Abstract
The effects of insulin on the suppression of lipolysis are neither fully understood nor quantified. We examined a variety of mathematical models analogous to the minimal model of glucose disposal (MMG) to quantify the combined influence of insulin on lipolysis and glucose disposal during an insulin-modified frequently sampled intravenous glucose tolerance test. The tested models, which include two previously published ones, consisted of separate compartments for plasma free fatty acids (FFA), glucose, and insulin. They differed in the number of compartments and in the action of insulin to suppress lipolysis that decreased the plasma FFA level. In one category of models, a single insulin compartment acted on both glucose and FFA simultaneously. In a second category, there were two insulin compartments, each acting on FFA and glucose independently. For each of these two categories, we tested 11 variations of how insulin suppressed lipolysis. We also tested a model with an additional glucose compartment that acted on FFA. These 23 models were fit to the plasma FFA and glucose concentrations of 102 subjects individually. Using Bayesian model comparison methods, we selected the model that best balanced fit and minimized model complexity. In the best model, insulin suppressed lipolysis via a Hill function through a remote compartment that acted on both glucose and FFA simultaneously, and glucose dynamics obeyed the classic MMG.
Keywords: free fatty acids, insulin resistance, mathematical model
insulin resistance is a primary risk factor for several common diseases, including diabetes, cardiovascular disease, hypertension, and some forms of cancer. The mechanisms underlying insulin resistance are not completely understood. One important gap in our understanding relates to defects in insulin's ability to regulate lipolysis, leading to relative elevations of plasma free fatty acids (FFA). FFA elevation has been implicated in the modification of insulin action in various tissues as well as altering intermediates in the insulin signaling pathway and mitochondrial enzymes (6, 23). Therefore, methods to quantify insulin's effects on lipolysis and plasma FFA levels in various conditions of insulin resistance would be useful.
The pace of development of quantitative methods to assess insulin's effect on FFA has lagged behind that of quantifying insulin's action on glucose disposal. For example, the hyperinsulinemic euglycemic clamp and the minimal model of glucose disposal (MMG) (1, 8) provide a quantitative index of the glucoregulatory action of insulin as a single value, such as glucose infusion rate divided by steady-state insulin concentration (M/I) for the clamp and the insulin sensitivity index (SI) for the MMG.
The current method for quantifying insulin's effect on FFA is to use a stepwise low-dose insulin clamp to define various quantities such as the ED50 for FFA suppression by insulin (15). However, this method is technically demanding because of the extreme sensitivity of the suppression of lipolysis to insulin (i.e., has a lower ED50) compared with glucose disposal (27). Although stepwise clamps have provided great insight into the effect of insulin on lipolysis, questions remain. For example, insulin levels are not constant but vary throughout the day, particularly following meals. It is not known whether the time-dependent dynamics of insulin has an additional effect on lipolysis.
To complement steady-state studies (i.e., the clamp), it would be advantageous to quantitatively assess the influence of insulin on lipolysis suppression during a dynamic situation such as during an intravenous glucose challenge. In addition, the intravenous glucose tolerance test to assess glucose's sensitivity to insulin is a protocol that is widely used. Thus insulin's influence on glucose and FFA can be assessed simultaneously. However, during a dynamic situation, a functional relationship between lipolytic rate and insulin is not readily apparent. When a similar problem was encountered for assessing insulin's effect on glucose disposal, the MMG (2–4, 19, 20) was developed, which obviated the need to clamp the blood glucose level. Mathematical modeling was then used to extract the glucose-dependent insulin sensitivity from a frequently sampled intravenous glucose tolerance test (FSIGT).
Several FSIGT protocols have been introduced over the years, including the FSIGT with a single intravenous glucose injection and the insulin-modified (IM)-FSIGT (22). The IM-FSIGT differs from the glucose-only FSIGT by the addition at 20 min of insulin in the form of either a bolus or a 5-min infusion (22). It would be highly useful to develop a mathematical model of the effect of insulin on lipolysis using FFA levels obtained during the IM-FSIGT. Information on both glucose disposal and lipolysis could then be obtained from a single test, the IM-FSIGT.
Our goal was to develop a mathematical model that could describe the plasma FFA and glucose time course during an IM-FSIGT. We used an approach similar to that used in the development of the MMG. The minimal model approach has been attempted previously by Thomaseth and Pavan (31) and Roy and Parker (21). Given enough parameters, many models could potentially be developed that could fit a finite sample of data, and without prior physiological information, a wide range of plausible models could be proposed. Hence, our strategy was to consider a number of possible models, including the published models, and evaluate them using a Bayesian model comparison method. The advantage of a Bayesian method is that it balances maximizing the goodness of fit to the data with the complexity of the model. In addition, we fit the models to each individual of the study population separately as opposed to the mean data, as was done previously (21, 31). This made for a more stringent test of the models, since it must be applicable to a wide range of possible behaviors.
Different models of varying complexity were considered. The measured insulin concentrations at fixed time points during the IM-FSIGT were the “input” variables for the model, and the time courses of plasma FFA and glucose concentrations were the “output” variables. From this set of models, we identified a model that best balanced model complexity and the ability to reproduce the data of all the subjects in the study population. In this initial attempt to model insulin's effect on FFA, we wanted to study a population that was likely to have the greatest sensitivity to insulin as a fat regulatory hormone. We elected to study an African American population because, compared with whites, blacks are more obese and insulin resistant but have lower triglyceride (TG) levels (25, 26). Because TG levels are normal in African Americans even in the presence of insulin resistance (26–30), we postulated that African Americans are able to maintain sensitivity to insulin as a fat regulatory hormone over a very wide range of resistance to insulin as a glucoregulatory hormone (26, 28, 29).
