Abstract
We investigated the effect of 6 wk of strength training on maximal pressing (MVC) force, indexes of finger individuation (enslaving), and performance in accurate force production tests and in functional hand tests in healthy, physically fit, elderly individuals. Twelve participants (average age 76 yr) exercised with both hands. One of the hands exercised by pressing with the proximal phalanges (targeting mainly intrinsic hand muscles), whereas the other hand exercised by pressing with the finger tips (targeting mainly extrinsic hand muscles). Training led to higher MVC forces, higher enslaving indexes, and improved performance on the pegboard grooved test. Changes in an index of multi-finger force stabilizing synergy showed a significant correlation with changes in the index of force variability in the accurate force production test. Strong transfer effects were seen to the site that did not perform strength training exercise within each hand. Effects of exercise at the proximal site were somewhat stronger compared with those of exercise at the finger tips, although the differences did not reach significance level. Control tests showed that repetitive testing by itself did not significantly change the maximal finger force and enslaving. The results suggest that strength training is an effective way to improve finger strength. It can also lead to changes in finger interaction and in performance of accurate force production tasks. Adaptations at a neural level are likely to mediate the observed effects. Overall, the data suggest that strength training can also improve the hand function of less healthy elderly subjects.
Keywords: age, strength training, finger, force production, synergy
healthy aging is associated with changes at different levels of the neuromotor system. In particular, the documented decline in hand function (19, 24, 28, 53, 55) has been attributed to both peripheral changes, such as sarcopenia, a drop in the number of motor units, an increase in average size of motor units, general slowing down of muscle contractile properties (6, 13, 38), and changes in central commands to the motoneuronal pools (9, 10, 63, 64).
Previous studies of maximal voluntary contraction (MVC) tasks have suggested that elderly individuals show a disproportional loss of strength in the intrinsic hand muscles compared with the extrinsic hand flexors (8, 64, 65). It has also been suggested that the strength imbalance between these muscle groups may contribute to the impaired ability of elderly individuals to stabilize the total force and total moment of force in multi-finger tasks as well as to the decrease in dexterity with aging (63, 64–66). Finger coordination problems in the elderly may also be attributed to documented changes in indexes of finger interaction, in particular enslaving (force production by uninstructed fingers of the hand) (33, 45, 75), which show strong correlation with the finger MVC values (64, 65). Note that both very high and very low enslaving may have negative effects on the hand function. Very high enslaving limits individual finger control and may contribute to poor multifinger coordination (39). On the other hand, enslaving contributes to stabilization of the hand rotational action (76) that is crucial in a variety of everyday tasks; hence, low enslaving may contribute to the age-related decline in dexterity.
Strength training has been shown to be an effective way to improve the force-producing capacity of muscles in the elderly and to partially reverse the changes observed in the muscle architecture with age (29, 48, 56, 57). In this study, we asked the following specific questions: Will changes in finger strength lead to changes in indexes of finger interaction, bringing those closer to values observed in younger persons? What will be the effects of strength training on accurate force production tasks and tests of hand dexterity? Can strength training, targeting mostly intrinsic hand muscles, lead to an improved balance in the force-generating abilities between the extrinsic and intrinsic muscles?
The muscular design of the hand allows variation in the involvement of the intrinsic and extrinsic muscles in MVC and submaximal accurate force production tasks by varying the point of force application along the fingers, at the fingertips (the distal site), and at the proximal phalanges (the proximal site) (11, 46). We used this opportunity to vary the relative involvement of hand muscles during both strength-training exercise and hand tests.
We hypothesized that training would improve finger strength, and the effects would be larger when the intrinsic hand muscles are primarily involved during the training. We also expected enslaving [which is lower in the elderly (64–65)] to increase in parallel with finger strength (11). This prediction is based on strong correlations between strength and enslaving indexes observed in studies of finger fatigue, effects of gender, and effects of age (11, 64–66). Excessive enslaving could be expected to lead to a predominance of positive covariation among finger forces and, consequently, to worse performance in typical laboratory accurate force production tasks that are known to benefit from negative covariation of individual finger forces (23, 41) and, possibly, in hand dexterity tests (cf. “strength-dexterity trade-off” in Ref. 65). This negative prediction poses a potential ethical problem since it entails a possibility of negative effects of strength training on everyday hand function. Hence, we limited the duration of strength training in this first study to only 6 wk and enrolled in the study only healthy, physically fit elderly persons. We also explored transfer of the strength training effects to untrained sites of force application.
METHODS
Control Tests
To find out whether repetitive participation in finger tests by itself could lead to changes in maximal finger force and indexes of finger interaction, we tested a group of 14 subjects three times at 2-wk time intervals. For these control tests, we used the data from elderly subjects who participated in another series of experiments. In that experiment, subjects were required to participate in three visits to the laboratory and performed maximal force production tests with individual fingers (I, M, R, L) and all four fingers together (IMRL) at the beginning of each visit. Maximal finger forces and indexes of finger interaction were quantified and compared across the three tests. The methods and results of the control tests are described in more detail in the appendix.
Subjects
Twelve elderly individuals (6 men and 6 women) volunteered to participate in the study. Their average age, height, and weight were 76 ± 6 yr, 167.0 ± 9.1 cm, and 74.9 ± 11.4 kg. The subjects were all right-hand dominant according to their hand usage during writing and eating. The subjects were recruited from local retirement communities. To be eligible for participation in the studies, the elderly subjects had to pass a screening process that involved a cognition test (mini-mental status exam ≥24 points), a depression test (Beck depression inventory ≤20 points), a quantitative sensory test (monofilaments ≤3.22), and a general neurological examination.
We purposefully selected elderly subjects who exercised regularly and were generally in a good physical condition (self reported). All subjects gave informed consent according to the procedures approved by the Office for Research Protection of The Pennsylvania State University.
Apparatus
Experimental setup.
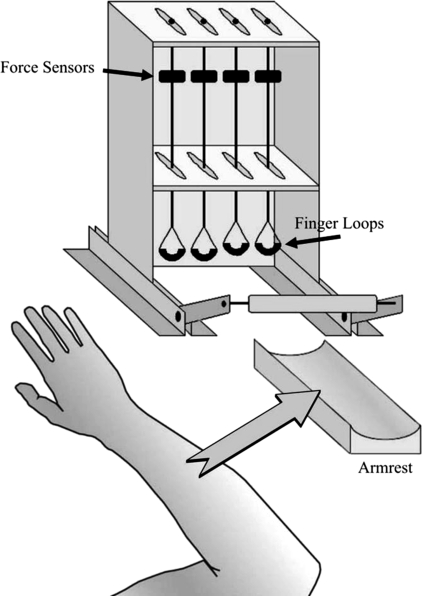
The experimental setup is illustrated in Fig. 1. Four unidirectional piezoelectric force sensors (model 208A03, PBC Piezotronics, Depew, NY) amplified by AC/DC conditioners (M482M66, PCB Piezotronics, Depew, NY) were used to measure the vertical forces produced by the four fingers (index, I; middle, M; ring, R; and little, L). Each sensor was connected in series with a wire that was suspended through a slot from the top plate of an inverted U-shaped metal frame. A butterfly nut secured the attachment of the wires to the top plate. The slots were spaced 3.0 cm in the medio-lateral direction and allowed for forward-backward adjustments of the wires to fit individual subject anatomy. At the bottom of each wire was a rubber-coated loop that could be placed either at the fingertip/distal phalanx (distal site, DS) or at the proximal phalanx (proximal site, PS) of individual fingers.
Fig. 1.
The experimental setup.
During the experiment, subjects sat in a chair facing the testing table with shoulders at ∼45° of abduction and flexion, and elbows flexed ∼135°. The forearm of the hand being tested rested on a padded armrest and was secured with Velcro straps. The position of the hand was maintained stable by a padded metal “clasp” made up of a cylindrical bar (lower part) and a concave bar (upper part). The palm rested on the lower bar with the thumb below it and was secured by the upper concave bar just proximal to the metacarpophalangeal (MCP) joints on the back of the hand. During the tests, all precautions were taken to maintain a stable configuration of the forearm and hand. A 17-in. computer monitor, located ∼65 cm away in front of the subject, displayed the total force of all four fingers or the force of individual fingers, depending on the task. Both the right and left hands were tested.
A LabVIEW-based program was used for data acquisition. Sampling frequency was set at 200 Hz with a 12-bit resolution.
