Abstract
Intermittent, extended access to preferred diets increases their intake. However, the effects of such access on the acceptance and reinforcing efficacy of otherwise satisfying alternatives is less known. To investigate the role of nonnutritional contributions to the hypophagia that follows removal of preferred food, male Wistar rats were fed a chow diet (Chow A/I), preferred to their regular chow (Chow), which was equally consumed under 1-choice conditions to an even more preferred chocolate-flavored, sucrose-rich diet (Preferred). Rats then learned to obtain Chow A/I pellets under a progressive ratio schedule of reinforcement and were assigned to two matched groups. Each week, one group (n = 15) was diet-cycled, receiving Chow A/I for 5 days followed by the Preferred diet for 2 days. Controls received Chow A/I daily (n = 14). Progressive ratio sessions were performed daily during the 5 days that all subjects received Chow A/I in the home cage. Across 5 wk, diet-cycled rats progressively ate less of the otherwise palatable Chow A/I diet. Hypophagia was not due to greater prior intake or weight gain, motor impairment, or facilitated satiation and was associated with changes in progressive ratio performance that suggested a reduced reinforcing efficacy of the Chow A/I diet in diet-cycled animals. By week 4, diet-cycled animals began to overeat the preferred diet, especially during the first 6 h of renewed access, resembling a deprivation effect. The results suggest that intermittent access to highly preferred food, as practiced by many restrained eaters, may progressively decrease the acceptability of less palatable foods, and may promote relapse to more rewarding alternatives.
Keywords: limited access, food intake, negative contrast, deprivation, palatability
providing limited, rather than continuous, access to rewards is a procedure used to model excessive behaviors. For example, intermittent, but extended, access to substances of abuse, such as ethanol (8, 19, 24, 29, 66), nicotine (35, 66), cocaine (1, 2, 5, 49, 62, 69, 95), heroin (3, 56), and methamphetamine (48) induces behavioral and molecular adaptations in rodents which resemble features of drug dependence in humans (54, 99), including increased drug self-administration. Drug addicts themselves often show cyclic patterns of uncontrolled use vs. abstinence, a profile also modeled by intermittent access animal models.
Cycles of access to and deprivation from palatable foods also have been used to model the dysregulated food-directed behavior seen in some humans. Restrained eaters attempt to limit themselves to “safe” foods, typically less palatable than “forbidden” foods, to which they often return in bouts of overeating (37, 46, 50, 86, 98). Accordingly, it has been proposed that animals given intermittent access to sugary (24, 39–41) or fatty (18, 20, 21, 28, 32, 33) palatable, energy-dense foods or to sucrose solutions (6–9, 11, 12) show adaptations that may be relevant to the etiology of binge eating or obesity, and, more generally, to changes in the appetitive or satiating properties of specific foods.
The bases for the greater preferredness (in food choice paradigms) and acceptance (in single-food paradigms) of high-fat, high-sugar and energy-dense foods that develops in intermittent access models has received study (8, 17–21, 32, 61). However, less attention has been given to the underconsumption of less preferred, but otherwise acceptable, food that follows access to palatable, energy-dense food. The hypophagia of once acceptable food has often been interpreted to result from a corrective energy homeostasis mechanism to oppose weight gain (4, 18, 58) or caloric conditioning (13, 83, 90). Alternatively, some investigators have proposed nonnutritional contributions, including “negative contrast” (21, 24, 32, 77), due to recent experience of or the prospect of access to a more rewarding alternative (30, 31, 38, 79); “food withdrawal,” analogous to an aversive state of drug withdrawal (87); or opponent-process decrements in brain reward function (84).
The first aim of the present study was to test the hypothesis that the “energy compensation” explanation is sufficient to account for undereating of an otherwise acceptable diet following extended access to a more preferred diet. For this purpose, rats were provided cycles of 5-day access to an otherwise palatable diet followed by 2-day access to an even more preferred diet. The two diets were chosen because they maintain equal degrees of overeating, relative to standard vivarium chow, under 1-choice conditions, but with one diet strongly preferred over the other under 2-choice conditions. The presence of hypophagia following overeating and weight gain might still support an “energy compensation” explanation; conversely, the presence of hypophagia despite comparable prior caloric intake and weight gain might support a nonnutritional interpretation.
The present study also sought to test the hypothesis that the reinforcing efficacy of an otherwise acceptable food decreases in animals given a history of intermittent access to a more preferred alternative. The reinforcing efficacy of the diet was defined in subjects with no history of caloric deprivation using a progressive ratio schedule of reinforcement, in which ratio requirements increase with subsequent reinforcer deliveries (15, 34, 43, 44, 63, 81, 85, 97). The “breakpoint,” defined as the maximum effort an animal will expend to obtain the reinforcer, was used as an objective measure of reinforcer efficacy, sensitive both to the subject's incentive state and to the reinforcer's stimulus properties (15, 43, 44, 81, 85). It is known that animals will work less to obtain otherwise reinforcing gustatory rewards when withdrawn from access or exposure to highly motivating substances of abuse, including heroin (25, 100), methamphetamine (45, 80), nicotine (57), or d-amphetamine (10, 68). Here, the present study sought to test the hypothesis that rats withdrawn from intermittent access to highly preferred food showed deficits in their progressive ratio responding for a less preferred, but previously acceptable, food reinforcer.
MATERIALS AND METHODS
Subjects
Adolescent male Wistar rats (n = 29, 180–230 g, 41–47 days old), obtained from Charles River Laboratories (Raleigh, NC), were single-housed on arrival in wire-topped, plastic cages (19 × 10.5 × 8 inches) in a 12:12-h light-dark cycle (1000 lights off), humidity- (60%) and temperature-controlled (22°C) vivarium. Subjects were studied in two matched cohorts, balanced for treatment assignment, which yielded the same results. Rats had access to corn-based chow (Harlan LM-485 7012) and water ad libitum for 1 wk before experiments. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85–23, revised 1996) and the Principles of Laboratory Animal Care (http://www.nap.edu/readingroom/bookslabrats) and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Diet preference and baseline.
After at least 1 wk of acclimation, the regular Harlan chow diet was replaced with an AIN-76A-based diet, hereafter referred to as “Chow A/I.” In preliminary 2-choice, preference tests, the Chow A/I diet was found to be preferred to the Harlan chow diet, but less preferred than a third, chocolate-flavored sugary diet, which hereafter is referred to as “Preferred” (see Table 1). These preference tests were performed in three groups of ad libitum-fed, male Wistar rats (n = 6/preference test), distinct from the groups of experimental rats. Each group received a different 2-choice access condition (Harlan chow vs. Chow A/I, Harlan chow vs. Preferred, or Chow A/I vs. Preferred). After 1 day of concurrent access to two diets, preference during the next 24 h was calculated as the % of total intake (kcal). The diets are of similar macronutrient proportions and energy density (Table 1).
Table 1.