METHODS
The participants were 102 nondiabetic African Americans enrolled in the Triglyceride and Cardiovascular Risk in African Americans (TARA) study at the National Institutes of Health [(NIH); Bethesda, MD; see Table 1; 46 men and 56 women, age 34 ± 7 yr (mean ± SD; range 20–50 yr), body mass index (BMI) 30.7 ± 7.8 kg/m2 (range 19.5–56.9 kg/m2)]. FSIGTs from 19 of these subjects had previously been presented (24). Recruitment was achieved with flyers, newsletters, and the NIH website. All subjects self-identified as African Americans, were born in the United States, and reported that both parents were African Americans born in the United States. Enrollees denied a history of diabetes, anemia, thyroid, or liver or kidney disease. The subjects were not taking any medications known to affect either glucose or lipid metabolism. The women were premenopausal and were studied in the follicular phase of their cycle. The Institutional Review Board of NIH approved the study. Subjects gave informed consent.
Table 1.
Subject characteristics of the TARA population
| Mean | Range | |
|---|---|---|
| Age, yr | 34±7 | 20–50 |
| Male, % | 45 | |
| BMI, kg/m2 | 30.7±7.8 | 19.5–56.9 |
| Fasting glucose, mg/dl | 86±9 | 70–114 |
| Fasting FFA, μM | 484±170 | 102–930 |
| Fasting insulin, μU/ml | 8.8±5.4 | 1.9–29.6 |
| Glucose intolerant, % | 25 | |
| SI, μU·l−1·min−1 | 3.58±2.4 | 0.17–12.75 |
| Triglycerides, mg/dl | 70±35 | 23–212 |
Values are means ± SD; n = 102 subjects of the Triglyceride and Cardiovascular Risk in African Americans (TARA) study. “Glucose intolerant” was defined as either impaired fasting glucose (>100 mg/dl) or a 2-h glucose after oral glucose tolerance test between 140 and 199 mg/dl, or both. BMI, body mass index; FFA, free fatty acids; SI, insulin sensitivity index.
As an outpatient, each subject had an IM-FSIGT. The IM-FSIGT was performed in the morning after a 12-h overnight fast. Intravenous catheters were placed in both antecubital veins. Arterialized blood samples were collected for analysis. At time 0, glucose (0.3 g/kg) was injected over 1 min and insulin (4 mU·kg−1·min−1) was infused from 20 to 25 min. Samples for glucose, insulin, and FFA were obtained at −10, −1, 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 20, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 min. Glucose was determined at the bedside using the glucose oxidase method (Glucostat; Yellow Springs Instruments, Yellow Springs, OH). Blood for insulin was drawn into serum separator tubes. Blood for FFA was drawn into chilled EDTA tubes and centrifuged immediately, and the plasma was stored at −70°C. FFA assays were performed within 72 h.
Assay methods.
Insulin was measured with the double-antibody chemiluminescent sandwich assay (Diagnostic Products, Los Angeles, CA). The cross-reactivity between insulin and proinsulin in this assay is 8%. FFA concentrations were assayed with enzymatic colorimetric kits (Wako Chemicals USA, Richmond, VA). Glucose, insulin, and FFA concentrations were determined in duplicate. The coefficients of variation were 1.9, 3.2, and 4.9%, respectively.
Model rationale.
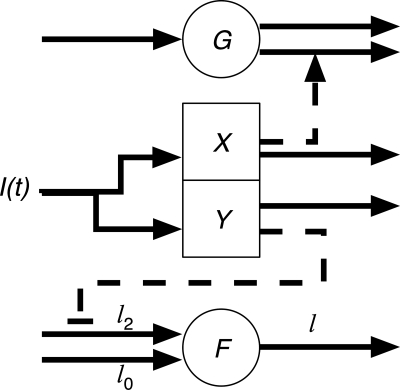
We proposed and evaluated 23 physiologically based candidate mathematical models of FFA kinetics in response to the changing insulin and glucose levels to account for FFA data (see Parameter fitting and model evaluation). Two of the models were previously published (21, 31). Twenty-two of the models, including the model of Thomaseth and Pavan (31), have a similar structure. In these models, insulin acts on glucose through a remote compartment and is modeled using the classic MMG (3). These models also assume that insulin suppresses lipolysis (and hence reduces the rate of appearance of FFA) through a remote insulin compartment and that FFA is cleared via a first-order reaction. The models differ in how insulin suppresses lipolysis and in the dynamics of the remote insulin compartment. We considered two schemes for the remote insulin compartment. In one category (which we call “X” models), insulin acts through a remote compartment that is identical to that of the MMG (i.e., determined only by fitting the MMG to the insulin and glucose data), which is historically denoted by X. In the second category (“Y” models), the action of insulin on FFA is through a remote compartment that is different from X (determined by fitting to the insulin and FFA data). Hence, X models have one remote insulin compartment that acts on glucose and FFA, and Y models have two remote insulin compartments, one that acts on glucose and the other that acts on FFA. Schematics of the X and Y models are shown in Figs. 1 and 2, respectively. The third category of model was proposed by Roy and Parker (21) and has an additional remote FFA compartment that affects glucose metabolism directly.
Fig. 1.
X models. Insulin [I(t)] acts on glucose (G) and free fatty acids (F) through a remote glucose compartment (X). The solid arrows indicate input and output fluxes to the compartments. The dashed arrow is an amplification of glucose removal by insulin. The dashed line with bar is the inhibition of lipolysis by insulin (l0, minimal lipolysis rate; l0 + l2, maximal lipolysis rate). FFA is cleared from the plasma as a first-order reaction with rate cf.
