Abstract
The anti-inflammatory cytokine interleukin (IL)-10 is important for regulating inflammation in the periphery and brain, but whether it protects against infection- or age-related psychomotor disturbances and fatigue is unknown. Therefore, the present study evaluated motor coordination, time to fatigue, and several central and peripheral proinflammatory cytokines in male young adult (3-mo-old) and middle-aged (12-mo-old) wild-type (IL-10+/+) and IL-10-deficient (IL-10−/−) mice after intraperitoneal injection of lipopolysaccharide (LPS) or saline. No age-related differences were observed; therefore, data from the two ages were pooled and analyzed to determine effects of genotype and treatment. LPS treatment increased IL-1β, IL-6, and TNFα mRNA in all brain areas examined in IL-10+/+ and IL-10−/− mice, but to a greater extent and for a longer time in IL-10−/− mice. Plasma IL-1β and IL-6 were increased similarly in IL-10+/+ and IL-10−/− mice 4 h after LPS but remained elevated longer in IL-10−/− mice, whereas TNFα was higher in IL-10−/− mice throughout after LPS treatment. Motor performance and motor learning in IL-10+/+ mice were not affected by LPS treatment; however, both were reduced in IL-10−/− mice treated with LPS compared with those treated with saline. Furthermore, although LPS reduced the time to fatigue in IL-10+/+ and IL-10−/− mice, the effects were exacerbated in IL-10−/− mice. Thus the increased brain and peripheral inflammation induced by LPS in IL-10−/− mice was associated with increased coordination deficits and fatigue. These data suggest that IL-10 may inhibit motor deficits and fatigue associated with peripheral infections via its anti-inflammatory effects.
Keywords: brain, proinflammatory cytokines, motor coordination, lipopolysaccharide
the proinflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNFα) are well known to induce sickness behavior in response to infection (7). In addition to sickness behavior, proinflammatory cytokines reduce muscle mass and strength (20, 30) and, also, may affect brain areas involved in motor coordination and fatigue (8). This outcome may be particularly important in the elderly, because inflammation tends to increase with age and is often paralleled by a decline in psychomotor function, largely marked by impaired coordination, balance, and strength (16). These motor and balance disruptions are the major causes of admission into hospitals and nursing homes (18), and it is estimated that more deaths in the elderly are caused by injuries from falls than by pneumonia or diabetes (12a). It is noteworthy that constitutive expression of several proinflammatory cytokines is increased in the periphery and brain of old, but otherwise healthy, animals (32). Moreover, when lipopolysaccharide (LPS) is administered to mimic a peripheral infection, old mice experienced an exaggerated proinflammatory cytokine response in the brain and exhibited signs of behavioral pathology, including prolonged anorexia (10), depressive-like behavior (11), and cognitive dysfunction (2), that were not seen in younger cohorts. Collectively, these studies support a linkage between inflammation and motor deficits and fatigue.
The anti-inflammatory cytokine IL-10 can inhibit the effector functions of macrophages and microglia by blocking proinflammatory cytokine synthesis, suppressing expression of receptors for proinflammatory cytokines, and inhibiting cytokine receptor activation (28). IL-10, therefore, is important for restricting the proinflammatory response during infection, and, not surprisingly, mice deficient in IL-10 have higher serum levels of proinflammatory cytokines after LPS administration (1). This finding is relevant to the geriatric population as well, because the age-associated increase in proinflammatory cytokines in the brain is inversely related to IL-10 expression in rats and mice (22, 32).
Given the purported relationship between proinflammatory cytokines and motor deficits and fatigue, the present study was conducted to determine whether IL-10 protects against infection- or age-related psychomotor disturbances and fatigue. Specifically, we evaluated motor coordination, time to fatigue, and several proinflammatory cytokine proteins and mRNAs in plasma and brain, respectively, in young adult and middle-aged wild-type and IL-10-deficient mice after intraperitoneal injection of LPS. Our hypothesis was that fatigue and motor deficits in older mice and mice challenged with LPS would be exacerbated in the absence of the anti-inflammatory cytokine IL-10.
