Abstract
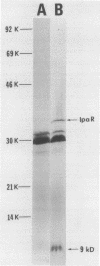
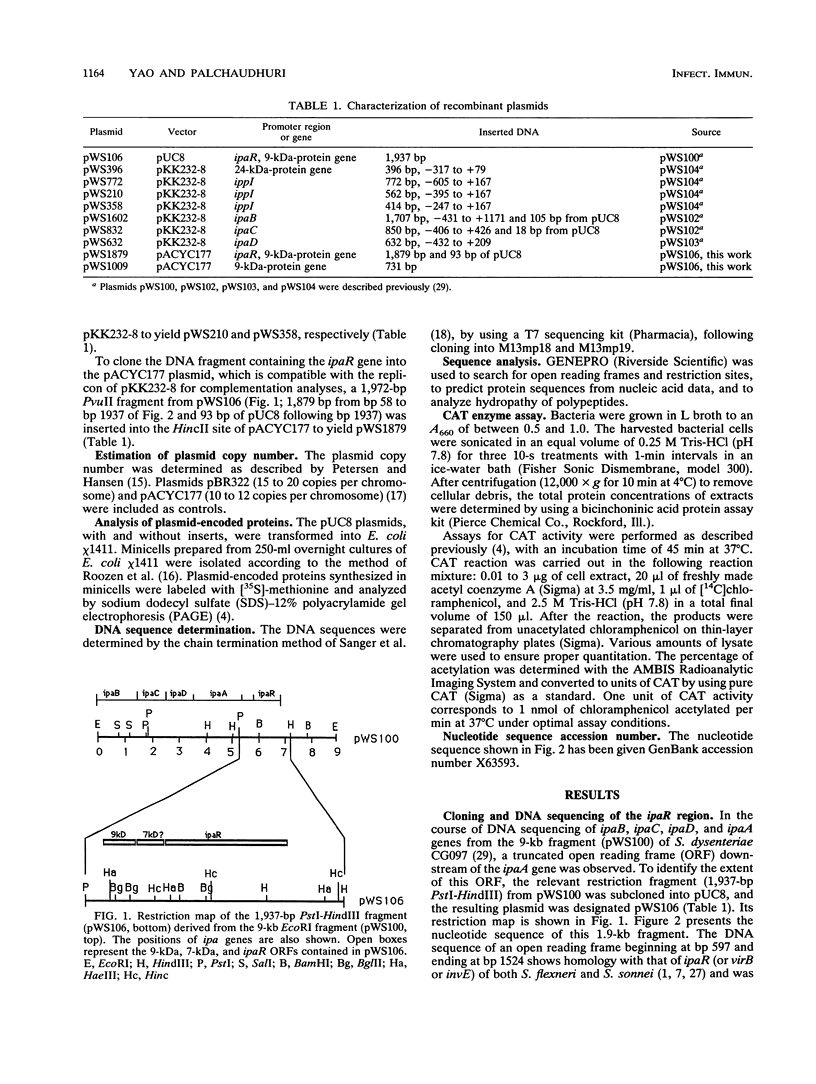
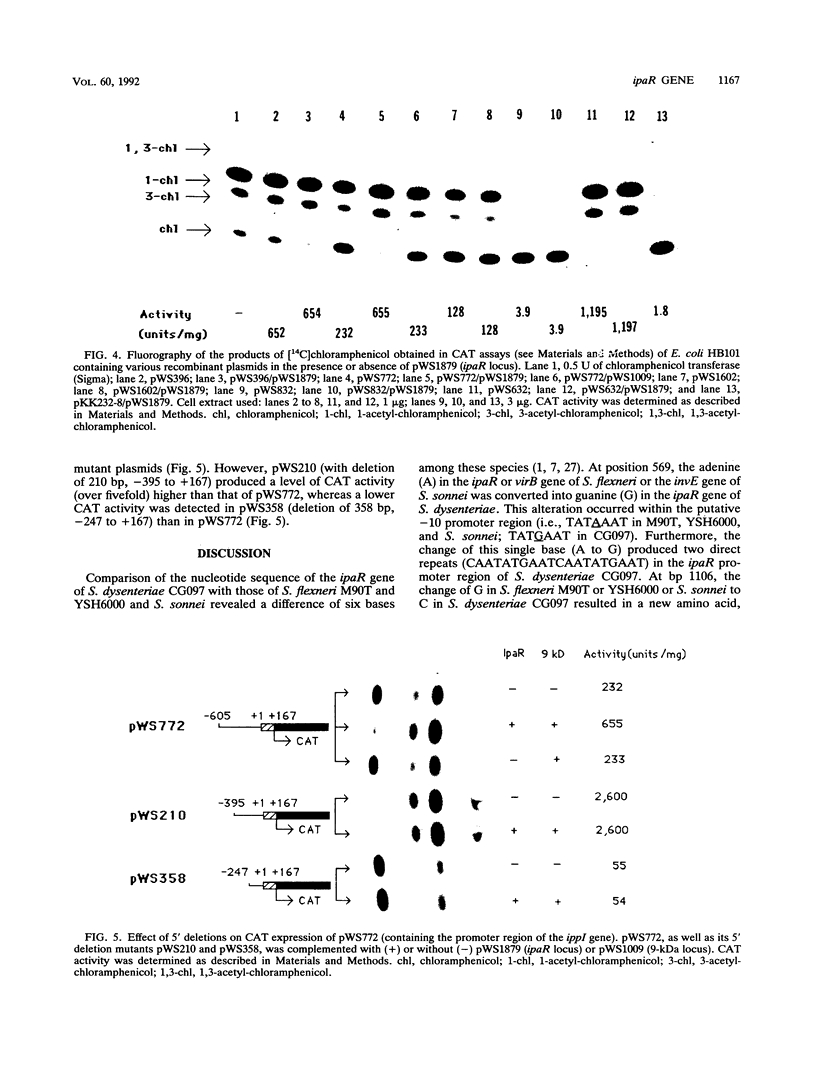
A 1,937 bp PstI-HindIII fragment containing the ipaR locus was cloned from the large invasion plasmid of Shigella dysenteriae CG097, and its nucleotide sequence was completely determined. The IpaR protein (35 kDa, calculated from the DNA sequence) was synthesized in Escherichia coli chi 1411 minicells containing the 1,937-bp PstI-HindIII fragment. To determine the regulatory role of ipaR for ipa genes, we applied genetic complementation experiments using chloramphenicol acetyltransferase (CAT) as reporter. Analyses of CAT activity of the recombinant plasmids containing the 5' flanking sequences of the 24-kDa-protein gene and the ippI, ipaB, ipaC, and ipaD genes defined strong promoters upstream of the 24-kDa-protein gene and ipaD gene, weak promoters upstream of the ippI and ipaB genes, and the absence of any promoter activity for the ipaC gene. Complementation analyses showed that the CAT activity only under direction of the ippI promoter region increased 1.8-fold in the presence of IpaR protein. On the basis of our data, we suggest that an operon comprising ippI, ipaB, and ipaC is positively regulated by IpaR protein which has a trans effect on a DNA sequence upstream of the ippI promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Sasakawa C., Tobe T., Makino S., Komatsu K., Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989 May;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Baudry B., Kaczorek M., Sansonetti P. J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988 May;4(5):345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- Baudry B., Maurelli A. T., Clerc P., Sadoff J. C., Sansonetti P. J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987 Dec;133(12):3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse J. M., Venkatesan M., Mills J. A., Oaks E. V. Molecular characterization of a trans-acting, positive effector (ipaR) of invasion plasmid antigen synthesis in Shigella flexneri serotype 5. Microb Pathog. 1990 Mar;8(3):197–211. doi: 10.1016/0882-4010(90)90047-t. [DOI] [PubMed] [Google Scholar]
