Abstract
Tumor necrosis factor-α (TNF-α) is associated with sleep regulation in health and disease. Previous studies assessed sleep in mice genetically deficient in the TNF-α 55-kDa receptor. In this study, spontaneous and influenza virus-induced sleep profiles were assessed in mice deficient in both the 55-kDa and 75-kDa TNF-α receptors [TNF-2R knockouts (KO)] and wild-type (WT) strain controls. Under baseline conditions the TNF-2R KO mice had less non-rapid eye movement sleep (NREMS) than WTs during the nighttime and more rapid eye movement sleep (REMS) than controls during the daytime. The differences between nighttime maximum and daytime minimum values of electroencephalogram (EEG) delta power during NREMS were greater in the TNF-2R KO mice than in WTs. Viral challenge (mouse-adapted influenza X-31) enhanced NREMS and decreased REMS in both strains roughly to the same extent. EEG delta power responses to viral challenge differed substantially between strains; the WT animals increased, whereas the TNF-2R KO mice decreased their EEG delta wave power during NREMS. There were no differences between strains in body temperatures or locomotor activity in uninfected mice or after viral challenge. Analyses of cortical mRNAs confirmed that the TNF-2R KO mice lacked both TNF-α receptors; these mice also had higher levels of orexin mRNA and reduced levels of the purine P2X7 receptor compared with WTs. Results reinforce the hypothesis that TNF-α is involved in physiological sleep regulation but plays a limited role in the acute-phase response induced by influenza virus.
Keywords: cytokine, hypothermia, electroencephalogram delta power, purine receptor, orexin
tumor necrosis factor-α (TNF-α) is a cytokine well-characterized for its role in physiological sleep regulation (reviewed in Ref. 28) and in infection-induced pathologies, including the fever and sleep disturbances that form part of the acute-phase response (APR) (1). Administration of TNF-α enhances non-rapid eye movement sleep (NREMS) while inhibition of TNF-α acutely reduces duration of spontaneous NREMS (28). TNF-α and its receptors are expressed in brain and immunocytes. In brain, TNF-α mRNA levels vary with sleep propensity, e.g., it increases during sleep deprivation and is at its highest levels at the onset of daylight, the period of greatest spontaneous sleep in rats. TNF-α is expressed by glia and neurons; in neurons expression is dependent on prior neural activity (18). TNF-α enhances sleep if injected into sleep-regulatory circuits such as the anterior hypothalamus (29) or the locus ceruleus (13). In contrast, microinjection of a TNF-α soluble receptor into the anterior hypothalamus inhibits spontaneous sleep (29). TNF-α also unilaterally enhances sleep intensity, as measured by electroencephalogram (EEG) delta wave power, if applied to one side of the cerebral cortex (49). Further, if applied locally to the cortex it enhances the amplitudes of cortical column-evoked response potentials to levels that characterize a sleeplike state of cortical columns (8). In contrast, if TNF-α is reduced in one cerebral hemisphere but not the other by use of a TNF-α small interfering RNA, the affected hemisphere has reduced EEG delta power during NREMS (44). Mice lacking the TNF 55-kDa receptor have NREMS and rapid eye movement sleep (REMS) deficits during the transition from dark to light extending about 6 more hours into the light period. At other times of the day, duration of sleep is normal (17). Collectively, such data strongly implicate TNF-α in physiological sleep regulation.
TNF-α levels also change during inflammatory states, and systemic inflammatory states are associated with changes in sleep propensity. For example, after A/Puerto Rico/8/34 (H1N1) influenza virus challenge, sleep and hypothalamic levels of TNF-α mRNA are enhanced (1). Further, within hours of influenza virus intranasal challenge, TNF-α mRNA is enhanced in the olfactory bulb (32) and TNF-α protein expression is enhanced in olfactory bulb neurons (Leyva-Grado VH, Churchill L, Wu M, Williams TJ, Taishi P, De A, Majde JA, and Krueger JM, unpublished observations). These changes occur concurrently with changes in body temperature and sleep. Bacterial challenge is also associated with changes in TNF-α and sleep; e.g., systemic injection of endotoxin, a Gram-negative cell wall product, enhances plasma TNF and sleep in humans (33). Many pathological conditions in humans, for example, myocardial infarction, post-viral fatigue syndrome, insomnia, chronic fatigue syndrome, AIDS, preeclampsia, postdialysis fatigue, alcoholism, and sleep apnea, are characterized by enhanced circulating TNF levels and sleep or sleepiness (28). Further, a TNF polymorphic variant, G-308A, is linked to metabolic syndrome (42) and sleep apnea (38). Such data suggest that TNF-α is likely to be a key mediator of sleep responses to pathological challenges, including viral challenge.
In the present work, we investigate the role of the TNF system in physiology and in the APRs induced by influenza virus by using mice that lack both the TNF 55-kDa and 75-kDa receptors. These receptors share some amino acid homology in their extracellular domains but not in their intracellular moieties. They have distinct biological actions (35). We report that the mice lacking these receptors have less spontaneous sleep under control conditions and their EEG delta wave responses to viral challenge during NREMS are decreased rather than increased. Results are consistent with the hypotheses that TNF-α plays a key role in the regulation of spontaneous NREMS, and in the sleep response occurring during the APR induced by influenza.
MATERIALS AND METHODS
Animals.
TNF-α p55 and p75 double-receptor knockout mice (B6;129S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J, or TNF-2R KOs) and wild-type (WT) control (B6;129S F2/J) male mice (12–16 wk of age) were purchased from Jackson Laboratories (Bar Harbor, ME). The WT controls were designated as appropriate by Jackson staff and were previously used in sleep studies in our laboratory (7). Mice were housed individually in environmental chambers that were set on a 12:12-h light-dark (LD) cycle (lights on at 0800) and maintained at 29–30°C for sleep studies and 23°C for temperature-activity studies. Mice were given free access to food and water. All experimental protocols were approved by the Washington State University Animal Care and Use Committee and were in compliance with National Institutes of Health guidelines.
Influenza virus.
Studies employed a marginally lethal dose of mouse-adapted influenza X-31, a recombinant human strain comprised of six internal genes from A/Puerto Rico/8/34 (H1N1) and two surface protein genes from A/Aichi/2/68 (H3N2). In high doses the virus induces lethal pneumonitis in mice. Virus was purified from allantoic fluid of specific pathogen-free chicken embryos (SPAFAS, North Franklin, CT) by centrifugation through a sucrose gradient as described previously (7). The purified virus pellet was suspended in Dulbecco's phosphate buffered saline with Ca2+ and Mg2+ plus 0.2% bovine serum albumin (Sigma, St. Louis, MO) at a concentration of 200 μg/ml of viral protein, aliquoted and stored at −80°C. The heat-inactivated virus stock tested free of endotoxin and mycoplasma contamination. Viral titers were measured following 72-h incubation by endpoint dilution in Madin-Darby canine kidney cells cultured with residual trypsin from cell transfer and expressed in terms of median tissue culture infectious doses (TCID50) (7).
Viral infections.
