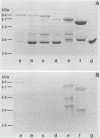
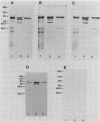
Abstract
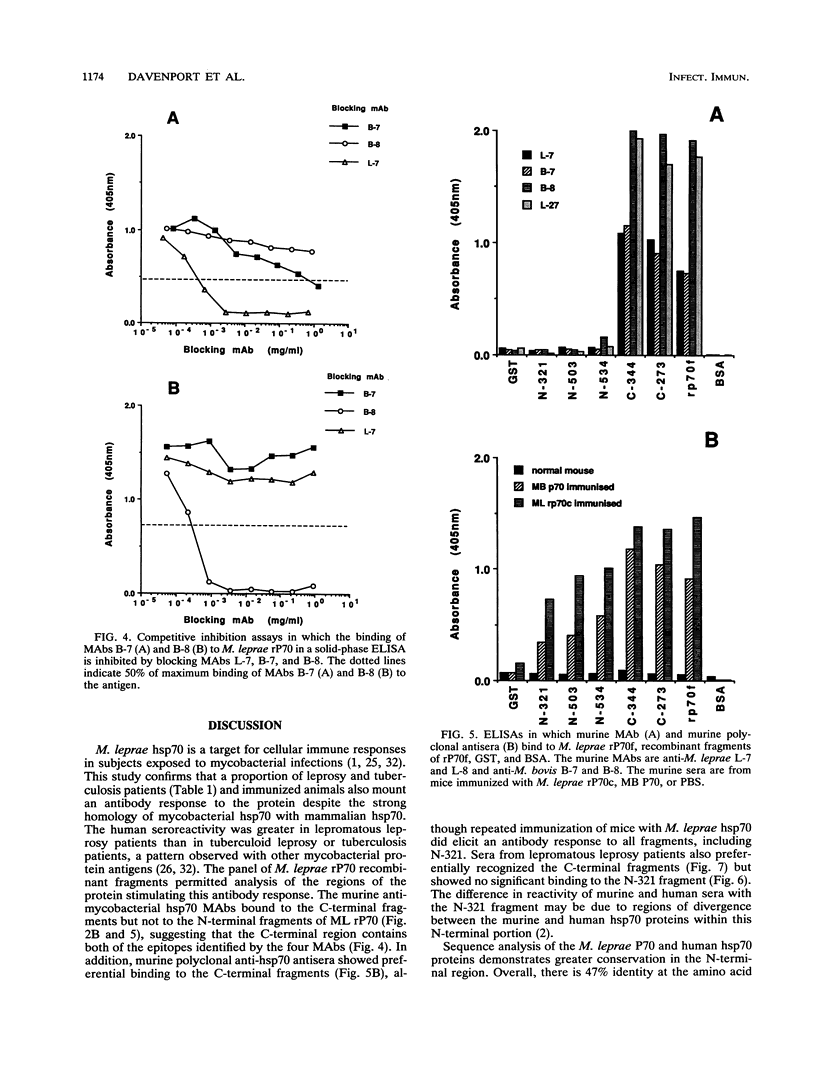
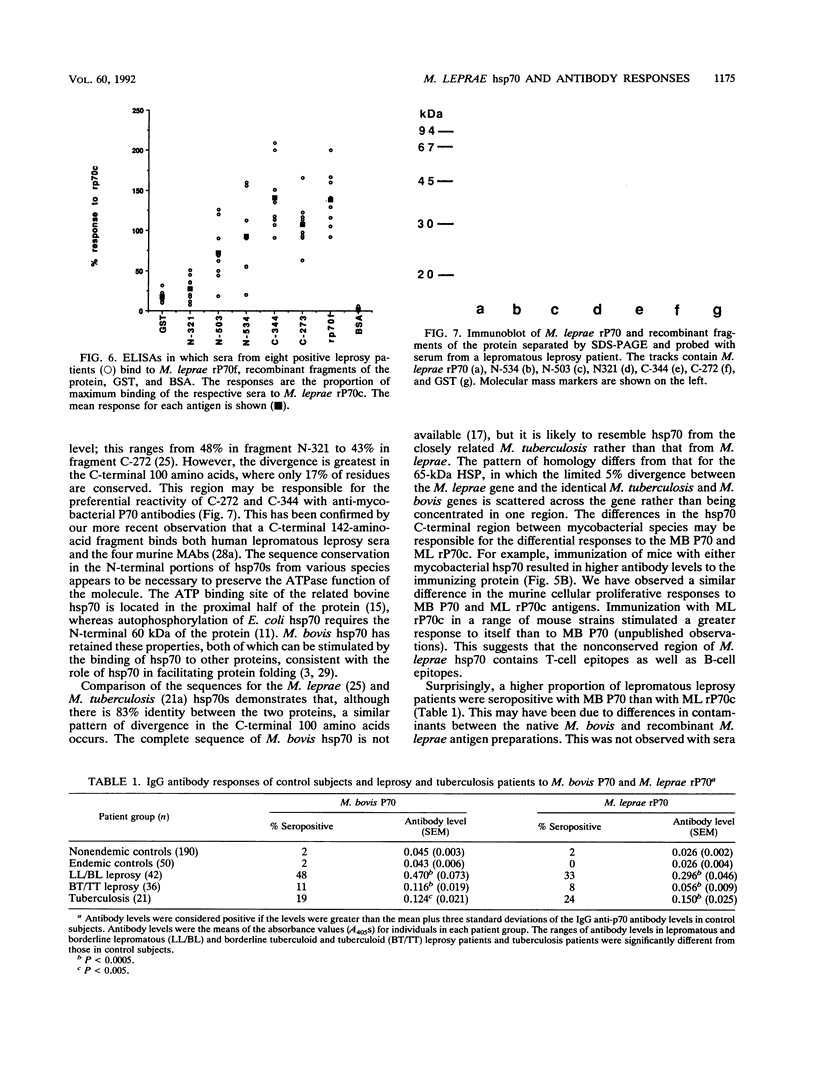
The 70-kDa heat shock protein of Mycobacterium leprae has a high degree of homology with the human hsp70 protein, yet it still elicits T-lymphocyte responses in subjects infected with M. leprae or vaccinated with the related Mycobacterium bovis BCG. We examined the serological responses to this protein by using recombinant protein fragments expressed from mutants with deletions of the M. leprae p70 gene. Monoclonal antibodies raised against either M. bovis or M. leprae p70 reacted with the C-terminal fragments but not the N-terminal fragments in a solid-phase enzyme-linked immunosorbent assay and an immunoblot assay. Inhibition enzyme-linked immunosorbent assays confirmed that two separate epitopes were defined by these monoclonal antibodies. Murine polyclonal sera also showed stronger binding to the C-terminal fragments. Sera from 33 and 48% of lepromatous leprosy patients reacted with M. leprae and M. bovis p70. This reactivity was mycobacterium specific, since few sera from control subjects in the same leprosy-endemic region were seropositive. The levels of anti-mycobacterial hsp70 antibodies were higher in patients with lepromatous leprosy than in those with tuberculoid leprosy or tuberculosis. The reactivity of sera from patients with leprosy was maximal with the C-terminal fragments. Therefore the C-terminal portion of M. leprae hsp70, which includes the region of maximum divergence from human hsp70, is the major target for the humoral immune response to the protein.