- Dillard J. P., Yother J. Analysis of Streptococcus pneumoniae sequences cloned into Escherichia coli: effect of promoter strength and transcription terminators. J Bacteriol. 1991 Aug;173(16):5105–5109. doi: 10.1128/jb.173.16.5105-5109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. A., Friedman S. A., Austin S. J. Partition site of the P1 plasmid. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8544–8547. doi: 10.1073/pnas.84.23.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S. K., Hansen F. G. A missense mutation in the rpoC gene affects chromosomal replication control in Escherichia coli. J Bacteriol. 1991 Aug;173(16):5200–5206. doi: 10.1128/jb.173.16.5200-5206.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Hale T. L. Plasmid-associated adherence of Shigella flexneri in a HeLa cell model. Infect Immun. 1989 Aug;57(8):2580–2582. doi: 10.1128/iai.57.8.2580-2582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Adler B., Tobe T., Okada N., Nagai S., Komatsu K., Yoshikawa M. Functional organization and nucleotide sequence of virulence Region-2 on the large virulence plasmid in Shigella flexneri 2a. Mol Microbiol. 1989 Sep;3(9):1191–1201. doi: 10.1111/j.1365-2958.1989.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Shimizu I., Kaji A. Identification of the promoter region of the ribosome-releasing factor cistron (frr). J Bacteriol. 1991 Aug;173(16):5181–5187. doi: 10.1128/jb.173.16.5181-5187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L. M., Jackson M. P., O'Brien A. D., Holmes R. K. Transcription of the Shiga-like toxin type II and Shiga-like toxin type II variant operons of Escherichia coli. J Bacteriol. 1990 Nov;172(11):6386–6395. doi: 10.1128/jb.172.11.6386-6395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M. Nucleotide sequence of invasion plasmid antigen gene ipaA from Shigella flexneri 5. Nucleic Acids Res. 1990 Mar 25;18(6):1648–1648. doi: 10.1093/nar/18.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Arakawa E., Ito K., Kato J., Nakamura A. Genetic analysis of an invasion region by use of a Tn3-lac transposon and identification of a second positive regulator gene, invE, for cell invasion of Shigella sonnei: significant homology of invE with ParB of plasmid P1. J Bacteriol. 1990 Feb;172(2):619–629. doi: 10.1128/jb.172.2.619-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. J., Palmer K. C., Leon M. A., Palchaudhuri S. Shigella dysenteriae 60R strain adheres to and invades tissue culture cells in the absence of virulence plasmid. FEMS Microbiol Lett. 1991 Oct 15;67(3):323–328. doi: 10.1016/0378-1097(91)90496-w. [DOI] [PubMed] [Google Scholar]
- Yao R., Palchaudhuri S. Nucleotide sequence of the ipaBCD structural genes of Shigella dysenteriae. Mol Microbiol. 1991 Sep;5(9):2217–2221. doi: 10.1111/j.1365-2958.1991.tb02151.x. [DOI] [PubMed] [Google Scholar]