Fig. 2.
Y models. Insulin acts on glucose through a remote glucose compartment X and on FFA through a separate remote adipose compartment Y.
The MMG describes insulin's action on plasma glucose concentrations. The dynamics obey
 |
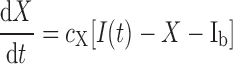 |
(1) |
where G is the plasma glucose concentration, X(t) is the effect of insulin in a remote glucose compartment, I(t) is the plasma insulin concentration, and Gb, SG, SI, cX, and Ib are free parameters (3, 9).
The FFA compartment in the X models has the form
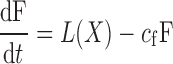 |
(2) |
where X is given by Eq. 1 and L(X) is the insulin-dependent rate of lipolysis, which models the insulin-suppressible plasma FFA input flux. FFA is cleared from the plasma as a first-order reaction with rate cf (14). We considered 11 different types of L(X), which are listed in Table 2. The Y models have the form
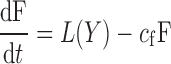 |
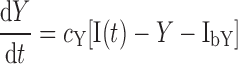 |
(3) |
where Y is the insulin action in a remote compartment that is distinct from X, and the rate of lipolysis L(Y) has the same functional form as those for the X models but with X replaced by Y. Our models also predict that for an insulin-clamp experiment, the lipolysis rate will depend on insulin through the function L(I − Ib), which can be compared directly with insulin-clamp experiments.
Table 2.
Lipolysis functions used in X models
| Model Type | L(X) | Parameters |
|---|---|---|
| TP | 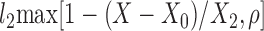 |
l2, X0, X2, ρ |
| H | 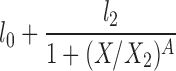 |
l0, l2, X2, A |
| P | 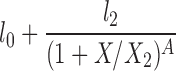 |
l0, l2, X2, A |
| H(X2 = 14.7) | 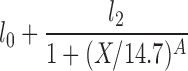 |
l0, l2, A |
| P(X2 = 14.7) | 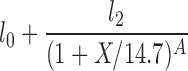 |
l0, l2, A |
| H(A = 1) | 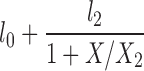 |
l0, l2, X2 |
| H(l0 = 0) | 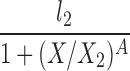 |
l2, X2, A |
| P(l0 = 0) | 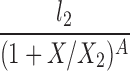 |
l2, X2, A |
| H(l0 = 0, X2 = 14.7) | 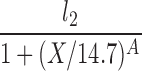 |
l2, A |
| P(l0 = 0, X2 = 14.7) | 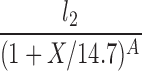 |
l2, A |
| H(l0 = 0, A = 1) | 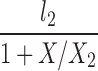 |
l2, X2 |
X is replaced by Y for Y models. TP, Thomaseth and Pavan model; H, Hill function; P, power law function.
The lipolysis functions in Table 2 are variations of three basic families. In one family, according to Thomaseth and Pavan (31), lipolysis is suppressed linearly by insulin, but not below a fixed minimum rate. Although these authors did not consider a Y version of their model, we have included their original model and the Y version of it for completeness. The two other families are motivated by insulin-clamp experiments, which show that the effect of insulin on lipolysis could be modeled by a power law function; i.e., L(I) ∝ I−A (17). However, given that a power law is infinite for zero insulin, we considered generalized power law functions that were well behaved for zero insulin. We considered two generalized forms. The first is the Hill function L(X) = l0 + l2/[(1 + X/X2)A], where X2 is the Hill constant, A is the Hill coefficient, l0 is the minimal lipolysis rate, and l0 + l2 represents the maximal rate of lipolysis. The second is a power law function with an offset of the form L(X) = l0 + l2/(1 + X/X2)A, where X2 is the offset parameter and A is the exponent. Both of these families have four parameters each. To reduce the number of parameters, we considered versions of these two families where one or two of the parameters l0, A, or X2 are fixed.
Parameter fitting and model evaluation.
We fitted each model individually to data from each of the 102 subjects. We let yi,j,tM(θM) represent the prediction of model M for the plasma concentrations of either FFA (j = 1) or glucose (j = 2) at time point t for subject i, where θM denotes the set of free parameters and initial conditions for model M. The prediction yi,j,tM(θM) was obtained by numerically integrating the differential equations for glucose and FFA concentrations (i.e., Eqs. 1 and 2 for class I models, Eqs. 1 and 3 for class II models, and four equations for the Roy and Parker model) using a fourth-order Runge-Kutta method. The time-dependent insulin function was estimated by linearly interpolating between the measured data points. The parameters were estimated for each subject using a Markov chain Monte Carlo (MCMC) method with parallel tempering as described by Gregory (10). The posterior probability (probability distribution) for the parameters conditional on the data D was obtained using Bayes's rule, given by P(θM|D) ∝ P(D|θM)P(θM), where the likelihood function was given by P(D|θM) = exp(−χ2/2) with χ2 = ∑i=1102 ∑t=129 ∑j=12 (yi,j,tData− yi,j,tModel)2/σ2. For σ2, we took one-half the mean (over the population) of the squared difference between a representative continuous curve chosen to smooth the experimental data and the experimental data. We used singular spectrum analysis to obtain this representative smoothing of the data (32). We considered uniform prior distributions between parameter-dependent minima and maxima (see Tables 3 and 4). From the IM-FSIGT, the glucose, insulin, and FFA data set we used was the 29 time points between 10 and 120 min. The rationale for using this time range from 10 to 120 min, rather than the entire data set from −10 to 180 min, was that our goal was to determine the minimal model that best modeled the suppressive action of insulin on FFA. In all of the subjects, plasma FFA levels did not start declining until 10 min and were maximally suppressed by 120 min. The advantage of using the MCMC method with parallel tempering was that the parameters could be determined and the models could be compared in the same numerical procedure. The method computes the posterior probability for all the parameters as well as the posterior model probability P(M|D) (i.e., the probability that the model describes the data). From Bayes's theorem, P(M|D) ∝ P(D|M)P(M), where P(D|M) = ∫ P(D|θM)P(θM)dθM is the likelihood function of the model marginalized over the parameters and P(M) is the prior probability for the model. If the prior probabilities of all the models are the same, then the Bayes factor Bij = P(D|Mi)/P(D|Mj) gives the odds ratio between models i and j. The model with the largest Bayes factor with respect to the other models is the simplest model that best fits the data. It is sufficient to compute the Bayes factor of all the models compared with just one model to determine the best model. In the parallel tempering method, multiple copies of the simulation are run (with occasional swapping of states between copies), each with the likelihood function raised to a different tempering parameter β, where 0 < β ≤ 1. It then can be shown that the marginalized likelihood function can be computed using ln[P(D|M)] = ∫01 E{ln[P(D|θM)β]}dβ, which then can be used in the Bayes factor (10).