Hand training device.
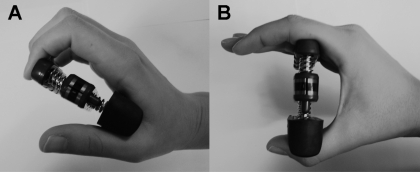
The hand training device, Digi-Flex (IMC Products, Hicksville, NY) is shown in Fig. 2. The devices come in five levels of resistance that are color coded. A yellow device has the resistance of 22.5 N for the four fingers; red = 44.1 N; green = 71.5 N; blue = 101.9 N; and black = 138.2 N for the fully compressed springs. The training devices were used in two different ways corresponding to the two positions of the fingers illustrated in Fig. 2. Figure 2A shows how the fingertips were used to press down (DS training), and Fig. 2B shows the position when the proximal phalanges of the fingers were used (PS training).
Fig. 2.
The Digi-Flex hand training device and the two finger positions used: pressing with fingertips (training at the distal site; A) and pressing with bases of fingers (training at the proximal site; B).
Experimental Procedure
General.
Participation of each subject in the experiment took 6 wk; during that time, each subject was tested four times, at the beginning of the study, after week 2, after week 4, and at the end of the study. Hand functional tests were administered only in the first and the last testing sessions.
Hand functional tests.
To quantify functional manual ability, all subjects filled out the ABILHAND questionnaire (51) and performed two clinical tests: the Grooved Pegboard test (58) and the Jebsen-Taylor Hand function test (30). The Grooved Pegboard test requires subjects to put key-shaped pegs into keyholes on a small board as fast as they can, and the time needed to complete the task is recorded. The tests were performed by both dominant and nondominant hand in a balanced order. The Jebsen-Taylor Hand function test has seven tasks involving manipulation of objects of various sizes under the instructions of performing the tasks as fast as possible. For each task, the total time required to complete the task is recorded. The ABILHAND questionnaire requires the participants to rate their perception of the difficulty of 23 everyday tasks, ranging from “impossible” to “difficult” to “easy.”
MVC and ramp tasks.
Every testing session included a maximal force production (MVC) task and an accurate force production (Ramp) task performed by each hand and at each site of force production, DS and PS. Before each trial, the subject sat with the fingers of one of the hands resting in the loops, which were positioned either against the fingertips (DS) or against the proximal phalanges (PS). The computer generated two brief beeps that indicated “get ready” and a cursor representing the total force of the instructed fingers started to move across the screen at a constant speed.
During the MVC tasks, the maximal force produced by each of the fingers individually (I, M, R, L) and by all four fingers acting together (IMRL) was measured. During these trials, subjects were instructed to “press as hard as possible” with the instructed finger(s) in a self-paced manner after the cursor reached a vertical line at the 2.5-s mark. Subjects were given up to 4.5 s to reach peak force and could relax at any time once they had done so. Each trial lasted 10 s. Two trials were recorded for each of the instructed finger(s), and the trial with the larger force magnitude was used for analysis. During single-finger MVC tests, the subjects were explicitly required not to pay attention to possible force production by other, noninstructed fingers as long as the instructed finger (the master finger) produced maximal force. They were also told not to lift any fingers in any tests.
The ramp task required the subjects to produce a ramp pattern of force from a resting level to 25% of MVC over 4 s by pressing down either with one finger at a time (I, M, R, L) or with all four fingers together (IMRL). An oblique blue line was shown on the screen, starting 3 s after the initiation of the trial, and the subject's task was to trace this line in time with the cursor. Each trial lasted 10 s. Two trials were collected for the single-finger ramps (these data were used later for the uncontrolled manifold analysis, see Uncontrolled manifold analysis below), and 12 trials were collected for the four-finger ramp tasks. The intervals between trials in both the MVC and the ramp tasks were at least 30 s for a series of trials within a testing site, at least 5 min between the two sites, and at least 10 min between the two hands.
Training
Subjects were divided randomly into two equal groups, 1) “right-DS and left-PS training,” and 2) “left-DS and right-PS training.” After the first testing session, the subjects were assigned training devices; this was done by calculating 50% of their four-finger MVC (MVCIMRL) of the corresponding hand-site combination, and the device with the resistance closest to that value was used. This process was repeated after each testing session to adjust to possible strength increments over the time of the study. Subjects were instructed to train twice daily, except on the days when they visited the laboratory to be tested, with two sets of 10 repetitions within a training session. The explicit instruction on the method of training was: “The strengthening exercise should consist of a slow and controlled squeeze of the DIGI-FLEX device until the springs from all fingers are fully compressed, holding the squeeze for 2 s, and then releasing it.” During the first testing session, the subjects received instruction on the usage of the devices (see Fig. 2) and an instructional sheet with photographs to take home and use as a reminder of the correct positioning of the hand on the device and method of training, as well as a training calendar where they marked off each time they trained. To further ensure correct training, the experimenter reminded the subjects by phone or visited them at home as needed. During each subsequent visit, subjects were also asked to demonstrate their training technique.
Data Analysis
All the data were analyzed using Matlab 7.0 (MathWorks, Natick, MA) and Excel (Micorsoft, Redmond, WA) software. In this paper, only the data obtained in test 1 (before training) and test 4 (posttraining) are presented.
Clinical functional tests.
For the Grooved Pegboard test and the Jebsen-Taylor test, the total score for the dominant hand and nondominant hand was calculated separately for test 1 and test 4.
MVC task.
For each MVC trial, the forces produced by individual fingers were measured at the time when the task finger(s) (I, M, R, L, or IMRL) reached maximum value. These data were used to calculate the sum of maximal individual finger forces (ΣMVCi = MVCI + MVCM + MVCR + MVCL), the maximal total force in the four-finger task (MVCTOT), and the enslaving forces during single-finger trials for each hand and each site of force application separately. Enslaved forces are forces produced by noninstructed fingers in single-finger tasks. The enslaved force of each finger was expressed as percentage of its own MVC force when it acted as the instructed finger in its single-finger task. By doing so for each single-finger trial (I, M, R, L), an enslaving matrix was generated for both sites (DS and PS) and both hands. For further comparisons, the enslaving indexes were averaged across all slave fingers to yield a grand mean for each site/hand combination.
Performance in the ramp task.
For each site and testing session, subject's performance during the four-finger task was quantified by calculating the root mean square (RMS) of the difference between the target (template) ramp and actual ramps over the whole ramp interval.
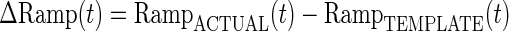 |
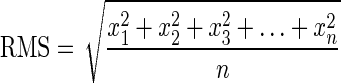 |
where x(t) = ΔRamp(t), and n is the number of samples.
Since force variability is known to depend on force magnitude (reviewed in Ref. 48), for between-tests comparisons, we computed RMS during the pre- and posttraining sessions using time intervals that corresponded to identical force ranges. For this purpose, a 1-s time interval (starting 1.5 s before the end of the ramp) was selected for each subject for the pretraining (test 1) trials. The absolute force range (in Newtons) was defined for that interval and, for the posttraining (test 4) trials, a 1-s interval was selected that spanned the same force range.
For further analysis, the average RMS scores over the whole ramp duration were further averaged across both hands and sites, and the difference between the RMS indexes in test 1 and test 4 was calculated as
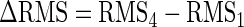 |
Uncontrolled manifold analysis.
The data from the ramp task was analyzed within the framework of the uncontrolled manifold (UCM) hypothesis (Ref. 62; reviewed in Refs. 42, 43). The UCM hypothesis offers a method to compute an index of stabilization of certain performance variables produced by a redundant set of elements; in our study, we use it to compute an index of total force stabilization by covaried changes in commands to individual fingers. The method of the UCM hypothesis may be formally expressed as follows: The space of elemental variables is divided into two subspaces, one corresponding to a fixed value of the performance variable (the UCM) and the other leading to changes in this variables (orthogonal to the UCM). Furthermore, variance across trials is compared within the two subspaces (per dimension); if more variance lies within the UCM, a conclusion is drawn that a multi-element synergy stabilizes the performance variable.
Due to the mentioned phenomenon of enslaving (32, 45, 75), individual finger forces covary positively across tasks and force values. To analyze task-specific patterns of covariation, forces have to be converted into finger modes (42), which can hypothetically be changed by the controller one at a time. This was done using the corresponding enslaving matrix, E.
