Abstract
Acute episodes of severe hypoxia are among the most common stressors in neonates. An understanding of the development of the physiological response to acute hypoxia will help improve clinical interventions. The present study measured ACTH and corticosterone responses to acute, severe hypoxia (8% inspired O2 for 4 h) in neonatal rats at postnatal days (PD) 2, 5, and 8. Expression of specific hypothalamic, anterior pituitary, and adrenocortical mRNAs was assessed by real-time PCR, and expression of specific proteins in isolated adrenal mitochondria from adrenal zona fascisulata/reticularis was assessed by immunoblot analyses. Oxygen saturation, heart rate, and body temperature were also measured. Exposure to 8% O2 for as little as 1 h elicited an increase in plasma corticosterone in all age groups studied, with PD2 pups showing the greatest response (∼3 times greater than PD8 pups). Interestingly, the ACTH response to hypoxia was absent in PD2 pups, while plasma ACTH nearly tripled in PD8 pups. Analysis of adrenal mRNA expression revealed a hypoxia-induced increase in Ldlr mRNA at PD2, while both Ldlr and Star mRNA were increased at PD8. Acute hypoxia decreased arterial O2 saturation (SPo2) to ∼80% and also decreased body temperature by 5–6°C. The hypoxic thermal response may contribute to the ACTH and corticosterone response to decreases in oxygen. The present data describe a developmentally regulated, differential corticosterone response to acute hypoxia, shifting from ACTH independence in early life (PD2) to ACTH dependence less than 1 wk later (PD8).
Keywords: adrenal cortex, pituitary, ontogeny, oxygen, body temperature
hypoxia may develop in the neonate due to the lack of lung development following preterm birth, or from a variety of cardiopulmonary disease states, such as patent ductus arteriosus (17, 29). Paradoxically, hypoxia may also be precipitated by bronchopulmonary dysplasia caused by prior therapy with hyperoxia (9). Hypoxia in the premature neonate is a common and often devastating condition requiring mechanical ventilation, oxygen therapy, corticosteroids, and other supplementary therapies (39, 51, 58, 59). This is of particular concern, as the number of preterm births in the United States has increased by 30% over the last two decades (12% of all live births in 2003) (30). Coordinated physiological and metabolic responses to hypoxia, and a complete understanding of the development of these responses, are crucial for positive clinical outcomes (19, 21, 34, 36).
Acute, severe hypoxia in newborns may occur as a result of apnea, respiratory infection, or other conditions (24, 26, 31). A coordinated hypothalamic-pituitary-adrenal (HPA) axis response may confer a variety of protective mechanisms aimed at coping with brief periods of severe hypoxia. For example, hypoxia-induced increases in plasma glucocorticoids may facilitate the closing of the ductus arteriosus, improve vasoconstrictor responses to circulating catecholamines, and promote maturation of the immature lung (11, 33, 67). Although acute hypoxia-induced increases in plasma corticosterone have been reported in neonatal rats (62, 64), a complete understanding of the development, timing, and mechanisms of the HPA axis response to this stimulus has not been elucidated.
The primary goal of the present study was to evaluate plasma ACTH and corticosterone responses to acute hypoxia in rats at three critical ages in neonatal development [postnatal days (PD) 2, 5, and 8]. Previous studies analyzed the pituitary-adrenal response to a shorter, more severe exposure to hypoxia (5% O2 for 20 min), reporting the absence of a plasma ACTH response until PD14, whereas plasma corticosterone displayed a U-shaped, age-dependent response to hypoxia (PD2-PD21) (62). Our laboratory has extensively examined HPA axis responses to chronic hypoxia in neonatal rats (5, 7, 44–46, 48, 50). Since episodes of acute, severe hypoxia are more likely to occur in the clinical setting, we have shifted our focus accordingly. We hypothesized that our acute hypoxia paradigm would elicit age-dependent differences in pituitary-adrenal responses. Furthermore, we hypothesized that these differences may be associated with changes in gene expression in the hypothalamus (e.g., Crh, Nr3c1, Nr3c2, Hsd11b2), anterior pituitary (e.g., Pomc, Crhr1, Hsd11b1), and the adrenal gland (e.g., Star, Ldlr, Cyp11a, Cyp21a1, Mc2r). Protein expression analyses were also performed on some of these gene products. Pulse oximetry, body temperature, and heart rate measurements were assessed to characterize cardiopulmonary responses to acute hypoxia.
METHODS
Animal treatment.
The Institutional Animal Care and Use Committee of Aurora Health Care approved the animal protocol. Timed-pregnant Sprague-Dawley rats at 15 days gestation or dams with their litters at PD 1 (Harlan Sprague Dawley, Indianapolis, IN; n = 64 dams) were received and maintained on a standard diet with water available ad libitum in a controlled environment (0600-1800 lights on). The size of litters born in-house was normalized to 12 pups per litter (mixed sexes). To allow acclimatization to the animal facility, pups received at PD1 were studied at PD5 or PD8. On the morning of PD2, PD5, or PD8, litters (without dams) were placed in an environmental chamber. Litters were maintained together but were separated with plastic cage dividers, allowing the pups to nest and huddle on an adequate amount of bedding. During an initial 30-min period, room air (21% O2) was supplied to the chamber at a rate of ∼8 l/min. For baseline measurements, 2 or 3 pups from each litter were removed from the chamber and decapitated (control). Immediately following this, the chamber was closed and the input O2 concentration was decreased to 8% (room air + N2; ∼8 l/min). Ambient O2 concentration in the chamber reached the desired 8% within 10 min. At each subsequent time point (1, 2, 3, and 4 h), 2–6 pups/litter were removed from the chamber and killed.
Blood and tissue collection.
Trunk blood from 2 or 3 pups was pooled in an EDTA-plasma tube and treated as one sample. Whole adrenal glands for real-time PCR analyses, or adrenal zona fasciculata/reticularis (ZFR) for immunoblot analyses, were pooled and frozen on dry ice (4–12 adrenals/sample). Anterior pituitary glands were dissected, pooled (2–6 pituitaries/sample), and frozen on dry ice. Whole brains were frozen separately (1 brain/sample) by immersion in a container of 2-methylbutane (Thermo Fisher Scientific, Waltham, MA) chilled on dry ice. All plasma and tissue samples were stored at −70°C until analyses were performed. Real-time PCR and immunoblot analyses were performed on tissues from PD2 and PD8 pups. Intact hypothalami were obtained from whole brains, frozen on dry ice, and manually dissected with a scalpel in the coronal plane aided with a dissecting microscope. Landmarks were determined based on the atlas of Paxinos and Watson (42). Briefly, brains slices containing the hypothalamic paraventricular nucleus extended from a level immediately caudal to the crossing of the anterior commissure to the level of the dorsomedial hypothalamic nucleus. Sections were blocked dorsally at the dorsal edge of the third ventricle, ventrally at the border of the optic chiasm, and laterally at a position midway between the fornix and lateral border of the section (1 hypothalamus = 1 sample). Dissected tissue was immediately processed for extraction of total RNA (see RNA isolation below).
