measurements and histology on both a global (i.e., over the whole lung) and localized basis (see Table 3). Figure 7 shows good distinction between the saline controls and elastase-dosed animals for both the global MRI results and histological measurements, with most of the elastase-dosed animals lying outside the saline control 95% confidence interval. Therefore, we conclude that both MRI and the histological measurements are sensitive to air space enlargement in the elastase animal model.
On a local level, a highly significant correlation between the localized size-weighted indexes D1 and D2, and Dave was found, as shown in Fig. 8. Table 3 also shows that the correlation between the MRI results and the histological measurements improves as higher-order moments are considered, implying that the macroscopic MRI measurements contain information about the microscopic air space size distribution, more so than they do about the mean diameters (μ). We speculate that this is because the higher moments are weighted by air space size, and MRI is essentially a volume-weighted imaging technique; proportionally more signal within each voxel comes from the larger air spaces. These results provide the most compelling evidence yet that 3He MRI diffusivity results are accurate on a local basis, suggesting that in vivo 3He MRI can be used as a noninvasive histological tool to assess disease. The results also suggest that D1 and D2 are more sensitive to localized tissue destruction than a numerical average of air space size. This supports the assertion of Parameswaran et al. (27), who showed that the presence of a few large blebs, which may be indicative of early emphysema, surrounded by many small air spaces will be masked by a numerical average or by traditional point-counting methods. We note that, although Rmean correlated somewhat better with D2 than with μ, the local correlation between Rmean and histology was not significant. We attribute this to the low SNR in many of the image sets. Since Rmean is the result of fitting two complicated functions, fit uncertainties are expected to be higher as SNR decreases. Because Rmean was calculated on a pixel-by-pixel basis, random variations in Rmean are likely to be averaged out globally while they remain pronounced locally. With improved SNR, one could better test the assertion of Fichele et al. (11), whose computer simulations indicated that Rmean may not be a good indicator of mild emphysema.
The Yablonskiy model (42) is a two-parameter model that assumes a locally homogeneous distribution of air space sizes. We have previously shown that the model describes the MRI diffusion data exceedingly well in healthy rats (15), where the assumption is more rigorously satisfied than in the current study. However, we have continued to use this model to enable consistent comparisons with previous work. Although fit parameters in emphysematous rats are not readily interpreted in terms of Yablonskiy's original model, the strong correlation between histology and MRI data shown herein suggests that the Yablonskiy model is able to describe the signal decay for a broader range of conditions than the specific case used for derivation.
Localized correlations in this study were successful, although only 12 regions from the 101 histology slides were deemed suitable for comparison. Generally, incomplete use of histological samples was dictated by the difficulty in confidently matching specific locations with those in corresponding 3He images. In practice, this difficulty arose from two problems. First, the in situ shape of the lung was not well preserved, so the shapes of the lung sections did not well match those of the 3He images. Lungs that have been removed from the chest can change significantly from the in situ shape defined by the thoracic cavity (14, 30). Second, the lungs were not rigid during sectioning. The nonrigidity of the fixed lungs allowed the lobes to mislocate from their correct relative in situ positions (cf. Fig. 1). More rigid lung fixation, done preferably in situ and perhaps accomplished with long-term (i.e., >3 wk) soaking in a formalin bath, may facilitate future local correlation studies. Freezing the lungs for sectioning, as performed by Woods et al. (41), may also be a viable option.
In this study the lung inflation levels were different for imaging and histology. However, a valid correlation between 3He diffusivity and histology is possible because no attempt was made to quantitatively calibrate the 3He diffusion coefficient with an absolute airway size. Furthermore, the lung inflation level does not affect the intrinsic tissue structure, and hence does not affect air space size distribution; only the average airway size is affected. Once the MRI data were corrected for variations in EEP, the data were assumed to fairly represent each rat at some constant level of inflation for which EEP = 0 cmH2O. With the lungs all inflated to the same pressure for fixation, a qualitative comparison between the histology and MRI could then be made.
The strong dependence of the 3He diffusivity on the small differences in EEP observed in this study was unanticipated, although previous observations by the authors and those reported by others (5) have indicated that large differences in breath hold level significantly affect measured 3He diffusivity. Although the cause of the variations in EEP was not clear, we propose that variations in lung compliance may have played a role. It has been reported that rat-to-rat variability in pulmonary compliance and functional residual capacity (FRC, the lung volume after full, passive exhalation) exists, even within healthy groups (17, 26). If it is assumed that FRC is measured at EEP = 0 cmH2O, then an EEP > 0, such as reported herein, can only be obtained when there is incomplete exhalation. This may be the case when insufficient time is allowed for full exhalation, and it would be particularly pronounced in rats with relatively high lung compliance (or low elasticity). In this work, all rats were allowed to exhale for the same fixed duration. Hence, we speculate that natural variations in compliance may have contributed to the differences in EEP. In this case, a simple, empirical correction based on the results of the saline controls was implemented to allow fair assessment of the data. A proposed experimental solution to this problem may be to acquire 3He data at a controlled breath hold level above EEP. However, a correction to the data may still be necessary due to lung compliance differences. This is because the degree of tissue distension or alveolar recruitment—both of which affect air space size and 3He diffusivity—at any given inflation pressure or volume may be affected by the lung compliance, particularly in a severe disease state (21). How such a correction would be made remains unclear and remains to be the subject of future study. Therefore, acquiring data for all animals at the same point in the breathing cycle (i.e., EEP) may currently be the best method of ensuring experimental consistency.