Single-finger trials were used to generate E. For each single-finger trial, a linear regression of the forces produced by individual fingers against the total force produced by all four fingers over a 3-s time interval in the middle of the ramp was computed. The ratios between the changes in individual finger forces and the change in the total force were used to construct an enslaving matrix for each subject as follows:
 |
where Δfj,k is the change of individual finger forces j (j = I, M, R, and L), and ΔFk are the changes of the respective total forces produced during the ramp when finger k (k = I, M, R, and L) is the instructed finger. This matrix is a linear approximation of a matrix containing partial derivatives ∂fj,k/Fk where ∂fj,k and ∂Fk are the infinitesimal changes of individual and total finger forces.
The force data from the four finger ramp trials were converted to mode magnitudes by using the E matrix: mi = [E]−1·Fj, where j = I, M, R, L.
According to the UCM hypothesis, more variance is expected within the manifold (UCM, three-dimensional) that corresponds to a constant value of total force than in an orthogonal complement to the UCM (one-dimensional). For each subject and for each time sample, ti, the average across trials mode vector mAV, was computed. Then, for each trial, k, deviations (Δmk) between mk and mAV, were computed. Variance of the Δmk data set was then computed along a direction orthogonal to the UCM determined for an average value of the total force observed across trials at that particular time slice. This index is referred to as VORT. This is done using the Raleigh fraction (21)
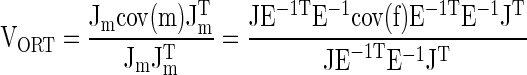 |
where J is the Jacobian matrix relating small changes in modes (Jm) or forces (J) to changes in the total force, cov(m) is the covariance matrix in the mode space, cov(f) is the covariance matrix in the finger force space, and T is the sign of transpose. For total force J = [1,1,1,1]; Jm = JE−1T. The difference between the total amount of variance (VTOT) and VORT corresponds to variance that does not affect the average value of the performance variable (variance within the UCM, VUCM; cf. Ref. 41), VUCM = VTOT − VORT. To compare the amounts of variance per dimension in the two subspaces, an index ΔV was used: ΔV = [(VUCM/3) − VORT]/(VTOT/4). In more intuitive terms, this index in our study reflects the relative amount of “good variance” (variance that does not affect total force, VUCM) compared with “bad variance” (variance that does affect total force, VORT).
Normalization of the ΔV index by the total variance per dimension was done to be able to compare the data across subjects that might show different amounts of the total variance. Note that positive values of ΔV correspond to proportionally more VUCM, that is, to proportionally more variance compatible with a constant value of the total force. Therefore, positive ΔV can be interpreted as a sign of a multi-mode synergy stabilizing total force.
The ΔV index has fixed limits. On the one hand, if all the variance falls within the UCM space, ΔV reaches its maximum of +1.33; on the other hand, if all the variance falls within the orthogonal space, ΔV reaches its minimum of −4.
Due to these limits, the ΔV data were not normally distributed, and, therefore, a z-transformation was used to normalize the data and make parametric statistics applicable
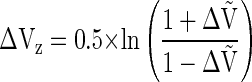 |
where ΔṼ = a × ΔV + b; a = 2/5.33; and b = 1 − [(2.33/5.33) × 1.33]. For further analysis, ΔVZ was averaged over the four 1-s intervals of the ramp and further averaged over both sites and hands. After averaging the ΔVZ index over the whole duration of the ramp, the difference between test 1 and test 4 was calculated, ΔΔVZ = ΔVZ4 − ΔVZ1.
Statistical Analysis
The analysis was performed using SPSS (SPSS, Chicago, IL). Standard methods of parametric statistics were used. The level of significance was set at P = 0.05.
Results of the clinical functional tests were analyzed using three-way repeated-measures ANOVA with within-subjects factors test (two levels, test 1 and test 4), hand (two levels, right and left), and site-of-training (two levels, distal and proximal). To estimate the effects of training on ΣMVCi, MVCTOT, enslaving, and RMS, four-way repeated-measures ANOVA was run with factors test (two levels, test 1 and test 4), hand (two levels, right and left hands), site (two levels, PS and DS), and training (two levels, trained and untrained sites). Test, hand, and site were within-subjects factors, and training was a between-subjects factor. In the comparisons, the different combinations of hands and sites will be abbreviated as RD (right hand, distal site), RP (right hand, proximal site), LD (left hand, distal site), and LP (left hand, proximal site). For ΔVZ analysis, a two-way repeated-measures ANOVA with within-subjects factors test and time interval (four levels corresponding to four consecutive 1-s intervals of the ramp) was used (collapsed across sites and hands). Significant effects were further analyzed using multiple comparisons with Bonferroni corrections. In cases of sphericity violations, the Huynh-Feldt corrections for the degrees-of-freedom were used.
To study possible links between the change of ΔVZ (the difference between ΔVZ of test 1 and test 4) and the change in ΔRMS (the difference between RMS of test 1 and test 4), a linear regression analysis was used.
RESULTS
This section is organized as follows. First, we describe changes in the functional clinical tests with training followed by analysis of changes in indexes of finger forces and interaction in the MVC tests. Furthermore, the performance in the accurate force production task (the ramp task) is described, including analysis performed within the UCM hypothesis. Although strength training led to significant changes in various performance indexes described further in this section, these changes were modest in magnitude and significant only at the completion of the training protocol. The intermediate tests (test 2 and test 3) showed smaller changes in the outcome variables that did not reach significance; therefore, we have decided not to present those data. Note that both extrinsic and intrinsic hand muscles were involved in exercise at both distal and proximal sites, albeit to different degrees. For brevity, we will continue to address the two sites of force application as “trained” (if a site matches the exercise site) and “untrained” (if it does not) to avoid using a more exact but clumsy pair of terms such as “more trained” and “less trained.”
Functional Clinical Tests
Grooved Pegboard test.
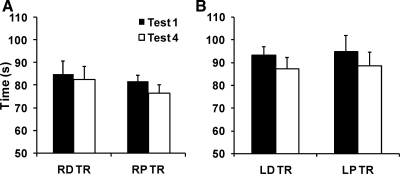
Subjects were generally faster at completing the task with their right, dominant hand than their left, nondominant hand. After the training (by test 4), their average time decreased in both hands, regardless of whether they had trained distally or proximally. For the right hand, this decrease was from 84.8 ± 5.9 to 82.4 ± 5.9 s in distally trained hands but from 81.8 ± 2.7 to 76.4 ± 3.8 s in proximally trained hands; for the left hand, it was from 93.2 ± 3.7 to 87.3 ± 4.7 s and from 94.7 ± 7.0 to 88.7 ± 6.0 s, respectively. Figure 3 shows the average time it took the subjects to complete the test separately for test 1 and test 4 and for the two training sites. Figure 3A shows the results for the right hand and Fig. 3B the left hand. A three-way repeated-measures ANOVA with factors test, hand, and site-of-training showed a significant effect of test [F(1,5) = 22.55; P < 0.01] and hand [F(1,5) = 22.55; P < 0.05] but not site-of-training [F(1,5) = 0.29; P = 0.62]; there were no significant interactions.
Fig. 3.
The results of the Grooved Pegboard test. Both hands improved their performance after the training (test 4) whether they had trained at the distal or proximal site. The dominant hand (A) was faster than the nondominant (B) hand both before and after training. RD TR, training at the right distal site; RP TR, training at the right proximal site; LD TR, training at the left distal site; LP TR, training at the left proximal site.
Jebsen-Taylor hand function test.
The total time subjects needed to complete all seven tasks of the Jebsen-Taylor test was ∼20 s less for the right hand compared with the left hand. After the training, the total time showed a modest decrease in both hands when they had been trained at the distal site (right hand: 51.5 ± 4.3 to 48.8 ± 3.8 s; left hand: 73.1 ± 5.1 to 69.7 ± 5.3 s) but only in the right hand when the hands were trained at the proximal site (54.8 ± 4.9 to 51.9 ± 4.7 s). In the left hand, training at the proximal site led, on average, to a slight increase in the total time (70.9 ± 5.1 to 71.5 ± 8.6 s). A three-way repeated-measures ANOVA with factors hand, site-of-training, and test confirmed the difference between the two hands [F(1,5) = 27.91; P < 0.01], but no significant effects were found for the other two factors [training: F(1,5) = 0.32, P = 0.60; test: F(1,5) = 1.47, P = 0.28]; there were no significant interactions.