Selected characteristics of the experimental diets
| Diet |
Preference Ratio, % |
Energy Density, kcal/g | Macronutrient Composition, kcal %
|
||||
|---|---|---|---|---|---|---|---|
| Harlan LM-485 (Regular Chow) | 5TUM Diet (Chow A/I) | 5TUL Chocolate Diet (Preferred) | Carbohydrate | Protein | Fat | ||
| Harlan LM-485 (Regular Chow)* | 13.9±7.0‡ | 9.3±3.6‡ | 3.41 | 66.0 | 21.0 | 13.0 | |
| 5TUM Diet (Chow A/I)† | 86.1±7.0‡ | 8.8±3.7‡ | 3.30 | 65.5 | 24.1 | 10.4 | |
| 5TUL Chocolate Diet (Preferred)† | 90.7±3.6‡ | 91.2±3.7‡ | 3.48 | 66.8 | 20.5 | 12.7 | |
Preferences are expressed as means ± SE and were calculated as the % of total intake (kcal) by acclimated male Wistar rats (n = 6/preference test) in two-choice 24-h preference tests where preference ratio = 100 × Row/(Row + Column) of intakes. (row vs. column). Diet specifications are available in Supplemental Table 1 and as follows: Harlan LM-485 (Regular Chow; http://www.teklad.com/standardrodentdiet/r7012.asp), 5TUM Diet (Chow A/I; http://www.testdiet.com/PDF/5TUM.pdf), and 5TUL Chocolate Diet (Preferred; http://www.testdiet.com/PDF/5TUL.pdf).
Harlan Teklad, Indianapolis, IN.
TestDiet, Richmond, IN.
P < 0.001.
After 1 wk of maintenance on the Chow A/I diet, daily intake of experimental subjects (n = 29) was stable (means ± SE: 5.7+1.9% variability over 4 days), and operant food self-administration test sessions were initiated. To acquire operant self-administration of food and acclimate fully to the test apparatus, rats were housed (∼23 h/day) in previously described test cages (22×22×35 cm) (22, 23, 101, 102), in which they could obtain nosepoke-contingent (0.5 s) food and water on a fixed ratio 1 (FR1) continuous reinforcement schedule. Cages had a wire-mesh floor and were located in ventilated, sound-attenuating enclosures with a 1.1 W bulb synchronized to the vivarium light cycle. Food reinforcers during training, delivered by a pellet dispenser (Med Associates, St. Albans, VT), were 45-mg precision pellets (Test Diets) that were identical in composition to the Chow A/I diet that rats received in the home cage as ∼5 g pellets. Water reinforcers were 100 μl, delivered by a solenoid (W.W. Grainger, Lincolnshire, IL), into a reservoir adjacent to the nosepoke hole. After attainment of stable food and water self-administration (>400 food and >200 water responses/day with <20% variation in nocturnal responses for 3 days), subjects resumed home cage housing with Chow A/I diet, and progressive ratio schedule self-administration sessions were initiated.
Under the progressive ratio schedule, the number of responses required to produce a food pellet increased with successive food reinforcers based on the following shallow exponential progression: response ratio = [4·(e# of reinforcer*0.075) −3.8], rounded to the nearest integer. To avoid unintended session starts (e.g., due to exploratory rather than food-directed activity), the first reinforcement required three responses. Thus, the progressive ratio schedule was 3, 1, 1, 2, 2, 2, 3, 3, 4, 5, 5, 6, 7, 8, 9, 9, 11, 12, 13, 14, 16, 17, 19, 20, 22, 24, 27, 29, etc. responses. Sessions ended when subjects had not completed a ratio for 14 min. This criterion was used because, within meals, male Wistar rats do not voluntarily wait longer than 14 min without eating a pellet in this apparatus (101). Thus, sessions involved rats initiating a meal but with the meal ending prematurely (prior to full satiation), when escalating response requirements surpassed the rats' breakpoint. The dependent measures were as follows: 1) breakpoint, last ratio completed by a subject prior to the end of the session; 2) total responses, total number of reinforced and non-reinforced responses; 3) reinforcers, total number of reinforced responses; 4) latency, the interval from the time the rat was placed into the test chamber until the time it earned its first reinforcer; and 5) session duration, the time between completion of the first ratio and the end of the session. The latency was set to a maximum of 2 h. At the end of each session, subjects were returned to their home cage where Chow A/I was always available ad libitum. Sessions were conducted on five consecutive days each week beginning at dark cycle onset. After attaining stable baseline responding, defined as <10% variation in the number of food reinforcers earned across three consecutive sessions, experimental testing began.
Testing.
For testing, rats were divided into two groups matched for body weight, food intake, and progressive ratio measures from the previous five days, a period designated as baseline performance. One group then continued to receive the Chow A/I diet 7 days/wk (Chow A/I / Chow A/I, n = 14), whereas the second group was provided Chow A/I for 5 days each week followed by 2 days of access to the more preferred, chocolate-flavored, high-sucrose diet (“Preferred”) (Chow A/I / Preferred, n = 15). For brevity, the first 5 days (Chow A/I only) and last 2 days (Chow A/I or Preferred per experimental group) of each week are referred to as C and P phases. Progressive ratio sessions were performed daily during the 5 days of the C phase, during which all subjects received Chow A/I in the home cage. Data from these five sessions were averaged within each of 5 wk of diet cycling for statistical analysis; day-by-day results are also provided in the Supplemental Results.
Note that progressive-ratio sessions were only performed during the 5 days that all subjects received Chow A/I chow diet (C phase) to avoid several potential confounds. Sessions for Chow A/I pellets during the preferred diet cycle (P phase) would have changed the nature of diet-cycling for the Chow A/I / Preferred treatment group. Similarly, sessions for “Preferred” pellets would have changed the diet condition of the Chow A/I / Chow A/I group. Alternatively, if sessions had been performed daily, reinforced by the rat's home cage-appropriate diet, then performance across groups would have been confounded by only one group having received different reinforcers at the same magazine as a result of the same operant response (92, 93). Thus, the present design, summarized in Supplemental Fig. 1 (found in the online version of this article), sought to assess whether the reinforcing efficacy of an otherwise acceptable diet decreased in rats that had intermittently received extended access to a more preferred diet on other days.
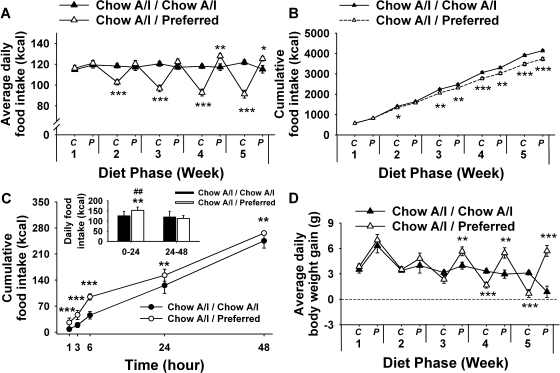
Fig. 1.