MATERIALS AND METHODS
Animals
Male C57BL/6J (WT; IL-10+/+) and IL-10-knockout B6.129P2-II10tm1Cgn (IL-10−/−) mice were purchased from Jackson Laboratories (Bar Harbor, ME) at 2 mo of age and maintained at the University of Illinois under specific pathogen-free conditions to minimize the development of colitis (14). Mice were housed in polypropylene cages and maintained at 23°C under a reverse-phase 12:12-h light-dark cycle with ad libitum access to water and rodent chow. To test the mice when they are naturally active, all experimental procedures were carried out during the dark phase of the light-dark cycle under infrared lighting. Mice were 3–5 or 10–12 mo old when studies were conducted. Animals that exhibited signs of colitis (e.g., perianal ulceration, diarrhea, rectal prolapse, or weight loss) were removed from the study (n = 2). All procedures were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals and were approved by the University of Illinois Laboratory Animal Care and Use Committee.
Experimental Design
To determine the effect of age, LPS, and IL-10 on motor coordination and fatigue, 5- and 11-mo-old IL-10+/+ and IL-10−/− mice were injected intraperitoneally with 0.33 mg/kg (∼10 μg/mouse) Escherichia coli LPS (serotype 0127:B8, Sigma, St. Louis, MO) or sterile saline (n = 8/group). Mice were tested on the rotarod treadmill in three consecutive trials at 4 and 24 h after injection. At the end of the last rotarod trial at the 24-h time point, mice were allowed to rest for 1 h and then placed on a motorized treadmill and tested for time to fatigue. Immediately after the exhaustive fatigue test, mice were killed by CO2 asphyxiation, blood was collected by cardiac puncture, and the brain was quickly dissected for collection of the hippocampus, striatum, cortex, and cerebellum. Brain tissue was placed in RNAlater, and tissue and plasma were stored at −80°C until assaying for peripheral and central proinflammatory cytokines. Effects of LPS on the inflammatory response at a time point corresponding to the behavioral test were determined in a separate study: IL-10+/+ and IL-10−/− mice (n = 6/group) were injected with LPS or saline and killed 4 h thereafter for collection of blood and brain.
Behavioral Tests
Motor function.
A rotarod treadmill (Med Associates, St Albans, VT) was used to assess motor coordination. Mice were placed on a 4-cm-diameter horizontal rod positioned 16.5 cm above a cushioned base. Immediately before the first test session, mice were allowed a 30-s acclimation period, during which the speed of the rotarod was held constant at 4 rpm. Mice had to maintain their footing and balance as the rotating drum accelerated. During the test sessions, the speed of rotation was increased by continuous acceleration from 0 to 40 rpm for 5 min, and latency to fall was measured. Animals that reached maximum speed were allowed to stay on the rotarod for a total of 500 s and then were removed from the apparatus. Each test session consisted of three trials with a 1-h intertrial interval.
Exhaustive fatigue.
For assessment of volitional fatigue, mice were placed on a motorized treadmill (Jog-a-Dog, Ottawa Lake, MI) in individual lanes separated by plastic dividers 24 h after LPS or saline injection. For each test, mice ran for 10 min at 6 m/min and then for an 8-min period, during which the speed was gradually increased to 18 m/min. The final speed corresponded to an intensity of ∼80–85% of their maximal oxygen uptake (23). The mice ran until volitional exhaustion, which was defined as 10 s of continual nonrunning at the back of the treadmill lane, where a foam sponge was placed. Electric shock was not used as negative reinforcement to induce running behavior.
Cytokine Determination
Plasma cytokines.
Plasma samples were assayed for IL-1β, IL-6, and TNFα with use of a multiplex bead-based immunoassay kit combined with a cytokine reagent kit as described by the manufacturer (Bio-Rad, Hercules, CA). IL-10 was assayed with the use of a quantikine mouse IL-10 immunoassay kit as described by the manufacturer (R&D Systems, Minneapolis, MN). The assays were sensitive to <3 pg/ml for IL-1β, IL-6, TNFα, and IL-10. The inter- and intra-assay coefficients of variation were <8%.
Cytokine mRNA in brain.
Total RNA was isolated from homogenized brain regions using the TriReagent protocol (Sigma). A Quanti Tect reverse transcription kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer's protocol. Briefly, RNA samples were mixed with gDNA Wipeout Buffer and RNase-free water and incubated at 42°C for 2 min. Quantiscript reverse transcriptase, Quantiscript RT buffer, and RT primer mix were added to samples, which were incubated at 42°C for 15 min and then incubated at 95°C for 3 min to inactivate Quantiscript reverse transcriptase. Quantitative real-time PCR was performed using the Assay-on-Demand gene expression protocol (Applied Biosystems, Foster City, CA). Briefly, cDNA was amplified by PCR, where a target cDNA (Mm00434228_ml for IL-1β, m00443258_ml for TNFα, and Mm00446190_ml for IL-6) and a reference cDNA (Mn99999915_gl for glucose-3 phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′-fluorescent reporter dye (6-carboxyfluorescein) and a 3′-nonfluorescent quencher dye. PCRs were performed under the following conditions: 50°C for 2 min and 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method as previously described (5), and results are expressed as fold change compared with saline controls.