ABILHAND questionnaire.
No difference was found in the score of this questionnaire pre- and posttraining. In both tests, subjects ranked 22–23 out of 23 tasks on the list as “easy.” The task subjects most commonly reported as “difficult” was “threading a needle,” and in many cases subjects commented that decreased eyesight rather than manipulation skills was to blame.
MVC Task
ΣMVCi and MVCTOT.
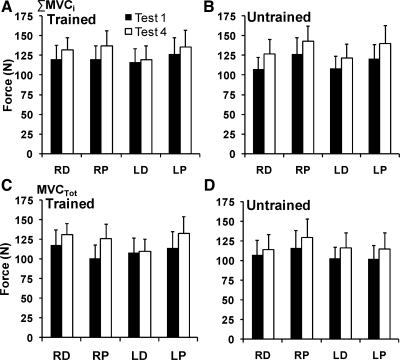
The average total force values produced at PS and DS, by the left and right hands, over both tests for the trained and untrained sites are displayed in Fig. 4. Figure 4, A and B, shows the results for ΣMVCi, and Fig. 4, C and D, shows the MVCTOT data.
Fig. 4.
Total force produced before (test 1) and after (test 4) training at the trained and untrained distal (D) and proximal (P) sites of the left (L) and right (R) hands, averaged across subjects with standard error bars for maximal individual finger force (ΣMVC1) (A: trained sites; B: untrained sites) and maximal total force in the four-finger task (MVCTOT) (C: trained sites; D: untrained sites). The filled bars represent the pretraining testing session, and the open bars show the posttraining session.
The sum of the peak forces produced in the single-finger tasks was, on average, larger than the four-finger force both pre- (test 1) and posttraining (test 4) (see Fig. 4). Before the training, the total forces produced at the proximal site (PS) in the single finger tasks (ΣMVCi) were on average larger than those produced by the distal sites (DS) by ∼9.2% (DS: 113.2 ± 7.6 N; PS:123.6 ± 8.9 N). In the four-finger tasks (MVCTOT), the forces produced at the two sites were, on average, the same (DS: 109.3 ± 8.2 N; PS 108.6 ± 9.0 N). After the training, the total force produced at both PS and DS of both hands increased for both indexes, ΣMVCi and MVCTOT, regardless of whether that site had been trained or not (Fig. 4, A and B). This increase ranged from 2.5 to 17.8% for ΣMVCi and from 1.3 to 25.5% for MVCTOT; for both indexes, larger forces were produced at PS than at DS.
For ΣMVCi, a four-way repeated-measures ANOVA with factors test, site, hand, and training showed significant effects of test [F(1,10) = 24.73; P < 0.001] and site [F(1,10) = 12.65; P < 0.05] but not of hand [F(1,10) = 0.44; P = 0.52] or training [F(1,10) = 0.01; P > 0.9]; there were no significant interactions.
For MVCTOT, a four-way repeated-measures ANOVA with the same factors showed a significant effect of test [F(1,10) = 13.20; P < 0.01] but not of site [F(1,10) = 0.95; P = 0.35], hand [F(1,10) = 1.43; P = 0.26], or training [F(1,10) = 0.09; P < 0.077] and no significant interactions. In general, MVCTOT, on average, increased after the training at both trained and untrained sites as can be seen in Fig. 4, C and D.
Enslaving.
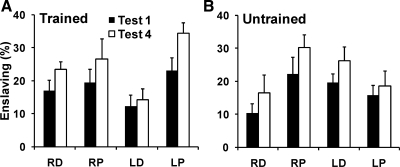
The enslaved forces were produced in single-finger MVC tasks by fingers that were not instructed to produce force. These forces were expressed as percentage of their respective maximal force when acting as instructed fingers; the enslaving indexes were further averaged across fingers. The average enslaving indexes at the DS and PS for both hands, trained and untrained, pre- (test 1) and posttraining (test 4) are shown in Fig. 5. Figure 5A shows the results for the trained sites, and Fig. 5B shows the results for the untrained sites.
Fig. 5.
Average enslaving indexes with standard error bars. A: average enslaving at the trained distal and proximal sites of both hands produced pre- (test 1) and posttraining (test 4). B: average enslaving at the untrained distal and proximal sites of both hands produced pre- (test 1) and posttraining (test 4). RD, distal site of right hand; RP, proximal site of right hand; LD, distal site of left hand; LP, proximal site of left hand. Filled bars represent pretraining tests (test 1), and open bars show posttraining tests (test 4).
After the training (by test 4), subjects produced larger enslaving forces than before training (test 1). For the right hand, on average, enslaving increased from 17.0 ± 3.1 to 23.4 ± 2.4% at trained DS and from 10.4 ± 2.7 to 16.5 ± 5.4% at untrained DS, whereas the increase at the trained PS was from 19.4 ± 4.1 to 26.6 ± 6.4% and at the untrained PS it was from 22.2 ± 5.1 to 30.1 ± 3.9%. Respectively, the increase in the left hand enslaving increased at trained DS from 12.4 ± 3.3 to 14.3 ± 3.2%, at untrained DS from 19.6 ± 2.7 to 26.3 ± 4.1%, at trained PS from 23.2 ± 3.8 to 34.5 ± 3.1%, and at untrained PS from 15.8 ± 2.9 to 18.6 ± 4.4%.
A four-way repeated-measures ANOVA with factors test, site, hand, and training showed significant effects of test [F(1,10) = 29.77; P < 0.001], site [F(1,10) = 12.95; P < 0.01], and the hand × site × training interaction [F(1,10) = 11.88; P < 0.05] but no significant effects of hand [F(1,10) = 1.43; P = 0.26], training [F(1,10) = 0.09; P > 0.9] or any of the other interactions.
Ramp Task
Performance.
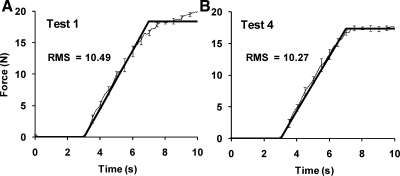
All subjects were able to perform the ramp task with relative ease, both before (test 1) and after (test 4) the training. Figure 6 shows the average performance of a representative subject with standard deviations when force was generated at PS by the right hand. The thin black lines show the subject's average performance, and the thick black lines show the target templates. The results of test 1 and test 4 are shown in Fig. 6, A and B, respectively. Note how the quality of performance showed a modest improvement (the thin black line is better matched up with the template) in the posttraining test despite the performance already being quite accurate before the training. RMS over the ramp duration also improved marginally from 10.49 to 10.27 N.
Fig. 6.
The average performance of a representative subject in the ramp task when producing total force at the proximal site of the right hand before training (test 1; A) and after training (test 4; B) with standard error bars. Thick lines represent the task template, and the thin lines show the average performance. The RMS values computed over the whole ramp were 10.49 and 10.27 N at test 1 and test 4, respectively.
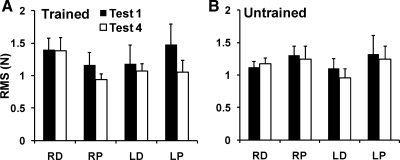
To quantify the performance of subjects during the ramp task, the RMS index was calculated to reflect the difference between the actual force time profiles the subjects produced and the target ramp. RMS was then averaged over 1-s time intervals that corresponded to equal force ranges over the test 1 and test 4 trials. On average, RMS during performance at both trained and untrained sites showed a tendency to decrease after the training. These differences did not, however, reach statistical significance, since the effect of test in a four-way repeated-measures ANOVA was under the level of significance [F(1,10) = 2.22; P > 0.1]. Effects of site [F(1,10) = 0.31; P = 0.59], hand [F(1,10) = 0.24; P = 0.63], and training [F(1,10) = 0.02; P = 0.88] were not significant, with no significant interactions. Figure 7 shows the average RMS of each hand, site, and test; Fig. 7A shows the trained sites, and Fig. 7B shows the untrained sites.
Fig. 7.
The average RMS during the accurate force production task at the trained and untrained distal and proximal sites of both hands pre- (test 1) and posttraining (test 4) with standard error bars. A: trained sites. B: untrained sites. Filled bars represent the pretraining tests, and open bars show the posttraining tests.
UCM analysis.
As a reminder, variance in the space of four finger modes (hypothetical commands to individual fingers) was quantified in two subspaces, the UCM (where an average total force value did not change) and orthogonally to the UCM (where the total force changed). Furthermore, an index ΔV was computed such that its positive values reflected predominance of variance within the UCM that we interpret as a force stabilizing four-finger synergy; this index was transformed into z scores for statistical purposes, ΔVz.