Separate sets of PD8 litters were used to analyze possible sex differences in hormonal responses to hypoxia, as well as to provide a time control for the possible effects of maternal separation. Pups were separated by sex using anogenital distance as a visual marker (25) and exposed to hypoxia as described in Animal treatment. Visual sex determinations were confirmed by verifying the presence (or absence) of testes post mortem. Time control pups were placed into the chamber but were exposed to room air for the 4-h study duration. This experiment was performed to rule out effects of separation from the dam on plasma ACTH and corticosterone. Sampling methods were the same as described in Blood and tissue isolation.
Hormone assays, pulse oximetry, and body temperature measurement.
Plasma ACTH and corticosterone were measured by radioimmunoassay (MP Biomedicals, Orangeburg, NJ), as previously described (46). Arterial O2 saturation (SPo2; %) and heart rate (bpm) were measured using the MouseOx pulse oximetry unit (Starr Life Sciences, Allison Park, PA). This unit has been validated for use in rat pups per the vendor's literature. One sentinel pup from each experiment (PD8 only; n = 4) was instrumented by fastening sensor clips to each side of the head. The pup was kept near, but isolated, from the other pups in the chamber during the hypoxic exposure. Data from the MouseOx were gathered continuously (at each heartbeat) for the duration of the experiment. Over 50,000 data points were gathered over the 4-h experimental period. To analyze and summarize the data, 30 SPo2 and heart rate measurements were selected at each time point, chosen at the point in the experiment immediately prior to pup removal from the chamber. The mean value of these 30 measurements was used as one datum, so each experiment yielded five data points (baseline, 1, 2, 3, and 4 h). In a separate group of rats (n = 5 litters), body temperature was measured using a RET-3 rectal microprobe and a BAT-12 thermometer (Physitemp Instruments, Clifton, NJ) in PD2 and PD8 pups. Body temperature was recorded every 15 min during a 4-h exposure to 8% O2 or 21% O2 (normoxic time control). Instrumented pups were allowed to huddle with their littermates, and ambient air temperatures remained constant.
RNA isolation and real-time PCR assays.
Total RNA for real-time PCR was isolated from whole adrenals, anterior pituitaries, and whole hypothalami using the RNeasy Mini Protocol (Qiagen, Valencia, CA). The concentration of RNA was quantified using the absorbance value at 260 nm, and the quality of the sample preparation was assessed using the A260/A280 ratio. All RNA preparations were diluted to a final concentration of 10–20 ng/μl, so that equal amounts were loaded across wells. Real-time PCR was performed using the Taqman One-Step RT-PCR Protocol [Applied Biosystems (ABI), Foster City, CA]. Premade primers and probes were purchased from ABI (see Table 1). The final reaction volume of 25 μl consisted of 1X AmpliTaq Gold DNA Polymerase mix, 1× RT enzyme mix containing MultiScribe Reverse Transcriptase and RNase Inhibitor, 1× primer/probe mix, and 50–100 ng of total RNA. Amplification and detection were performed with the ABI Prism 7300 Sequence Detection System with the following thermal cycler conditions: 48°C for 30 min (RT), 95°C for 10 min, and 40 cycles at 95°C for 0.25 min and 60°C for 1 min. Each sample was assayed in triplicate. DNA digestion (ABI) was carried out on separate aliquots of RNA sample when the primer/probe mix for a specific gene could possibly detect genomic DNA (see Table 1).
Table 1.
TaqMan gene expression assays used for real-time RT-PCR
| Gene | Symbol | Assay ID# |
|---|---|---|
| Steroidogenic acute regulatory protein | Star | Rn00580695_m1 |
| Cytochrome P-450, subfamily 11A | Cyp11a | Rn00568733_m1 |
| Cytochrome P-450, subfamily 21A, polypeptide 1 | Cyp21a1 | Rn00588996_g1 |
| Melanocortin 2 receptor (ACTH receptor) | Mc2r | Rn02082290_s1 |
| Low-density lipoprotein receptor | Ldlr | Rn00598438_m1 |
| Hypoxia-inducible factor 1α | Hif1a | Rn00577560_m1 |
| Corticotropin-releasing hormone receptor 1 | Crhr1 | Rn00578611_m1 |
| Proopiomelanocortin | Pomc | Rn00595020_m1 |
| FBJ murine osteosarcoma viral oncogene homolog | Fos | Rn02396759_m1 |
| Corticotropin-releasing hormone | Crh | Rn01462137_m1 |
| Glucocorticoid receptor (GR) | Nr3c1 | Rn00561369_m1 |
| Mineralocorticoid receptor (MR) | Nr3c2 | Rn00565562_m1 |
| Hydroxysteroid 11β dehydrogenase 1 | Hsd11b1 | Rn00567167_m1 |
| Hydroxysteroid 11β dehydrogenase 2 | Hsd11b2 | Rn00492539_m1 |
| Neuropeptide Y | Npy | Rn00561681_m1 |
| Neuropeptide Y receptor 1 | Npy1r | Rn01402912_g1 |
| Actin, β | Actb | Rn00667869_m1 |
Each gene expression assay consisted of a mix of sequence-specific forward/reverse primers and a FAM-labeled probe. Note: Primer/probe sets that cross an exon-exon boundary are designated “m1” at the end of the Assay ID#. Primer/probe sets designated “g1” cross an exon-exon boundary but may still detect genomic DNA, while an “s1” designation describes an assay target found within a single exon (i.e., detects genomic DNA). RNA samples used for assays with “g1” or “s1” designations were treated with DNase prior to PCR.
Presentation of gene expression data.
Real-time PCR data are presented as the number of cycles needed to cross a predetermined cycle threshold (Ct), as previously published (6). The Ct value is set at a point at which an increase in fluorescence (and therefore cDNA concentration) is exponential. Assuming 100% PCR reaction efficiency, the amount of cDNA doubles after each cycle. For example, a difference of 3.3 cycles between a control sample and an experimental sample translates to a 10-fold difference in mRNA expression. Note that the lower the Ct value, the greater the concentration of starting material (i.e., mRNA) in the sample of interest. We chose to analyze 18S rRNA expression as an internal control (i.e., reference gene) in a subset of real-time PCR assays. We were then able to calculate normalized, relative changes in target mRNA expression (vs. baseline) using the 2−ΔΔCt equation (28).
Isolation of adrenal mitochondria and the 100,000 g membrane fraction.
Adrenal glands were dissected and quickly decapsulated to remove the outer zona glomerulosa/capsular layer. Subcapsules (adrenal ZFR and medulla) were then immediately frozen on dry ice and pooled (1 litter; 24 subcapsules/sample). Samples were homogenized on ice for 45 s in ice-cold Tris (50 mM)/sucrose (0.25 M) buffer (“mito buffer”), pH 7.5, with an IKA T10 Basic Ultra-Turrax Dispersing Tool (IKA Works, Wilmington, NC). Mitochondria were isolated by differential centrifugation, as previously described (48). The supernatant from mitochondrial processing was further processed via differential centrifugation (100,000 g for 1 h). The resulting pellet, in addition to containing plasma membranes, also contained membranes from the following organelles: endoplasmic reticulum, microsomes, peroxisomes, Golgi apparatus, endosomes, and lysosomes (12). These samples will be referred to as “membrane” samples. Membrane and mitochondrial pellets were resuspended in mito buffer, and the concentration of total protein was determined using a Qubit Fluorometer (Invitrogen, Carlsbad, CA). Following protein quantification, samples were centrifuged (10,000 rpm for 15 min) and pellets were resuspended in loading buffer. Final protein concentration was normalized across samples on any given gel. Samples were boiled for 10 min and frozen for subsequent immunoblot analyses.