Effects of repeated cycles of 5-day access to Chow A/I (C phase) alternated with 2-day access (P phase) to either Chow A/I (Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) in male Wistar rats. A: average daily food intake. B: cumulative food intake (note that error bars are smaller than symbols). C: time course of food intake during 2 days of access to the preferred diet. Food intake was measured 1, 3, 6, 24, and 48 h after restoration of preferred diet access after 20 wk of diet cycling. Inset depicts food intake of the first (0–24 h) vs. second (24–48 h) day of access to preferred diet. Inset scale differs from main panel. D: average daily change in body weight during each diet phase of each week. *Significant difference from Chow A/I / Chow A/I, P < 0.05, **P < 0.01, ***P < 0.001.
Ingestion and Body Weight
Home cage food intake was measured daily during the chow phase and at the end of the 2-day “preferred food” phase. Body weights were recorded at the end of each diet phase at the dark-cycle onset. Measures were obtained using a scale of 0.1 g precision. Food intake was measured as the difference in food weight from one measurement point to the next, after accounting for recovered spillage in the bedding. Calculated total daily intake included pellets from the progressive ratio session. To determine whether intake of preferred food was constant across days or increased following the 5-day “deprivation” periods, intake of the second cohort of subjects (n = 9 Chow A/I / Chow A/I; 7 Chow A/I / Preferred) was measured 1, 3, 6, 24, and 48 h after restoration of preferred diet access after 20 wk of diet cycling.
Rate of Progressive Ratio Responding
To test the hypothesis that alternating, extended access to a more preferred food reduced the rate of responding for the less preferred food, the duration of interresponse intervals (IRIs) that followed reinforced vs. nonreinforced responses under the progressive ratio schedule were compared between the 1st vs. 5th wk of diet cycling (22, 23). Preferred orosensory reinforcers (e.g., higher vs. lower concentrations of sucrose, milk, or saccharin) often increase free-running operant response rates under ratio schedules that promote short interresponse intervals (16, 27, 47). Therefore, differences in the duration of the interresponse intervals that follow food-reinforced responses may also serve as a measure of differential reinforcement efficacy under the present conditions. Interresponse intervals were approximately log-normally distributed in duration, so the values were ln-transformed for statistical analysis (89, 101).
Statistics
Group comparisons used the general linear model, including ANOVA or analysis of covariance (ANCOVA). Daily intake of Harlan chow vs. Chow A/I was compared by paired Student's t-test. Average daily food intake and weight gain were analyzed by three-way mixed ANOVAs with diet schedule as a between-subjects factor and week and phase as within-subject factors. Incremental food intake was analyzed by split-plot ANOVAs with diet schedule as a between-subjects factor and week or hour as a within-subject factor. Measures of progressive ratio performance were analyzed by split-plot ANCOVAs with diet schedule as a between-subjects factor, week as a within-subject factor, and baseline performance as a covariate. Time course of responding was analyzed by split-plot ANOVAs with diet schedule as a between-subjects factor and hour a within-subject factor. The duration of reinforced and nonreinforced interresponse intervals were analyzed by two-way ANOVAs with diet schedule and week as between-subjects factors. Following significant omnibus effects (P < 0.05), pairwise comparisons used Student's t-tests for split-plot ANOVAs and Newman-Keuls comparisons for factorial ANOVAs. Linear regressions were used to quantify relations between breakpoint, reinforcers earned, total responses, latency, and progressive ratio session duration on the one hand with the duration of reinforced and nonreinforced interresponse intervals on the other. The statistical packages used were Instat 3.0 and GraphPad Prism 4.0 (GraphPad, San Diego, CA, USA), Systat 11.0 and SPSS 11.5 (SPSS, Chicago, IL).
RESULTS
Effects of Preferred Diet Alternation on Daily Food Intake and Body Weight
When rats were switched from the Harlan chow diet to the relatively preferred Chow A/I diet, daily intake increased significantly (115.8 ± 2.8 vs. 97.7 ± 2.5 kcal, P < 0.001, means ± SE), as intended. The elevated (∼20%) level of intake was maintained in Chow A/I-fed controls through acclimation and the subsequent 5-wk diet cycling period (Fig. 1A).
To address the alternative interpretation that differences in food intake between Harlan chow and Chow A/I diet conditions might reflect differential unrecovered diet spillage, a follow-up study was performed in wire-mesh cages. For this, 8 male Wistar rats (41–47 days old) were acclimated to wire-mesh cages (20×25×36 cm) and then provided regular Harlan chow ad libitum. After 4 days, rats were switched to Chow/A/I diet for 3 days. Spillage was collected daily and dried under a fume hood for 48 h. Intake was defined as the difference in food weights after accounting for dried spillage. Rats ate significantly more of the Chow A/I diet than the Harlan chow, with values resembling those seen in the tub cages (means ± SE; 117.6 ± 6.1 vs. 101.1 ± 3.3 kcal/day, P < 0.01). Intakes of Chow A/I diet did not differ between the first and third days of access, consistent with a stable hyperphagia (not shown).
Figure 1A shows that alternating access to the chocolate-flavored, sugary, preferred diet progressively altered daily food intake of male rats in a diet-specific manner [Week×Diet Phase×Diet Schedule: F (4,108) = 88.98, P < 0.001]. After each access to the preferred diet, Chow A/I / Preferred rats underate Chow A/I. Linear trend analysis showed that the Chow A/I intake of diet-cycled rats progressively decreased with successive diet cycles [F (1,14) = 53.59, P < 0.001; Fig. 1A, chow phases, from 116.2 to 91.4 kcal]. In contrast, Chow A/I intake of controls slightly increased across the same period [linear contrast: F (1,13) = 6.72, P < 0.03, from 114.7 to 121.9 kcal]. As intended, Chow A/I / Preferred rats did not eat more than controls during their first 3 accesses to the preferred diet (Fig. 1A, P phases of weeks 1–3). Beginning from the fourth access to the preferred diet; however, diet-cycled rats began to show increased intake of the preferred diet relative to Chow A/I / Chow A/I rats (Fig. 1A; P phases of weeks 4 and 5). Because chow hypophagia of diet-cycled rats preceded and exceeded their overeating of preferred diet, their cumulative energy intake was increasingly less than that of Chow A/I- fed controls beginning from week 2 [Fig. 1B; Week×Diet Phase×Diet Schedule: F (4,108) = 33.99, P < 0.001].
As shown in Fig. 1C (main panel), the degree to which diet-cycled rats overate the preferred diet when it was returned to them was greatest during the first 6 h of access [Time×Diet Schedule: F (4,56) = 4.67, P < 0.02; diet schedule: F (1,14)=7.59, P < 0.02]. Their cumulative intake was 318 ± 56% that of controls after 1 h, 242 ± 24% at 3 h, 208 ± 7% at 6 h, 122 ± 5% at 24 h, and 108 ± 1% at 48 h (means ± SE). Thus, incremental overeating was specific to the first 6 h of renewed access and accounted for the excess cumulative 24- and 48-h cumulative intake. Accordingly, hyperphagia was present during the first, but not second, 24 h of access to the preferred diet (Fig. 1C, inset).