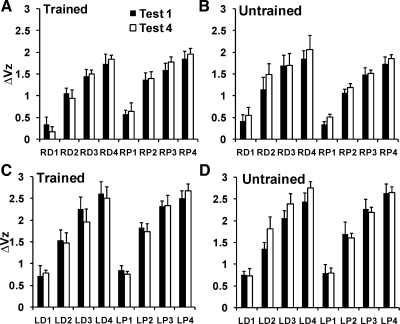
After the training period (test 4), the transformed delta variance index (ΔVz) showed a general increase for all four 1-s time intervals of the force ramp. This index increased over the time of the ramp and was higher in the left hand. However, there were no significant effects of training and no significant differences between the sites of force application. Figure 8 shows the average ΔVz in tests 1 and 4, for the four time intervals, the two hands (right, Fig. 8, A and B; left, Fig. 8, C and D), trained and untrained sites, and distal (D) and proximal (P) sites. A five-way repeated-measures ANOVA showed main effects of hand [F(1,10) = 45.52; P < 0.001], time interval [F(1.75,17.46) = 317.24; P < 0.001], the hand × time interval interaction [F(2.07,20.65) = 18.49; P < 0.001] but no effects of site [F(1,10) = 0.77; P = 0.40], test [F(1,10) = 0.54; P = 0.48], training [F(1,10) = 0.02; P = 0.89], or any other interactions.
Fig. 8.
ΔVz (delta variance converted to z score) pre- (test 1; filled bars) and posttraining (test 4; open bars) for the four time intervals, the two hands [R, right (A and B); L, left (C and D)], trained and untrained sites, and distal (D) and proximal (P) sites. The data were averaged over each of the four 1-s time intervals (standard error bars across subjects are shown).
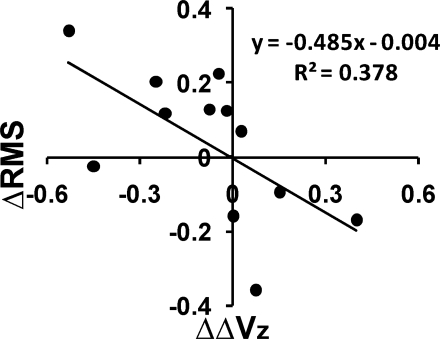
Given the high variability of the effects of training on both the RMS index of performance in the ramp task and the ΔV index, we investigated the relation between the change in subjects' performance (RMS, averaged across both hands and sites) with training and the change in the synergy index (ΔV, also averaged across both sites and hands over the whole ramp interval). For this purpose, the differences between the values in test 1 and test 4 were calculated for RMS (ΔRMS) and ΔVz (ΔΔVz) for each subject.
Figure 9 shows that some subjects improved both indexes (negative ΔRMS and positive ΔΔVz), whereas others showed an opposite effect (positive ΔRMS and negative ΔΔVz). In other words, those who did show better indexes of stabilization of total force by covaried changes of finger modes also showed more accurate task performance. This was supported by a significant linear correlation between ΔRMS and ΔΔVz (R = 0.62, P < 0.05).
Fig. 9.
The relationship between a change (ΔRMS) in the RMS index from test 1 to test 4 (ΔRMS = RMS4 − RMS1) and a change (ΔΔVz) in the index of multi-finger synergy (ΔΔVz = ΔVz4 − ΔVz1) with a linear regression line and equation.
DISCUSSION
To summarize, following the training, we saw an increase in strength at both the proximal and distal sites of both hands. These effects at the trained sites may be viewed as predictable. However, a comparable increase in finger strength at the untrained sites is a novel and unexpected result. In the control study (see the appendix), no difference was found in the magnitude of MVC produced during the three testing sessions that were spaced by similar time intervals without strength training. These results support an argument that the changes in strength observed in the present study can be attributed to the training rather than to familiarity with the testing protocol.
In agreement with our second hypothesis, enslaving showed a general increase with training (but not in the control study). We hypothesized that larger enslaving might induce a decrease in the accuracy of performance by promoting positive covariation among finger forces, but the results did not support this hypothesis. In contrast, the index of performance accuracy (RMS) did not worsen, and the relatively small changes in the index of multi-finger synergy were also in the direction of improvement. In addition, subjects performed significantly better on the Grooved Pegboard test, suggesting no detrimental effects of the increased enslaving on hand dexterity. Furthermore, we discuss implications of the findings for such issues as mechanisms involved in effects of strength training on finger force and finger individuation, and the relation between strength and dexterity.
Mechanisms of Force Increase with Strength Training and Effects of Transfer
Gains in voluntary muscle force production following a strength training program may result from two main factors, muscle hypertrophy and adaptations at a neural level (for review, see Ref. 15). The importance of neural adaptations has been supported by observations of the increase in the voluntary strength of untrained (in particular, contralateral) muscles (27, 73) and by the effectiveness of mental practice for improved performance (50). These effects might be mediated, in particular, by changes in the cortical and cortico-spinal excitability, as suggested by studies using transcranial magnetic stimulation (17, 67, 69).
Our experiments showed strong effects of transfer of the exercise to the untrained site. Note that both extrinsic and intrinsic hand muscles are activated during many daily activities, such as grip and pinch (12, 35). The different anatomical points of attachment of extrinsic and intrinsic muscles (2) present an opportunity to vary the relative involvement of these muscle groups by changing the point of force application. Extrinsic flexors (flexor digitorum profundus, FDP, and flexor digitorum superficialis, FDS) are multi-digit muscles and focal flexors at the distal and proximal interphalangeal joints, respectively, whereas intrinsic muscles act as digit-specific focal flexors at the MCP joints in addition to their extensor action at distal joints (37, 47). Hence, when a person presses with fingertips, extrinsic flexors are focal force generators whereas intrinsic muscles participate in balancing moments at the MCP joints. When a person presses with proximal phalanges, intrinsic digit-specific muscles become focal force generators, whereas extrinsic flexors balance the action of the extensor mechanism at IPs (1, 7).
Although both hands participated in the exercise program, the difference in the site of force application during the exercise allowed expecting different involvement of the extrinsic and intrinsic hand muscles. According to existing estimates (25, 46), when humans produce large forces at the fingertips, the extrinsic muscles are activated close to their maximal level, whereas the intrinsic muscles produce under 30% of their maximal force. In contrast, pressing strongly with the proximal phalanges is expected to be associated with very high forces produced by the intrinsic muscles and relatively low forces (under 30% of maximum) produced by the extrinsic muscles that balance the action of the extensor mechanics at the interphalangeal joints (1, 46). Based on these estimates, we expected practice at the distal site to lead to proportionally much higher forces by the extrinsic muscles, whereas practice at the proximal site was expected to lead to proportionally higher forces by the intrinsic hand muscles. The large effects of transfer between the two sites of force application (approaching 100%) suggest that the effects of training were primarily mediated by changes at a relatively high neural level, not by changes in properties of muscles or involved motoneuronal pools.
A similar phenomenon of transfer between the distal and proximal sites of finger force application was seen in studies of fatigue in young persons, where fatigue was induced by producing MVC force at one site or the other (11). Performing a fatiguing exercise at a site caused MVC at both sites to drop. Taken together with the current results, these data suggest a strong neural component in the effects of both exercise and fatigue on maximal finger force. This conclusion is in line with a few earlier studies (34, 56, 72).
Even healthy young persons cannot reach the absolutely maximal muscle force during MVC tasks; this phenomenon has been addressed as neural deficit (reviewed in Ref. 20), and its quantitative estimates are typically over 10% (54, 77). The neural deficit is significantly larger in the elderly (70). It is possible, therefore, that effects of short-term resistance exercise on MVC can be interpreted as overcoming the neural deficit and using the potential of the involved muscles to a fuller degree. This conclusion is supported by the effectiveness of the relatively short strength-training program in our study as well as by earlier studies that showed an increase in strength following strength-training exercise of a comparable (29) or even shorter duration (34).