Table 3.
χ2 and log of the Bayes factor (compared with model XH) for the 23 models
| Model Type | χ2/2 | ln Bij | ||
|---|---|---|---|---|
| X models | ||||
| XTP | 6,598 | −4,562 | ||
| XH | 1,953 | 0 | ||
| XP | 2,178 | −187 | ||
| XH(X2 = 14.7) | 2,173 | −216 | ||
| XP(X2 = 14.7) | 2,212 | −200 | ||
| XH(A = 1) | 2,366 | −431 | ||
| XH(l0 = 0) | 1,990 | −39 | ||
| XP(l0 = 0) | 2,206 | −190 | ||
| XH(l0 = 0, X2 = 14.7) | 2,203 | −203 | ||
| XP(l0 = 0, X2 = 14.7) | 2,235 | −192 | ||
| XH(l0 = 0, A = 1) | 2,370 | −387 | ||
| Y models | ||||
| YTP | 6,573 | −4,539 | ||
| YH | 1,950 | −26 | ||
| YP | 2,183 | −212 | ||
| YH(X2 = 14.7) | 2,163 | −231 | ||
| YP(X2 = 14.7) | 2,210 | −222 | ||
| YH(A = 1) | 2,369 | −455 | ||
| YH(l0 = 0) | 1,993 | −54 | ||
| YP(l0 = 0) | 2,204 | −208 | ||
| YH(l0 = 0, X2 = 14.7) | 2,174 | −198 | ||
| YP(l0 = 0, X2 = 14.7) | 2,237 | −215 | ||
| YH(l0 = 0, A = 1) | 2,360 | −402 | ||
| RP model | ||||
| 4,520 | −2,681 | |||
RP, Roy and Parker model. The X and Y prefixes indicate X and Y versions of each model type.
Table 4.
Population statistics of subject posterior parameter expectation values and SD for model XH
| Parameter | Mean | SD | Median | Q2–Q3 | Minimum | Maximum | Prior Minima | Prior Maxima |
|---|---|---|---|---|---|---|---|---|
| −ln[P(M | D)] | 27.7745098 | 1.61E+01 | 2.31E+01 | 1.59E+01 | 1.00E+01 | 1.30E+02 | ||
| E(χsubj2/2) | 19.14254902 | 1.48E+01 | 1.47E+01 | 1.56E+01 | 3.89E+00 | 1.16E+02 | ||
| SD(χsubj2/2) | 2.258137255 | 3.43E−01 | 2.17E+00 | 4.20E−01 | 1.77E+00 | 3.66E+00 | ||
| E[F(t = 0)] | 470.5686275 | 1.52E+02 | 4.70E+02 | 2.37E+02 | 1.53E+02 | 6.98E+02 | 1.50E+02 | 7.00E+02 |
| SD[F(t = 0)] | 16.74058824 | 7.56E+00 | 1.74E+01 | 1.04E+01 | 1.66E+00 | 3.94E+01 | ||
| E[G(t = 0)] | 209.9803922 | 2.74E+01 | 2.10E+02 | 3.35E+01 | 1.55E+02 | 2.82E+02 | 1.00E+02 | 4.00E+02 |
| SD[G(t = 0)] | 7.682254902 | 3.20E+00 | 7.68E+00 | 4.48E+00 | 2.16E+00 | 1.75E+01 | ||
| E[X(t = 0)] | 17.34259804 | 1.18E+01 | 1.78E+01 | 2.12E+01 | 2.72E−01 | 4.31E+01 | 1.00E−01 | 5.00E+01 |
| SD[X(t = 0)] | 9.311813725 | 4.82E+00 | 1.14E+01 | 8.49E+00 | 1.68E−01 | 1.54E+01 | ||
| E(l0) | 2.448098039 | 9.21E−01 | 2.50E+00 | 1.44E+00 | 4.37E−01 | 4.34E+00 | 0.00E+00 | 5.00E+00 |
| SD(l0) | 1.15472549 | 2.74E−01 | 1.21E+00 | 3.15E−01 | 4.31E−01 | 1.72E+00 | ||
| E(X2) | 11.2587451 | 8.67E+00 | 8.82E+00 | 8.02E+00 | 8.42E−01 | 4.08E+01 | 1.00E−01 | 5.00E+01 |
| SD(X2) | 4.805872549 | 3.30E+00 | 3.46E+00 | 4.39E+00 | 6.03E−01 | 1.62E+01 | ||
| E(l2) | 42.85588235 | 1.36E+01 | 4.16E+01 | 1.82E+01 | 1.38E+01 | 6.86E+01 | 1.00E+01 | 7.00E+01 |
| SD(l2) | 11.27333333 | 4.68E+00 | 1.19E+01 | 6.66E+00 | 1.35E+00 | 1.96E+01 | ||
| E(cf) | 0.083893137 | 4.23E−02 | 7.42E−02 | 5.37E−02 | 1.59E−02 | 2.11E−01 | 1.00E−02 | 2.50E−01 |
| SD(cf) | 0.015491863 | 9.94E−03 | 1.34E−02 | 1.31E−02 | 3.38E−03 | 4.86E−02 | ||
| E(cX) | 0.054098039 | 3.22E−02 | 4.61E−02 | 3.28E−02 | 1.15E−02 | 1.55E−01 | 1.00E−02 | 2.50E−01 |
| SD(cX) | 0.029360588 | 2.17E−02 | 2.20E−02 | 2.06E−02 | 1.55E−03 | 8.39E−02 | ||