Mice were inoculated intranasally using a 100 μl micropipette with 250 TCID50 X-31 in a volume of 50 μl/mouse (25 μl each nostril) under light methoxyflurane (Metofane, Pitman-Moore, Mundelein, IL) inhalation anesthesia. This dose of virus induces severe illness (48) but was sublethal for both mouse strains kept at 29–30°C. When mice were kept at 23°C ambient temperature, 4 of 12 infected mice died on day 8 during the 14-day observation period. All inoculations were performed within 1 h of light onset. Due to limited access to the TNF-2R KO mice, boiled virus controls were not used in these studies. However, past studies in our laboratory have shown that intranasal boiled virus produces no changes in any of the APR parameters studied in these experiments.
Surgeries.
For sleep studies, mice (n = 8 for TNF-2R KO and n = 10 for WT controls) were implanted with two electromyogram (EMG) electrodes and three EEG electrodes (Plastics One, Roanoke, VA) (17). Surgeries for electrode implantation used intraperitoneal ketamine-xylazine anesthesia (87 and 13 mg/kg, respectively). Electrodes for EEG recordings were placed over the frontal and parietal cortexes and over the cerebellum. Electrodes for EMGs were placed in the dorsal neck muscles. Following surgery, mice were allowed to recover for 7 days before sleep recording. Baseline wake (W), NREMS, and REMS were established by recording for 48 h before infection.
Surgical preparation for temperature and locomotor activity studies was performed using the same anesthesia in five TNF-2R KO and seven WT mice. Minimitter biotelemetry transmitters (Minimitter, Bend, OR) were chemically sterilized and implanted intraperitoneally. Mice were allowed to recover for at least 1 wk following surgeries.
Body temperature and locomotor activity analyses.
Body temperature and locomotor activity measurements were performed using biotelemetry as described in Ref. 48. Data were recorded at 6-min intervals, and data points representing those values were averaged over 2- or 6-h intervals for temperature and activity. The mice were kept at 23°C for these experiments because body temperature responses to influenza challenge are larger at this ambient temperature (25). Two mice from each strain died on day 8, and thus data obtained from days 8–14 are not graphed.
Sleep scoring.
EEG and EMG signals were digitized (128-Hz sampling rate) and stored on digital media. W, NREMS, and REMS were scored by hand in 10-s epochs by defining NREMS as high-amplitude EEG slow waves and low-tone muscle activity, REMS as highly regular theta EEG activity and loss of muscle tone with occasional twitches, and W as EEG activities similar to, but often less regular and with lower amplitude than, those in REMS and high-EMG activity. Time spent in each state was tabulated into 2-h intervals and graphed. In infected mice, sleep was analyzed over 24-h intervals before infection (baseline) or 1 day (virus 1), 2 days (virus 2), 4 days (virus 4), or 8 days (virus 8) following intranasal X-31 challenge (see Figs. 4 and 5). These scoring days were selected based on body temperature changes and previous studies in this model (48) and represent the asymptomatic infection prodrome (virus 1 and virus 2), the acute phase (virus 4), and the early recovery phase (virus 8) of the infection, respectively. An additional nonbiased automated computer-based scoring method (37) was used to determine duration of NREMS under baseline conditions. These data (Fig. 1, inset) are not further developed herein, but they did confirm the major results that the TNF-2R KOs had less NREMS than the WT strain under baseline conditions, and that both strains had more NREMS after viral challenge lasting up to 8 days postchallenge (see results).
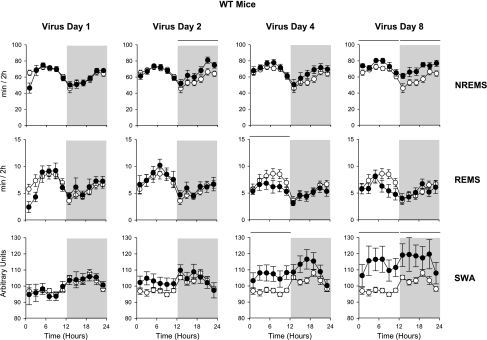
Fig. 4.
NREMS, REMS, and SWA of WT mice in response to virus infection. ○, Baseline day (uninfected); •, postinoculation days; horizontal bars at the top of the columns, significant difference between baseline and virus days (ANOVA, P < 0.005). See legend to Fig. 1 for details. The mice were intranasally challenged with influenza virus during the beginning of the light period on day 1 of the recording. Mice were then continuously recorded from for the next 8 days. Results from postchallenge days 1, 2, 4, and 8 are shown. By day 2 postinoculation, NREMS was elevated during the dark period although REMS was not affected at this time. By day 4, REMS decreased. On day 8, NREMS was increased during both the light and dark periods. EEG delta wave power after viral challenge increased on days 4 and 8, thereby confirming prior findings (25, 48).
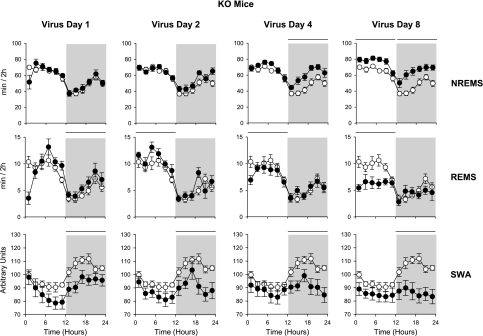
Fig. 5.
NREMS, REMS, and SWA of KO mice in response to virus infection. See legend to Fig. 4 for details. On day 8, NREMS was increased during both the light and dark periods, and REMS was reduced during the light period only. EEG SWA values, as in control mice, were higher during the dark period than during the light period. After influenza virus challenge, there was a rapid decrease in EEG delta wave power that began on day 1 and then persisted through postinoculation day 8. The daily light-dark differences in EEG delta power during NREMS were substantially less by day 8. The decrease EEG delta power response in the TNF-2R KO mice was in the opposite direction as that observed in the WT mice.
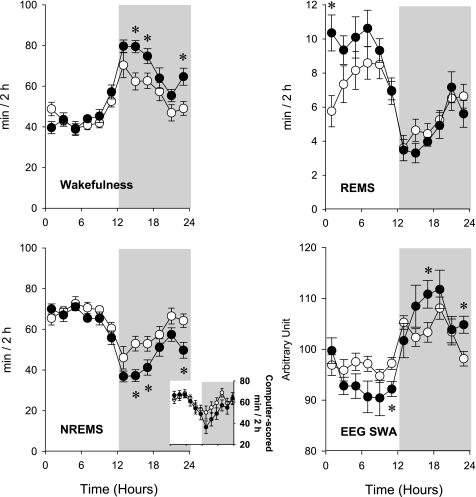
Fig. 1.
Spontaneous wakefulness, non-rapid eye movement sleep (NREMS), rapid eye movement sleep (REMS), and electroencephalogram (EEG) slow-wave activity during NREMS (SWA) of wild-type (WT; ○) and TNF-α double-receptor knockout (TNF-2R KO; •) mice. Inset: results from automated NREMS scoring. Data points represent 2-h averages. Error bars, SE; gray shaded area, dark phase of the day. Time 0 on the x-axis indicates light onset. The KO mice had less NREMS during the dark period and more REMS during the light period. EEG slow-wave activity baseline values were normalized to 100 for the 24-h period; values for individual 2-h time blocks are shown. EEG SWA during NREMS showed the typical enhanced values during the dark period and reduced values during the light period (43, 48). *Significant difference between WT and KO groups (univariate tests of significance for planned comparison, P < 0.05).