The design of our experiments was based on earlier reports suggesting that intrinsic muscles lose proportionally more strength with aging than the extrinsic muscles (8, 64, 65). One consequence of this process may be that the disproportional weakening of the intrinsic muscles does not allow the extrinsic muscles to be activated to their full potential because of the requirements of balancing moments of force in all the finger joints. Hence, training of the intrinsic muscles may be expected not only to improve their force-producing abilities but, in addition, to allow the distal muscles to be activated to a larger degree during force production at the finger tips. This mechanism could lead to an increase in the maximal force produced at the untrained distal sites observed in our study.
This interpretation of the effects of transfer is corroborated by a trend toward stronger effects of training (including stronger transfer effects) following exercise at the proximal site compared with exercise at the distal site. However, this interpretation is apparently incomplete since exercise at the distal site also led to increased MVC force and showed effects of transfer to the untrained proximal site. Besides, the aforementioned trend did not reach significance level possibly due to the small number of subjects and the relatively short exercise protocol.
We would like to note that the two strength-training exercises differed in more than one way. In particular, the thumb was explicitly involved in the exercise at the proximal site but not in the exercise at the distal site (Fig. 2). Note that the intrinsic muscles controlling the thumb are known to show a substantial loss in force with aging (5) similar to other intrinsic hand muscles (8, 64, 65). It is possible, therefore, that the larger thumb involvement in the proximal site exercise (see Fig. 2), which was expected to target primarily extrinsic muscles, could affect the outcome and decrease the effects of the exercise site.
Mechanisms of Changes in Finger Individuation
Earlier studies of both young and elderly individuals have shown that the magnitude of enslaving is strongly positively correlated with the magnitude of MVC force (11, 64, 65). In particular, enslaving is larger in men than in women (65), larger in young than in elderly persons (64, 65), decreased with fatigue (11), and increased after practice in individuals with Down syndrome (39).
In our experiments, enslaving increased at both sites of both hands after the training; these effects were more notable at the trained proximal sites, similar to the effects on MVC force. Before the training, we saw values of enslaving that were larger than those observed for elderly subjects by Shinohara and colleagues (64), but the elderly people in this study were also overall much stronger than those tested by Shinohara et al. After the training, the observed values were closer to those seen for the young group in the previous study (64). All these findings are indirectly supporting the mentioned general idea that the index of enslaving (expressed in percent of the maximal force) correlates closely with maximal force magnitude.
Even though there was more enslaving at the proximal sites than at the distal sites, at all sites there was an increase in enslaving after training. This is a non-trivial observation that provides further support for the notion of a central origin of the phenomenon of enslaving (also see Refs. 40, 75). Indeed, force production at the proximal sites uses the intrinsic hand muscles as focal force generators, muscles that are finger specific and do not show substantial passive force transmission to other digits (37, 47). Hence, such factors as multi-digit action of the extrinsic multi-tendon muscles that are sometimes invoked to explain the lack of finger independence (33, 44) are not expected to play a big role.
Enslaving reflects to what degree the fingers can produce force independently of one another and is commonly viewed as a detrimental factor for finger action, something that hurts dexterity (39). This opinion is probably valid for tasks that require prestidigitation, such as playing musical instruments, typing, etc. The idea of a strength-dexterity trade-off was introduced in an earlier study (64). Good dexterity is in general language associated with good individual control of the fingers and, by that definition, the increased enslaving observed with increased strength can be expected to lead to a decrease in dexterity. This led us to hypothesize that an increase in enslaving due to training might actually have negative effects on the performance of the subjects in the accurate force production tasks, a potentially very dangerous hypothesis that questions the ethics of performing strength training exercise of the hand.
There are three points worth mentioning. First, in many other tasks, including such everyday activities as drinking from a glass or eating soup with a spoon, a healthy amount of enslaving appears to be beneficial because it contributes to stabilization of the rotational action (total moment of force) applied by the hand onto the hand-held object (74, 75). Second, we enrolled in the study healthy, highly functioning elderly participants to mitigate the mentioned possible negative effects of strength training on finger individual control. Third, even if one considers tasks that require accurate individual finger control, it is not at all obvious that an increase in strength with exercise would lead, as an unavoidable by-product, to a drop in dexterity. This leads us to the next subsection on possible changes in the strength-dexterity trade-off with exercise.
Is There an Unavoidable Strength-Dexterity Trade-Off?
Consider typical effects of practice on velocity and accuracy of movements performed under the instruction to be “as fast and accurate as possible.” Without practice, there is a well known speed-accuracy trade-off. On the one hand, movement time increases with an increase in movement distance and a decrease in target size; this relation is known as Fitts' law (Ref. 18; reviewed in Ref. 52). On the other hand, when people move faster to a target, they show a larger scatter of the final position (61). Practice, however, can lead to a parallel improvement in both speed and accuracy (reviewed in Ref. 60) in a seeming violation of the speed-accuracy trade-off. Can practice show similar effects on strength and dexterity?
Several earlier studies have reported a parallel improvement in force production and indexes of force variability with strength training (4, 34, 36). Such results have also been reported in studies of elderly persons (26, 53). In a recent study, improvement in both finger-pinch force variability and targeting error, as well as in finger-pinch force, was observed in the elderly following nonspecific strength-training protocol targeting the whole arm (31).
On the other hand, other studies failed to confirm improved control of submaximal forces following strength training. In particular, an increase in strength was not accompanied by better control of submaximal forces in a study by Bellew (3); whereas in another study, the force fluctuations of the quadriceps femoris muscles during anisometric contractions but not during isometric contractions were reduced following exercise (71). Neuromuscular adaptations to strength training are known to be highly specific to the training protocols used (14, 16, 59). So, the diversity of the mentioned reports may reflect the diversity of study protocols. In addition, a recent study has come up with a conclusion that age-related changes in force variability are fundamentally due to the association between maximal force and force variability (68). This conclusion is more in line with our pessimistic prediction that strength training may have detrimental effects on performance in accurate force production and clinical tests that require dexterous manipulation.
Our hypothesis on a decline in performance following the training was not supported. After the training period, subjects performed significantly better on the Grooved Pegboard test, whereas the score on the other functional tests did not change. These tests may not be adequate tools to estimate manipulating skills in such a highly functioning population. Before the training, all subjects reported the tasks on the ABILHAND questionnaire as “easy” except for “threading a needle,” which was attributed to decreased eyesight. The accurate force production task also showed a general tendency toward improvement in the RMS index. We would like to admit that the Grooved Pegboard test data are weakened by the lack of a control group that would go through the same sequence of tests without strength training. On the other hand, we are not aware of any published data that allow us to expect the results of the Grooved Pegboard test to change significantly when it is administered twice within a 6-wk interval. We would like to conclude, therefore, that the association between strength and dexterity (the strength-dexterity trade-off) can be modified by exercise similarly to the mentioned effects of practice on movement speed and accuracy: Both strength-dexterity and speed-accuracy pairs can improve in parallel. This conclusion carries an optimistic message that allows expecting beneficial effects of such exercise not only on finger force but also on a range of everyday tasks that require accurate force control.
Multi-Finger Synergies and Accuracy of Performance
Multi-finger synergies, as reflected in indexes of covariation of commands to individual fingers, have been described as the means to ensure low variability of a performance variable such as total force (reviewed in Ref. 42) and as the means to allow a multi-finger system to perform several tasks simultaneously (76). According to this latter view, changes in an index of a force-stabilizing multi-finger synergy does not have to correlate with accuracy of performance (23). In our study, subjects showed modest changes in the indexes of both accurate force production tasks (RMS) and of multi-finger synergies stabilizing total force (ΔV) with exercise. The across-subjects variability prevented most of these changes from reaching statistical significance. However, the changes in the two indexes showed a significant correlation such that a drop in the RMS index (more accurate performance) was associated with an increase in ΔV (stronger force-stabilizing synergy). This observation corroborates the idea of synergies leading to more reproducible performance and supports using the ΔV index to assess such changes.
Concluding Comments
To test the main specific hypotheses related to the effects of strength training on finger strength and coordination, we selected a group of elderly individuals who exercised regularly and were generally in excellent physical condition. This is reflected, in particular, in the maximal finger force values produced before training that were 25–40% larger than typical values reported in previous studies (64–66). As a result, our participants showed relatively modest age-related changes in the finger force and the indexes of finger interaction and, hence, could be expected to have little room for improvement with exercise. Hence, we view the significant positive effects of exercise in this group as highly promising and allowing even stronger effects in the general aged population.