Immunoblot analyses.
Mitochondrial and membrane protein samples were fractionated by one-dimensional SDS-PAGE on a 12% acrylamide gel (Invitrogen) (60 min at 200 V). An equal amount of protein was loaded per well, and molecular weight markers (Precision Plus Protein WesternC Standards; Bio-Rad Laboratories, Hercules, CA) were run on each gel. Protein bands were transferred onto a 0.45-μm nitrocellulose membrane using the XCell II Blot Module (Invitrogen) (60 min at 30 V). Membrane blots were blocked for nonspecific absorption using Blocker Blotto in TBS (5% w/v nonfat powdered milk; Thermo Scientific, Rockford, IL). Mitochondrial blots were probed using anti-StAR (1:1,000; kindly provided by Douglas Stocco at Texas Tech University, Lubbock, TX), anti-CYP11A (1:5,000; Millipore, Temecula, CA), and anti-β-actin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), while membrane blots were probed using anti-low-density lipoprotein receptor (LDLR) (1:300; Cayman Chemical, Ann Arbor, MI) and anti-β-actin. Donkey enchanced chemiluminescence anti-rabbit IgG-horseradish peroxidase (1:15,000; GE Healthcare, Buckinghamshire, UK) was used as a secondary antibody for detection of the proteins of interest. Precision Protein StrepTactin-HRP Conjugate (1:5,000; Bio-Rad) was used as a secondary antibody for the detection of the molecular weight markers. Chemiluminescent detection was achieved using SuperSignal West Pico Luminescent Substrate (ThermoScientific) and imaging with an Ultra-Lum camera (Ultra-Lum, Claremont, CA). Data were acquired by calculating area density of each protein band using the UltraQuant 6.0 software (Ultra-Lum).
Statistical analyses.
Data from pulse oximetry and heart rate measurements were analyzed by one-way ANOVA for repeated measures, with P < 0.05 considered significant. Data from plasma ACTH and corticosterone assays and body temperature measurements were analyzed by two-way ANOVA for repeated measures, with P < 0.05 considered significant. The ACTH and corticosterone response of each litter to 4 h of hypoxia was integrated as area under curve (AUC) above baseline by the trapezoidal rule and treated as one datum. Data from AUC calculations were log transformed and analyzed by one-way ANOVA. The Ct values obtained from real-time PCR and densitometric values obtained from Western blot analyses were analyzed by one-way ANOVA. All post hoc analyses were performed by Student-Newman-Keuls method for multiple comparisons (SigmaStat 2.03).
RESULTS
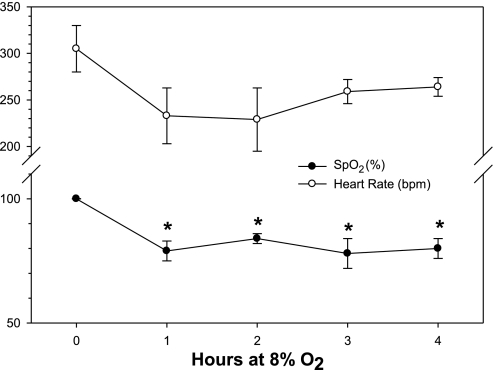
Pulse oximetry data for PD8 rats are shown in Fig. 1. Thirty SPo2 or heart rate measurements were selected at each time point and were chosen at the point in the experiment immediately prior to the chamber being opened. The mean value of these 30 measurements was used as one datum. Arterial O2 saturation (SPo2) was significantly decreased at all time points during exposure to 8% O2, compared with SPo2 values at baseline (P < 0.05). There were no differences between subsequent time points. Exposure to an 8% O2 environment consistently decreased SPo2 to around 80%, from a baseline value of nearly 100%. There was only a tendency for hypoxia to have an effect on heart rate, with rates decreasing from 305 ± 25 bpm at baseline to 229 ± 34 bpm at 2 h (P = 0.254).
Fig. 1.
Pulse oximetry measurements from PD8 pups exposed to 8% O2 for 4 h. Arterial O2 saturation (SPo2; %) and heart rate (bpm) were measured by fastening sensor clips to each side of the head. One sentinel pup from each experiment was instrumented. Data were gathered continuously (at each heartbeat) for the duration of the experiment. Thirty SPo2 or heart rate measurements were selected at each time point, chosen at the point in the experiment immediately prior to the chamber being opened. The mean value of these 30 measurements was used as one datum, so each experiment yielded five data points (baseline, 1, 2, 3, and 4 h); n = 4 at each time point. *Significant difference from baseline with P < 0.05.
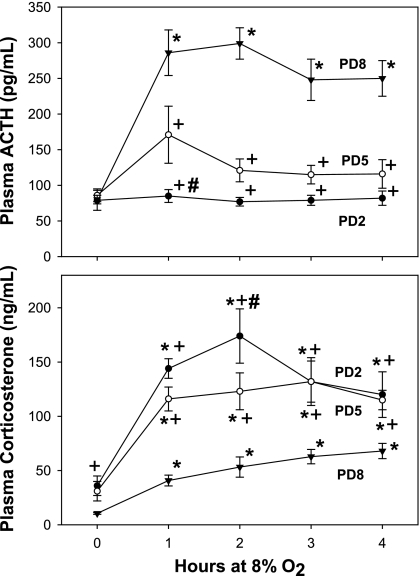
Figure 2 shows plasma ACTH (top) and corticosterone (bottom) measurements from PD2, 5, and 8 pups exposed to 8% O2 for 0–4 h. Plasma ACTH concentrations were significantly increased, compared with baseline, after 1 h in PD8 rats, and remained significantly elevated throughout the study (P < 0.001). Acute hypoxia did not elicit significant changes in plasma ACTH concentrations at PD2 (P = 0.977). Although there was only a tendency for ACTH to increase in PD5 pups by ANOVA, there was a significant increase after 1 h of hypoxia when the data were log transformed and analyzed by t-test (P = 0.04). Two of the five litters at PD5 showed a robust ACTH response to hypoxia, while the other three did not. This did not correlate with the magnitude of the corticosterone response. Plasma corticosterone was significantly increased at all time points compared with baseline in all three age groups (P < 0.006). The most robust corticosterone response to hypoxia was in PD2 pups, where peak plasma concentrations of this hormone were nearly three times greater than in PD8 pups. In addition, baseline plasma corticosterone concentrations in PD2 pups were significantly greater than those in PD8 pups when the data were analyzed by t-test (P = 0.008). Plasma ACTH was unchanged from baseline (49 ± 3 vs. 50 ± 3 pg/ml) in pups exposed to 21% O2 as a time control for 4 h of maternal separation (P = 0.383). Maternal separation also had no effect on plasma corticosterone (9 ± 1 vs. 10 ± 1 ng/ml; P = 0.264). Female pups at PD8 exhibited a more robust plasma ACTH response at 4 h at 8% O2 compared with males of the same age (from 85 ± 2 to 151 ± 26 vs. 81 ± 2 to 115 ± 10 pg/ml; P = 0.025). There were no sex differences in the corticosterone response to acute hypoxia (P = 0.148; data not shown).