EEG delta (1/2–4 Hz) wave power analysis.
On-line fast Fourier transformation was performed on EEG data in 2-s epochs. Fast Fourier transformation spectral analysis generated power values from 0.25 to 63.5 Hz in 0.5-Hz bins that were integrated for 1-Hz bands. The power values were averaged for every 10 s. Mean power spectra between 1 and 20 Hz were obtained from 1.0-Hz power values in artifact-free, uninterrupted 10-s NREMS, REMS, or W epochs during each day and were averaged for each mouse. EEG slow-wave activity (SWA) during NREMS was calculated from these values and averaged over 2-h time blocks during baseline (Fig. 1) and after viral challenge (see Figs. 4 and 5). On the baseline day, EEG power spectra were calculated in 0.5-Hz bins in the 0.5- to 20-Hz frequency range for the entire 24-h period and also separately for the light and the dark periods. On day 8 after virus infection, EEG power spectra were computed for the entire 24-h period. EEG power in 0.5-Hz bins was expressed as percentage of total power.
mRNA analyses.
Real-time polymerase chain reaction (qPCR) was used to analyze levels of cortical mRNAs in uninfected mice of both strains for prolactin, orexin, period 1, period 2, cryptochrome 1, TNF-α, interleukin-1β (IL-1β), IFN-α-consensus sequence, 2′,5′-oligoadenlyate synthase 1a (OAS), TNF 55-kDa and TNF 75-kDa receptors, purine P2X7 and P2Y1 receptors, and nitric oxide synthases 1 and 2. These genes were chosen because each has been implicated in either sleep regulation or the immune response. Cortical tissue was chosen because it is abundant and there is evidence that a slow component (<1 Hz) of the EEG delta band originates within the cortex. For these studies six mice from each strain were killed between 0700 and 0900. RNA was extracted and cDNA prepared as previously described (3). Levels of specific RNAs were compared with cyclophilin A mRNA as previously described (3). Relative gene expression was calculated by a comparative threshold cycle method (3). Primers for factors other than for purine receptors were described previously (3, 6, 34, 47). The primers for the P2X7 and P2Y1 receptors were GCCAACTATGAACGGCTCTT (forward) and TACCCATGATTCCTCCCTGA (reverse) for the P2X7 receptor, and GGCAGGCTCAAGAAGAAGAA (forward) and AGATCAGCACCAAAGGGATG (reverse) for the P2Y1 receptor.
Statistics.
Statistical analysis was performed by using ANOVA (see Table 1 for details). One WT animal had artifacts in the EEG; therefore it was excluded from EEG SWA analysis (n = 9 for EEG SWA). Also, univariate tests of significance for planned comparison were performed a priori: 1) between the baseline day and the virus days within one genotype; 2) between the baseline days of the two genotypes; and 3) between the corresponding virus infection days of the two genotypes. For comparisons of mRNA levels, the two-tailed Student's t-tests were used. P values <0.05 were considered significant.
Table 1.
ANOVA performed on W, NREMS, REMS, and EEG SWA
| Analysis | Type of ANOVA | Independent Factor | Repeated Factor |
|---|---|---|---|
| Comparison of baseline W, NREMS, REMS, and EEG SWA between genotypes | 2-way ANOVA across 24 h on 2-h data blocks | Genotype | Time |
| Effects of virus infection on NREMS and REMS | 3-way ANOVA across 5 days on 12-h data blocks | Genotype | Light-dark, day |
| Sleep episode numbers and durations | 2-way ANOVA across 5 days on 24-h averages | Genotype | Day |
| Comparison of EEG power spectrum on the baseline day between genotypes | 3-way ANOVA | Genotype, frequency | Light-dark |
| Effect of virus infection on EEG power spectrum on virus day 8 | 3-way ANOVA | Genotype, frequency | Treatment |
| Comparison of baseline body temperature between genotypes | 2-way ANOVA across 24 h on 2-h data blocks | Genotype | Time |
| Effects of virus infection on body temperature and activity | 3-way ANOVA across 8 days on 6-h data blocks | Genotype | Time of the day, day |
W, wakefulness; NREMS, non-rapid eye movement sleep; REMS, rapid eye movement sleep; EEG, electroencephalogram; SWA, slow-wave activity.
RESULTS
Spontaneous sleep of WT mice compared with TNF-2R KO mice.
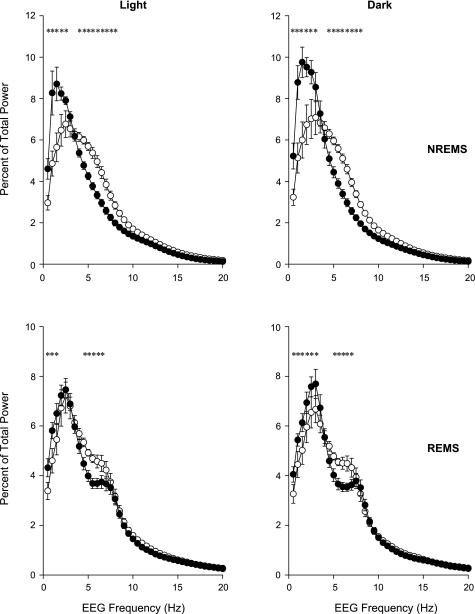
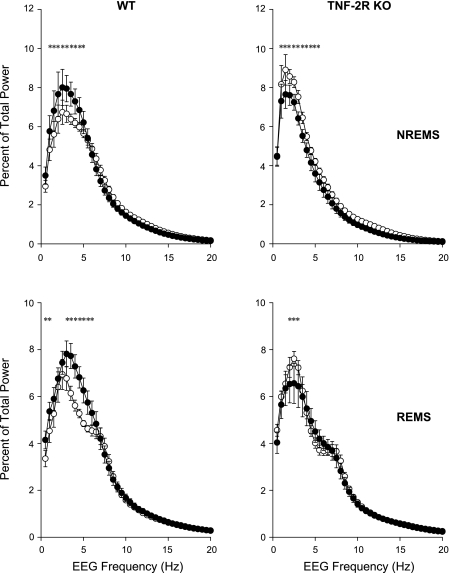
Durations of spontaneous NREMS and REMS in uninfected mice were greater during daylight hours and less during the night in both strains of mice (Fig. 1). Using 2-h time blocks and two-way ANOVA, the amounts of NREMS, REMS, and W and the circadian distribution of EEG SWA showed significant differences between the WT and KO animals [genotype × time interaction for NREMS: F(11,176) = 2.3, P < 0.05; REMS: F(11,176) = 3.1, P < 0.01; W: F(11,176) = 2.9, P < 0.005; SWA: F(11, 165) = 2.2, P < 0.05]. There was no difference in time spent in NREMS between WT mice or TNF-2R KO mice during the light period. In contrast, during the dark period, TNF-2R KO mice had less spontaneous NREMS than control animals. REMS was enhanced in the mutant TNF-2R KO mice during the light period compared with the WT mice. In the first 8 h of the light period, WT animals had 29.8 ± 3.4 min REMS vs. 40.4 ± 3.0 min in KOs (Student's t-test, P = 0.037). During the dark period, REMS did not differ between the two strains. Diurnal variation, as defined by the difference between sleep amounts in the 12-h light and 12-h dark periods, of NREMS and REMS in TNF-2R KO mice was about twice as large as in WT mice (diurnal variation of NREMS: 67.5 ± 16.0 min in WT vs. 121.1 ± 6.4 min in KO animals, Student's t-test P = 0.011; REMS: 14.2 ± 4.2 in WT vs. 28.3 ± 3.1 min in KO, Student's t-test P = 0.019). EEG SWA in both genotypes showed characteristic diurnal rhythm [SWA time effect: F(11,165) = 11.8, P < 0.001]; the amplitude of this rhythm was significantly enhanced in KO mice (Fig. 1). There were also differences in the distribution of the fraction of EEG power during NREMS [genotype × frequency interaction NREMS: F(39,600) = 8.4, P < 0.001; (Fig. 2)]. The TNF-2R KO mice had a greater concentration of EEG power in the lower frequency bands compared with the WT mice. These differences were evident during both the light and dark hours [genotype × frequency × light-dark interaction: F(39,600) = 0.9, not significant (NS)]. (Fig. 2). Similar, although less robust, differences were observed in power spectra of the two genotypes during REMS [genotype × frequency interaction: F(39,600) = 2.1, P < 0.001] (Fig. 2). On the baseline day, the TNF-2R KO mice had longer waking episodes than did the WT mice (Table 2). The TNF-2R KO mice also had fewer and shorter NREMS episodes, although the difference did not reach the level of significance, which accounts for the reduced duration of NREMS on baseline days (Table 2).