Data Analysis
Statistical Analysis Systems general linear model procedures were used for data analysis. Data were subjected to two-way (genotype × treatment) or three-way (genotype × treatment × age) ANOVA or a repeated-measures ANOVA in which test day was a within-subjects measure (i.e., repeated measure) and LPS (0 μg or 10 μg), genotype (IL-10+/+ or IL-10−/−), and age (adult and middle-aged) were between-subjects measures to determine significant main effects as well as interactions between these main effects. Values are means ± SE.
RESULTS
Psychomotor Deficits in IL-10+/+ and IL-10−/− Mice After LPS Administration
Effects of age, peripheral immune stimulation, and IL-10 deficiency on psychomotor performance and fatigue were determined in 5- and 11-mo-old IL-10+/+ and IL-10−/− mice intraperitoneally injected with LPS. Although increased brain cytokine production and a more severe sickness behavior syndrome had been reported in older (22- to 24-mo-old) rodents injected with LPS (6, 9, 10, 12, 29), contrary to our hypothesis, no effects of age were observed for any measurement. Therefore, data from the two age groups were pooled and analyzed for assessment of genotype and treatment effects.
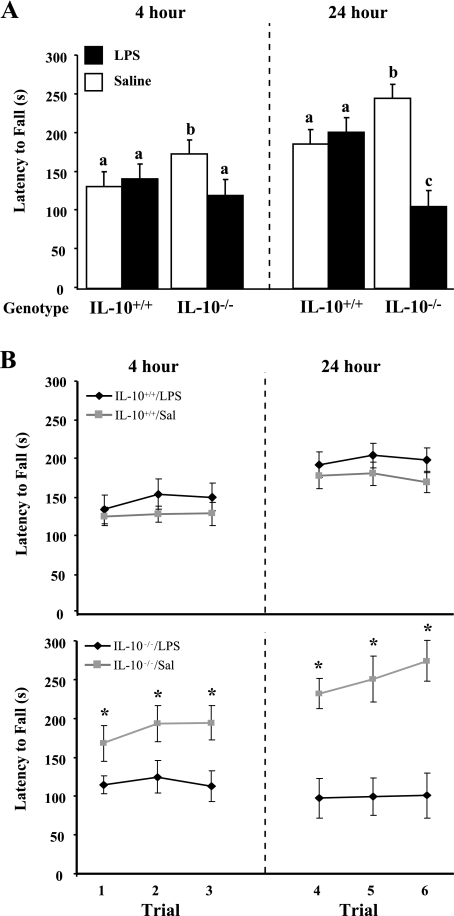
Motor coordination was assessed on the rotarod treadmill 4 and 24 h after injection, and the average latency to fall during the three trials conducted at each time point was determined (Fig. 1A). ANOVA of latency to fall from the rotarod revealed main effects of time [F(1,28) = 9.19, P = 0.003] and treatment [F(1,28) = 13.47, P = 0.0004] and a treatment × genotype [F(1,28) = 28.51, P < 0.0001] interaction. There was a tendency for a treatment × time [F(1,28) = 2.81, P = 0.0962] and treatment × genotype × time [F(1,28) = 3.27, P = 0.0731] interaction. Post hoc analysis indicated that LPS did not affect performance of IL-10+/+ mice on the rotarod at 4 h (P = 0.2643), whereas performance of IL-10−/− mice was worse after LPS injection than in saline-treated controls (P = 0.0069). Moreover, IL-10+/+ mice showed improvement in motor coordination 24 h after LPS, but motor deficits were still apparent in IL-10−/− mice (P < 0.0001). Furthermore, only LPS-treated IL-10−/− mice failed to demonstrate motor learning by improving performance over consecutive trials conducted at 4 and 24 h (Fig. 1B). Consecutive training on an accelerating rotarod can be regarded as a valid paradigm for motor skill learning (4). Interestingly, the saline- and LPS-treated IL-10+/+ mice and the saline-treated IL-10−/− mice improved performance on the rotarod; among all the treatment groups, the saline-treated IL-10−/− mice seemed to exhibit the greatest improvement. These results indicate that the psychomotor disturbances associated with LPS treatment were more pronounced and persistent in IL-10−/− mice.