Localized disease assessment may allow for improved early diagnosis for COPD patients. It may also be a useful research tool in preclinical pulmonary studies requiring screening of large populations of animals. In addition, accurate, localized detection of disease distribution may facilitate treatment with innovative new therapies, such as magnetically targeted delivery of aerosolized pharmaceuticals (7, 31).
GRANTS
This work was funded by Battelle and by National Heart, Lung, and Blood Institute Grant RO1-HL-073598.
Acknowledgments
We gratefully acknowledge the animal preparation by A. Woodstock of Pacific Northwest National Laboratory (PNNL) and lung section and slide preparation by K. Gideon and K. Studniski of Battelle Toxicology Northwest. We thank R. Corley and J. Carson of PNNL for helpful conversations, and C. Bilskis of PNNL for use of microscope and camera. We also thank B. Saam of the University of Utah for supplying spin-exchange optical pumping cells. A portion of the research was performed using EMSL, a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research located at PNNL.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Babcock E, Nelson I, Kadlecek S, Driehuys B, Anderson LW, Hersman FW, Walker TG. Hybrid spin-exchange optical pumping of He-3. Phys Rev Lett 91: 123003, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Briggs DD Jr. Chronic obstructive pulmonary disease overview: prevalence, pathogenesis, and treatment. J Manag Care Pharm 10: S3–S10, 2004. [PubMed] [Google Scholar]
- 3.Chann B, Babcock E, Anderson LW, Walker TG, Chen WC, Smith TB, Thompson AK, Gentile TR. Production of highly polarized He-3 using spectrally narrowed diode laser array bars. J Appl Phys 94: 6908–6914, 2003. [Google Scholar]
- 4.Chen BT, Brau ACS, Johnson GA. Measurement of regional lung function in rats using hyperpolarized 3-helium dynamic MRI. Magn Reson Med 49: 78–88, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen XJ, Hedlund LW, Möller HE, Chawla MS, Maronpot RR, Johnson GA. Detection of emphysema in rat lungs by using magnetic resonance measurements of He-3 diffusion. Proc Natl Acad Sci USA 97: 11478–11481, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conradi MS, Yablonskiy DA, Woods JC, Gierada DS, Jacob RE, Chang YLV, Choong CK, Sukstanskii AL, Tanoli T, Lefrak SS, Cooper JD. He-3 diffusion MRI of the lung. Acad Radiol 12: 1406–1413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dames P, Gleich B, Flemmer A, Hajek K, Seidl N, Wiekhorst F, Eberbeck D, Bittmann I, Bergemann C, Weyh T, Trahms L, Rosenecker J, Rudolph C. Targeted delivery of magnetic aerosol droplets to the lung. Nature Nanotechnol 2: 495–499, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Dugas JP, Garbow JR, Kobayashi DK, Conradi MS. Hyperpolarized He-3 MRI of mouse lung. Magn Reson Med 52: 1310–1317, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Emami K, Cadman RV, Macduffie Woodburn JP, Fischer MC, Kadlecek SJ, Zhu J, Pickup S, Guyer RA, Law M, Vahdat V, Friscia ME, Ishii M, Yu J, Gefter WB, Shrager JB, Rizi RR. Early changes of lung function and structure in an elastase model of emphysema—a hyperpolarized 3He MRI Study. J Appl Physiol 104: 773–786, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Fain SB, Panth SR, Evans MD, Wentland AL, Holmes JH, Korosec FR, O'Brien MJ, Fountaine H, Grist TM. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology 239: 875–883, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fichele S, Paley MNJ, Woodhouse N, Griffiths PD, van Beek EJR, Wild JM. Finite-difference simulations of He-3 diffusion in 3D alveolar ducts: comparison with the “cylinder model”. Magn Reson Med 52: 917–920, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Hedlund LW, Cofer GP, Owen SJ, Johnson GA. MR-compatible ventilator for small animals: computer-controlled ventilation for proton and noble gas imaging. Magn Reson Imaging 18: 753–759, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol 97: 204–212, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Jacob RE, Chang YV, Choong CK, Bierhals A, Hu DZ, Zheng J, Yablonskiy DA, Woods JC, Gierada DS, Conradi MS. F-19 MR imaging of ventilation and diffusion in excised lungs. Magn Reson Med 54: 577–585, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Jacob RE, Laicher G, Minard KR. 3D MRI of non-Gaussian 3He gas diffusion in the rat lung. J Magn Reson 188: 357–366, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Jacob RE, Morgan SW, Saam B. He-3 spin exchange cells for magnetic resonance imaging. J Appl Phys 92: 1588–1597, 2002. [Google Scholar]