Full text
PDF
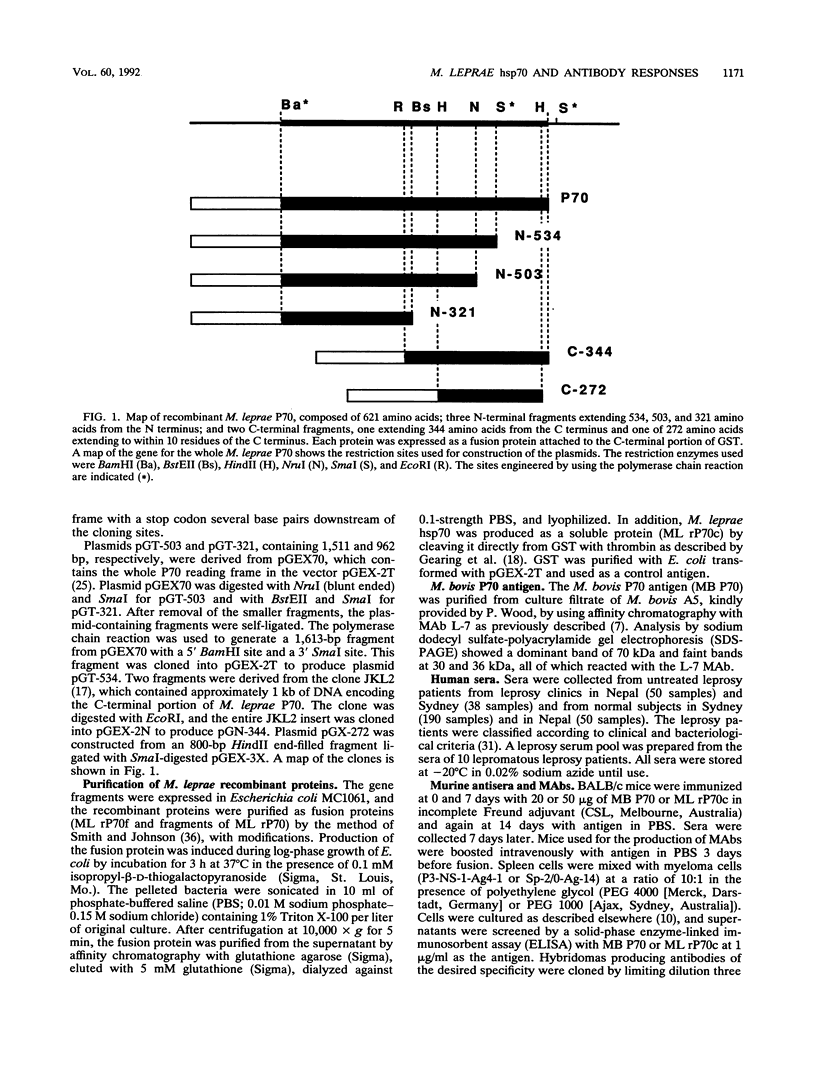

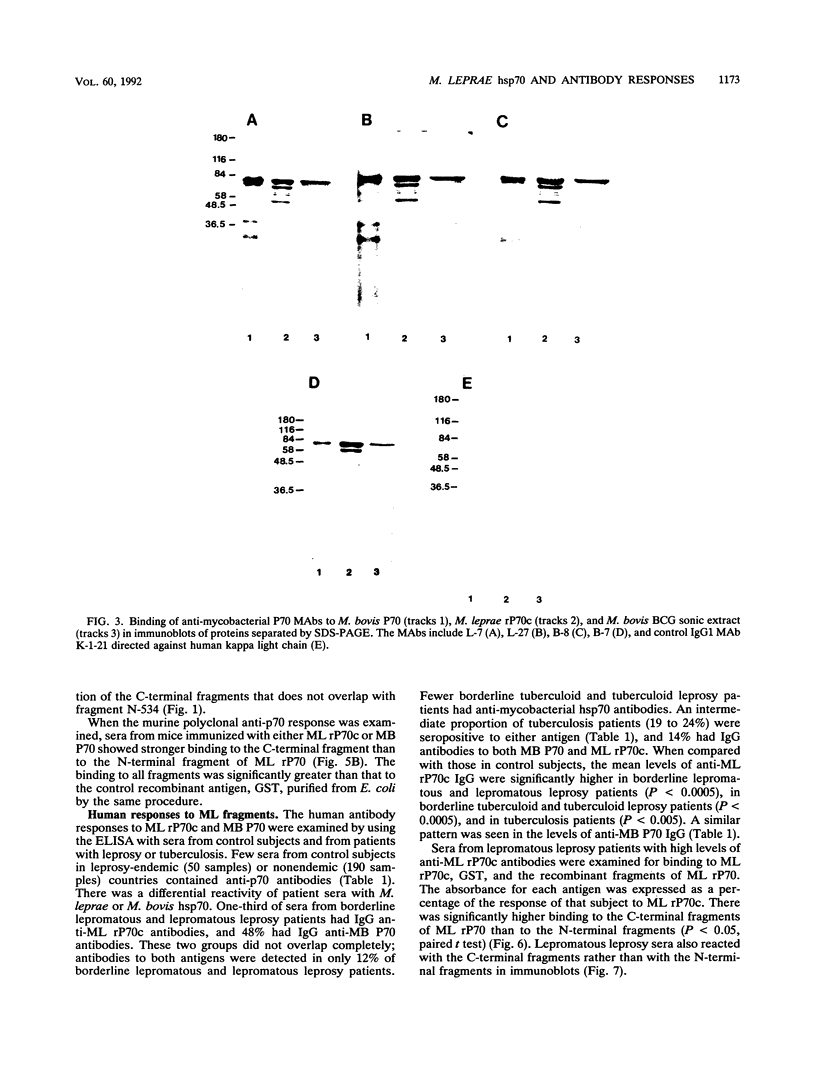


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Garsia R. J., Hellqvist L., Holt P., Basten A. T cell reactivity to the purified mycobacterial antigens p65 and p70 in leprosy patients and their household contacts. Clin Exp Immunol. 1990 May;80(2):206–212. doi: 10.1111/j.1365-2249.1990.tb05235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranovsky A. A., Boyko V. P., Karasev A. V., Koonin E. V., Dolja V. V. Putative 65 kDa protein of beet yellows closterovirus is a homologue of HSP70 heat shock proteins. J Mol Biol. 1991 Feb 20;217(4):603–610. doi: 10.1016/0022-2836(91)90517-a. [DOI] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Characterization of native and recombinant 75-kilodalton immunogens from Chlamydia trachomatis serovar L2. Infect Immun. 1989 Sep;57(9):2683–2690. doi: 10.1128/iai.57.9.2683-2690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Bothamley G., Beck J. S., Britton W., Elsaghier A., Ivanyi J. Antibodies to Mycobacterium tuberculosis-specific epitopes in lepromatous leprosy. Clin Exp Immunol. 