Fig. 2.
Plasma ACTH (top) and corticosterone (bottom) responses to acute hypoxia in neonatal rats at three different ages. Pups were placed in an environment chamber and exposed to a gas mixture consisting of 8% O2. At each time point, 2–6 pups were removed and immediately killed. Blood from 2 or 3 pups was pooled and treated as one sample; n = 5–7 measurements per age group (at each time point) for each analyte measured. *Significant difference from baseline within the same age group with P < 0.05. +Significant difference from PD8 within the same time point with P < 0.05. #Significant difference from PD5 within the same time point with P < 0.05.
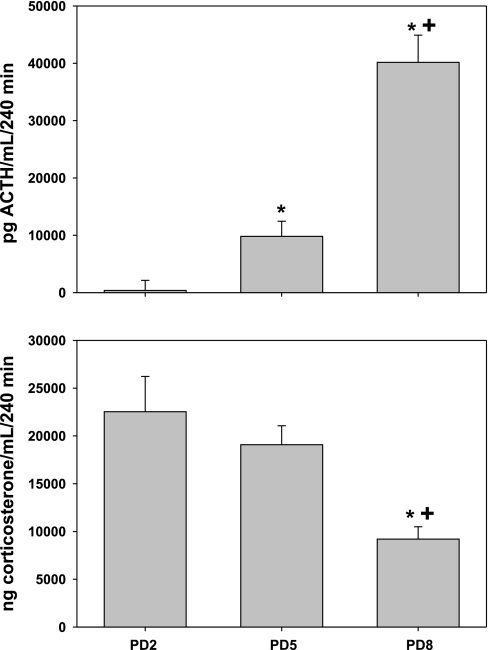
Figure 3 shows the integrated ACTH and corticosterone response to acute hypoxia (calculated from data shown in Fig. 1). Note that the ACTH response to hypoxia increased with age, whereas the corticosterone response decreased with age. These results illustrate age-dependent decreases in adrenal sensitivity to ACTH and an age-dependent disinhibition from corticosterone negative feedback of the ACTH response to hypoxia.
Fig. 3.
Integrated plasma ACTH and corticosterone responses to acute hypoxia. Data were calculated as area under the curve for each age group using the trapezoidal rule, from individual litter responses shown in Fig. 1; n = 5–7 litters per age group for each analyte measured. *Significant difference from PD2 with P < 0.05. +Significant difference from PD5 with P < 0.05.
The expression of genes with known or putative roles in adrenocortical function was analyzed by real-time PCR (Table 2). A decrease in the Ct value, compared with baseline, corresponds to an increase in mRNA expression. Normalized, relative fold changes in mRNA expression (vs. baseline), calculated using the 2−ΔΔCt equation, are included for a subset of genes. Exposure of PD2 and PD8 pups to 4 h of hypoxia (8% O2) significantly increased the expression of Ldlr mRNA (P < 0.002). Interestingly, hypoxia increased Star mRNA expression in adrenals from PD8 pups only. Hypoxia tended to increase adrenal Npy mRNA in PD2 rats, but the change was not significant (P = 0.087). The expression of 18S rRNA (reference gene) was not affected by hypoxia (data not shown). In the anterior pituitary gland, hypoxia for 4 h caused a significant decrease in the expression of Crhr1 mRNA in PD8 pups only (P < 0.001). There were no effects of hypoxia on pituitary Pomc or Hsd11b1 mRNA expression (P > 0.05). Hypoxia decreased Crhr1 and Npy1r expression and increased Fos expression in PD2 hypothalami (P < 0.04). In PD8 hypothalami, the only hypoxia-induced change was a decrease in Nr3c1 (GR) mRNA expression (P < 0.02).
Table 2.
Real-time RT-PCR analysis of adrenal, anterior pituitary, and hypothalamic gene expression in PD2 and PD8 pups exposed to acute hypoxia
| Tissue | Gene Symbol |
Postnatal Day 2 Cycles to Threshold |
Postnatal Day 8 Cycles to Threshold
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 h | P | 2−ΔΔCt | Baseline | 4 h | P | 2−ΔΔCt | ||
| Adrenal | Star | 23.71±0.23 | 23.65±0.10 | 0.800 | 0.81 | 23.98±0.15 | 22.84±0.14* | 0.001 | 2.10 |
| Ldlr | 31.40±0.40 | 29.72±0.41* | 0.002 | 2.46 | 30.59±0.10 | 28.07±0.08* | 0.001 | 5.46 | |
| Cyp11a | 23.20±0.10 | 23.44±0.08 | 0.097 | 0.76 | 23.55±0.08 | 23.88±0.27 | 0.282 | 0.90 | |
| Cyp21a1 | 25.41±0.08 | 25.35±0.14 | 0.764 | 1.22 | 19.78±0.14 | 19.59±0.18 | 0.433 | 1.26 | |
| Mc2r | 31.89±0.18 | 31.94±0.09 | 0.799 | ND | 32.28±0.39 | 32.46±0.49 | 0.780 | 1.34 | |
| Hif1a | 26.83±0.13 | 27.11±0.09 | 0.110 | 0.78 | 26.27±0.04 | 26.77±0.21 | 0.061 | 0.67 | |
| Npy | 19.62±0.16 | 19.10±0.20 | 0.087 | ND | 19.67±0.49 | 19.43±0.40 | 0.716 | ND | |
| Anterior | Pomc | 25.38±0.07 | 25.61±0.19 | 0.159 | ND | 22.02±0.22 | 22.01±0.33 | 0.980 | ND |
| Pituitary | Crhr1 | 34.29±0.26 | 35.00±0.13 | 0.255 | ND | 29.42±0.05 | 30.50±0.17* | 0.001 | ND |
| Hsd11b1 | 32.65±0.19 | 32.80±0.24 | 0.822 | ND | 32.08±0.25 | 32.63±0.22 | 0.171 | ND | |
| Hypothalamus | Crh | 34.37±0.19 | 34.26±0.24 | 0.731 | ND | 33.44±0.18 | 33.62±0.16 | 0.461 | ND |
| Fos | 26.96±0.11 | 26.25±0.17* | 0.005 | ND | 26.76±0.07 | 26.57±0.17 | 0.317 | ND | |
| Crhr1 | 27.43±0.07 | 27.73±0.10* | 0.032 | ND | 29.02±0.09 | 29.11±0.09 | 0.475 | ND | |
| Nr3c1 (GR) | 23.73±0.06 | 23.89±0.09 | 0.190 | ND | 23.41±0.04 | 23.72±0.09* | 0.011 | ND | |
| Nr3c2 (MR) | 27.33±0.06 | 26.94±0.22 | 0.115 | ND | 29.40±0.05 | 29.51±0.09 | 0.301 | ND | |
| Hsd11b2 | 29.27±0.14 | 29.10±0.08 | 0.282 | ND | 29.44±0.17 | 29.53±0.08 | 0.640 | ND | |
| Npy | 22.35±0.15 | 22.70±0.08 | 0.060 | ND | 22.70±0.10 | 22.76±0.11 | 0.686 | ND | |
| Npy1r | 24.14±0.06 | 24.40±0.08* | 0.020 | ND | 25.95±0.08 | 26.13±0.07 | 0.138 | ND | |
| Actb | 13.78±0.08 | 13.81±0.08 | 0.797 | ND | 16.57±0.08 | 16.73±0.11 | 0.282 | ND | |
Whole adrenal glands (4–12 adrenals/sample) and anterior pituitary glands (2–6 pituitaries/sample) were pooled. Whole hypothalami were dissected and treated as one sample; n = 3–6 per tissue type for each gene of interest (at each age). RNA concentrations were normalized to 10–20 ng/μl, and each sample was assayed in triplicate for PCR analysis. A decrease in the cycle threshold (Ct) value at 4 h, as compared to baseline, translates into an increase in mRNA expression for that particular gene. Comparisons between age groups were not performed.