Diet-alternated rats showed cycling of body weight changes beginning from week 3 [Fig. 1D; Week×Diet Phase×Diet Schedule: F (4,108) = 7.19, P < 0.001]. However, their absolute body weight (501.0 ± 12.7 vs. 491.3 ± 11.3 g, P = 0.57; means ± SE) and cumulative weight gain (117.7 ± 5.9 vs. 118.7 ± 5.7 g, P = 0.90) did not differ from those of controls at study completion (week 20, C or P phase) or at any earlier measured timepoint (not shown).
Effects of Preferred Diet Alternation on Progressive Ratio Responding for Chow A/I Food
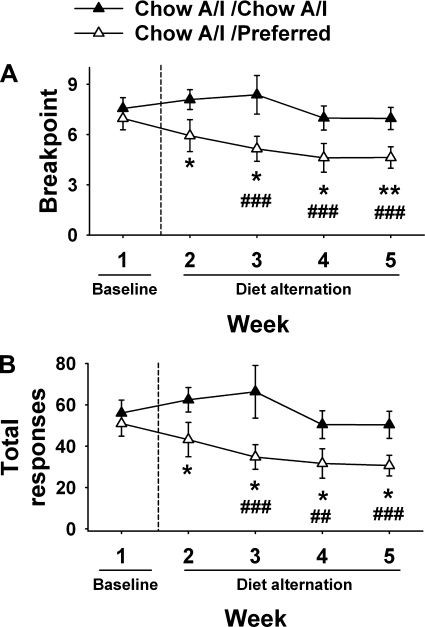
As shown in Fig. 2, diet-cycled rats worked less to obtain the otherwise palatable, but less preferred, Chow A/I diet during the days that they were withdrawn access to the highly preferred diet. The breakpoint [Diet Schedule: F (1,26) = 12.32, P < 0.005; Week×Diet Schedule: F (4,104) = 3.15, P 0.05], total responses emitted [Diet Schedule: F (1,26) = 11.03, P < 0.005; Week×Diet Schedule: F (4,104) = 2.91, P < 0.05], and reinforcers earned [Diet Schedule: F (1,26) = 18.24, P < 0.001; Week×Diet Schedule: F (4,104) = 4.54, P < 0.005; Table 2] all were reduced after the first access to the preferred diet and decreased further with additional cycles. Diet schedule also altered session duration [F (1,26) = 4.98, P < 0.05], with sessions ending sooner in rats that had received intermittent palatable diet access (Table 2).
Fig. 2.
Effects of repeated cycles of 5-day access to Chow A/I alternated by 2-day access to either Chow A/I (Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) on progressive ratio responding for Chow A/I pellets in male Wistar rats. Rats were tested daily at the dark cycle onset during the C (Chow A/I) Phase. Values represent the average of all 5 days from each phase. Values are presented as means ± SE. A: breakpoint, the last ratio completed by a subject prior to the end of the session. B: total responses, the number of reinforced and nonreinforced responses. *Significantly different from Chow A/I / Chow A/I P < 0.05, **P < 0.01. ##Significantly different from Chow A/I / Preferred baseline (week 1) P < 0.01, ###P < 0.001.
Table 2.
Progressive ratio responding for chow A/I pellets
| Parameter |
Latency, ln s [s] |
Reinforcers
|
Session Duration, ln min [min]
|
|||
|---|---|---|---|---|---|---|
| Group | Chow A/I / Chow A/I | Chow A/I / Preferred | Chow A/I / Chow A/I | Chow A/I / Preferred | Chow A/I / Chow A/I | Chow A/I / Preferred |
| Baseline (Week 1) | 4.97±0.22 [144–28.4] | 4.99±0.40 [146.9–48.4] | 13.2±0.7 | 12.5±0.9 | 3.21±0.06 [24.8–1.4] | 3.1±0.06 [22.2–1.3] |
| Week 2 | 4.47±0.18 [87.4–14.4] | 4.90±0.39 [134.3–43.4] | 14.0±0.7 | 10.6±1.3*# | 3.38±0.06 [29.4–1.7] | 3.07±0.08 [21.5–1.7]** |
| Week 3 | 4.43±0.20 [83.9–15.2] | 5.03±0.42 [152.9–52.4] | 14.0±1.1 | 9.8±1.2**### | 3.31±0.09 [27.4–2.4] | 3.01±0.06 [20.3–1.2]**# |
| Week 4 | 4.46±0.22 [86.5–17.1] | 4.99±0.48 [146.9–56] | 12.6±0.9 | 8.8±1.3*### | 3.23±0.07 [25.3–1.7] | 3.05±0.07 [21.1–1.4]* |
| Week 5 | 4.27±0.26 [71.5–16.4] | 4.96±0.48 [142.6–54.4] | 12.7±0.7 | 9.1±1.1**### | 3.19±0.08 [24.3–1.9] | 2.97±0.05 [19.5–1.0]*## |
Effects of repeated cycles of 5-day access to Chow A/I alternated by 2-day access to either Chow A/I (Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) on progressive ratio responding for Chow A/I pellets in male Wistar rats. Values show the means ± SE of the 5-day averages from each week. Bracketed values show the backtransformed means ± SE to facilitate interpretation.
Significant difference from Chow A/I / Chow A/I P < 0.05,
P < 0.01.
Significant difference from respective baseline (week 1) P < 0.05,
P < 0.01,
P < 0.001.
The average latency to obtain the first pellet during sessions also tended to be longer in diet-cycled rats [diet schedule: F (1,26) = 3.75, P = 0.06; Table 2], reflecting that diet-cycled subjects took significantly and progressively longer than controls to obtain the first reinforcer after being switched from the preferred to Chow A/I diet. Day-by-day analysis showed that effects for each progressive-ratio measure were greatest and highly significant the first day that rats were withdrawn from the preferred diet. For the breakpoint, reinforcers, and total responses measures, these changes were still partly evident up to 5 days later (see Supplemental Fig. 2 in the online version of this article).
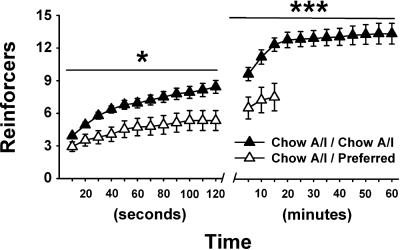
Time course analysis of the first session after rats were withdrawn from the preferred diet in week 5 showed that diet-cycled rats had already obtained fewer reinforcers than controls 10 s after each had obtained their first pellet (2.9 ± 0.4 vs. 3.9 ± 0.2 reinforcers, P < 0.05). The early onset of reduced responding, the low quantity of food obtained by that point (equivalent to 0.13 ± 0.02 vs. 0.18 ± 0.01 g, or 0.43 ± 0.02 vs. 0.59 ± 0.01 kcal), and the increased latency of diet-cycled rats to obtain their first reinforcer (68.5 + 27.8 vs. 305.1 – 123.0 s, P < 0.05) collectively point to a reduced reinforcing efficacy of the Chow A/I pellets in diet-cycled rats, rather than a facilitation of satiation resulting from earned reinforcers. As shown in Fig. 3, diet-cycled rats obtained fewer reinforcers than controls across the entire session.
