Abstract
Oxidative stress impairs endothelium-dependent dilation (EDD) with aging in healthy sedentary adults. Increased cytochrome P-450 2C9 (CYP 2C9) signaling can contribute to oxidative stress-mediated suppression of EDD, but its role in aging is unknown. We hypothesized that inhibition of CYP 2C9 signaling with sulfaphenazole would improve EDD in older, but not young, healthy sedentary adults. At baseline, increases in forearm blood flow (FBF; venous occlusion plethysmography) in response to brachial artery infusions of ACh (1, 2, 4, and 8 μg·100 ml forearm volume−1·min−1), an endothelium-dependent dilator, were smaller in older [n = 14, 63 ± 1 (SE) yr] than in young (n = 11, 23 ± 2 yr) adults (P < 0.05), with a reduction in peak FBF of 32% (11.8 ± 1.7 vs. 17.3 ± 2.3 ml·100 ml tissue−1·min−1). Infusion of sulfaphenazole at doses that block CYP 2C9 signaling in humans did not affect the FBF responses to ACh in the older (peak FBF = 13.0 ± 4.3 ml·100 ml tissue−1·min−1, P = 0.41) or the young (peak FBF = 17.1 ± 1.9 ml·100 ml tissue−1·min−1, P = 0.55) adults. Coadministration of the nitric oxide inhibitor l-NMMA and sulfaphenazole decreased the FBF response to ACh in young and older subjects (P < 0.05); the effect was smaller in the older subjects, but group differences in EDD remained (P < 0.05). Endothelium-independent dilation assessed with sodium nitroprusside was not different in the young and older subjects. These results provide the first support for the concept that increased CYP 2C9 signaling does not contribute to impairments in EDD with aging in healthy adults.
Keywords: endothelium-dependent dilation, oxidative stress, arterial aging
vascular endothelial function declines with aging, even in healthy adults, as indicated by a reduction in endothelium-dependent dilation (EDD) (3, 30). The decrease in EDD with aging is mediated by oxidative stress (10, 13, 31), which reduces nitric oxide (NO) bioavailability (31). In older animals, oxidative stress decreases NO bioavailability, at least in part via increased production of superoxide anions (32), which rapidly react with NO to form peroxynitrite (25). However, little information is available regarding the sources of vascular superoxide that contribute to impaired EDD in older adults. Our recent work rules out a possible contribution of the oxidant enzyme xanthine oxidase to reduced EDD with aging in healthy humans (12).
Endothelial cytochrome P-450 epoxygenase 2C9 (CYP 2C9) is a dynamic enzyme that produces endothelium-hyperpolarizing factors (11,12-epoxyeicosatrienoic acid) and reactive oxygen species (ROS), depending on the substrates available (16, 33). Endothelial CYP 2C9-generated superoxide anions can react with NO to form peroxynitrite and reduce NO bioavailability and EDD (17). Consistent with this, CYP 2C9 inhibition with sulfaphenazole improves impaired EDD in patients with coronary artery disease without influencing EDD in healthy controls with normal baseline function (15). The improvement in EDD with sulfaphenazole in the patients was mediated by an increase in NO bioavailability because coinfusion with an inhibitor of NO production abolished the effect.
In the present study, we tested the hypothesis that CYP 2C9 contributes to the impairment of baseline EDD observed in older healthy adults. We also sought to determine whether any improvement in EDD in response to inhibition of CYP 2C9 was mediated by an increase in NO bioavailability.
METHODS
Subjects.
Data were obtained from 25 healthy men and women: 11 young (18–30 yr) and 14 older (58–75 yr) adults. Subjects had resting blood pressure <140/90 mmHg and were free of cardiovascular diseases, diabetes, and other clinical disorders as assessed by medical history, physical examination, and blood chemistries. Subjects >40 yr of age were further screened for cardiovascular disease using ECG and blood pressure responses to incremental treadmill exercise performed to volitional exhaustion. Subjects were nonsmokers, not taking medications or dietary supplements (including antioxidants), and not regularly exercising. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained prior to participation.
Study procedures.
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center after an overnight fast and a 24-h abstention from alcohol and physical activity. All assays were performed by the University of Colorado General Clinical Research Center core laboratory.
Subject characteristics.