Significant difference in Ct value. Where indicated, the 2−ΔΔCt equation was used to calculate the fold change in target mRNA expression (vs. baseline). The 2−ΔΔCt equation uses a single reference gene (18S rRNA in this case) for the normalization of relative changes in target mRNA expression. See Table 1 for description of gene symbol abbreviations. ND, not determined.
Data from adrenal ZFR immunoblot analyses are summarized in Table 3. Hypoxia did not affect mitochondrial StAR or P450scc protein expression, nor did it affect LDLR protein expression in membrane samples (P > 0.05). Interestingly, hypoxia significantly decreased β-actin protein expression in membrane samples from PD8 rats (P = 0.008) and tended to decrease β-actin expression in mitochondrial samples from PD2 rats (P = 0.089). This effect obviated the use of β-actin as a loading control. While the data suggest a possible effect of age per se on the expression of StAR, P450scc, LDLR, and β-actin, statistical analyses could not be performed for this comparison because of the changes in β-actin expression.
Table 3.
Immunoblot analyses of adrenal subcapsule protein expression in PD2 and PD8 pups exposed to acute hypoxia (8% O2)
| Cellular Fraction From Adrenal Subcapsules | Protein |
Area Density, AU Postnatal Day 2 |
Area Density, AU Postnatal Day 8
|
||||
|---|---|---|---|---|---|---|---|
| Baseline | 4 h | P | Baseline | 4 h | P | ||
| Mitochondria | StAR | 4.47±0.27 | 5.60±0.99 | 0.314 | 8.04±0.48 | 8.97±1.14 | 0.492 |
| CYP11A | 4.28±0.20 | 3.65±0.43 | 0.232 | 12.90±2.14 | 10.87±2.13 | 0.527 | |
| β-actin | 2.40±0.25 | 1.49±0.37 | 0.089 | 4.07±0.70 | 3.09±0.56 | 0.315 | |
| Membranes | LDLR | 0.99±0.11 | 0.68±0.22 | 0.307 | 3.51±1.12 | 3.19±0.44 | 0.797 |
| β-actin | 4.18±1.11 | 4.80±0.16 | 0.544 | 2.12±0.39 | 0.51±0.15* | 0.008 | |
Adrenal subcapsules were pooled (1 litter/sample) and immediately frozen. Comparisons between age groups were not performed due to a significant effect of hypoxia on the loading control (β-actin); n = 4 per cellular fraction for each protein of interest (at each age).
Significant difference with P < 0.05. See Table 1 for descriptions of abbreviations. Note that samples titled “Membranes” consist of plasma membranes and membranes from the following organelles: endoplasmic reticulum, microsomes, peroxisomes, Golgi apparatus, endosomes, and lysosomes. AU, arbitrary units; LDLR, low-density lipoprotein receptor.
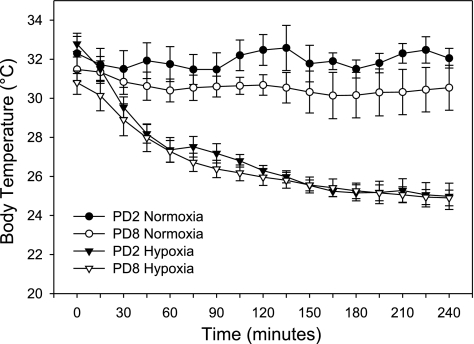
Figure 4 shows the effects of acute hypoxia on body temperature. The data show no effect of maternal separation (21% O2 for 4 h) on body temperature. Interestingly, PD2 pups had significantly higher baseline (T = 0) body temperatures than those of PD8 pups (32.8 ± 0.4 vs. 30.8 ± 0.6°C; P < 0.05). Hypoxia (8% O2) decreased body temperature in PD2 and PD8 pups, with significant changes measured after 30–45 min of hypoxic exposure (P < 0.05). A further significant decrease in body temperature only occurred at the 4-h time point (as compared with values at 30 min; P < 0.05). There was no significant interaction between age and hypoxia on body temperature (P > 0.05).
Fig. 4.
Body temperature (°C) in PD2 and PD8 rats exposed to acute hypoxia. One pup from each litter was instrumented with a microprobe rectal thermometer and allowed to huddle with its littermates. Body temperature was monitored continuously during the entire hypoxic exposure, and data were recorded every 15 min; n = 4 or 5 instrumented pups per age group per exposure (21% O2 or 8% O2 for 4 h). Body temperature significantly decreased from baseline values after 30 min at 8% O2, and values at 240 min were significantly lower than those at 30 min (PD2 and PD8; P < 0.05). Exposure to 21% O2 for 4 h had no effect on body temperature in pups at either age (P > 0.05).
DISCUSSION
The present study characterized the early development of the HPA axis response to acute, severe hypoxia in neonatal rats. The results support the hypothesis that ACTH and corticosterone responses to acute hypoxia are age dependent. Most remarkable was the shift in adrenocortical response to acute hypoxia from ACTH-independent in PD2 pups to ACTH-dependent in PD8 pups, with PD5 pups showing an intermediate, transitional ACTH response. We also demonstrated the development of adrenal hyporesponsiveness to hypoxia-induced increases in ACTH at PD8. Analysis of mRNA revealed hypoxia-induced increases in Ldlr expression in PD2 and PD8 adrenals, while Star expression was increased only in PD8 adrenals.
We have previously described the effects of chronic hypoxia on the HPA axis of newborn rats. Moderate, continuous hypoxia (12% O2) from birth to PD7 caused an ACTH-independent increase in plasma corticosterone concentrations (44, 49, 50). This increase in steroidogenesis was at least partially attributed to increased expression of both Star mRNA and StAR protein, as well as increased expression of Ldlr mRNA (7, 44). Interestingly, the corticosterone response to chronic hypoxia could be attenuated via blockade of postganglionic sympathetic neurotransmission using guanethidine (50). We also described hypoxia-induced changes in the adrenal lipid profile that may play a role in increased steroid production (8). Analysis of central mechanisms revealed that chronic hypoxia attenuated the plasma ACTH response to exogenous corticotropin-releasing hormone (CRH), indicating intact negative feedback due to increased adrenal steroidogenesis (46).