Fig. 1.
A: rotarod treadmill performance of IL-10+/+ and IL-10−/− mice 4 and 24 h after peripheral injection of saline or LPS. Average latency to fall from the rotarod was determined for each animal in 3 trials conducted 4 h after injection and another 3 trials conducted 24 h after injection. Values are means ± SE. Means with different letters (a, b, c) are significantly different (P < 0.05). B: motor learning in IL-10+/+ and IL-10−/− mice 4 and 24 h after peripheral injection of saline or LPS. Motor learning was considered if mice exhibited improved performance over consecutive trials. Values are means ± SE. *Significantly different from LPS (P < 0.05).
Fatigue in IL-10+/+ and IL-10−/− Mice After Peripheral LPS Injection
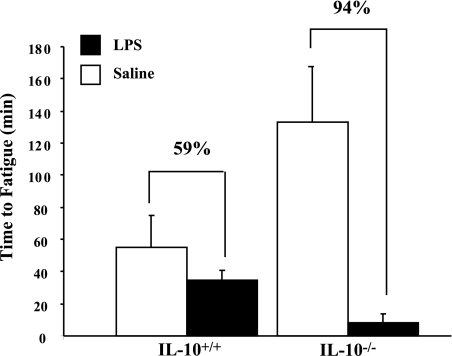
To determine whether IL-10 affected exhaustive fatigue following peripheral immune activation, time to fatigue on a motorized treadmill was determined for IL-10+/+ and IL-10−/− mice 24 h after LPS injection (Fig. 2). ANOVA of time to fatigue revealed a significant main effect of LPS [F(1,28) = 14.44, P = 0.0007] as well as an LPS × genotype [F(1,28) = 7.46, P = 0.0108] interaction. Moreover, in IL-10+/+ mice, LPS treatment tended to reduce time to fatigue compared with saline-treated IL-10+/+ mice (P = 0.0950), whereas in IL-10−/− mice time to fatigue was markedly depressed (P < 0.0001). Although not statistically significant, LPS treatment reduced time to fatigue in IL-10+/+ mice by 59%, whereas time to fatigue in IL-10−/− mice was reduced 94%. The reduced time to fatigue in IL-10−/− mice after LPS injection was particularly striking, because saline-treated IL-10−/− mice resisted fatigue. Thus, in the absence of peripheral immune stimulation, IL-10 deficiency seemed to enhance motor function and time to fatigue. When LPS is administered to mimic a peripheral infection, the findings indicate that IL-10 deficiency leads to gross motor impairments and fatigue.
Fig. 2.
Exhaustive fatigue in IL-10+/+ and IL-10−/− mice 24 h after treatment with saline or LPS. Time to fatigue was determined in mice that ran on a motorized treadmill until volitional exhaustion. Values are means ± SE.
Proinflammatory Cytokines in IL-10+/+ and IL-10−/− Mice After Peripheral LPS Injection
Plasma IL-1β, IL-6, and TNFα levels were measured in IL-10+/+ and IL-10−/− mice 4 and 24 h after injection of saline or LPS (Table 1). Furthermore, because the striatum, cerebellum, and motor cortical regions of the frontal lobe are important for the acquisition and/or retention of skilled motor behaviors (21), proinflammatory cytokine mRNA levels were measured in the cerebellum, cortex, and striatum (Table 1). Proinflammatory cytokine mRNA levels were also determined in the hippocampus, because this area is associated with LPS-induced deficits in learning and memory. Plasma and brain proinflammatory cytokine data were subjected to ANOVA, and the significance of the main effects (genotype and treatment) and interaction of main effects (genotype × treatment) at each time point are presented in Table 1.
Table 1.