Fig. 3.
Effects of repeated cycles of 5-day access to Chow A/I alternated by 2-day access to either Chow A/I (Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) on the time course by which 45-mg Chow A/I pellet reinforcers were obtained by male Wistar rats under a progressive ratio schedule of reinforcement. Testing occurred on the first day of the Chow A/I (C) phase of week 5. Values are presented as means ± SE. *Significantly different from Chow A/I / Chow A/I, P < 0.05, ***P < 0.001. The rate at which subjects earned reinforcers per unit time is shown. Each symbol represents the mean number of cumulative reinforcers earned by subjects through that time point. The time course is continued until each subject in the group has stopped obtaining reinforcers, and the graph therefore does not represent average session durations.
Effects of Preferred Diet Alternation on the Rate of Progressive Ratio Responding
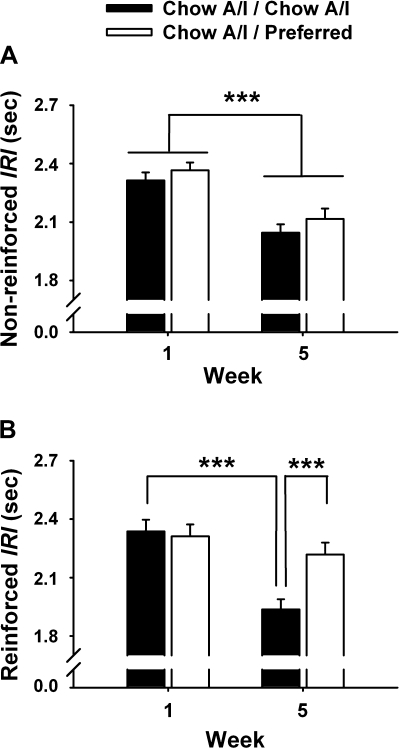
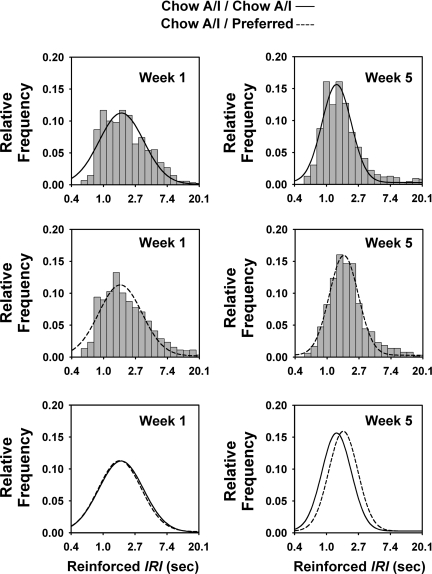
Because diet-cycled rats not only showed reduced breakpoints to work for Chow A/I diet but also less rapidly earned successive reinforcers during the session, their rates of reinforced and nonreinforced responding during progressive ratio sessions were compared with those of controls. From week 1 to week 5, intervals that followed nonreinforced responses became ∼0.3 s briefer in both diet-cycled and control rats, a general increase in performance over time [Fig. 4A; Week: F (1,8846) = 34.42, P < 0.001]. In contrast, intervals that followed reinforced responses became ∼0.4 s briefer only in control rats and did not change in diet-cycled rats, resulting in a Week×Diet Schedule interaction [F (1,3308) = 7.42, P < 0.01] (Fig. 4B). As illustrated in frequency histogram analysis (Fig. 5), Chow A/I / Preferred rats had longer postreinforcement intervals than controls after, but not before, 5 wk of diet cycling. This was evident as a relative rightward shift of their interresponse interval distribution.
Fig. 4.
Effects of repeated cycles of 5-day access to chow alternated by 2-day access to either Chow A/I (Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) on the duration of interresponse intervals (IRIs) following reinforced (A) or nonreinforced responses (B) during progressive ratio responding for 45-mg Chow A/I pellets in male Wistar rats. Values are presented as means ± SE. IRI durations were ln-transformed for statistical analysis, and bars represent back-transformed values. Data were cumulated from 5 sessions during each of weeks 1 and 5. *Significantly different from Chow A/I / Chow A/I, P < 0.05, ***P <0.001.
Fig. 5.
Effects of repeated cycles of 5-day access to chow alternated by 2-day access to either Chow A/I(Chow A/I / Chow A/I, n = 14) or highly preferred chocolate-flavored sugary diet (Chow A/I / Preferred, n = 15) on the duration of interresponse intervals (IRIs) following reinforced responses during progressive ratio responding for 45-mg Chow A/I pellets in male Wistar rats. Relative frequency histograms (bars) and fit log-Gaussian distributions (lines) (22, 23), show the relation of diet schedule to the duration of IRIs after Chow A/I-reinforced responses. Before diet cycling (left, week 1), distributions were similar between groups, whereas after 5 wk of diet cycling (right), the relative frequency histogram was shifted to the right in diet-alternated rats compared with controls, indicating longer postreinforcement intervals. Nonreinforced intervals were not similarly shifted (not shown). The frequency histogram shows IRIs of between e-1 and e3 s in duration (0.4–20.1 s) using a bin width of e0.2 (note ln-scale of x-axis). The time scale shown accounts for a similarly large majority of IRIs in diet-cycled rats (98%) and controls (98%). Tick labels indicate the beginning of the bin. Data were cumulated from 5 sessions during each of weeks 1 and 5.
To assess the construct validity of the duration of postreinforcement intervals under the progressive ratio schedule as a measure related to reinforcement efficacy, correlation analysis was performed on PR measures during week 5. Table 3 shows that the measures of breakpoint, reinforcers earned, and total responses emitted all highly intercorrelated (r = 0.92 to 0.99), as expected, since they are functions of one another. These measures also correlated directly with session duration (r = 0.84 to 0.89) and, inversely, with the latency to earn the first reinforcer (r = −0.57 to −0.79). The mean duration of postreinforcement intervals showed comparably strong and significant correlations with each progressive ratio measure, accounting for on average 44% of their variance (|r| = 0.53 to 0.76; mean r = 0.67). In contrast, the mean duration of interresponse intervals following nonreinforcement did not correlate significantly with any progressive ratio measure, accounting for on average only 9% of their variance (|r| = 0.23 to 0.34; mean r = 0.30).
Table 3.