The study had several limitations that might have influenced the outcome. The training period lasted only 6 wk, and it is likely that training over a longer period of time would have resulted in more dramatic changes. However, based on the expectation of an increase in enslaving and its possible detrimental effects of hand dexterity (see the Introduction), we did not feel comfortable running a full-scale strength-training study using a long-term exercise protocol. After the promising results of this study, we will feel safe in applying long-term exercise to a more typical group of elderly persons, not only exceptionally fit ones. The short training period combined with the relatively small number of participants likely affected our ability to detect significant changes in some of the indexes. Nevertheless, even under those limiting factors, the study demonstrated significant effects of the exercise. Some of the tests used in the main study, including the Pegboard test, were not used in the control study (described in the appendix). We hope to overcome this limitation in a future full-scale study that would use an untrained control group.
GRANTS
The study was in part supported by National Institutes of Health Grants AG-018751, NS-035032, AR-048563, and M01 RR-10732.
Acknowledgments
We are very grateful to the Foxdale Village and Village at Penn State retirement communities for their participation in the study. The screening process of the elderly subjects was conducted at the General Clinical Research Center (The Pennsylvania State University). Special thanks are given to Dr. Simon Goodman for invaluable help with the UCM analysis and to Stacey Gorniak for help with editing the manuscript.
APPENDIX
The purpose of the control tests was to examine possible effects of repetitive testings on the maximal voluntary force and finger individuation.
Methods
Subjects.
Fourteen elderly (7 men and 7 women) individuals volunteered to participate in the study. Their average age, height, and weight was 77 ± 4 yr, 175.7 ± 6.6 cm, and 84.8 ± 12.1 kg for the men and 77 ± 4 yr, 160.4 ± 10.1 cm, and 60.5 ± 7.8 kg, respectively, for the women. Due to illness, one female subject dropped out of the study after the first testing session, and thus her data is not included in the results.
Apparatus.
Four unidirectional piezoelectric force sensor (model 208A03, PCB Piezotronics, Depew, NY) amplified by AC/DC conditioners (M482M66, PCB Piezotronics) were used to measure the vertical force produced by the four fingers (I, M, R, L). The sensors were placed in a metal frame, sitting in a grove on a wooden board. The sensors were medio-laterally spaced 3.0 cm apart. Their position in forward-backward direction was adjustable within 6.0 cm to fit each subject's hand anatomy. Once the appropriate position of the sensors was determined, double-sided tape was placed under the bases of the sensors to prevent them from moving from that position. During the experiments, the subjects were seated in a chair that faced the testing table with the right shoulder at ∼45° of abduction and flexion, and the elbow flexed ∼135°. MCP joints were flexed ∼20°, and all interphalangeal joints slightly flexed such that the hand formed a dome. A wooden piece, shaped to fit comfortably under the subjects palm, helped to maintain a constant configuration of the hand and fingers. Velcro straps were used to attach the subject's forearm to the board. A 17-in. computer screen, located ∼65 cm away from the subject, displayed the total force produced by the instructed fingers.
A LabVIEW-based program was used for data acquisition. Sampling frequency was set at 1,000 Hz with a 12-bit resolution.
Experimental procedure.
The experiment lasted 5 wk and, during that time, each subject was tested three times, in weeks 1, 3, and 5. During the experiment, the subject sat relaxed with the fingertips of the right hand resting on the sensors. The computer generated two beeps, a “get ready” and “trial starting” signal, then a cursor showing the total force produced by the instructed fingers started to move across the screen. Subjects produced maximal voluntary force (MVC) with each of the fingers individually (I, M, R, L) and with all four fingers together (IMRL). In these trials, subjects were instructed to press “as hard as possible” with the task finger(s), not to lift the other fingers and not to pay attention to the force they might produce. Each trial lasted 10 s, and subjects were asked to produce peak force within a 3-s time interval marked with two vertical lines on the screen. Two trials were collected for each task finger(s), and the one with higher peak force was used. All subjects had two practice trials to get familiar with the task and 30-s rests between pairs of trials to avoid fatigue. Note that this experimental design involved the force production at the distal site only.
Data analysis.
The data were processed offline using Matlab 7.0 and Excel.
In each trial, the force produced by individual fingers was measured at the time when the force produced by the task finger(s) reached peak value. These values were used to calculate the enslaving forces for single-finger trials. Enslaving forces are forces produced by noninstructed fingers. Enslaving of each finger was expressed as percentage of its own MVC when acting as the task finger. By calculating enslaving for each individual finger, an enslaving matrix was generated. For further comparison, the indexes of enslaving were averaged across the slave fingers.
Statistics.
Standard descriptive statistics were used. Repeated-measures ANOVAs were used to test the effects of multiple testing on peak force and enslaving. Factors were test (three levels, test 1, 2, and 3) and finger (four levels, I, M, R, and L). Multiple comparisons with Bonferroni correction were used to analyze significant effects. Level of significance was set at P = 0.05.
Results
Overall, the performance of subjects showed no changes during the course of the experiment. During both the single- and four-finger trials, the I and M fingers produced the largest peak forces, followed by the R and L fingers. The peak forces of individual fingers reached during the four-finger trials were smaller than those produced during single-finger trials. The sum of the peak forces during single-finger trials was, on average, ∼110 N across all testing sessions; the average peak force during the four-finger trials was ∼83 N. Figure 10A shows the sum of the maximal forces produced by individual fingers during the four-finger trials for the three testing sessions.
Fig. 10.
The control experiment. A: summed peak force of fingers in the single-finger tasks (ΣMVCi). B: average enslaving during single-finger MVC tasks. Data for the three testing sessions are shown with filled, open, and hatched bars, respectively. Averaged across-subjects data with standard error bars are shown.
The lack of effects of the repetitive testing on the total peak force during single- and four-finger trials was confirmed by a two-way repeated-measures ANOVAs with factors finger and test that showed no effects of test during the single-finger [F(2,20) = 0.76; P = 0.48] and four-finger [F(2,20) = 1.52; P = 0.24] trials. The ANOVA showed a significant effect of finger [single-finger: F(1.5,15.5) = 35.4; P < 0.001; four-finger F(3,30) = 37.5; P < 0.001] but not of interaction.
In the single-finger trials, The R and L finger were the most enslaved, whereas the I finger was the most independent finger. Figure 10B shows average enslaving averaged across all fingers (All) for the three testing sessions. The total enslaving index was ∼16–17% during all three testing sessions. A two-way ANOVA with repeated measures with factors test and finger showed no effect of test [F(1.4,14.1) = 0.38; P = 0.62] but did show the expected main effects of finger [F(3,30) = 9.64; P < 0.001].
Overall, the results of the control study indicate that repetitive testing does not by itself lead to changes in finger peak force and enslaving index.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.An KN, Chao EY, Cooney WP, Linscheid RL. Forces in the normal and abnormal hand. J Orthop Res 3: 202–211, 1985. [DOI] [PubMed] [Google Scholar]
- 2.Basmajian JV, De Luca CJ. Muscles Alive (5th ed.). Baltimore, MD: Williams & Wilkins, 1985.