| E(Ib) | 10.50163725 | 6.91E+00 | 9.05E+00 | 9.02E+00 | 3.31E−01 | 3.34E+01 | 1.00E−01 | 5.00E+01 |
| SD(Ib) | 6.667519608 | 3.59E+00 | 6.20E+00 | 5.28E+00 | 2.31E−01 | 1.54E+01 | ||
| E(A) | 1.882598039 | 5.26E−01 | 2.04E+00 | 6.65E−01 | 5.69E−01 | 2.89E+00 | 5.00E−01 | 3.00E+00 |
| SD(A) | 0.477834314 | 1.54E−01 | 4.93E−01 | 1.59E−01 | 6.85E−02 | 8.23E−01 | ||
| E(SGGb) | 2.392215686 | 9.49E−01 | 2.22E+00 | 1.46E+00 | 5.34E−01 | 4.66E+00 | 1.00E−01 | 5.00E+00 |
| SD(SGGb) | 0.627264706 | 2.34E−01 | 6.03E−01 | 3.50E−01 | 1.98E−01 | 1.33E+00 | ||
| E(SG) | 0.035062745 | 1.70E−02 | 2.99E−02 | 2.27E−02 | 1.14E−02 | 8.41E−02 | 1.00E−02 | 1.00E−01 |
| SD(SG) | 0.006529412 | 2.17E−03 | 6.56E−03 | 2.49E−03 | 1.26E−03 | 1.34E−02 | ||
| E(SI) | 1.20E−04 | 3.53E−05 | 1.19E−04 | 5.07E−05 | 5.08E−05 | 1.89E−04 | 5.00E−06 | 2.00E−04 |
| SD(SI) | 4.43E−05 | 1.23E−05 | 4.67E−05 | 1.75E−05 | 9.73E−06 | 6.16E−05 |
See text for detailed definitions of parameters.
The MCMC process was carried out over 2 × 106 iterations for each of 10 uniformly spaced values of β, with swapping between processes about once every 20 steps. The parameter steps during equilibration were selected at random up to a maximum of ±5% of the prior range for that parameter. The final 2 × 105 iterations after equilibration had no swapping, and parameter steps were selected at random up to a maximum of ±1% of the prior ranges. All results concerning posterior parameter estimates are obtained from these final 2 × 105 iterations.
RESULTS
Figure 3 shows the time course of the mean values of glucose, insulin, and FFA over the TARA population. We fit each of the models to each individual subject. Table 3 shows the χ2 value for the 23 models. We see that model YH (Y model with Hill function) had the lowest χ2 value, implying it had the highest maximum likelihood. However, model XH (X model with Hill function) had the highest Bayes factor, although just slightly over that of model YH. Simplifying the model by fixing one or two of the parameters in the lipolysis rate function led to lower Bayes factors (less favorable). Thus it seems that including all of the parameters in the lipolysis function of model XH was justified. However, the models without a minimal lipolysis rate only performed marginally worse than the winning model. All the Hill function and offset power law models performed better than the models of Thomaseth and Pavan and of Roy and Parker.
Fig. 3.
Mean insulin (μU/ml; ▪), FFA (μM; ▴), and glucose (mg/dl; •) plasma concentration time courses.
For each model, the MCMC method computed a parameter posterior probability distribution for each subject. Tables 4 and 5 give statistics over the TARA population of the subject expectation value and standard deviations of the parameters for model XH and the Thomaseth and Pavan model. We found that all the models had some posterior weakly identifiable parameters as evidenced by very wide posterior probability distributions.
Table 5.