Arterial blood pressure was measured over the brachial artery during seated rest using a semiautomated device (Dynamap XL, Johnson and Johnson). Fasting plasma metabolic factors were determined by standard assays. Plasma samples were analyzed for oxidized LDL, a marker of systemic oxidative stress, and serum samples were analyzed for total antioxidant status, a measure of systemic antioxidant defenses, as previously described (27). Serum concentrations of C-reactive protein were measured by an Olympus AU400e Chemistry Analyzer. Leisure time activity was estimated to provide a measure of habitual physical activity (11).
Blood flow measurements.
Forearm blood flow (FBF) was measured in the experimental (nondominant) and the control (dominant) forearm with strain-gauge venous occlusion plethysmography (D. E. Hokanson), as previously described (8). Briefly, a mercury-Silastic strain gauge was placed around the forearm, and a cuff was placed around each upper arm and repeatedly inflated to 50 mmHg to occlude venous outflow for 7 s. A second cuff was placed around the wrist and inflated to suprasystolic pressures to exclude the hand circulation during FBF measures. Flows were measured during the last minute of each 3-min dose, and the mean value was reported. All FBF values are presented in milliliters per 100 milliliters of forearm volume per minute. Forearm vascular conductance (FVC) was calculated from intra-arterial blood pressure and FBF (FVC = FBF/mean arterial pressure) to account for any differences in arterial pressure between groups and within an experimental protocol.
EDD and endothelium-independent dilation.
Forearm volume was determined by the water displacement method. Drug infusion rates were normalized per 100 ml of forearm tissue and infused at <4 ml/min by a syringe pump (Harvard). EDD and endothelium-independent dilation were determined as the FBF responses to an incremental intrabrachial artery infusion of ACh (1.0, 2.0, 4.0, and 8.0 μg·100 ml forearm tissue−1·min−1) and sodium nitroprusside (0.5, 1, and 2.0 μg·100 ml forearm tissue−1·min−1), respectively.
Inhibition of CYP 2C9.
The contribution of CYP 2C9 to EDD in young and older subjects was determined by coinfusion of sulfaphenazole (2 mg/min, 10-min loading dose) into the brachial artery with ACh (1.0, 2.0, 4.0, and 8.0 μg·100 ml forearm tissue−1·min−1). This dose of sulfaphenazole inhibits CYP 2C9 ROS production and improves EDD in patients with coronary artery disease, but not in healthy controls with normal function (15). Furthermore, systemic infusion of sulfaphenazole for 1 h reduces oxidized protein products in plasma of patients with coronary artery disease, but not in healthy controls (15). To determine the effects of CYP 2C9 blockade on the NO contribution to EDD in young and older adults, we infused sulfaphenazole (2 mg/min, 10-min loading dose) with both NG-monomethyl-l-arginine (l-NMMA, 5 mg/min, 10-min loading dose) and ACh (doses above). This dose of l-NMMA inhibits NO production by competitive inhibition of endothelial NO synthase in the human forearm without causing systemic effects (9).
Data analysis.
Statistical analyses were performed with SPSS (version 11.0.3). Group differences were determined by t-tests for independent sample comparisons, and FBF responses to incremental doses of ACh and sodium nitroprusside were analyzed by repeated-measures ANOVA. Pearson correlation analysis was used to assess bivariate relations of interest. Statistical significance for all analyses was set at P < 0.05.
RESULTS
Subject characteristics.
Characteristics of the young and older subjects are shown in Table 1. Resting blood pressure and fasting plasma total and LDL cholesterol were higher in the older subjects (all P < 0.05), but all values were within clinically normal ranges. Body mass, body mass index, leisure time physical activity, and fasting plasma HDL cholesterol and glucose were not different between the groups. Plasma oxidized LDL was greater and total antioxidant status was lower in the older subjects (both P < 0.05), whereas C-reactive protein was not significantly different between groups.
Table 1.
Subject characteristics
| Young (n = 11) | Older (n = 14) | P | |
|---|---|---|---|
| Male/Female | 9/2 | 10/4 | |
| Age, yr | 23±2 | 64±1 | <0.01 |
| Body mass, kg | 77±4 | 76±3 | 0.93 |
| BMI, kg/m2 | 23.7±0.7 | 25.6±0.9 | 0.13 |
| BP, mmHg | |||
| Systolic | 110±3 | 123±3 | <0.01 |
| Diastolic | 71±2 | 78±2 | 0.03 |
| Cholesterol, mg/dl | |||
| Total | 157±9 | 194±8 | <0.01 |
| LDL | 89±7 | 115±7 | 0.02 |
| HDL | 48±4 | 57±4 | 0.15 |
| Fasting glucose, mg/dl | 88±2 | 94±2 | 0.32 |
| Leisure physical activity, MET, h/wk | 26±9 | 37±9 | 0.42 |
| OxLDL, U/l | 39.1±4.3 | 61.4±5.1 | 0.01 |
| Total antioxidant status, mmol/l | 1.43±0.04 | 1.26±0.04 | 0.02 |
| C-reactive protein, mg/l | 0.42±0.10 | 0.75±0.18 | 0.20 |
Values are means ± SE. BMI, body mass index; BP, blood pressure; OxLDL, oxidized LDL; METs, metabolic equivalents.