Proinflammatory cytokine mRNA and protein in brain and plasma, respectively, of IL-10+/+ and IL-10−/− mice 4 and 24 after peripheral injection of saline or LPS
|
IL-10+/+ Mice |
IL-10−/− Mice
|
P
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | LPS | Saline | LPS | G | T | Age | G*T | ||||||||
| 4 hours | |||||||||||||||
| Cerebellum cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 1.65±0.40* | 19.36±2.90† | 0.86±0.07 | 28.15±4.61‡ | 0.07 | 0.001 | 0.145 | 0.08 | |||||||
| IL-6 | 1.35±0.13* | 58.09±18.75† | 1.28±0.24* | 105.36±27.89† | 0.112 | 0.001 | 0.599 | 0.167 | |||||||
| IL-1β | 1.28±0.18* | 56.85±9.57† | 2.41±0.35* | 91.58±12.10‡ | 0.008 | 0.001 | 0.135 | 0.03 | |||||||
| Cortex cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 0.848±0.32* | 17.33±2.93† | 4.61±2.07* | 18.52±4.56† | 0.717 | 0.001 | 0.861 | 0.691 | |||||||
| IL-6 | 1.46±0.42* | 10.12±2.17* | 4.52±1.96* | 28.97±6.61† | 0.004 | 0.001 | 0.437 | 0.04 | |||||||
| IL-1β | 1.12±0.17* | 39.15±10.50†‡ | 13.28±7.65*‡ | 57.56±13.44† | 0.18 | 0.001 | 0.934 | 0.757 | |||||||
| Hippocampus cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 0.955±0.07* | 26.67±4.73† | 1.31±0.17* | 46.56±4.43‡ | 0.01 | 0.001 | 0.624 | 0.004 | |||||||
| IL-6 | 1.24±0.15* | 12.34±3.07† | 1.34±0.30* | 32.46±4.68‡ | 0.002 | 0.001 | 0.326 | 0.001 | |||||||
| IL-1β | 1.07±0.13* | 69.55±16.19† | 2.52±0.43* | 121.32±10.23‡ | 0.02 | 0.001 | 0.498 | 0.014 | |||||||
| Striatum cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 1.57±0.29* | 39.87±6.80† | 1.66±0.23* | 69.79±10.79‡ | 0.05 | 0.001 | 0.509 | 0.05 | |||||||
| IL-6 | 1.07±0.11* | 6.24±1.17* | 0.81±0.13* | 23.29±3.94† | 0.001 | 0.001 | 0.809 | 0.001 | |||||||
| IL-1β | 1.16±0.17* | 53.17±11.18† | 2.44±0.47* | 103.58±17.73‡ | 0.04 | 0.001 | 0.295 | 0.05 | |||||||
| Plasma cytokine measurements, pg/ml | |||||||||||||||
| TNFα | 2.59±0.16* | 304.69±209.65* | 2.80±0.35* | 2,566.80±774.93† | 0.005 | 0.001 | 0.453 | 0.005 | |||||||
| IL-6 | 6.90±0.49* | 4,933.42±816.32† | 15.87±4.91* | 6,861.98±1,230.50† | 0.194 | 0.0001 | 0.642 | 0.198 | |||||||
| IL-1β | 12.16±0.25* | 38.14±5.28† | 11.32±1.02* | 51.63±9.49† | 0.243 | 0.0001 | 0.875 | 0.187 | |||||||
| IL-10 | 5.09±0.27* | 40.81±11.63† | ND | ND | |||||||||||
| 24 hours | |||||||||||||||
| Cerebellum cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 1.94±0.84* | 3.94±0.59† | 1.39±0.55*‡ | 4.42±0.80†‡ | 0.894 | 0.01 | 0.779 | 0.50 | |||||||
| IL-6 | 1.54±0.53* | 0.874±0.25* | 0.682±0.16* | 16.61±6.92† | 0.02 | 0.09 | 0.771 | 0.06 | |||||||
| IL-1β | 1.76±0.61* | 9.28±1.05† | 3.02±0.66* | 9.19±1.98 | 0.907 | 0.001 | 0.586 | 0.650 | |||||||
| Cortex cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 1.21±0.19* | 8.78±0.93† | 1.84±0.26* | 14.04±2.65‡ | 0.06 | 0.001 | 0.197 | 0.218 | |||||||
| IL-6 | 1.08±0.13* | 0.401±0.06* | 1.08±0.13* | 4.53±1.31† | 0.002 | 0.125 | 0.404 | 0.019 | |||||||
| IL-1β | 1.14±0.14* | 6.47±0.74† | 1.78±0.40* | 5.28±1.15† | 0.447 | 0.001 | 0.388 | 0.335 | |||||||
| Hippocampus cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 1.23±0.13* | 8.32±1.01† | 1.43±0.41* | 9.76±1.77† | 0.585 | 0.001 | 0.364 | 0.663 | |||||||
| IL-6 | 0.476±0.05* | 0.194±0.02* | 0.397±0.04* | 2.02±0.68† | 0.009 | 0.155 | 0.657 | 0.03 | |||||||
| IL-1β | 1.21±0.16* | 9.08±1.15† | 1.95±0.68* | 8.77±1.75† | 0.871 | 0.001 | 0.349 | 0.727 | |||||||
| Striatum cytokine mRNA, fold increase | |||||||||||||||
| TNFα | 0.212±0.21* | 0.606±0.14* | 0.262±0.19* | 0.688±0.13* | 0.690 | 0.02 | 0.611 | 0.925 | |||||||
| IL-6 | 0.788±0.38* | 0.338±0.29* | 0.656±0.42* | 2.08±0.28† | 0.001 | 0.207 | 0.261 | 0.01 | |||||||
| IL-1β | 1.39±2.00* | 6.86±1.55† | 1.00±2.45* | 6.80±1.48† | 0.805 | 0.006 | 0.341 | 0.932 | |||||||
| Plasma cytokine measurements, pg/ml | |||||||||||||||