Fig. 2.
EEG power spectra in uninfected mice during NREMS (top) and REMS (bottom) separated into light (left) and dark (right) periods. ○, WT mice; •, TNF-2R KO mice; error bars, SE. *Significant difference between WT and KO groups (univariate tests of significance for planned comparison, P < 0.05).
Table 2.
Average episode numbers and durations of W, NREMS, and REMS on the baseline day and on days after virus infection in WT and TNF-2R KO mice
| Baseline | Virus 1 | Virus 2 | Virus 4 | Virus 8 | |
|---|---|---|---|---|---|
| Sleep episode numbers | |||||
| W | |||||
| WT | 6.38±0.33 | 5.83±0.40 | 6.17±0.42 | 8.34±0.98* | 8.15±0.76 |
| KO | 5.49±0.34 | 5.44±0.41 | 6.64±0.71* | 8.03±0.89* | 10.46±1.45* |
| NREMS | |||||
| WT | 11.19±0.70 | 10.49±0.44 | 12.41±0.98 | 14.55±1.23* | 15.64±0.83* |
| KO | 10.79±0.79 | 10.82±0.57 | 13.17±1.20* | 15.01±1.33* | 16.31±1.17* |
| REMS | |||||
| WT | 2.32±0.18 | 2.27±0.20 | 2.52±0.22 | 2.05±0.22 | 2.06±0.25 |
| KO | 2.50±0.15 | 2.61±0.21 | 2.86±0.19* | 2.36±0.18 | 1.63±0.20* |
| Sleep episode durations | |||||
| W | |||||
| WT | 5.16±0.36 | 6.45±0.69 | 5.19±0.67 | 4.67±0.92 | 3.28±0.33 |
| KO | 7.82±0.65† | 7.85±1.02 | 5.84±1.12* | 4.45±0.74* | 3.97±1.76* |
| NREMS | |||||
| WT | 3.17±0.17 | 3.29±0.15 | 3.01±0.15 | 2.60±0.21* | 2.52±0.14* |
| KO | 2.91±0.23 | 2.88±0.21 | 2.58±0.26* | 2.49±0.28* | 2.37±0.25* |
| REMS | |||||
| WT | 1.41±0.06 | 1.49±0.05 | 1.37±0.07 | 1.35±0.09 | 1.35±0.11 |
| KO | 1.48±0.04 | 1.55±0.05 | 1.49±0.03 | 1.49±0.03 | 1.72±0.07* |
Values are means ± SE. Sleep episode numbers are hourly average numbers, and sleep episode durations are average durations during the entire 24-h recording periods. Postchallenge days: virus 1 and virus 2, asymptomatic infection prodrome on days 1 and 2; virus 4, acute phase of infection on day 4; virus 8, early recovery phase of infection on day 8.
Significant difference from baseline;
significant difference between wild-type (WT) and TNF-α double-receptor (TNF-2R) knockout (KO) (univariate tests of significance for planned comparisons, P < 0.05).
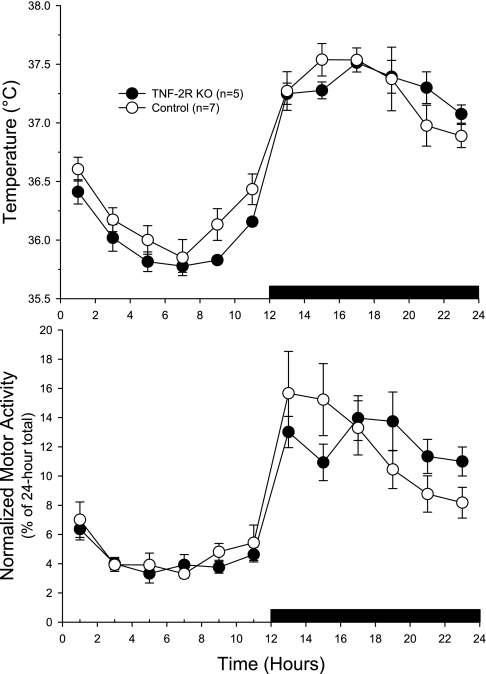
Baseline temperatures did not differ between the two strains of mice; both strains had higher body temperatures during the dark period and when they were more active than during the light period (Fig. 3) [genotype effect: F(1,10) = 0.8, NS; genotype × time interaction: F(11,110) = 1.1, NS]. Similarly, the normalized locomotor activity, expressed as a percentage of the total 24-h activity, was not different between strains (Fig. 3). Both strains demonstrated the greatest activity level just after the lights went out, and minimal activity was seen during the early hours of the light period.
Fig. 3.
Baseline body temperatures (top) and baseline locomotor activity (bottom) of WT (○) and TNF-2R KO mice (•). Values shown were obtained from 2 days of recording and averaged for each time bin (±SE). There were no significant differences between the strains for either temperature or activity. Black horizontal bar, dark period.
Sleep responses of X-31-infected WT mice compared with infected TNF-2R KO mice.
Using 12-h time blocks and three-way ANOVA, there was no significant genotype effect [F(1,16) = 3.0, NS] on NREMS but the genotype × LD interaction [F(1,16) = 12.2, P < 0.005] indicates that there were significant differences in NREMS between the two genotypes depending on the phase of the day. Univariate tests for planned comparisons revealed that TNF-2R KO mice had decreased NREMS during the dark compared with WT mice [F(1,16) = 6.8, P < 0.05] when data from all recording days were collapsed; daily comparisons confirmed that this difference was due to decreased NREMS on the baseline and the first two post-viral inoculation days. Viral infection caused increases in NREMS over the course of all recording days (Figs. 4 and 5) [day effect: F(4,64) = 14.0, P < 0.001]; these effects were not different in the WT and TNF-2R KO genotypes as indicated by the nonsignificant day × genotype interaction [F(4,64) = 0.7] but were dependent on the phase of the day [day × LD interaction: F(4,64) = 79.2, P < 0.001]. NREMS was significantly above baseline levels in the dark phase of the second and the fourth postinoculation days in WT and TNF-2R KO animals, respectively. On postinoculation day 8, NREMS was elevated for the entire 24-h period in both genotypes.