- 17.Lai YL, Hildebrandt J. Respiratory mechanics in anesthetized rat. J Appl Physiol 45: 255–260, 1978. [DOI] [PubMed] [Google Scholar]
- 18.LeaWoods JC, Choong CC, Yablonskiy DA, Chino K, Pierce JA, Scheske J, Cooper JD, Conradi MS, Hogg J. Measuring changes in the severity of emphysema with hyperpolarized He-3 diffusion MRI. FASEB J 18: A949, 2004. [Google Scholar]
- 19.Lee KM, Renne RA, Harbo SJ, Clark ML, Johnson RE, Gideon KM. 3-Week inhalation exposure to cigarette smoke and/or lipopolysaccharide in AKR/J mice. Inhal Toxicol 19: 23–35, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Leff RD COPD: clinical significance of early diagnosis. J Manag Care Pharm 11: S8–S11; quiz S20–S22, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q, Rouby JJ. Measurement of pressure-volume curves in patients on mechanical ventilation: methods and significance. Crit Care 4: 91–100, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mata JF, Altes TA, Cai J, Ruppert K, Mitzner W, Hagspiel KD, Patel B, Salerno M, Brookeman JR, de Lange EE, Tobias WA, Wang HTJ, Cates GD, Mugler JP. Evaluation of emphysema severity and progression in a rabbit model: comparison of hyperpolarized He-3 and Xe-129 diffusion MRI with lung morphometry. J Appl Physiol 102: 1273–1280, 2007. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin RF, Tueller EE. Anatomic and histologic changes of early emphysema. Chest 59: 592, 1971. [DOI] [PubMed] [Google Scholar]
- 24.NIST/SEMATECH. e-Handbook of Statistical Methods. http://www.itl.nist.gov/div898/handbook/, 2006.
- 25.O'Halloran RL, Holmes JH, Altes TA, Salerno M, Fain SB. The effects of SNR on ADC measurements in diffusion-weighted hyperpolarized He-3 MRI. J Magn Reson 185: 42–49, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Palecek F Measurement of ventilatory mechanics in the rat. J Appl Physiol 27: 149–156, 1969. [DOI] [PubMed] [Google Scholar]
- 27.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol 100: 186–193, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Peces-Barba G, Ruiz-Cabello J, Cremillieux Y, Rodriguez I, Dupuich D, Callot V, Ortega M, Arbo MLR, Cortijo M, Gonzalez-Mangado N. Helium-3 MRI diffusion coefficient: correlation to morphometry in a model of mild emphysema. Eur Respir J 22: 14–19, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Peters DC, Korosec FR, Grist TM, Block WF, Holden JE, Vigen KK, Mistretta CA. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med 43: 91–101, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Phalen RF, Yeh HC, Raabe OG, Velasque DJ. Casting Lungs in-Situ. Anat Rec 177: 255–263, 1973. [DOI] [PubMed] [Google Scholar]
- 31.Plank C Nanomagnetosols: magnetism opens up new perspectives for targeted aerosol delivery to the lung. Trends Biotechnol 26: 59–63, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Rasband WS ImageJ. Bethesda, MD: National Institutes of Health, 1997. –2008. http://rsb.info.nih.gov/ij/.
- 33.Saam BT, Yablonskiy DA, Kodibagkar VD, Leawoods JC, Gierada DS, Cooper JD, Lefrak SS, Conradi MS. MR imaging of diffusion of He-3 gas in healthy and diseased lungs. Magn Reson Med 44: 174–179, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Swift AJ, Wild JM, Fichele S, Woodhouse N, Fleming S, Waterhouse J, Lawson RA, Paley MNJ, Van Beek EJR. Emphysematous changes and normal variation in smokers and COPD patients using diffusion He-3 MRI. Eur J Radiol 54: 352–358, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Thevenaz P, Unser M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc Res Tech 70: 135–146, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Thurlbeck WM, Muller NL. Emphysema—definition, imaging, quantification. Am J Roentgenol 163: 1017–1025, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Modern Phys 69: 629–642, 1997. [Google Scholar]
- 38.Waters B, Owers-Bradley J, Silverman M. Acinar structure in symptom-free adults by helium-3 magnetic resonance. Am J Respir Crit Care Med 173: 847–851, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Weibel ER Stereological Methods. New York: Academic, 1979.
- 40.West JB Pulmonary Pathophysiology—The Essentials. Baltimore, MD: Williams and Wilkins, 1998, p. 198.
- 41.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized He-3 diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 56: 1293–1300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized He-3 diffusion MRI. Proc Natl Acad Sci USA 99: 3111–3116, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]