EDD and endothelium-independent dilation.
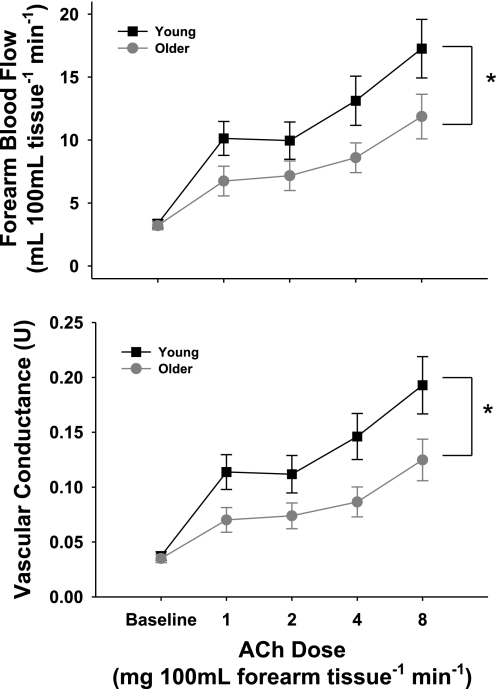
The FBF and FVC responses to ACh were smaller in the older subjects (Fig. 1; P < 0.05). At the highest dose of ACh, FBF was ∼32% lower in the older than in the young group: 11.8 ± 1.7 vs. 17.3 ± 2.3 ml·100 ml tissue−1·min−1.
Fig. 1.
Forearm blood flow (FBF, top) and forearm vascular conductance (FVC, bottom) in response to intrabrachial artery infusion of ACh in healthy young and older adults. Values are means ± SE. *P < 0.05 vs. young.
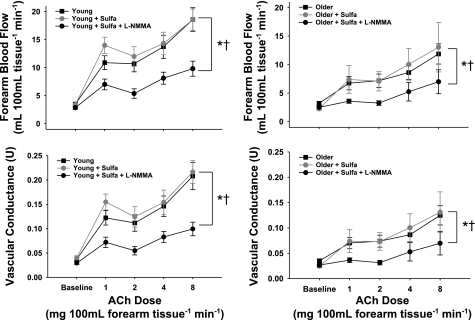
Inhibition of CYP 2C9 with sulfaphenazole did not alter (P > 0.05) baseline FBF or FVC in the young (FBF = 3.3 ± 0.3 and 3.7 ± 0.5 ml·100 ml tissue−1·min−1 at baseline and with sulfaphenazole, respectively) or older (FBF = 2.6 ± 0.2 and 2.3 ± 0.4 ml·100 ml tissue−1·min−1 at baseline and with sulfaphenazole, respectively) subjects. Sulfaphenazole did not affect the FBF or FVC responses to ACh in either group (P > 0.40; Fig. 2). Administration of the NO inhibitor l-NMMA with sulfaphenazole reduced the FBF and FVC responses to ACh in both groups (P < 0.05). The effect was smaller in the older subjects, but age group differences in EDD remained (P < 0.05, Fig. 2).
Fig. 2.
FBF (top) and FVC (bottom) in response to intrabrachial artery infusion of ACh in the absence and presence of the nitric oxide inhibitor NG-monomethyl-l-arginine (l-NMMA, 5 mg/min) and the cytochrome P-450 2C9 inhibitor sulfaphenazole (Sulfa, 2 mg/min) in young (left) and older (right) adults. Values are means ± SE. *P < 0.05 vs. ACh. †P < 0.05 vs. ACh + Sulfa.