| TNFα | 24.19±3.92* | 41.33±4.11* | 33.95±3.85* | 184.75±44.23† | 0.001 | 0.007 | 0.274 | 0.025 | |||||||
| IL-6 | 25.11±21.09* | 466.98±332.73* | 16.07±5.32* | 78,224.60±3,639.97† | 0.021 | 0.084 | 0.257 | 0.08 | |||||||
| IL-1β | 61.95±16.11* | 70.31±12.80* | 61.09±18.75* | 474.25±23.62† | 0.089 | 0.189 | 0.898 | 0.197 | |||||||
| IL-10 | 2.99±3.57* | 22.72±2.53† | ND | ND | — | — | — | — | |||||||
Values are means ± SE. G, genotype; T, treatment; ND, not determined. Meanswithin a row with different symbols are significantly different (P < 0.05).
In IL-10−/− mice, plasma IL-10 concentration was below assay sensitivity in the absence and presence of LPS stimulation. However, IL-10 was detectable in plasma of saline-treated IL-10+/+ mice, and levels increased after LPS injection. Plasma IL-1β and IL-6 were increased in LPS-treated IL-10+/+ and IL-10−/− mice 4 h after peripheral immune stimulation compared with saline controls. Plasma TNFα, however, was increased in LPS-treated IL-10−/− mice but was not significantly increased in IL-10+/+ mice at 4 h, probably because, in wild-type mice, TNFα levels peak 1–2 h after LPS treatment and return to baseline shortly thereafter (1). At 24 h after injection, cytokine levels were returning to baseline in LPS-treated IL-10+/+ mice, yet the proinflammatory cytokines remained elevated in similarly treated IL-10−/− mice. Thus the LPS-induced elevation in plasma proinflammatory cytokines was prolonged in the absence of IL-10.
Treatment with LPS increased IL-1β, TNFα, and IL-6 mRNA at 4 h in all brain areas in IL-10+/+ and IL-10−/− mice; however, the increase was often greater in IL-10−/− mice. For example, mRNA levels for the three proinflammatory cytokines were markedly higher in three of the four brain areas sampled in LPS-treated IL-10−/− mice than in LPS-treated IL-10+/+ mice. In LPS-treated mice, IL-1β and TNFα mRNA levels in all brain regions examined returned toward baseline at 24 h but were still higher than in saline-treated mice, irrespective of genotype. Interestingly, IL-6 mRNA level in the four brain regions returned to baseline at 24 h in LPS-treated IL-10+/+ mice but was still higher in IL-10−/− mice, especially in the cerebellum. Taken together, these data indicate that, in brain areas important for skilled motor behaviors, IL-10 mediates the magnitude and duration of the proinflammatory cytokine response during peripheral immune activation.
DISCUSSION
Anti-inflammatory cytokines play an important role in regulating inflammation in the periphery and brain (27, 32). Because IL-10 has been reported to be decreased in the brain of aged humans and mice, allowing inflammatory cytokines to increase (22, 32), one aim of the present study was to determine whether IL-10 deficiency would hasten the onset of age-associated psychomotor deficits. If performance in fatigue and motor coordination tasks is poorer in older than in younger IL-10−/− mice, we thought this strain might be useful for investigation of psychomotor aging. Unfortunately, we found no age effect on any measurement, perhaps because the groups differed in age by just 6 mo. In some instances, we saw improved performance in the older IL-10−/− mice compared with the younger IL-10−/− mice (e.g., fatigue test in saline-treated mice). It was difficult to expand the age difference further because of the age-associated risk that the IL-10−/− mice might develop colitis even while maintained under specific pathogen-free conditions (14). Clinical signs of chronic inflammatory bowel disease are diarrhea, perianal ulceration, rectal prolapse, and intestinal bleeding (3, 14). Indeed, two animals exhibited rectal prolapses and were removed from the study. Thus the IL-10−/− mice did not prove to be useful for studying age-related psychomotor deficits, as we had hoped.