Population statistics of subject posterior parameter expectation values and SD for TP model
| Parameter | Mean | SD | Median | Q2–Q3 | Minimum | Maximum | Prior Minima | Prior Maxima |
|---|---|---|---|---|---|---|---|---|
| −ln[P(M | D)] | 74.34 | 5.90E+01 | 6.16E+01 | 6.25E+01 | 1.15E+01 | 3.34E+02 | ||
| E(χsubj2/2) | 66.72 | 5.81E+01 | 5.43E+01 | 6.12E+01 | 6.50E+00 | 3.24E+02 | ||
| SD(χsubj2/2) | 2.04 | 2.99E−01 | 2.02E+00 | 3.10E−01 | 1.40E+00 | 3.14E+00 | ||
| E[F(t = 0)] | 389.1 | 1.64E+02 | 3.60E+02 | 2.43E+02 | 1.51E+02 | 6.99E+02 | ||
| SD[F(t = 0)] | 8.6 | 4.26E+00 | 8.12E+00 | 5.28E+00 | 5.56E−01 | 2.04E+01 | ||
| E[G(t = 0)] | 210.3 | 2.75E+01 | 2.10E+02 | 3.50E+01 | 1.53E+02 | 2.79E+02 | ||
| SD[G(t = 0)] | 7.07 | 3.09E+00 | 6.40E+00 | 4.28E+00 | 1.84E+00 | 1.81E+01 | ||
| E[X(t = 0)] | 35.37 | 1.06E+01 | 4.04E+01 | 1.03E+01 | 5.46E+00 | 4.47E+01 | ||
| SD[X(t = 0)] | 4.13 | 3.04E+00 | 3.05E+00 | 3.92E+00 | 2.92E−01 | 1.23E+01 | ||
| E(X2) | 7.74 | 7.28E+00 | 5.54E+00 | 5.38E+00 | 2.01E+00 | 5.78E+01 | 2.00E+00 | 1.00E+02 |
| SD(X2) | 0.59 | 5.87E−01 | 3.67E−01 | 5.13E−01 | 9.68E−03 | 2.62E+00 | ||
| E(l2) | 41.78 | 1.34E+01 | 4.36E+01 | 2.05E+01 | 1.43E+01 | 6.67E+01 | ||
| SD(l2) | 9.63 | 4.21E+00 | 8.71E+00 | 5.52E+00 | 2.64E+00 | 2.27E+01 | ||
| E(cf) | 0.12 | 5.48E−02 | 1.21E−01 | 9.84E−02 | 3.14E−02 | 2.19E−01 | ||
| SD(cf) | 0.04 | 1.68E−02 | 3.58E−02 | 2.13E−02 | 8.09E−03 | 8.94E−02 | ||
| E(cX) | 0.185 | 6.37E−02 | 2.13E−01 | 8.05E−02 | 2.31E−02 | 2.46E−01 | ||
| SD(cX) | 0.021 | 1.30E−02 | 1.90E−02 | 1.89E−02 | 3.07E−03 | 5.61E−02 | ||
| E(X0) | 22.74 | 1.04E+01 | 2.31E+01 | 1.48E+01 | 5.67E+00 | 4.49E+01 | 5.00E+00 | 4.50E+01 |
| SD(X0) | 2.08 | 1.88E+00 | 1.52E+00 | 1.74E+00 | 1.35E−01 | 1.21E+01 | ||
| E(ρ) | 0.99 | 2.27E−01 | 9.97E−01 | 3.49E−01 | 5.77E−01 | 1.41E+00 | 5.00E−01 | 1.50E+00 |
| SD(ρ) | 0.17 | 6.91E−02 | 1.65E−01 | 9.45E−02 | 3.73E−02 | 3.51E−01 | ||
| E(SGGb) | 1.99 | 1.09E+00 | 1.71E+00 | 1.46E+00 | 5.06E−01 | 4.50E+00 | ||
| SD(SGGb) | 0.48 | 1.89E−01 | 4.61E−01 | 2.19E−01 | 1.56E−01 | 1.02E+00 | ||
| E(SG) | 0.0325 | 1.76E−02 | 2.72E−02 | 2.03E−02 | 1.12E−02 | 8.36E−02 | ||
| SD(SG) | 0.0051 | 2.02E−03 | 5.11E−03 | 2.60E−03 | 9.42E−04 | 1.07E−02 | ||
| E(SI) | 1.10E−04 | 5.07E−05 | 1.07E−04 | 9.38E−05 | 1.96E−05 | 1.90E−04 | ||
| SD(SI) | 2.80E−05 | 1.25E−05 | 2.57E−05 | 1.77E−05 | 8.63E−06 | 6.91E−05 |
Refer to Table 4 for priors with blank entries.
On the basis of this analysis, we propose that model XH (lipolysis function is a Hill function with nonzero minimal rate, and insulin acts through a remote compartment that is identical to the one for glucose disposal) is the simplest model that best fits the FFA kinetics and glucose kinetics in response to changes in insulin for the TARA population. However, considering a separate remote compartment for insulin or excluding a minimal lipolysis rate could also be reasonable models to use. Further testing on other populations would be necessary to better separate the models.
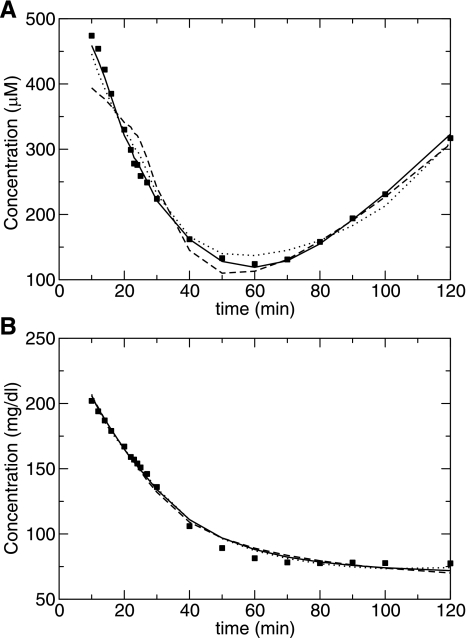
Figure 4 shows the mean over the TARA population of the fits and the data for the XH, Thomaseth and Pavan, and Roy and Parker models. In Fig. 5, the mean fractional residual differences at each time point between the data and the model estimates are shown. We note that the systematic deviations in the residuals for glucose arise from the fitting of the MMG model directly and are not influenced by the FFA fits. However, the magnitude of the fractional residuals for the glucose fits is reasonably small, as shown in Fig. 4B.