There was no general genotype effect on REMS [F(1,16) = 1.2, NS], but the genotype × LD interaction [F(1,16) = 38.9, P < 0.005] shows that there were significant differences between the two genotypes, depending on the light-dark phase (Figs. 4 and 5). Univariate tests for planned comparisons indicate that TNF-2R KO mice, in general, had more REMS than WT mice in the light phase [F(1,16) = 4.8, P < 0.05]; daily comparisons showed that the differences were significant in the light periods of postinoculation days 1, 2, and 4. Virus treatment led to an overall decrease in REMS [day effect: F(4,64) = 16.5, P < 0.001] equally in both genotypes [day × genotype interaction: F(4,64) = 1.6, NS]. The effect of virus depended on the phase of the day [day × LD interaction: F(4,64) = 7.4, P < 0.001]. In WT mice, REMS was significantly suppressed below baseline during the light period of postinoculation day 4. TNF-2R KO animals showed biphasic REMS responses to the infection. There was a slight but significant increase in the dark phase of the day of virus treatment and the subsequent light period. On postinoculation days 4 and 8, REMS was suppressed during the light phases.
The two genotypes showed strikingly different EEG SWA responses to virus infection [genotype effect: F(1,15) = 12.6, P < 0.005]. In WT mice EEG SWA during NREMS was elevated on postinoculation days 4 and 8, while in the TNF-2R KO animals EEG SWA during NREMS was suppressed throughout the entire course of the virus infection (Figs. 4 and 5). The distribution of EEG power across frequency bands (Fig. 6) was also different between the strains on postinoculation day 8 [day × genotype interaction: F(1,560) = 19.6, P < 0.001]. In the TNF-2R KO mice the virus-induced reductions in EEG power were mostly confined to the frequency range of 2–5 Hz, and this was evident during both the light and dark periods (data not shown). In the control mice, the virus-induced increases in EEG power were evident in the same range of frequencies (Fig. 6). During REMS, the EEG power spectra were also, but differently, affected in the two genotypes [day × genotype interaction: F(1,560) = 36.4, P < 0.001]. In the control mice there was an increase in EEG power during REMS in the 0.5- to 1.5-Hz and 3- to 6.5-Hz frequency ranges, while in KO animals EEG power was decreased in the 2- to 3.5-Hz band.
Fig. 6.
EEG power spectra during NREMS and REMS on the baseline day (○) and day 8 post-viral inoculation (•). *Significant difference between baseline and postinoculation (univariate tests of significance for planned comparison, P < 0.05). Error bars, SE.
After viral challenge, the number of NREMS episodes increased in both strains of mice, although the effect tended to be larger in the TNF-2R KO mice (Table 2). In contrast, the duration of NREMS episodes decreased. The number of REMS episodes decreased and the average episode duration increased in compared with baseline in the TNF-2R KO mice on the last day of recording (Table 2).
Body temperature and locomotor activity responses to viral challenge.
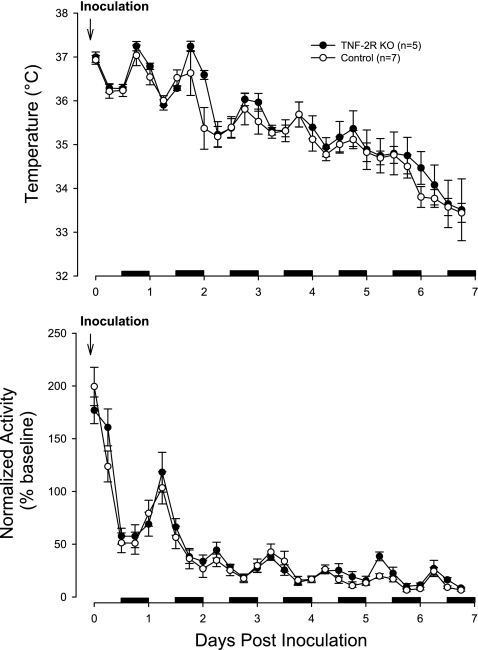
Of the five TNF-2R KO and seven WT mice used for the temperature/activity study, two of each strain died on day 8 of the recording. The body temperature responses to the viral challenge were not different between the two strains [Fig. 7; genotype effect: F(1,10) = 0.6, NS; genotype × day interaction: F(7,70) = 0.3, NS; day effect: F(7,70) = 61.1, P < 0.001]. By the end of day 2, both strains showed reduced body temperatures. From day 2 until day 7, temperatures continued to decline (Fig. 7). Body temperatures of the mice that survived began to increase on day 8 and continued back toward normal until the end of the recording (day 14) (data not shown). The locomotor activity responses to the viral challenge were more rapid in onset than the temperature responses in both strains of mice [genotype effect: F(1,10) = 1.7, NS; genotype × day interaction: F(7,70) = 0.2, NS; day effect: F(7,70) = 86.4, P < 0.001]. Initially, both strains showed an increase in activity after the intranasal inoculation of virus that lasted ∼4 h (Fig. 7). By the end of the light period on the day they were inoculated, both strains had reduced activity compared with their baseline values. These reductions continued for several more days in both strains. Both strains showed increased activity from days 11 until the end of the recording, although there was a great deal of variation in this response between mice (data not shown).
Fig. 7.
Temperature (top) and locomotor activity responses to virus challenge. Baseline recordings were obtained for 48 h (data not shown), and then mice were challenged on the morning of day 1. WT mice (○) and TNF-2R KO mice (•) responded with a reduction in body temperature. The control mice appeared to respond earlier with reductions in body temperature evident during the dark period of day 2 whereas the mutant mice did not respond until the light period of day 3 post-viral challenge. After day 2 postchallenge, the control mice showed a larger reduction in body temperature. Activity was normalized to baseline values taken from each strain for each 6-h time bin. After the viral challenge, activity increased for ∼6 h and then it decreased below baseline values in both strains for the next 10 days. On day 11, the mutant mice, but not the control mice, began to recover. Both strains showed increased activity during days 12–16 post-virus challenge. Black horizontal bars, dark periods.
Virus-associated mortality.
Mortality (4/12) was seen in influenza-infected mice (2 WT and 2 TNF-2R KOs) maintained at 23°C but not in animals maintained at 29–30°C (n = 16). These data are consistent with the observation that influenza-infected mice demonstrate more slow wave sleep and amelioration of illness at 30°C compared with mice maintained at 22°C (25).
mRNA expression.
Most of the mRNA species examined in the cortex did not significantly differ in their relative levels from each other in the two strains of mice used (Table 3). As expected, the TNF 55-kDa and 75-kDa receptor mRNAs were very low in the mutant mice, suggesting we reached the limits of detection. The purine P2X7 receptor mRNA was reduced by 31% in the TNF-2R KO mice compared with WT mice (P < 0.01). In contrast, cortical orexin expression was ∼30% higher in the TNF-2R KO mice than in the WT mice (P < 0.02). Although IL-1β was ∼45% higher in the mutant mice compared with the WT mice, this increase was only a trend (P < 0.08).