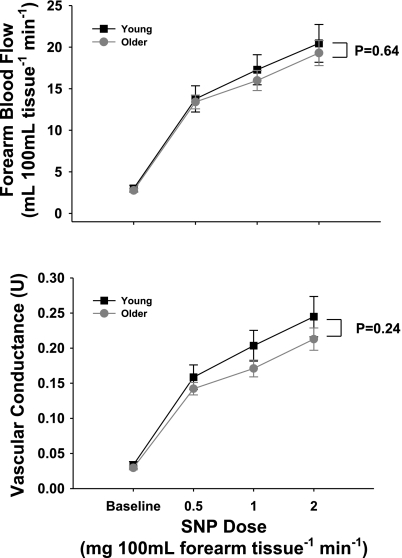
In contrast to EDD, the responses to sodium nitroprusside were not different in the young and older subjects: P = 0.62 for FBF and P = 0.24 for FVC (Fig. 3).
Fig. 3.
FBF (top) and FVC (bottom) responses to intrabrachial artery infusion of the nitric oxide donor sodium nitroprusside (SNP) in young and older adults. Values are means ± SE.
DISCUSSION
Consistent with previous findings from our laboratory and others (3, 8, 10, 13, 30), in the present study baseline EDD was lower in healthy older adults in the absence of age-related differences in the FBF and FVC responses to sodium nitroprusside, confirming endothelial dysfunction in the presence of intact endothelium-independent dilation. Our results extend current insight into the mechanisms contributing to the impairment of EDD with aging in humans. The key new finding was that inhibition of CYP 2C9 does not improve EDD in older adults. As such, our results suggest that CYP 2C9 signaling does not contribute to reductions in peripheral resistance artery EDD with aging in healthy humans.
CYP 2C9 and its closely related isoforms are membrane-bound heme-containing terminal oxidases that metabolize arachidonic acid and other cellular lipids (retinoic and linoleic acids) to produce epoxyeicosatrienoic acids. These molecules can act as endothelium-derived hyperpolarizing factors (26), but reduction of their central heme iron also produces ROS, such as superoxide, hydrogen peroxide, and hydroxyl radical (17). In several settings of oxidative stress-associated endothelial dysfunction, inhibition of this enzyme with sulfaphenazole increases the sensitivity of arteries to ACh-mediated vasodilation by reducing synthesis of ROS and enhancing NO bioavailability (15, 17, 22). Because aging is a state of impaired vascular endothelial function mediated by oxidative stress and reduced NO bioavailability (3, 13, 28, 30, 31), we reasoned that increased CYP 2C9 signaling might be involved.
In agreement with our previous work (10, 12, 19, 27), the older adults in our study demonstrated greater circulating concentrations of oxidized LDL, a marker of systemic oxidative stress, which, in the present sample, was associated with a slightly, but significantly, lower plasma total antioxidant status (Table 1). Previously, we (13) and others (31) demonstrated that acute reduction of this baseline oxidative stress in older adults via systemic administration of supraphysiological concentrations of the potent antioxidant ascorbic acid (vitamin C) improves EDD, indicating a tonic oxidative stress-mediated suppression of EDD with aging. However, the source of superoxide production responsible for this oxidative stress-associated impairment of EDD in older adults is unknown. The fact that sulfaphenazole did not improve EDD in the healthy older subjects in the present study, despite a clear impairment of baseline EDD, suggests that CYP 2C9 is not a functionally significant source of superoxide production and oxidative stress contributing to impaired EDD with aging. We do not believe that our results can be explained by an insufficient dose of sulfaphenazole because we infused a concentration that improves ACh-evoked increases in FBF in patients with cardiovascular disease and impaired baseline vascular endothelial function (21, 24). Rather, our results are more consistent with findings that sulfaphenazole does not enhance the FBF responses to ACh in middle-aged patients with essential hypertension (29). However, in the present study, we do not have measures of oxidative stress in the forearm vasculature to determine whether sulfaphenazole reduced oxidative stress locally in resistance vessels. Sustained infusions of the dose used in our study reduces systemic markers of oxidative stress (15), but only the effects of local intrabrachial artery infusion were assessed in the present investigation.
A recent investigation by our laboratory demonstrated that inhibition of xanthine oxidase, another oxidant enzyme implicated in oxidative stress-driven endothelial dysfunction (2), does not improve EDD in healthy older adults (12). Given the present results, what other sources of superoxide may contribute to oxidative stress and reduced vascular endothelial function with aging in humans? One possibility is the oxidant enzyme NADPH oxidase, the expression of which is increased in the vasculature of older animals and patients with cardiovascular disease (1, 5, 6). Recently, we demonstrated greater protein expression of NADPH oxidase in endothelial cells obtained from older than young healthy men (10). Moreover, inhibition of NADPH oxidase reduces ROS to a greater extent in middle-aged than young rodents (20). However, the role of this enzyme system in mediating age-associated endothelial dysfunction cannot be determined directly in humans because there is no safe, specific inhibitor.