Fig. 4.
Mean individual maximum likelihood fits plotted with the mean data for FFA (A) and glucose (B) for models XH (solid line), XTP (dashed line), and Roy and Parker (dotted line).
Fig. 5.
Residuals (mean differences between the data and the individual maximum likelihood fits) for FFA (A) and glucose (B) for models XH (solid line), XTP (dashed line), and Roy and Parker (dotted line).
The parameters of the rate of lipolysis function L(X) provide a means to assess the efficacy of insulin on suppressing lipolysis. The lipolysis function decreases faster as a function of insulin for an increase in Hill coefficient A and for a decrease in the Hill constant X2. The ED50 for insulin's action on lipolysis has been suggested as a measure of insulin sensitivity for FFA previously (17). For model XH, we can derive an effective ED50 that has the form X2 + Ib. This measures the insulin dose that would suppress FFA to half maximum (halfway between l0 + l2 and l0). Our prediction would be that this model-derived ED50 would be significantly correlated to the ED50 obtained from an insulin-clamp study. The correlation coefficient between our estimated ED50 value and SI was r = 0.12 (P = 0.2), and between ED50 and BMI, it was r = 0.14 (P = 0.16). It is not surprising in African Americans that the correlation between ED50 and SI was not significant. In African Americans, the lack of association between SI and TG levels suggests this outcome (25, 26, 29). Similarly in African Americans, TG and BMI are only weakly correlated (25, 26, 29). Figure 6 shows two example FFA time courses for one subject with an effective ED50 = 11 μU/ml and one with ED50 = 46 μU/ml.
Fig. 6.
Example plots of FFA data (▴), model fits (solid line), and insulin (▪) for subjects with 46 μM (A) and ED50 = 11 μU/ml (B).
DISCUSSION
The purpose of this investigation was to quantitatively assess the response of plasma FFA to insulin. We postulated that a mathematical model transforming a time course of plasma insulin measurements into a time course of FFA measurements from 10 to 120 min would be described by parameters that would serve as quantitative measures of the regulation of lipolysis by insulin action. Within three categories, we considered 23 different mathematical models of varying complexity and mechanisms of action. We selected and discriminated between these models, using a Bayesian model comparison method that balances goodness of fit and parameter space required to describe the model.
Hill function models clearly outperformed all other models, in terms of both fit and Bayes factor. Among the top-performing models, the analysis selected XH as the best model. However, given the small margin of victory, we feel that a superior model is not unambiguously decided by this data set. In model XH, lipolysis is suppressed by insulin through a Hill function with a nonzero minimal rate, and insulin acts on lipolysis through the glucose remote compartment of the glucose minimal model (illustrated in Fig. 1).
Physiologically, it may be surprising that insulin affects FFA dynamics from the remote glucose compartment from which insulin modulates glucose levels. The pathways through which insulin regulates glucose involve the insulin receptor on the cell surface and, ultimately, glucose transporters. In contrast, insulin regulates lipolysis by initiating a chain of events that lead to inhibition of lipolysis, by promoting the dephosphorylation of both hormone-sensitive lipase and the protein perilipin (30). However, models YH and XH had nearly identical Bayes factors, implying that both remote compartments had similar enough dynamics that one could serve for the other with little effect on the model fit. This may be because insulin action in the remote compartment is a rate-limiting step so that adipose tissue and muscle in close proximity would receive similar signals. Alternatively, it may indicate that the physiology of glucose and FFA regulation may have unknown mechanisms ensuring coordinated insulin response even though the pathways are prima facie distinct.
Previous studies indicate that there may be a maximally suppressible level of FFA plasma appearance (11, 12, 15, 16, 18). This would be manifested as a nonzero insulin-independent lipolysis rate (i.e., l0 ≠ 0). Our model confirms this claim, although weakly. Fixing the minimal rate to zero only reduced the Bayes factor marginally. Additional data may be required to fully resolve the insulin-independent minimum rate.
Our model predicts that the lipolysis rate during an insulin clamp will depend on insulin via a Hill function. Jensen and Nielsen (17) showed that their clamped lipolysis rate could be fit by a power law function, and the properties of this function such as the ED50 might be a measure of the sensitivity of insulin's action on lipolysis. Since a power law function is a special case of our function (with a Hill constant of zero), our model is consistent with the clamp results. Hence, our minimal FFA model provides a possible means to obtain the dependence of lipolysis for stepwise clamped insulin levels with a dynamic IM-FSIGT. Analogously, the parameters of the lipolysis function describing insulin's influence on lipolysis might provide a measure for the sensitivity of lipolysis on insulin. For example, we can derive an effective ED50 for insulin's action on lipolysis. The model-derived value would be a prediction of the ED50 that would be measured from an insulin-clamp experiment on the same subject. We note that the ED50 could not be predicted from the data without the benefit of a model. Future studies are required to test whether our predicted rate of lipolysis function and ED50 match the results obtained during an insulin clamp in the same subject.
We focused primarily on fit to data with minimal complexity and did not address issues of identifiability of the parameters. Some of the parameters in the models had fairly broad posterior distributions, indicating that multiple parameter sets could possibly fit the data equally well. This may be indicative of an interaction between parameters. For example, the exponent A can be partially compensated by the offset X2. Future work is required to optimize the model for identifiability of parameters.