Table 3.
Expression of mRNA for various peptides in the cortex
| WT | TNF-2R KO | |
|---|---|---|
| Prolactin | 1.01±0.23 | 1.11±0.08 |
| Orexin | 1.01±0.06 | 1.32±0.09* |
| OAS | 1.02±0.12 | 1.58±0.32 |
| TNFR 1 | 1.01±0.06 | 0.31±0.03* |
| TNFR 2 | 1.01±0.08 | 0.22±0.03* |
| NOS 1 | 1.06±0.18 | 1.34±0.11 |
| NOS 2 | 1.02±0.09 | 1.20±0.13 |
| mPER 1 | 1.04±0.12 | 1.14±0.11 |
| mPER 2 | 1.04±0.13 | 1.22±0.21 |
| Cry 1 | 1.05±0.14 | 1.18±0.07 |
| TNF-α | 1.02±0.12 | 1.37±0.42 |
| IL-1β | 1.03±0.12 | 1.46±0.18 |
| IFNacon | 1.03±0.10 | 1.20±0.14 |
| P2X7 | 1.00±0.07 | 0.70±0.04* |
| P2Y1 | 1.00±0.04 | 0.84±0.08 |
Values are means ± SE. mRNA levels are normalized to and expressed as fold difference from WT values. OAS, 2′,5′-oligoadenylate synthase 1a; TNFR1 and TNFR2, TNF receptors 1 and 2; NOS1 and NOS2, nitric oxide synthase 1 and 2; Cry 1, cryptochrome 1; IFNacon, IFN-α-consensus sequence.
Significant difference between WT and TNF-2R KO (Student's t-test, P < 0.05).
DISCUSSION
The present results indicate that the mice lacking both TNF-α receptors have less spontaneous NREMS than controls; similar results were obtained whether EEG recordings were scored by an experienced sleep researcher or by using a computer program. That spontaneous NREMS results were similar by these two methods is important because there are differences between the present and prior work. The present results are partially consistent with the previous report that mice lacking the TNF 55-kDa receptor had less NREMS during the last 4 h of night hours and during the first 8 h of daylight (17). However, the deficits in NREMS in the TNF-2R KO mice occurred predominantly during the dark period. We do not know the reason for the differences in the timing of the NREMS deficits between the single- and double-receptor knockout strains. TNF signaling is complex, involving multiple adaptor proteins for both receptors (reviewed in Ref. 24). The dynamics of the interactions of the adaptor proteins with the TNF 75-kDa receptor in the 55-kDa receptor KO mice may have been different from that of a normal mouse and absent in the TNF-2R KO mice. Further, the TNF receptor may itself act as a ligand and the 26-kDa transmembrane form of TNF as a receptor (15). The role that the soluble TNF receptor plays in sleep is also unknown although it is a normal constituent of cerebrospinal fluid (36) and has the capacity to reduce spontaneous NREMS in rats, rabbits, and humans (reviewed in Ref. 28). The soluble receptors would be absent in the TNF-2R KO mice since they are normally shed from the membrane-bound receptors. Regardless, their role in sleep regulation remains unknown although intuitively one might anticipate that they would have the opposite action as the membrane-bound forms of the receptors.
There were also differences between the single- and double-TNF receptor knockouts in the amount of REMS each strain had relative to their controls. The TNF 55-kDa receptor KO mice had less REMS than controls and this occurred at the same time of day as the NREMS deficits (17). In contrast, the TNF-2R KO mice had more REMS during the first 8 h of daylight compared with controls. Why the loss of one vs. two receptors would give the opposite result is unknown especially since mice injected with doses of TNF-α that promote NREMS have normal REMS duration (17). Another of the present findings further confounds the REMS observations: in the cortex orexin mRNA levels were enhanced in the TNF-2R KO mice. The absence of orexin is responsible for narcolepsy, a disease characterized by rapid onset of REMS.
Both of the studies with single (17) and double (present study) TNF receptor KO mice differ substantially from results reported by Ref. 14. Using TNF 55-kDa receptor KO mice, that group failed to get significant differences in baseline sleep between the mutant mice and controls. However, there were several problems with that study. They failed to demonstrate either genotypically or phenotypically that the mice from which sleep recordings were taken lacked the indicated gene. They also compared sleep of the knockout mice to control mice obtained from a different source and did not indicate if the mice were male or female. Both of these parameters affect sleep (e.g., see Refs. 17 and 27). Further, they did not specify the ambient temperature at which the mice were kept. TNF is involved in thermoregulation (10) and ambient temperatures affect the duration of both NREMS and REMS (25, 39). Despite these shortcomings there was a hint in their data that the TNF 55-kDa receptor knockout mice had less NREMS during the first 4 h of the dark period. That result would be consistent with the results published here for the double-receptor knockouts but not with the previously published results with the TNF 55-kDa KO mice (17). However, due to the complexity of TNF signaling, predictions as to the direction of an effect in animals lacking one of the components of the TNF system are not obvious. Nevertheless, there is a relatively large literature in support of the hypothesis that TNF is involved in the regulation of physiological sleep and in the sleep disturbances associated with pathologies. The present results are consistent with this hypothesis to the extent that spontaneous sleep was altered in the mice lacking both TNF receptors.
EEG SWA during NREMS was substantially different in the TNF-2R KO mice than in the WT mice during baseline conditions and in response to influenza viral challenge. During baseline conditions the TNF-2R KO mice tended to have much more pronounced day/night differences in their EEG SWA during NREMS, and their EEG power spectra were different from those observed in the WT mice. The mechanisms that determine EEG SWA during day and night are unknown. Our data suggest that intact TNF signaling may be required for the normal diurnal distribution of EEG SWA. Further, after viral challenge, the TNF-2R KO mice decreased EEG SWA while the WT mice increased EEG SWA during NREMS. The results from the WT mice replicate earlier findings (25). Results from the KO mice clearly indicate a potential role for TNF-α in this sleep phenotype. However, the relationships between TNF-α and EEG SWA are complex. Thus intracerebroventricular, intravenous (41), or intrapreoptic (29) injection of TNF-α enhances EEG SWA during NREMS. Further, direct unilateral application of TNF-α onto the surface of the cortex enhances EEG SWA during NREMS ipsilaterally (49). In contrast intraperitoneal injection of TNF-α in WT mice inhibits EEG SWA during NREMS, and this effect was absent in TNF 55-kDa receptor KO mice (17). Inhibitors of TNF-α also affect EEG SWA, but these results also suggest complex relationships between TNF-α and EEG SWA. Thus unilateral application of a TNF-α siRNA to the cortex ipsilaterally inhibits EEG SWA during NREMS but not during waking or REMS (44). Similarly, application of the TNF soluble receptor to the surface of the cortex attenuates sleep deprivation-enhanced EEG SWA during NREMS (49). In contrast, the TNF soluble receptor did not have an effect on EEG SWA if microinjected directly into the preoptic anterior hypothalamus (29). It seems likely that these differences are due to the multiple actions of TNF-α. Some of these actions are known to affect EEG SWA, e.g., blood flow (21). Further, TNF-α enhances production of downstream molecules such as nitric oxide and adenosine, both of which affect EEG SWA and cerebral blood flow.