Similarly, the effects of other sources of increased endothelial superoxide synthesis on endothelial dysfunction with aging such as uncoupled endothelial NO synthase and mitochondria, have not been experimentally isolated in humans because of the lack of suitable pharmacological approaches. Both of these mechanisms are believed to play a role in endothelial oxidative damage (2, 34). However, in particular, little direct evidence exists for a contribution of uncoupled endothelial NO synthase to the age-related decrease in EDD. Reduced bioavailability of tetrahydrobiopterin, a key cofactor for NO synthesis by endothelial NO synthase, causes uncoupling of the enzyme and reduced NO production (23). Thus, the fact that administration of tetrahydrobiopterin restores EDD in healthy older adults (14) and to aged arterioles from rats (7) may provide indirect evidence for uncoupling of endothelial NO synthase as a mechanism in oxidative stress-associated endothelial dysfunction in human aging. It has been reported that p66shc, a mitochondrial protein implicated in ROS production and signaling in settings of oxidative stress, mediates endothelial dysfunction with aging in rodents, thus providing compelling evidence for a mitochondrial origin of superoxide in vascular aging (4, 18). No data are available concerning this mechanism in humans.
In the present study, administration of l-NMMA with sulfaphenazole caused reductions in the FBF and FVC responses to ACh in both groups, with a lesser effect observed in the older adults. This is similar to the effects of l-NMMA alone on the FBF responses to ACh reported previously (31) because sulfaphenazole had no baseline effects on EDD in either group.
In summary, the results of the present study show that administration of sulfaphenazole does not improve EDD in healthy older adults with baseline vascular endothelial dysfunction. As such, our findings provide the first experimental support for the idea that increased CYP 2C9 signaling does not contribute to impairments in NO bioavailability and EDD with aging in healthy humans.
GRANTS
This work was supported by National Institutes of Health Grants AG-006537, AG-013038, AG-022241, AG-000279, HL-007851, AG-029337, and RR-00051.
Acknowledgments
We thank Rhea Chiang, Cassandra Roeca, and Brooke Lawson for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 100: 292–298, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer D, Sorensen K, Bull C, Robinson J, Deanfield J. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol 24: 1468–1474, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 28: 622–628, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol 170: 388–398, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dietz NM, Engelke KA, Samuel TT, Fix RT, Joyner MJ. Evidence for nitric oxide-mediated sympathetic forearm vasodilatation in humans. J Physiol 498: 531–540, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ Res 100: 1659–1666, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Eskurza I, Donato AJ, Moreau KL, Seals DR, Tanaka H. Changes in maximal aerobic capacity with age in endurance-trained women: 7-yr follow-up. J Appl Physiol 92: 2303–2308, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichtlscherer S, Dimmeler S, Breuer S, Busse R, Zeiher AM, Fleming I. Inhibition of cytochrome P450 2C9 improves endothelium-dependent, nitric oxide-mediated vasodilatation in patients with coronary artery disease. Circulation 109: 178–183, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Fleming I Cytochrome p450 and vascular homeostasis. Circ Res 89: 753–762, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Michaelis UR, Bredenkotter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res 88: 44–51, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110: 2889–2895, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Hunter AL, Bai N, Laher I, Granville DJ. Cytochrome p450 2C inhibition reduces post-ischemic vascular dysfunction. Vascul Pharmacol 43: 213–219, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Katusic ZS Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 281: H981–H986, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF Jr, Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 93: 1107–1113, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263: 681–684, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Miura H, Gutterman DD. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxygenase and Ca2+-activated K+ channels. Circ Res 83: 501–507, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Moreau KL, DePaulis AR, Gavin KM, Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. J Appl Physiol 102: 890–895, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taddei S, Versari D, Cipriano A, Ghiadoni L, Galetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome P450 2C9-derived endothelium-derived hyperpolarizing factor in essential hypertensive patients. J Am Coll Cardiol 48: 508–515, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, Pinto S, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension 28: 576–582, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 32.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199: 316–331, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol 292: H2023–H2031, 2007. [DOI] [PubMed] [Google Scholar]