That there were no differences attributed to age does not lessen the importance of the dissimilar responses of IL-10+/+ and IL-10−/− mice to peripheral immune stimulation. In the present study, LPS-treated IL-10−/− mice exhibited increased motor deficits and a shorter time to fatigue than similarly treated IL-10+/+ mice. The fatigue and motor deficits in LPS-treated IL-10−/− mice were associated with higher plasma inflammatory cytokine levels and increased expression of inflammatory cytokine genes in brain areas important for skilled motor behaviors. We interpret these findings to suggest that IL-10 can inhibit psychomotor deficits related to peripheral infections via its anti-inflammatory effects. The results are less clear about the role of IL-10 in motor performance and fatigue in healthy animals, inasmuch as IL-10−/− mice treated with saline seemed to perform better. Nonetheless, these findings are significant, because psychomotor functions used to perform day-to-day tasks that are necessary for independent living (e.g., stair climbing and walking) are often impaired in chronically infected patients. By extension, the present findings are also relevant to the geriatric population, because the age-related increase in inflammation is associated with a decrease in anti-inflammatory cytokines, including IL-10 (22, 32). It has been estimated that approximately one-third of community-dwelling elderly people suffer at least one fall each year, and this number increases among institutionalized patients (15). In addition, functional and cognitive deterioration entails a greater risk of falls (24). There is evidence of decreased motor performance in individuals with cognitive impairment and dementia when performing an additional cognitive task (26).
In the present study, the rotarod apparatus tested balance and coordination and motor learning, whereas the motorized treadmill tested time to fatigue. The fact that IL-10−/− mice performed poorly compared with IL-10+/+ mice when challenged with LPS is consistent with a linkage between inflammatory cytokines and loss of motor function. In one study, higher cytokine levels were associated with lower muscle mass and lower muscle strength (30). Specifically, IL-6 and TNFα have been shown to cause a loss of muscle mass and strength (20, 30), and these two proinflammatory cytokines, along with IL-1β, remained elevated in peripheral blood of IL-10−/− mice 24 h after LPS injection. In a related study, we recently found IL-6 to be higher in skeletal and cardiac muscle of LPS-treated IL-10−/− mice (17).
It is also noteworthy that the central expression of inflammatory cytokines was typically higher in IL-10−/− mice after LPS treatment. We were particularly interested in the central cytokine compartment because of its potential role in the pathogenesis of immunologically mediated fatigue (25). Chronic fatigue is considered to be the seventh most common symptom in primary healthcare (13). Chronic fatigue syndrome is often diagnosed for patients who present ≥6 mo of unexplained fatigue with other characteristic symptoms that are centrally mediated and known to be inducible by inflammatory cytokines, including cognitive dysfunction (e.g., impaired memory and concentration, depression, and irritability), malaise, poor sleep, and problems maintaining balance. A recent study of patients with postinfective fatigue syndrome that examined the relationship between fatigue symptoms and serum cytokines and cytokines secreted in vitro by isolated peripheral blood mononuclear cells concluded that fatigue was not associated with altered cytokine production (31). However, this study was unable to account for inflammatory cytokines within the central nervous system, where feelings of fatigue are likely to originate. Indeed, the authors suggested an alternative hypothesis for postinfective fatigue syndrome based on inflammatory mediators that are produced by activated brain glial cells. A key role for central inflammatory cytokines in fatigue is supported by a recent study of the perception of exercise-induced fatigue in mice (19). Inhibition of TNFα in the brain, but not in the periphery, increased voluntary exercise, suggesting that the perception of fatigue was delayed, whereas injection of recombinant TNFα intracerebroventricularly reduced voluntary exercise, suggesting that the perception of fatigue was enhanced. Thus inflammatory cytokines produced within the brain may be at least as important as those produced in the periphery for induction of fatigue, although from the present study we cannot distinguish effects of peripheral cytokines from effects of central cytokines. Nonetheless, to the extent that inflammatory cytokines contribute to exhaustive fatigue and deficits in motor coordination in elderly subjects or subjects with an infection, the model described here is useful for exploring ways to prevent or treat fatigue and motor complications.