Although TNF-α clearly affects EEG SWA during NREMS, the relationship of EEG SWA to sleep is anything but clear despite the fact that EEG SWA is the parameter used to model process S in the two-process model of sleep. As a model parameter, EEG SWA works remarkably well for modeling spontaneous sleep or homeostatic sleep responses to sleep loss. Briefly, increasing SWA in the dark period in nocturnal species correlates with increased sleep pressure, which is due to the relative sleep loss during the behaviorally active phase. In the light (predominantly sleep) phase, declining SWA correlates with the gradual discharge of this sleep pressure. Nevertheless, there is a substantial literature indicating that this EEG phenotype is also regulated independently from NREMS. There is a growing body of evidence indicating that multiple factors affect EEG delta power, e.g., blood flow, age, ventilation, etc., all somewhat independently of sleep. Pharmacological studies also suggest that the regulatory events responsible for NREMS are separate from those of EEG SWA. Thus systemic atropine induces high-amplitude EEG slow waves without affecting state (4, 40). Conversely, benzodiazepines enhance NREMS but inhibit EEG SWA (2). These suggest that changes in EEG SWA in response to pharmacological manipulations (or viral inoculation) have limited meaning about the intensity/quality of NREMS. The present data clearly show that in TNF-2R KO mice the direction of change in EEG SWA during NREMS induced by the virus is opposite to that observed in WT mice, while both strains increase their duration of NREMS. These findings are consistent with prior data indicating separate control mechanisms. For instance, if neonatal rabbits are given muramyl dipeptide, a substance that promotes both NREMS and EEG SWA in adults, only NREMS is enhanced (11). Similarly, if rats are given a cafeteria diet, they exhibit excessive NREMS during the days they are on the diet; in contrast during that time their EEG SWA values are reduced (19). If basal forebrain cholinergic neurons are lesioned using 192 IgG-saporin, there is a permanent reduction of EEG SWA without much long-term effect on duration of NREMS (26).
The minor differences in the sleep duration responses to virus were unexpected because previously TNF-α was implicated in responses to virus (see below), and we had shown in a variety of other KO models that removal of a gene can indeed alter sleep responses to virus. For example, mice lacking a functional growth hormone-releasing hormone sleep less after influenza viral challenge rather than more (1). Similarly, mice lacking either neural or inducible nitric oxide synthase exhibit a reduced duration of NREMS response to viral challenge (7). In that same study, both knockout strains had a reduced EEG SWA response to virus, and that response is consistent with the results reported here with the TNF-2R KO mice. The above models employed a lethal dose of a more virulent influenza strain, and the kinetics of the response were different than in the model used in the present study. In a study of mice lacking the type I interferon receptor (IFN-RI KOs) and using a X-31 dose comparable to that employed in the present study, we also observed blunted duration of NREMS responses in the KO mice later in the infection (48). Those IFN-RI KO mice had elevated increases in EEG SWA compared with controls after viral challenge (48). Although those responses were distinct from what is reported here, they again emphasize that independent mechanisms are regulating duration of state and EEG delta power.
TNF-α is produced in substantial quantities in influenza-infected tissue of mice given high doses of virus (9, 20). Regardless of viral dose, influenza consistently induces hypothermia in mice (9, 16, 46, 48), and TNF-α is thought to play a role in the induction of hypothermia (30). However, the absence of the two recognized receptors for TNF-α has a minimal effect on the hypothermic response to low dose X-31 (Fig. 7); the double receptor KO mice display a slight delay in the onset of hypothermia, but once hypothermia is initiated, the KO and WT responses are the same. This minimal effect on hypothermia contrasts with the substantial effect of mutating the type I interferon receptor (IFN-RI) in the same model (48). It also contrasts with the role of TNF-α in acute-phase responses to bacterial products (30).
Reduced locomotor activity is also consistently seen in acute influenza infections (9, 46). Locomotor activity levels were also suppressed in the X-31-infected mice in this study, but no differences were seen between the WT and KO strains with respect to this acute-phase parameter (Fig. 7). These data suggest that TNF-α plays little, if any, role in regulation of locomotor activity in response to influenza virus.
One important difference between these studies in TNF-2R KOs and our previous studies with the same infection model in IFN-RI KOs is the strain of mouse employed. The IFN-RI KO studies employed 129 SvEv mice (47, 48), which we have shown to be more susceptible to X-31 than C57BL/6 mice (unpublished data). The B6;129S F2/J WT mice used in these TNF-2R KO studies appeared less ill with double the dose of virus used in the 129 SvEv WT mice in the previous study (48). We doubled the dose of virus because we anticipated that the C57BL/6 component of the genome of both the WT and KO strains employed in these studies would increase resistance to the virus, and that appeared to be the case. It is possible that yet a higher dose of X-31 virus would have resulted in more separation between the non-SWA sleep parameters in the WT and KO mice used in this study, and that the EEG SWA parameter is more sensitive to the virus dose employed than the other sleep parameters.
Another insight into the role of TNF-α in viral disease is the analysis of disease parameters in humans expressing different TNF-α polymorphisms. Such studies reveal a role for TNF-α in susceptibility to chronic hepatitis infections (12), but only two studies have been performed in acute viral infections similar to influenza. These studies [one in infants infected with respiratory syncytial virus (RSV) (22) and the other in adults infected with RSV (23)] both demonstrated that TNF-α polymorphisms do not influence acute disease parameters. In adults, however, TNF-α polymorphisms do affect antibody levels after recovery (23). Thus the limited data available suggest that TNF-α plays a role in viral acquired immunity but not in viral disease.
The mRNA analyses failed to demonstrate many changes in gene expression. This may have been due to the time of day the mice were killed. When these experiments were conducted we did not know the results of the sleep studies and thus the mice were killed at a convenient time during daylight hours. As already mentioned, the increase in orexin mRNA was unexpected because during the daytime REMS duration increased in the TNF-2R KO mice above the WT level. We investigated the purine P2X7 mRNA because this receptor is involved in TNF release from glia induced by ATP (31). There is a large rich literature demonstrating co-release of ATP with neurotransmitters (reviewed in Ref. 5). There is an independent large literature demonstrating ATP-enhanced release of cytokines from immunocytes via purine P2 receptors. Those literatures led us to posit that ATP released during neurotransmission and the associated release of glial TNF was a mechanism by which the brain kept track of prior activity (28). We also recently showed that P2X7 mRNA levels have a distinct diurnal variation with highest levels occurring between daytime and nighttime (45). That the spontaneous levels of P2X7 mRNA were lower in the TNF-2R KO mice is consistent with the idea that these receptors are involved in the TNF-sleep-related mechanisms. Regardless of such speculation, further studies are needed before solid conclusions related to causal mechanisms are firm.
GRANTS
This work was supported, in part, by National Institutes of Health Grants NS-31453 and HD-36520.