We did not explore the possibility of direct action of glucose on FFA levels. There is some experimental evidence supporting our decision (13). We also did not include the direct effects of insulin or glucose on FFA clearance. Although it has been found that FFA uptake increases with glucose infusion (7), we note that in the minimal model framework, an increase in clearance is not distinguishable from a decrease in appearance. Thus this effect is partially accounted for by our rate of lipolysis function. Future modeling work could incorporate these effects explicitly. Our philosophy of employing a minimal model also meant that we did not incorporate the direct effects of catecholamines, corticosteroids, and glucagon. Presumably, these hormones contribute to the variability not accounted for by the model. Because FFAs promote resistance to insulin's ability to regulate glucose (5, 23), this model could potentially lead to a greater insight into our understanding of why some individuals with obesity are resistant to insulin's effect on glucose and others are sensitive.
Perspectives and Significance
The ability of insulin to modulate glucose and FFA levels has a major impact on many disease processes, including diabetes, heart disease, and cancer. In contrast to our ability to assess the effect on insulin on glucose disposal, there is no clinically relevant tool to assess the effect of insulin on FFA. To close this gap, we examined a variety of mathematical models analogous to the minimal model of glucose disposal to quantify the combined influence of insulin on lipolysis and glucose disposal during an insulin-modified frequently sampled intravenous glucose tolerance test. With insulin as the input and FFA as the output and employing Bayesian model comparison methods, we identified a single model that best balanced fit and minimized model complexity. For the model to have clinical relevance, an index of FFA sensitivity needs to be derived. Development of an index will be a major undertaking and will require testing in a broad range of subjects with a wide range of age, ethnic background, and body mass index.
GRANTS
This work was supported by the Intramural Research Program of National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). In addition, support was provided by NIDDK Grants DK27619 and DK29867 (to R. N. Bergman) and the Veterans Affairs Merit Review (to G. L. Vega).
Acknowledgments
We thank Kevin Hall for insightful comments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bergman RN Toward physiological understanding of glucose tolerance, minimal-model approach. Diabetes 38: 1512–1527, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Cobelli C. Minimal modeling, partition analysis, and the estimation of insulin sensitivity. Fed Proc 39: 110–115, 1980. [PubMed] [Google Scholar]
- 3.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab Gastrointest Physiol 236: E667–E677, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Bergman RN, Steil GM, Bradley DC, Watanabe RM. Modeling of insulin action in vivo. Annu Rev Physiol 54: 861–883, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Laakso M. Lipids and glucose in type 2 diabetes, what is the cause and effect? Diabetes Care 27: 2253–2259, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 32, Suppl 3: 14–23, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol Endocrinol Metab 276: E233–E240, 1999. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 9.Getty L, Hamilton-Wessler M, Ader M, Dea MK, Bergman RN. Biphasic insulin secretion during intravenous glucose tolerance test promotes optimal interstitial insulin profile. Diabetes 47: 1941–1947, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Gregory PC Bayesian Logical Data Analysis for the Physical Sciences: a Comparative Approach With Mathematical Support. Cambridge, UK: Cambridge University Press, 2005.
- 11.Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and non-insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 72: 96–107, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins M, Tonelli J, Kishore P, Stein D, Ragucci E, Gitig A, Reddy K. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes 52: 2748–2758, 2003. [DOI] [PubMed] [Google Scholar]
- 14.House JE Principles of Chemical Kinetics. Amsterdam: Elsevier/Academic, 2007.
- 15.Jensen MD, Caruso M, Heiling V, Miles JM. Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes 38: 1595–1601, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest 79: 207–213, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism 56: 68–76, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Nurjhan N, Campbell PJ, Kennedy FP, Miles JM, Gerich JE. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 35: 1326–1331, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 23: 113–122, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Pacini G, Finegood DT, Bergman RN. A minimal-model-based glucose clamp yielding insulin sensitivity independent of glycemia. Diabetes 31: 432–441, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Roy A, Parker RS. Dynamic modeling of free fatty acid, glucose, and insulin: an extended “minimal model”. Diabetes Technol Ther 8: 617–626, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Saad MF, Steil GM, Riad-Gabriel M, Khan A, Sharma A, Boyadjian R, Jinagouda SD, Bergman RN. Method of insulin administration has no effect on insulin sensitivity estimates from the insulin-modified minimal model protocol. Diabetes 46: 2044–2048, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Savage DB, Petersen KF, Shulman GI. Mechanisms of insulin resistance in humans and possible links with inflammation. Hypertension 45: 828–833, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Sumner AE, Bergman RN, Vega GL, Genovese DJ, Cochran CS, Pacak K, Watanabe RM, Boston RC. Multiphasic free fatty acid profile during the frequently sampled glucose tolerance test in adults. Metabolism 53: 1202–1207, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis 196: 696–703, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med 165: 1395–1400, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Sumner AE, Kushner H, Sherif KD, Tulenko TN, Falkner B, Marsh JB. Sex differences in African Americans in sensitivity to insulin's glucoregulatory and antilipolytic actions. Diabetes Care 22: 71–77, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Sumner AE, Kushner H, Tulenko TN, Falkner B, Marsh JB. The relationship in African-Americans of sex differences in insulin-mediated suppression of nonesterified fatty acids to sex differences in fasting triglyceride levels. Metabolism 46: 400–405, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism 54: 902–909, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. IUBMB Life 56: 379–385, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Thomaseth K, Pavan A. Model-based analysis of glucose and free fatty acid kinetics during glucose tolerance tests. In: Mathematical Modeling in Nutrition and Toxicology, edited by Hargrove J and Berdanier C. Athens, GA: Lulu, 2005, p. 21–40.
- 32.Vautard R, Yiou P, Ghil M. Singular-spectrum analysis: a toolkit for short, noisy chaotic signals. Physica D 58: 95–126, 1992. [Google Scholar]