Life-threatening episodes of severe, acute hypoxia may be frequently encountered in both term and preterm newborn humans. The causes may range from respiratory distress brought on by diminished lung function to apneic episodes of known or unknown etiology (24, 26, 31, 32). Increased adrenal glucocorticoid production in response to hypoxemia serves a number of adaptive roles, such as augmenting vasoconstrictor responses to circulating catecholamines and promoting lung maturation (11, 33). A maximal adrenal response to hypoxemia is often lacking in these patients, necessitating frequent administration of steroids such as dexamethasone and hydrocortisone (66–68).
We used a model of severe, acute hypoxia to examine the ontogeny of the HPA axis response in neonatal rats. Pulse oximetry verified the severity of hypoxemia, as SPo2 values were consistently around 80%. Heart rate and body temperature were decreased during the hypoxic exposure; however, only the latter reached statistical significance. Previous studies by Mortola and colleagues (18, 34, 35) have described a hypometabolic phenomenon (e.g., decreased body temperature and heart rate) in neonatal rats exposed to hypoxia. It was suggested that decreased body temperature was due to hypoxic inhibition of shivering and nonshivering thermogenesis, due directly to effects of low oxygen or indirectly to increased circulating levels of corticosterone (35, 55, 55, 56). Therefore, a decrease in body temperature during hypoxia could have interacted with hypoxia itself to alter the function of the HPA axis in our studies. A decrease in body temperature may be a beneficial response to hypoxia to allow a decrease in metabolism when oxygen is scarce. This may be compromised by artificially maintaining the body temperature at “normal” (i.e., isothermic) values. Most important, the age of the pups did not alter the decrease in body temperature in response to hypoxia. We feel the different ACTH and corticosterone responses between PD2 and PD8 were not significantly confounded by hypothermia.
The pituitary-adrenocortical response to hypoxia was highly dependent on age. Plasma ACTH concentration was significantly increased in pups at PD8, while pups at PD2 showed no changes. Pups at PD5 exhibited smaller average increases in plasma ACTH at 1 h, which only reached significance following log transformation and analysis by t-test or integration as AUC. This was due to a variable ACTH response that did not correlate with corticosterone responses. This suggests that PD5 is the critical time during which the adrenal switches from ACTH independence to dependence. Surprisingly, despite the lack of an ACTH response to hypoxia, PD2 pups exhibited the greatest increase in plasma corticosterone. Peak corticosterone concentrations in PD2 pups were more than three times greater than those measured in PD8 pups. It is clearly evident that a developmental pattern in the HPA axis response to hypoxia exists. Previous studies have described a period of attenuated HPA axis responses to various physiological and psychological stressors in neonatal rats (10, 27, 61, 62, 64, 65). During this stress hyporesponsive period (SHRP), basal plasma corticosterone concentrations are slightly elevated compared with older rats, while ACTH and corticosterone responses to stress are attenuated (62). Clearly, the stress hyporesponsiveness in PD8 rats exposed to acute hypoxia, as we have found in this study, is due to decreased adrenal sensitivity to ACTH (71).
Hypoxia, and other stressors, such as maternal deprivation, can overcome the physiological restraint placed on the HPA axis during the SHRP (60, 62, 64). A key component of the response to hypoxia is adrenomedullary catecholamine production, due to systemic (e.g., cardiovascular) effects and to the local stimulatory effect on adrenocortical function (3, 13, 52, 53). Splanchnic innervation of the adrenal medulla and cortex becomes complete by PD8 (16). Adrenomedullary chromaffin cells possess a cellular O2-sensing mechanism that, when activated by hypoxia, increase catecholamine production and release (38). This attribute is not evident in rats beyond PD8 (57). On the basis of these observations and our previous studies with chronic hypoxia and chemical sympathectomy (50), we hypothesize that augmented adrenomedullary activity during acute hypoxia may be at least partially responsible for increased plasma corticosterone in PD2 pups. Further experimentation will be necessary to evaluate this mechanism.
Real-time PCR analyses were employed to explore the molecular mechanism(s) of hypoxia-induced increases in plasma corticosterone. We examined the expression of mRNA for major enzymes and proteins in the steroid synthetic pathway. The expression of Star mRNA was increased by hypoxia in PD8 adrenals. This increase was likely a result of increased plasma ACTH concentrations in this age group, as ACTH has been shown to increase the expression of Star mRNA (2). Hypoxia increased Ldlr mRNA expression in PD2 and PD8 adrenals. This finding suggests that adrenal LDL uptake may have been augmented by hypoxia, supporting increased flux of cholesterol through the steroidogenic pathway (4). The expression of mRNA for other major mediators of adrenal steroidogenesis (i.e., CYP11A, CYP21A1, and MC2R) was not affected by hypoxia; however, there was a tendency for an increase in Npy mRNA expression in the PD2 adrenal. Although NPY has been shown to have little effect on adrenocortical function (14), an increase in its expression (likely of adrenomedullary origin) could reflect increased activation of the adrenal medulla (15). Although it is possible that the observed increases in mRNA expression were due to hypoxia-induced alterations in posttranscriptional processing or mRNA stability, it is likely that these increases are part of the physiological response to hypoxia. We chose to measure mRNA expression at the 4-h time point based on evidence suggesting that changes in mRNA expression can be detected as early as 1 h after the application of a stimulus (43).
Interestingly, immunoblot analyses could not detect hypoxia-induced changes in mitochondrial StAR or membrane LDLR protein expression. This lack of correlation with mRNA expression may be due to a lack of sensitivity of the immunoblot method. Alternatively, the expression and retention of LDLR protein in membranes, as analyzed by immunoblot, may not accurately reflect LDLR protein concentration in the whole cell. It has been suggested that active intracellular processing of LDL and its receptor may mask increases in LDLR protein expression expected upon increased Ldlr mRNA expression (37). Therefore, we hypothesize that hypoxia-induced corticosterone production in PD8 rats may be partially explained by increased expression of adrenal Ldlr and Star mRNA, likely a consequence of increased plasma ACTH concentrations in these rats. We also hypothesize that, in PD2 rats, hypoxia per se, or some other factor induced by hypoxia (e.g., catecholamines), increases adrenal Ldlr mRNA expression, LDL cholesterol uptake, and corticosterone production.
Although not an intended primary outcome, we found a hypoxia-induced decrease in β-actin protein expression in adrenal membrane samples from PD8 rats. Actin proteins have been implicated as a mediator of stimuli-driven steroid production in cells from the adrenal cortex and gonads (22, 23). Cytoskeletal actin is involved in the cellular rounding that occurs upon stimulation of adrenocortical cells with ACTH (1, 23). Through these actions, organelles involved in the steroidogenic pathway (e.g., endoplasmic reticulum, lipid droplets, mitochondria) are brought within closer proximity of each other. This allows efficient shuttling of steroid precursor (i.e., cholesterol) and steroid metabolites, and also mediates stimuli-induced intracellular Ca2+ signaling (40, 41). The hypoxia-induced decrease in membrane β-actin protein expression observed in the present study could be one mechanism by which the PD8 adrenal exhibits a relative insensitivity to ACTH stimulation.