Acknowledgments
We thank Richard Brown for help with these experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alt JA, Bohnet S, Taishi P, Duricka D, Obal F Jr, Traynor T, Majde JA, Krueger JM. Influenza virus-induced glucocorticoid and hypothalamic and lung cytokine mRNA responses in dwarf lit/lit mice. Brain Behav Immun 21: 60–67, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bastien CH, LeBlanc M, Carrier J, Morin CM. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep 26: 313–317, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bohnet SG, Traynor TR, Majde JA, Kacsoh B, Krueger JM. Mice deficient in the interferon type I receptor have reduced REM sleep and altered hypothalamic hypocretin, prolactin and 2′,5′-oligoadenylate synthase expression. Brain Res 1027: 117–125, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bringmann A Topographic mapping of the cortical EEG power in the unrestrained rat: peripheral effects of neuroactive drugs. Arch Ital Biol 133: 1–16, 1995. [PubMed] [Google Scholar]
- 5.Burnstock G Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci USA 104: 12843–12848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Duricka D, Nelson S, Mukherjee S, Bohnet SG, Taishi P, Majde JA, Krueger JM. Influenza virus-induced sleep responses in mice with targeted disruptions in neuronal or inducible nitric oxide synthases. J Appl Physiol 97: 17–28, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Churchill L, Rector D, Yasuda K, Rojas MJ, Schactler S, Fix C, Hall S, Yasuda T, Krueger JM. Tumor necrosis factor α increases surface evoked potentials in the barrel field by whisker deflection during sleep in rats. Sleep 29: A12–A13, 2006. [Google Scholar]
- 9.Conn CA, McClellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ. Cytokines and the acute phase response to influenza virus in mice. Am J Physiol Regul Integr Comp Physiol 268: R78–R84, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci 9: 1433–1449, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Davenne D, Krueger JM. Enhancement of quiet sleep in rabbit neonates by muramyl dipeptide. Am J Physiol Regul Integr Comp Physiol 253: R646–R654, 1987. [DOI] [PubMed] [Google Scholar]
- 12.de Andrade DR Jr, de Andrade DR. The influence of the human genome on chronic viral hepatitis outcome. Rev Inst Med Trop Sao Paulo 46: 119–126, 2004. [PubMed] [Google Scholar]
- 13.De Sarro G, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci 60: 555–564, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Deboer T, Fontana A, Tobler I. Tumor necrosis factor (TNF) ligand and TNF receptor deficiency affects sleep and the sleep EEG. J Neurophysiol 88: 839–846, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev 15: 353–366, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc Soc Exp Biol Med 210: 242–252, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNF alpha treatment. J Neurosci 17: 5949–5955, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fix C, Churchill L, Hall S, Kirkpatrick R, Yasuda T, Krueger JM. The number of tumor necrosis factor α-immunoreactive cells increases in layer IV of the barrel field in response to whisker deflection in rats. Sleep 29: A11, 2006. [Google Scholar]
- 19.Hansen MK, Kapás L, Fang J, Krueger JM. Cafeteria diet-induced sleep is blocked by subdiaphragmatic vagotomy in rats. Am J Physiol Regul Integr Comp Physiol 274: R168–R174, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hennet T, Ziltener HJ, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol 149: 932–939, 1992. [PubMed] [Google Scholar]
- 21.Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. J Neurosci 17: 4800–4808, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentile DA, Doyle WJ, Zeevi A, Howe-Adams J, Kapadia S, Trecki J, Skoner DP. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum Immunol 64: 338–344, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Gentile DA, Doyle WJ, Zeevi A, Piltcher O, Skoner DP. Cytokine gene polymorphisms moderate responses to respiratory syncytial virus in adults. Hum Immunol 64: 93–98, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T. Tumor necrosis factor receptor-associated factor (TRAF) family: Adapter proteins that mediate cytokine signaling. Exp Cell Res 254: 14–24, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Jhaveri KA, Trammell RA, Toth LA. Effect of environmental temperature on sleep, locomotor activity, core body temperature and immune responses of C57BL/6J mice. Brain Behav Immun 21: 975–987, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapás L, Obal F, Book AA, Schweitzer B, Wiley RG, Krueger JM. The effects of immunolesions of nerve growth factor-receptive neurons by 192 IgG-saporin on sleep. Brain Res 712: 53–59, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29: 1224–1231, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clin 2: 161–169, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res 932: 37–44, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Leon LR Hypothermia in systemic inflammation: role of cytokines. Front Biosci 9: 1877–1888, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Lister M, Sharkey J, Sawatzky D, Hodgkiss J, Davidson D, Rossi A, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm 4: 5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majde JA, Bohnet SG, Ellis GA, Churchill L, Leyva-Grado V, Wu M, Szentirmai E, Rehman A, Krueger JM. Detection of mouse-adapted human influenza virus in the olfactory bulb of mice within hours after intranasal infection. J Neurovirol 13: 399–409, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmacher T. Dose-dependent effects of endotoxin on human sleep. Am J Physiol Regul Integr Comp Physiol 278: R947–R955, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Obal F Jr, Garcia-Garcia F, Kacsoh B, Taishi P, Bohnet S, Horseman ND, Krueger JM. Rapid eye movement sleep is reduced in prolactin-deficient mice. J Neurosci 25: 10282–10289, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol 160: 943–952, 1998. [PubMed] [Google Scholar]
- 36.Puccioni-Sohler M, Rieckmann P, Kitze B, Lange P, Albrecht M, Felgenhauer K. A soluble form of tumor necrosis factor receptor in cerebrospinal fluid and serum of HTLV-I-associated myelopathy and other neurological diseases. J Neurol 242: 239–242, 1995. [DOI] [PubMed] [Google Scholar]
- 37.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res 1047: 45–55, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Riha RL, Brander P, Vennelle M, McArdle N, Kerr SM, Anderson NH, Douglas NJ. Tumour necrosis factor-α (−308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J 26: 673–678, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Roussel B, Turrillot P, Kitahama K. Effect of ambient temperature on the sleep-waking cycle in two strains of mice. Brain Res 294: 67–73, 1984. [DOI] [PubMed] [Google Scholar]
- 40.Schaul N, Gloor P, Ball G, Gotman J. The electromicrophysiology of delta waves induced by systemic atropine. Brain Res 143: 475–486, 1978. [DOI] [PubMed] [Google Scholar]
- 41.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol Regul Integr Comp Physiol 253: R142–R149, 1987. [DOI] [PubMed] [Google Scholar]
- 42.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor α gene variant and phenotypes associated with the metabolic syndrome. Obesity Res 13: 2122–2131, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. Spontaneous sleep and homeostatic sleep regulation in ghrelin knockout mice. Am J Physiol Regul Integr Comp Physiol 293: R510–R517, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Taishi P, Churchill L, Wang M, Kay D, Davis CJ, Guan X, De A, Yasuda T, Liao F, Krueger JM. TNF alpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res 1156: 125–132, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taishi P, Urza M, Jimenez L, Krueger JM. Diurnal variations in purine P2X7 and P2Y1 receptors correlate with sleep wake cycle in the cortex. Sleep 31: A363, 2008. [Google Scholar]
- 46.Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol 58: 89–99, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Traynor TR, Majde JA, Bohnet SG, Krueger JM. Sleep and body temperature responses in an acute viral infection model are altered in interferon type I receptor-deficient mice. Brain Behav Immun 20: 290–299, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Traynor TR, Majde JA, Bohnet SG, Krueger JM. Interferon type I receptor-deficient mice have altered disease symptoms in response to influenza virus. Brain Behav Immun 21: 311–322, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNF alpha. Brain Res 1009: 129–136, 2004. [DOI] [PubMed] [Google Scholar]