1991 Dec;86(3):426–432. doi: 10.1111/j.1365-2249.1991.tb02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Gaitanaris G. A., Papavassiliou A. G., Rubock P., Silverstein S. J., Gottesman M. E. Renaturation of denatured lambda repressor requires heat shock proteins. Cell. 1990 Jun 15;61(6):1013–1020. doi: 10.1016/0092-8674(90)90066-n. [DOI] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Schinski V., Agabian N., Newport G. Schistosome heat-shock proteins are immunologically distinct host-like antigens. Mol Biochem Parasitol. 1988 Jun;29(2-3):275–282. doi: 10.1016/0166-6851(88)90082-5. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Ostermann J., Shilling J., Neupert W., Craig E. A., Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990 Nov 8;348(6297):137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- Lathigra R. B., Butcher P. D., Garbe T. R., Young D. B. Heat shock proteins as virulence factors of pathogens. Curr Top Microbiol Immunol. 1991;167:125–143. doi: 10.1007/978-3-642-75875-1_8. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mattei D., Scherf A., Bensaude O., da Silva L. P. A heat shock-like protein from the human malaria parasite Plasmodium falciparum induces autoantibodies. Eur J Immunol. 1989 Oct;19(10):1823–1828. doi: 10.1002/eji.1830191010. [DOI] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Meeker H. C., Williams D. L., Anderson D. C., Gillis T. P., Schuller-Levis G., Levis W. R. Analysis of human antibody epitopes on the 65-kilodalton protein of Mycobacterium leprae by using synthetic peptides. Infect Immun. 1989 Dec;57(12):3689–3694. doi: 10.1128/iai.57.12.3689-3694.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen S. K. A look at world leprosy. Lepr Rev. 1991 Mar;62(1):72–86. doi: 10.5935/0305-7518.19910011. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., de Vries R. R. Antigen reactivity and autoreactivity: two sides of the cellular immune response induced by mycobacteria. Curr Top Microbiol Immunol. 1990;155:111–121. doi: 10.1007/978-3-642-74983-4_8. [DOI] [PubMed] [Google Scholar]
- Peake P., Basten A., Britton W. J. Characterization of the functional properties of the 70-kDa protein of Mycobacterium bovis. J Biol Chem. 1991 Nov 5;266(31):20828–20832. [PubMed] [Google Scholar]
- Peterson M. G., Crewther P. E., Thompson J. K., Corcoran L. M., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. A second antigenic heat shock protein of Plasmodium falciparum. DNA. 1988 Mar;7(2):71–78. doi: 10.1089/dna.1988.7.71. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Roche P. W., Britton W. J., Failbus S. S., Williams D., Pradhan H. M., Theuvenet W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int J Lepr Other Mycobact Dis. 1990 Sep;58(3):480–490. [PubMed] [Google Scholar]
- Scallon B. J., Bogitsh B. J., Carter C. E. Cloning of a Schistosoma japonicum gene encoding a major immunogen recognized by hyperinfected rabbits. Mol Biochem Parasitol. 1987 Jul;24(3):237–245. doi: 10.1016/0166-6851(87)90155-1. [DOI] [PubMed] [Google Scholar]
- Selkirk M. E., Denham D. A., Partono F., Maizels R. M. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J Immunol. 1989 Jul 1;143(1):299–308. [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]