Intercorrelations among progressive ratio variables
| Parameter | Reinforcers | Session Duration | Breakpoint | Total Responses | Mean Reinforced IRI | Mean Nonreinforced IRI |
|---|---|---|---|---|---|---|
| Latency | −0.79*** | −0.59** | −0.67** | −0.57** | 0.71*** | 0.23 |
| Reinforcers | 0.84*** | 0.97*** | 0.92*** | −0.76*** | −0.34 | |
| Session Duration | 0.89*** | 0.89*** | −0.53** | −0.25 | ||
| Breakpoint | 0.99*** | −0.68*** | −0.34 | |||
| Total Responses | −0.61** | −0.32 |
IRI, the duration of inter-response intervals following reinforced (“reinforced IRI”) or nonreinforced (“nonreinforced IRI”) responses. Results were calculated from data obtained during the first progressive ratio session of week 5 from male Wistar rats with a history of diet alternation (n = 15; Chow A/I / Preferred) or control Chow A/I conditions (n = 14).
P < 0.01, and
P < 0.001 vs. 0.
DISCUSSION
In the present study, rats that received intermittent, extended access to a highly preferred food progressively underate an otherwise palatable chow when it was the only available food. Hypophagia did not depend upon prior greater intake or weight gain, arguing against a compensatory, energy homeostatic interpretation (4, 18, 58). Changes in operant responding for the previously palatable chow suggested a decrease in reinforcing efficacy. After multiple cycles of intermittent access, rats began to overeat the highly preferred diet during the first, but not second, day of renewed access.
Hypophagia of Less Preferred Food
Hyophagia following access to preferred food has often been attributed to the excess caloric intake or weight gain that preferred food can induce or to caloric conditioning by the more energy-dense, preferred diet (13, 83, 90). Here, to equate levels of initial intake, regular chow diet of controls was replaced with a more preferred chow diet (86.1 ± 7.0% preference vs. Harlan chow), which promoted the same initial levels of energy intake and weight gain as an even more preferred chocolate-flavored, sugary diet. Diets were similar in energy density (within 4%) and macronutrient proportions. Despite ingesting similar energy as controls during the first 3 accesses to the preferred diet and less total energy by the second week, male rats receiving intermittent extended access to the highly preferred diet ate progressively less of the otherwise palatable chow. Thus, the initial development and progression of hypophagia occurred in the context of equal weight gain and less energy intake, suggesting a nonnutritional mechanism.
It might be argued that both diets promoted hyperphagia relative to standard chow and, therefore, that positive energy balance may be necessary, but not sufficient, to produce hypophagia of a less preferred alternative. Against this hypothesis, cyclic hypophagia of a less preferred diet also was seen in a previous study with female rats when the more preferred diet was Harlan chow (P. Cottone, V. Sabino, L. Steardo, and E. P. Zomilla, unpublished data). Furthermore, rats undereat chow after receiving long-term access to a palatable cafeteria diet, even if they are pair-fed to prevent greater weight gain (77).
Decreased Reinforcing Efficacy of Less Preferred Food
Decreases in the reinforcing efficacy of the less preferred, but previously accepted, diet accompanied the chow hypophagia. Specifically, diet-cycled subjects developed the following changes in operant responding for the less preferred food: 1) a longer latency to obtain their first reinforcer, 2) a reduced breakpoint under a progressive ratio schedule of reinforcement, 3) a decreased rate of responding from as early as 10 s into the session (i.e., a preabsorptive phase of responding), and 4) a slower rate of responding selectively after reinforced, but not nonreinforced, responses. These differences in progressive ratio operant responding do not support alternative explanations that greater rates of satiation within meals or nonspecific motor impairment were responsible for the chow hypophagia. Rather, findings are consistent with the interpretation that rats withdrawn from alternating access to the highly preferred, sugary, chocolate-flavored food showed decreases in the reinforcing efficacy of the relatively less preferred, but otherwise reinforcing, chow. Analogously, rats withdrawn from access to classic substances of abuse, including amphetamine (68), methamphetamine (45), morphine (100), or nicotine (57) show decreased breakpoints of responding for an otherwise reinforcing gustatory reinforcer, changes that have been interpreted to indicate a negative or hypohedonic-like affective state.
The present experimental conditions provided sufficient sensitivity to detect a 36% decrease in progressive ratio breakpoints for an alternate gustatory reinforcer, which is of similar magnitude to effects seen in rats withdrawn from classic drugs of abuse. Rats withdrawn from d-amphetamine showed 30–37% reductions in progressive ratio responding for sucrose solution reinforcement, with baseline breakpoints ranging from ∼40 (68) to ∼8 (10). Nicotine withdrawal likewise was associated with a ∼37% reduction in progressive ratio breakpoints for sucrose pellets (57).
The decreased reinforcing efficacy of Chow A/I pellets is difficult to explain as acute energy compensation because cumulative energy intake and weight gain of diet-cycled rats were similar to or less then those of controls at the onset of self-administration sessions. Rather, given that diet-cycled rats substantially reduced their own intake when chow was available and that caloric restriction increases the efficacy of gustatory reinforcers (14, 43, 44, 76), it might be said that chow remained less reinforcing in diet-cycled rats despite energy compensation mechanisms.
Potential nonnutritional, hedonically oriented mechanisms that might explain the collective results include “negative contrast” (21, 24, 32, 77), due to recent experience of or the prospect of access to a more rewarding alternative (30, 31, 38, 79); “food withdrawal,” analogous to an aversive state of drug withdrawal (8, 36, 87); or opponent-process allostatic shifts in brain reward function (84) to counter the effects of the highly preferred diet. The inability of subjects to access a known, more preferred reinforcer also might contribute to the progressive nature of behavioral changes by acting as a repeated stressor, which can impair brain reward function (65, 78). Future research may clarify the relative role, if any, of these mutually compatible explanations.
Reinforced vs. Nonreinforced Rates of Responding
Under the present experimental conditions, the rate of responding after reinforced, but not nonreinforced, responses directly correlated with other putative measures of reinforcement efficacy. Since Thorndike proposed the Law of Effect (1911, p. 244 of Ref. 88), the relation between free-running rates of responding for a reinforcer and the outcome's expected or empirical reinforcing efficacy has been studied extensively. There are clearly many conditions under which free-running rates of responding do not represent the reinforcing efficacy of an outcome (see 16, 27, 47, 60, 64, 73, 74, 82). However, the present findings support previous observations that preferred orosensory reinforcers (e.g., higher lower concentrations of sucrose, milk, or saccharin) maintain higher free-running operant response rates than do less preferred reinforcers under ratio schedules that promote short interresponse intervals. Here, however, only rates of responding after reinforced, and not after nonreinforced, responses consistently correlated with other measures of reinforcement efficacy. Because reinforced responses represent a minority of responses under the present conditions (∼25%), the present results may help further explain why the average rate of all responses does not more predictably indicate reinforcement efficacy under high ratio requirement conditions. That is, postreinforcement intervals better represent the reinforcer's ability to strengthen reinforcer-directed behavior via arousal or incentive mechanisms (see 16, 27, 47) than do the intervals that follow nonreinforced responses.