Molecular analyses at the hypothalamic-pituitary level measured limited changes in mRNA expression. In the anterior pituitary of PD8 rats, hypoxia caused a decrease in the expression of Crhr1 mRNA. This finding illustrates the fact that negative feedback at the level of the pituitary (via increased corticosterone production) is present in rats at this developmental age (63). It also supports previous studies that concluded that certain stressors (physiological or psychological) could override the restrictive effects of the pituitary component of the SHRP (62, 64). Hypothalamic expression of Crhr1 and Npy1r mRNA was decreased by hypoxia in PD2 rats. These changes may reflect an adaptation to the ACTH-independent, hypoxia-induced increases in plasma corticosterone. Hypothalamic expression of Fos mRNA was increased by hypoxia in PD2 rats, while this response was absent in PD8 rats. Previous studies measured increased hypothalamic Fos mRNA expression following a mild stressor in PD12 rats (54). This change occurred without increases in hypothalamic Crh mRNA expression or increases in plasma ACTH and corticosterone, suggesting HPA axis insensitivity to stress during the SHRP (54). Our findings confirm that stress-induced hypothalamic Fos mRNA expression and HPA axis responses to stress may occur independently and that these responses occur in a time- and stressor-specific fashion. Real-time PCR results from whole hypothalami must be interpreted with caution, since isolation of specific nuclei was not performed. Therefore, we cannot specifically evaluate hypoxia-induced changes in mRNA expression in the paraventricular nucleus (69).
Perspectives and Significance
Acute episodes of severe hypoxia may occur at any age and may cause significant morbidity and mortality. In adult humans, this has become abundantly clear as the consequences of obstructive sleep apnea are now being realized (20). During the perinatal period, episodes of severe hypoxia not only have an impact on health in the short term, but may also have lasting pathophysiological effects that persist into adulthood (47, 70). A thorough understanding of the mechanism and timing of the HPA axis response to acute hypoxia in neonates has yet to be fully elucidated. Decreased body temperature in response to hypoxia and its possible influence on the HPA axis deserves additional scrutiny, since standard of care in a typical neonatal ICU is to prevent severe hypothermia. Further studies will uncover the details of these physiological mechanisms, as well as possibly provide new insight into therapeutic interventions for hypoxia-induced morbidity.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-54685 to H. Raff and the Aurora St. Luke's Medical Center Medical Staff Summer Research Fellowship.
Acknowledgments
We thank Barbara Jankowski and Peter Homar for their expert technical assistance. Thanks to Douglas Stocco for donating StAR antibody, and to William Cullinan and Matthew Tector for their expert advice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Almahbobi G, Williams LJ, Hall PF. Attachment of mitochondria to intermediate filaments in adrenal cells: relevance to the regulation of steroid synthesis. Exp Cell Res 200: 361–369, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Ariyoshi N, Kim YC, Artemenko I, Bhattacharyya KK, Jefcoate CR. Characterization of the rat Star gene that encodes the predominant 3.5-kilobase pair mRNA ACTH stimulation of adrenal steroids in vivo precedes elevation of Star mRNA and protein. J Biol Chem 273: 7610–7619, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar M, Sarrieau A, Deschepper CF, Walker CD. Adrenal vasoactive intestinal peptide participates in neonatal corticosteroid production in the rat. Am J Physiol Regul Integr Comp Physiol 273: R1163–R1172, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Boggaram V, Funkenstein B, Waterman MR, Simpson ER. Lipoproteins and the regulation of adrenal steroidogenesis. Endocr Res 10: 387–409, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Bruder ED, Henderson LM, Raff H. Adrenal lipid profiles of chemically sympathectomized normoxic and hypoxic neonatal rats. Horm Metab Res 38: 807–811, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruder ED, Hoof JV, Young JB, Raff H. Epidermal growth factor and parathyroid hormone-related peptide mRNA in the mammary gland and their concentrations in milk: effects of postpartum hypoxia in lactating rats. Horm Metab Res 40: 446–453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruder ED, Lee JJ, Widmaier EP, Raff H. Microarray and real-time PCR analysis of adrenal gland gene expression in the 7-day-old rat: effects of hypoxia from birth. Physiol Genomics 29: 193–200, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruder ED, Lee PC, Raff H. Metabolomic analysis of adrenal lipids during hypoxia in the neonatal rat: implications in steroidogenesis. Am J Physiol Endocrinol Metab 286: E697–E703, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 30: 171–178, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Denenberg VH, Bell RW. Critical periods for the effects of infantile experience on adult learning. Science 131: 227–228, 1960. [DOI] [PubMed] [Google Scholar]
- 11.Deruelle P, Houfflin-Debarge V, Magnenant E, Jaillard S, Riou Y, Puech F, Storme L. Effects of antenatal glucocorticoids on pulmonary vascular reactivity in the ovine fetus. Am J Obstet Gynecol 189: 208–215, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dobrota MHR Conditions for density gradient separations. In: Preparative Centrifugation—A Practical Approach, edited by Rickwood D. Oxford: Oxford University Press, 1992, p. 77–142.
- 13.Ehrhart-Bornstein M, Bornstein SR, Gonzalez-Hernandez J, Holst JJ, Waterman MR, Scherbaum WA. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res 21: 13–24, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Ehrhart-Bornstein M, Haidan A, Alesci S, Bornstein SR. Neurotransmitters and neuropeptides in the differential regulation of steroidogenesis in adrenocortical-chromaffin co-cultures. Endocr Res 26: 833–842, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev 19: 101–143, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Engeland WC Functional innervation of the adrenal cortex by the splanchnic nerve. Horm Metab Res 30: 311–314, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Frankel L, Stevenson DK. Metabolic emergencies of the newborn: hypoxemia and hypoglycemia. Compr Ther 13: 14–19, 1987. [PubMed] [Google Scholar]
- 18.Frappell P, Lanthier C, Baudinette RV, Mortola JP. Metabolism and ventilation in acute hypoxia: a comparative analysis in small mammalian species. Am J Physiol Regul Integr Comp Physiol 262: R1040–R1046, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol 17: 106–121, 1993. [PubMed] [Google Scholar]
- 20.Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 43: 518–524, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Grongnet JF Metabolic consequences of induced hypoxia in newborn lambs. Ann Rech Vet 15: 17–28, 1984. [PubMed] [Google Scholar]
- 22.Hall PF On the mechanism of action of ACTH: the role of actin. Endocr Res 10: 431–461, 1984. [DOI] [PubMed] [Google Scholar]
- 23.Hall PF The roles of microfilaments and intermediate filaments in the regulation of steroid synthesis. J Steroid Biochem Mol Biol 55: 601–605, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Hermansen CL, Lorah KN. Respiratory distress in the newborn. Am Fam Physician 76: 987–994, 2007. [PubMed] [Google Scholar]
- 25.Hofstetter J, Suckow MA, Hickman DL. Morphophysiology. In: The Laboratory Rat, edited by Suckow MA, Weisbroth SH, and Franklin CL. New York: Elsevier, 2006, p. 93–127.