Perspectives and Significance
Fatigue and motor deficits in IL-10-deficient mice treated with LPS to mimic a peripheral infection were associated with higher plasma inflammatory cytokine levels and increased expression of inflammatory cytokine genes in brain areas important for skilled motor behaviors. These findings are interpreted to suggest that IL-10 can inhibit psychomotor deficits related to peripheral infections via its anti-inflammatory effects. These findings are significant, because psychomotor functions used to perform day-to-day tasks that are necessary for independent living are often impaired in chronically infected patients. Thus strategies to mitigate inflammatory cytokines in the periphery or brain may be useful for prevention of deficits in psychomotor behavior.
GRANTS
This research was supported by National Institutes of Health Grants AG-1670, AG-023580, and MH-069148.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agnello D, Villa P, Ghezzi P. Increased tumor necrosis factor and interleukin-6 production in the central nervous system of interleukin-10-deficient mice. Brain Res 869: 241–243, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging 27: 723–732, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest 98: 1010–1020, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short- and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem 81: 211–216, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22: 301–311, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-α and nitric oxide production in endotoxic mice. J Immunol 156: 1525–1530, 1996. [PubMed] [Google Scholar]
- 7.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis JM, Bailey SP. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 29: 45–57, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Foster KD, Conn CA, Kluger MJ. Fever, tumor necrosis factor, and interleukin-6 in young, mature, and aged Fischer 344 rats. Am J Physiol Regul Integr Comp Physiol 262: R211–R215, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 19: 1329–1331, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, Connor JO, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. In press. [DOI] [PMC free article] [PubMed]
- 12.Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. In press. [DOI] [PMC free article] [PubMed]
- 12a.Klein K, Ritzel DO. Falls Pose a Serious Threat to the Elderly. Washington, DC: National Safety Council, 2005.
- 13.Kroenke K, Wood DR, Mangelsdorff AD, Meier NJ, Powell JB. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA 260: 929–934, 1988. [PubMed] [Google Scholar]
- 14.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75: 263–274, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Lehtola S, Koistinen P, Luukinen H. Falls and injurious falls late in home-dwelling life. Arch Gerontol Geriatr 42: 217–224, 2006. [DOI] [PubMed] [Google Scholar]
- 16.McDowell K, Kerick SE, Santa Maria DL, Hatfield BD. Aging, physical activity, and cognitive processing: an examination of P300. Neurobiol Aging 24: 597–606, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Meador BM, Krzyszton CP, Johnson RW, Huey KA. Effects of IL-10 and age on IL-6, IL-1β, and TNFα responses in mouse skeletal and cardiac muscle to an acute inflammatory insult. J Appl Physiol 104: 991–997, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Menec VH The relation between everyday activities and successful aging: a 6-year longitudinal study. J Gerontol B Psychol Sci Soc Sci 58: S74–S82, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Kullberg BJ, Vonk AG, Verschueren I, Joosten LA, van der Meer JW. Increased voluntary exercise in mice deficient for tumour necrosis factor-α and lymphotoxin-α. Eur J Clin Invest 37: 737–741, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Roth SM, Metter EJ, Ling S, Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol 18: 625–630, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci 23: 393–415, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Saurwein-Teissl M, Blasko I, Zisterer K, Neuman B, Lang B, Grubeck-Loebenstein B. An imbalance between pro- and anti-inflammatory cytokines, a characteristic feature of old age. Cytokine 12: 1160–1161, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol 31: 387–392, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Shaw FE Falls in cognitive impairment and dementia. Clin Geriatr Med 18: 159–173, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Sheng WS, Hu S, Lamkin A, Peterson PK, Chao CC. Susceptibility to immunologically mediated fatigue in C57BL/6 versus Balb/c mice. Clin Immunol Immunopathol 81: 161–167, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer's disease. J Am Geriatr Soc 51: 1633–1637, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Strle K, McCusker RH, Tran L, King A, Johnson RW, Freund GG, Dantzer R, Kelley KW. Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFα-induced resistance to IGF-I in myoblasts. J Neuroimmunol 188: 48–55, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol 21: 427–449, 2001. [PubMed] [Google Scholar]
- 29.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun 64: 769–774, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57: M326–M332, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Vollmer-Conna U, Cameron B, Hadzi-Pavlovic D, Singletary K, Davenport T, Vernon S, Reeves WC, Hickie I, Wakefield D, Lloyd AR. Postinfective fatigue syndrome is not associated with altered cytokine production. Clin Infect Dis 45: 732–735, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. NeuroImmunoModulation 9: 183–192, 2001. [DOI] [PubMed] [Google Scholar]