Hyperphagia of More Preferred Food
Diet-cycled rats ultimately ate more of the preferred diet beginning from their fourth access. When restored access to the highly preferred diet, subjects selectively overate within the first 6 h, showing 3-fold greater intake than controls during the first hour. By the second day of access, intake normalized to levels of controls. The transient time course of overeating resembles patterns of excess intake seen after deprivation from alcohol (42, 71, 75), nicotine (35, 66), and other rewarding tastants such as sucrose, saccharin, salt, and high fat food (7, 32, 70, 91, 97). Such “deprivation effects” are proposed animal models of relapse in other contexts (52, 59, 75), and their progressive nature in the present model merits further study.
Because rats ultimately overate the preferred diet by week 4, it is possible that corrective energy homeostatic responses contributed to chow hypophagia in week 5. On the other hand, diet-cycled rats had still consumed ∼3 days worth less energy than chow-fed controls at the time that they were switched to the less preferred chow. Furthermore, subjects showed control levels of preferred diet intake during the 24 h before being switched back to chow diet. Nonetheless, once body weight and energy intake begin to cycle bidirectionally, energy homeostatic mechanisms may also contribute to a vicious circle of cycling energy intake. In this light, the transiently greater intake of the preferred diet beginning from week 4 may be partly driven by the preceding self-restriction from chow.
Perspectives and Significance
While intermittent, extended access to highly valued rewards is known to increase their consumption and positive reinforcing efficacy (8, 19, 24, 29, 35, 51, 66, 67, 94–96), less attention in food intake research has addressed the effects of such access on the acceptance and reinforcing efficacy of otherwise satisfying alternatives, and how those, in turn, influence the intake of more rewarding options. Here, intermittent, extended access to highly preferred food progressively reduced the acceptance of a less preferred, but otherwise palatable, food. Effects were not due to greater prior intake or weight gain, motor impairment, or facilitated satiation, but were associated with a reduced reinforcing efficacy of the chow in animals that were withdrawn access to the preferred diet. Over time, diet-cycled animals began to overeat the preferred food transiently when access was restored, resembling a “deprivation effect.” In drug abuse research, such changes are regarded as evidence of the “dark side” of addiction, wherein hypohedonia and a negative emotional state are seen during withdrawal from a substance of abuse and hypothesized to drive compulsive intake (53). The present results suggest that this theoretical model may also be relevant to the control of food intake, a hypothesis in its early stages of evaluation (8, 87). The present results also imply that intermittent access to highly preferred food, as practiced by many restrained eaters (26, 55, 72), may progressively decrease the acceptability and selection of perhaps more nutritious foods, and may contribute to relapse to more rewarding alternatives (36).
GRANTS
The work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK70118) and the National Institute of Drug Abuse (K99 DA23680).
Supplementary Material
Acknowledgments
This is manuscript 19472 from The Scripps Research Institute. We recognize the editorial assistance of Mike Arends, the administrative assistance of Mary Gichuhi, and the technical assistance of Bob Lintz and Lara Pockros.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–626, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Archer ZA, Rayner DV, Barrett P, Balik A, Duncan JS, Moar KM, Mercer JG. Hypothalamic energy balance gene responses in the Sprague-Dawley rat to supplementation of high-energy diet with liquid ensure and subsequent transfer to chow. J Neuroendocrinol 17: 711–719, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology 33: 1818–1826, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience 122: 17–20, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav 84: 359–362, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 32: 20–39, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience 139: 813–820, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology 141: 99–106, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res Bull 70: 422–429, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol 284: R1260–R1268, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Booth DA Conditioned satiety in the rat. J Comp Physiol Psychol 81: 457–471, 1972. [DOI] [PubMed] [Google Scholar]
- 14.Cabeza de Vaca S, Krahne LL, Carr KD. A progressive ratio schedule of self-stimulation testing in rats reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacology (Berl) 175: 106–113, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Cheeta S, Brooks S, Willner P. Effects of reinforcer sweetness and the D2/D3 antagonist raclopride on progressive ratio operant performance. Behav Pharmacol 6: 127–132, 1995. [PubMed] [Google Scholar]
- 16.Collier G, Johnson DF, Morgan C. The magnitude-of-reinforcement function in closed and open economies. J Exp Anal Behav 57: 81–89, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper SJ Palatability-dependent appetite and benzodiazepines: new directions from the pharmacology of GABA(A) receptor subtypes. Appetite 44: 133–150, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Corwin RL Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite 42: 139–142, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Corwin RL Bingeing rats: a model of intermittent excessive behavior? Appetite 46: 11–15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav 82: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav 65: 545–553, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Cottone P, Sabino V, Nagy TR, Coscina DV, Zorrilla EP. Feeding microstructure in diet-induced obesity susceptible versus resistant rats: central effects of urocortin 2. J Physiol 583: 487–504, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology 32: 1069–1081, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 33: 524–535, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 182: 579–587, 2005. [DOI] [PubMed] [Google Scholar]
- 26.de Castro JM The relationship of cognitive restraint to the spontaneous food and fluid intake of free-living humans. Physiol Behav 57: 287–295, 1995. [DOI] [PubMed] [Google Scholar]
- 27.de Villiers PA, Herrnstein RJ. Toward a law of response strength. Psychol Bull 83: 1131–1153, 1976. [Google Scholar]
- 28.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord 28: 436–445, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Files FJ, Lewis RS, Samson HH. Effects of continuous versus limited access to ethanol on ethanol self-administration. Alcohol 11: 523–531, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty CF Effect of anxiolytics and antidepressants on extinction and negative contrast. Pharmacol Ther 46: 309–320, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Flaherty CF, Coppotelli C, Grigson PS, Mitchell C, Flaherty JE. Investigation of the devaluation interpretation of anticipatory negative contrast. J Exp Psychol 21: 229–247, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Foltin RW “Tasting and wasting” behavior in non-human primates: aberrant behavior or normal behavior in “times of plenty”. Physiol Behav 89: 587–597, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Foltin RW, Haney M. Effects of the cannabinoid antagonist SR141716 (rimonabant) and d-amphetamine on palatable food and food pellet intake in non-human primates. Pharmacol Biochem Behav 86: 766–773, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner EL What we have learned about addiction from animal models of drug self-administration. Am J Addict 9: 285–313, 2000. [DOI] [PubMed] [Google Scholar]
- 35.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA 104: 17198–17203, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology 31: 2188–2196, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez VM, Vitousek KM. Feared food in dieting and non-dieting young women: a preliminary validation of the Food Phobia Survey. Appetite 43: 155–173, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Grigson PS, Spector AC, Norgren R. Microstructural analysis of successive negative contrast in free-feeding and deprived rats. Physiol Behav 54: 909–916, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord 34: 183–197, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord 22: 411–420, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav 77: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology 28: 1463–1471, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Hodos W Progressive ratio as a measure of reward strength. Science 134: 943–944, 1961. [DOI] [PubMed] [Google Scholar]