- 26.Knight DB The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomized trials. Semin Neonatol 6: 63–73, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol 24: 547–558, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatr 82: 433–437, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: final data for 2005. Natl Vital Stat Rep 56: 1–103, 2007. [PubMed] [Google Scholar]
- 31.Martin RJ, Miller MJ, Carlo WA. Pathogenesis of apnea in preterm infants. J Pediatr 109: 733–741, 1986. [DOI] [PubMed] [Google Scholar]
- 32.Miller MJ, Martin RJ. Apnea of prematurity. Clin Perinatol 19: 789–808, 1992. [PubMed] [Google Scholar]
- 33.Morales P, Rastogi A, Bez ML, Akintorin SM, Pyati S, Andes SM, Pildes RS. Effect of dexamethasone therapy on the neonatal ductus arteriosus. Pediatr Cardiol 19: 225–229, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Mortola JP How newborn mammals cope with hypoxia. Respir Physiol 116: 95–103, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Mortola JP Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir Physiol Neurobiol 141: 345–356, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol 61: 1329–1336, 1986. [DOI] [PubMed] [Google Scholar]
- 37.Ness GC, Zhao Z, Lopez D. Inhibitors of cholesterol biosynthesis increase hepatic low-density lipoprotein receptor protein degradation. Arch Biochem Biophys 325: 242–248, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Nurse CA, Buttigieg J, Thompson R, Zhang M, Cutz E. Oxygen sensing in neuroepithelial and adrenal chromaffin cells. Novartis Found Symp 272: 106–114, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Nye J Postnatal corticosteroids in the treatment of chronic lung disease in the preterm infant: past, present, and future. Neonatal Netw 26: 293–299, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Papadopoulos V, Brown AS, Hall PF. Calcium-calmodulin-dependent phosphorylation of cytoskeletal proteins from adrenal cells. Mol Cell Endocrinol 74: 109–123, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Papadopoulos V, Widmaier EP, Hall PF. The role of calmodulin in the responses to adrenocorticotropin of plasma membranes from adrenal cells. Endocrinology 126: 2465–2473, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, VT: Elsevier Academic, 2005.
- 43.Pocathikorn A, Taylor RR, James I, Mamotte CD. LDL-receptor mRNA expression in men is downregulated within an hour of an acute fat load and is influenced by genetic polymorphism. J Nutr 137: 2062–2067, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Raff H, Hong JJ, Oaks MK, Widmaier EP. Adrenocortical responses to ACTH in neonatal rats: effect of hypoxia from birth on corticosterone, StAR, and PBR. Am J Physiol Regul Integr Comp Physiol 284: R78–R85, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Raff H, Jacobson L. Glucocorticoid feedback control of corticotropin in the hypoxic neonatal rat. J Endocrinol 192: 453–458, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raff H, Jacobson L, Cullinan WE. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regul Integr Comp Physiol 285: R1224–R1230, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Raff H, Jacobson L, Cullinan WE. Augmented hypothalamic corticotrophin-releasing hormone mRNA and corticosterone responses to stress in adult rats exposed to perinatal hypoxia. J Neuroendocrinol 19: 907–912, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raff H, Jankowski BM, Bruder ED, Engeland WC, Oaks MK. The effect of hypoxia from birth on the regulation of aldosterone in the 7-day-old rat: plasma hormones, steroidogenesis in vitro, and steroidogenic enzyme messenger ribonucleic acid. Endocrinology 140: 3147–3153, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Raff H, Jankowski BM, Goodfriend TL, Baker JE, Papanek PE. Effect of exposure to hypoxia from birth on aldosterone in rabbits: role of unesterified fatty acids. Am J Physiol Regul Integr Comp Physiol 272: R1084–R1087, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. Basal and adrenocorticotropin-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology 145: 79–86, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Reyes ZC, Claure N, Tauscher MK, D'Ugard C, Vanbuskirk S, Bancalari E. Randomized, controlled trial comparing synchronized intermittent mandatory ventilation and synchronized intermittent mandatory ventilation plus pressure support in preterm infants. Pediatrics 118: 1409–1417, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Schinner S, Bornstein SR. Cortical-chromaffin cell interactions in the adrenal gland. Endocr Pathol 16: 91–98, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Seidler FJ, Slotkin TA. Adrenomedullary function in the neonatal rat: responses to acute hypoxia. J Physiol 358: 1–16, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith MA, Kim SY, van Oers HJ, Levine S. Maternal deprivation and stress induce immediate early genes in the infant rat brain. Endocrinology 138: 4622–4628, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 268: R183–R191, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Strack AM, Horsley CJ, Sebastian RJ, Akana SF, Dallman MF. Glucocorticoids and insulin: complex interaction on brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 268: R1209–R1216, 1995. [DOI] [PubMed] [Google Scholar]
- 57.Thompson RJ, Jackson A, Nurse CA. Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol 498: 503–510, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tin W Optimal oxygen saturation for preterm babies. Do we really know? Biol Neonate 85: 319–325, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Tin W, Wiswell TE. Adjunctive therapies in chronic lung disease: examining the evidence. Semin Fetal Neonatal Med 13: 44–52, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Walker CD Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol 268: R1281–R1288, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Walker CD, Aubert ML. Effects of early undernutrition and handling on the adrenocortical activity of neonatal rats. Life Sci 43: 1983–1990, 1988. [DOI] [PubMed] [Google Scholar]
- 62.Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology 118: 1445–1451, 1986. [DOI] [PubMed] [Google Scholar]
- 63.Walker CD, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology 119: 1816–1821, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology 128: 1385–1395, 1991. [DOI] [PubMed] [Google Scholar]
- 65.Walker CD, Sizonenko PC, Aubert ML. Modulation of the neonatal pituitary and adrenocortical responses to stress by thyroid hormones in the rat: effects of hypothyroidism and hyperthyroidism. Neuroendocrinology 50: 265–273, 1989. [DOI] [PubMed] [Google Scholar]
- 66.Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res 50: 190–195, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Watterberg KL, Gerdes JS, Gifford KL, Lin HM. Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 104: 1258–1263, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Watterberg KL, Scott SM, Backstrom C, Gifford KL, Cook KL. Links between early adrenal function and respiratory outcome in preterm infants: airway inflammation and patent ductus arteriosus. Pediatrics 105: 320–324, 2000. [DOI] [PubMed] [Google Scholar]
- 69.Watts AG, Tanimura S, Sanchez-Watts G. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology 145: 529–540, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig 12: 2–13, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Zilz A, Li H, Castello R, Papadopoulos V, Widmaier EP. Developmental expression of the peripheral-type benzodiazepine receptor and the advent of steroidogenesis in rat adrenal glands. Endocrinology 140: 859–864, 1999. [DOI] [PubMed] [Google Scholar]