- 3.Bellew JW The effect of strength training on control of force in older men and women. Aging Clin Exp Res 14: 35–41, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bilodeau M, Keen DA, Sweeney PJ, Shields RW, Enoka RM. Strength training can improve steadiness in persons with essential tremor. Muscle Nerve 23: 771–778, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci 58: 146–152, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao EY, Opgrande JD, Axmear FE. Three-dimensional force analysis of finger joints in selected isometric hand functions. J Biomech 9: 387–396, 1976. [DOI] [PubMed] [Google Scholar]
- 8.Cole KJ Age-related directional bias of fingertip force. Exp Brain Res 175: 285–291, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cole KJ, Rotella DL, Harper JG. Tactile impairements cannot explain the effect of age on a grasp and lift. Exp Brain Res 121: 263–269, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Cole KJ, Rotella DL, Harper JG. Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci 19: 3238–3247, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danion F, Latash ML, Li ZM, Zatsiorsky VM. The effect of fatigue on multifinger co-ordination in force production tasks in humans. J Physiol 523: 523–532, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darling WG, Cole KJ, Miller GF. Coordination of index finger movements. J Biomech 27: 479–491, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol 82: 93–101, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Dons B, Bollerup K, Bonde-Petersen F, Hancke S. The effect of weightlifting exercise related to muscle fiber composition and muscle cross-sectional area in humans. Eur J Appl Physiol Occup Physiol 40: 95–106, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Enoka RM Muscle strength and its development: New perspectives. Sports Med 6: 146–168, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Enoka RM Neural strategies in the control of muscle force. Muscle Nerve Suppl 5: S66–S69, 1997. [PubMed] [Google Scholar]
- 17.Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia 37: 147–158, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Fitts PM The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47: 381–391, 1954. [PubMed] [Google Scholar]
- 19.Francis KL, Spirduso W. Age differences in the expression of manual asymmetry. Exp Aging Res 26: 169–180, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gandevia SC Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Goodman SR, Shim JK, Zatsiorsky VM, Latash ML. Motor variability within a multi-effector system: experimental and analytical studies of multi-finger production of quick force pulses. Exp Brain Res 163: 75–85, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorniak S, Duarte M, Latash ML. Do synergies improve accuracy? A study of speed-accuracy trade-offs during finger force production. Motor Control 12: 151–172, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorniak S, Zatsiorsky VM, Latash ML. Hierarchies of synergies: an example of the two-hand, multi-finger tasks. Exp Brain Res 179: 167–180, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther 72: 373–377, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Harding DC, Brandt KD, Hillberry BM. Finger joint force minimization in pianists using optimization techniques. J Biomech 26: 1403–1412, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Hortobagyi T, Tunnel D, Moody J, Beam S, De Vita P. Low- or high-intensity strength training partially restores impaired quadriceps force accuracy and steadiness in aged adults. J Gerontol A Biol Sci Med Sci 56: 38–47, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Houston ME, Froese EA, Valeriote SP, Green HJ, Ranney DA. Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. Eur J Appl Physiol 51: 25–35, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Hughes S, Gibbs J, Dunlop D, Edelman P, Singer Chang RW R. Predictors of decline in manual performance in older adults. J Am Geriatr Soc 45: 905–910, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Izquierdo M, Hakkinen K, Ibanez J, Anton A, Garrues M, Ruesta M, Gorostiaga EM. Effects of strength training on submaximal and maximal endurance performance capacity in middle-aged and older men. J Strength Cond Res 17: 129–139, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil 50: 311–319, 1969. [PubMed] [Google Scholar]
- 31.Keogh JW, Morrison S, Barrett R. Strength training improves the tri-digit finger-pinch force control of older adults. Arch Phys Med Rehabil 88: 1055–1063, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kilbreath Gandevia SC SL Independent control of the digits: changes in perceived heaviness over a wide range of force. Exp Brain Res 91: 539–542, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Kilbreath SL, Gandevia SC. Limited independent flexion of the thumb and fingers in human subjects. J Physiol 479: 487–497, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornatz KW, Christou EA, Enoka RM. Practice reduces motor unit discharge variability in a hand muscle and improves manual dexterity in old adults. J Appl Physiol 98: 2072–2080, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Kozin SH, Porter S, Clark P, Thoder JJ. The contribution of the intrinsic muscles to grip and pinch strength. J Hand Surg 24A: 64–72, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Laidlaw DH, Hunter SK, Enoka RM. Nonuniform activation of the agonist muscle does not covary with index finger acceleration in old adults. J Appl Physiol 93: 1400–1410, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Landsmeer JMF, Long C. The mechanism of finger control, based on electromyograms and location analysis. Acta Anat (Basel) 60: 330–347, 1965. [DOI] [PubMed] [Google Scholar]
- 38.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol 45: 397–458, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Latash ML, Kang N, Patterson D. Finger coordination in persons with Down syndrome: atypical patterns of coordination and the effects of practice. Exp Brain Res 146: 345–355, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one- and two-hand force production at distal and proximal phalanges. Brain Res 924: 198–208, 2002b. [DOI] [PubMed] [Google Scholar]
- 41.Latash ML, Scholz JF, Danion F, Schöner G. Structure of motor variability in marginally redundant multifinger force production tasks. Exp Brain Res 141: 153–165, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Latash ML, Scholz JP, Schöner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30: 26–31, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Latash ML, Scholz JP, Schöner G. Toward a new theory of motor synergies. Motor Control 11: 275–307, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Leijnse JN, Snijiders CCJ, Bonte JE, Landsmeer JM, Kalker JJ, Van Der Meulen JC, Sonneveld GJ, Hovius SE. The hand of the musician: the kinematics of the bidigital finger system with anatomical restrictions. J Biomech 26: 1169–1179, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Li ZM, Latash ML, Zatsiorsky VM. Force sharing among fingers as a model of the redundancy problem. Exp Brain Res 119: 276–286, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin Biomech 15: 203–211, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Long C Intrinsic-extrinsic muscle control of the fingers. J Bone Jt Surg 50A: 973–984, 1965. [PubMed] [Google Scholar]
- 48.Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact 4: 161–164, 2004. [PubMed] [Google Scholar]
- 49.Newell KM, Carlton LG. Force variability in isometric responses. J Exp Psychol Hum Percept Perform 14: 37–44, 1993. [PubMed] [Google Scholar]
- 50.Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Penta M, Thonnard JL, Tesio L. ABILHAND: a Rasch-built measure of manual ability. Arch Phys Med Rehabil 79: 1038–1042, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Plamondon R, Alimi AM. Speed/accuracy trade-offs in target-directed movements. Behav Brain Sci 20: 1–31, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Ranganathan VK, Siemionow V, Sahgal V, Yue GH. Effects of aging on hand function. J Am Geriatr Soc 49: 1478–1484, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Ranganathan VK, Siemionow V, Liu JZ, Sahgal V, Yue GH. From mental power to muscle power: gaining strength by using the mind. Neuropsychologia 42: 944–56, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA 281: 558–560, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Reeves ND, Narici MV, Maganaris CN. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol 89: 675–689, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol 91: 483–498, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard tests. Percept Mot Skills 76: 1219–1230, 1993. [DOI] [PubMed] [Google Scholar]
- 59.Sale DG Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev 15: 95–151, 1987. [PubMed] [Google Scholar]
- 60.Schmidt RA, Wrisberg CA. Motor Learning and Performance (4th ed.). Champaign, IL: Human Kinetics, 2007.
- 61.Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT. Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86: 415–451, 1979. [PubMed] [Google Scholar]
- 62.Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Shim JK, Lay B, Zatsiorsky VM, Latash ML. Age-related changes in finger coordination in static prehension tasks. J Appl Physiol 97: 213–224, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol 95: 1361–1369, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol 94: 259–270, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Shinohara M, Scholz JP, Zatsiorsky VM, Latash ML. Finger interaction during accurate multi-finger force production tasks in young and elderly persons. Exp Brain Res 156: 282–292, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Sohn YH, Dang N, Hallett M. Suppression of corticospinal excitability during negative motor imagery. J Neurophysiol 90: 2303–2309, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Sosnoff JJ, Newell KM. Are age-related increases in force variability due to decrements in strength? Exp Brain Res 174: 86–94, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Sparing R, Mottaghy FM, Ganis G, Thompson WL, Töpper R, Kosslyn SM, Pascual-Leone A. Visual cortex excitability increases during visual mental imagery: a TMS study in healthy human subjects. Brain Res 938: 92–97, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve 27: 99–101, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Tracy BL, Byrnes WC, Enoka RM. Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol 96: 1530–1540, 2004. [DOI] [PubMed] [Google Scholar]
- 72.Vincent KR, Braith RW, Feldman RA, Magyari PM, Cutler RB, Persin SA, Lennon SL, Gabr AH, Lowenthal DT. Resistance exercise and physical performance in adults aged 60 to 83. J Am Geriatr Soc 50: 1100–1107, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Yasuda Y, Miyamura M. Cross-transfer effects of muscular training on blood flow in the ipsilateral and contralateral forearms. Eur J Appl Physiol 51: 321–329, 1983. [DOI] [PubMed] [Google Scholar]
- 74.Zatsiorsky VM, Latash ML. Prehension synergies. Exerc Sport Sci Rev 32: 75–80, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zatsiorsky VM, Li ZM, Latash ML. Enslaving effects in multi-finger force production. Exp Brain Res 131: 187–195, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, Scholz JP, Zatsiorsky VM, Latash ML. What do synergies do? Effects of secondary constraints on multi-digit synergies in accurate force-production tasks. J Neurophysiol 99: 500–513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zijdewind I, Toering ST, Bessem B, Van Der Laan O, Diercks RL. Effects of imagery motor training on torque production of ankle plantar flexor muscles. Muscle Nerve 28: 168–173, 2003. [DOI] [PubMed] [Google Scholar]