- 44.Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav 6: 387–392, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L. Effects of terguride, ropinirole, and acetyl-l-carnitine on methamphetamine withdrawal in the rat. Pharmacol Biochem Behav 83: 403–409, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Kales EF Macronutrient analysis of binge eating in bulimia. Physiol Behav 48: 837–840, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Killeen PR Economics, ecologics, and mechanics: The dynamics of responding under conditions of varying motivation. J Exp Anal Behav 64: 405–431, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology 186: 48–53, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Knackstedt LA, Kalivas PW. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322: 1103–1109, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Knight LJ, Boland FJ. Restrained eating: an experimental disentanglement of the disinhibiting variables of perceived calories and food type. J Abnorm Psychol 98: 412–420, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Koob GF Alcoholism: allostasis and beyond. Alcoholism Clin Exp Res 27: 232–243, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Koob GF Animal models of craving for ethanol. Addiction 95 Suppl 2: S73–S81, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side' of drug addiction. Nat Neurosci 8: 1442–1444, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. Behavioral and biological correlates of dietary restraint in normal life. Appetite 12: 83–94, 1989. [DOI] [PubMed] [Google Scholar]
- 56.Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology 32: 616–624, 2007. [DOI] [PubMed] [Google Scholar]
- 57.LeSage MG, Burroughs D, Pentel PR. Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav 83: 585–591, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Levin BE, Dunn-Meynell AA. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 282: R46–R54, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Li TK Clinical perspectives for the study of craving and relapse in animal models. Addiction 95 Suppl 2: S55–S60, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Loh EA, Roberts DC. Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology 101: 262–266, 1990. [DOI] [PubMed] [Google Scholar]
- 61.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav 91: 432–439, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin-releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology 195: 591–603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology 112: 163–182, 1993. [DOI] [PubMed] [Google Scholar]
- 64.McGregor A, Roberts DC. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Res 624: 245–252, 1993. [DOI] [PubMed] [Google Scholar]
- 65.Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol 2: 43–49, 1992. [DOI] [PubMed] [Google Scholar]
- 66.O'Dell LE, Koob GF. ‘Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav 86: 346–353, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28: 1676–1682, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Orsini C, Koob GF, Pulvirenti L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology 25: 789–792, 2001. [DOI] [PubMed] [Google Scholar]
- 69.Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14: 2229–2232, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav 16: 693–698, 1976. [DOI] [PubMed] [Google Scholar]
- 71.Pinel JP, Mucha RF, Rovner LI. Temporary effects of periodic alcohol availability. Behav Biol 16: 227–232, 1976. [DOI] [PubMed] [Google Scholar]
- 72.Polivy J, Herman CP. Dieting and binging. A causal analysis. Am Psychol 40: 193–201, 1985. [DOI] [PubMed] [Google Scholar]
- 73.Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology 15: 417–423, 1996. [DOI] [PubMed] [Google Scholar]
- 74.Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology 98: 408–411, 1989. [DOI] [PubMed] [Google Scholar]
- 75.Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79: 439–450, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Rodefer JS, Carroll ME. Progressive ratio and behavioral economic evaluation of the reinforcing efficacy of orally delivered phencyclidine and ethanol in monkeys: effects of feeding conditions. Psychopharmacology 128: 265–273, 1996. [DOI] [PubMed] [Google Scholar]
- 77.Rogers PJ Returning ‘cafeteria-fed' rats to a chow diet: negative contrast and effects of obesity on feeding behaviour. Physiol Behav 35: 493–499, 1985. [DOI] [PubMed] [Google Scholar]
- 78.Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162: 127–134, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Schroy PL, Wheeler RA, Davidson C, Scalera G, Twining RC, Grigson PS. Role of gustatory thalamus in anticipation and comparison of rewards over time in rats. Am J Physiol Regul Integr Comp Physiol 288: R966–R980, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Schwabe K, Koch M. Effects of aripiprazole on operant responding for a natural reward after psychostimulant withdrawal in rats. Psychopharmacology 191: 759–765, 2007. [DOI] [PubMed] [Google Scholar]
- 81.Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav 79: 663–670, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Smith A, Piercey M, Roberts DC. Effect of (−)-DS 121 and (+)-UH 232 on cocaine self-administration in rats. Psychopharmacology 120: 93–98, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Snowdon CT Motivation, regulation, and the control of meal parameters with oral and intragastric feeding. J Comp Physiol Psychol 69: 91–100, 1969. [DOI] [PubMed] [Google Scholar]
- 84.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81: 119–145, 1974. [DOI] [PubMed] [Google Scholar]
- 85.Spear DJ, Katz JL. Cocaine and food as reinforcers: effects of reinforcer magnitude and response requirement under second-order fixed-ratio and progressive-ratio schedules. J Exp Anal Behav 56: 261–275, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stirling LJ, Yeomans MR. Effect of exposure to a forbidden food on eating in restrained and unrestrained women. Int J Eat Disord 35: 59–68, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry 61: 1021–1029, 2007. [DOI] [PubMed] [Google Scholar]
- 88.Thorndike EL Animal Intelligence. New York: Macmillan, 1911.
- 89.Tolkamp BJ, Kyriazakis II. To split behaviour into bouts, log-transform the intervals. Anim Behav 57: 807–817, 1999. [DOI] [PubMed] [Google Scholar]
- 90.Treit D, Spetch ML. Caloric regulation in the rat: control by two factors. Physiol Behav 36: 311–317, 1986. [DOI] [PubMed] [Google Scholar]
- 91.Wayner MJ, Fraley S. Enhancement of the consumption of acclimated sapid solutions following periodic and prolonged withdrawal. Physiol Behav 9: 463–474, 1972. [DOI] [PubMed] [Google Scholar]
- 92.Weatherly JN, Nurnberger JT, Hanson BC. Investigating the procedural variables that determine whether rats will display negative anticipatory contrast or positive induction. Behav Proc 70: 10–18, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Weatherly JN, Nurnberger JT, Kristiansen-Moen LA. The role of place of reinforcer delivery in the appearance of positive induction when rats respond for 1% sucrose. Behav Proc 73: 156–163, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Wee S, Mandyam CD, Lekic DM, Koob GF. alpha(1)-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol 18: 303–311, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther 320: 1134–1143, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology 32: 2238–2247, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wojnicki FH, Roberts DC, Corwin RL. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol Biochem Behav 84: 197–206, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med 12: 62–66, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80: 65–119, 2007. [DOI] [PubMed] [Google Scholar]
- 100.Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W. Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addiction Biol 12: 152–157, 2007. [DOI] [PubMed] [Google Scholar]
- 101.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288: R1450–R1467, 2005. [DOI] [PubMed] [Google Scholar]
- 102.Zorrilla EP, Inoue K, Valdez GR, Tabarin A, Koob GF. Leptin and post-prandial satiety: acute central leptin more potently reduces meal frequency than meal size in the rat. Psychopharmacology 177: 324–335, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.