Abstract
Our objective was to determine whether neuronally induced atrial arrhythmias can be modified by α-adrenergic receptor blockade. In 30 anesthetized dogs, trains of five electrical stimuli (1 mA; 1 ms) were delivered immediately after the P wave of the ECG to mediastinal nerves associated with the superior vena cava. Regional atrial electrical events were monitored with 191 atrial unipolar electrodes. Mediastinal nerve sites were identified that reproducibly initiated atrial arrhythmias. These sites were then restimulated following 1 h (time control, n = 6), or the intravenous administration of naftopidil (α1-adrenergic blocker: 0.2 mg/kg, n = 6), yohimbine (α2-adrenergic blocker: 1 mg/kg, n = 6) or both (n = 8). A ganglionic blocker (hexamethonium: 1 mg/kg) was tested in four dogs. Stimulation of mediastinal nerves sites consistently elicited atrial tachyarrhythmias. Repeat stimulation after 1 h in the time-control group exerted a 19% decrease of the sites still able to induce atrial tachyarrhythmias. Hexamethonium inactivated 78% of the previously active sites. Combined α-adrenoceptor blockade inactivated 72% of the previously active sites. Bradycardia responses induced by mediastinal nerve stimulation were blunted by hexamethonium, but not by α1,2-adrenergic blockade. Naftopidil or yohimbine alone eliminated atrial arrhythmia induction from 31% and 34% of the sites (similar to time control). We conclude that heterogeneous activation of the intrinsic cardiac nervous system results in atrial arrhythmias that involve intrinsic cardiac neuronal α-adrenoceptors. In contrast to the global suppression exerted by hexamethonium, we conclude that α-adrenoceptor blockade targets intrinsic cardiac local circuit neurons involved in arrhythmia formation and not the flow-through efferent projections of the cardiac nervous system.
Keywords: neuromodulation, cardiac nervous system, ganglionic blockade
mediastinal nerves that are associated with pulmonary veins (20) and superior vena cava (7, 22) represent the caudal-most inputs from thoracic vagosympathetic nerves to the intrinsic cardiac nervous system (8, 17). These inputs to the intrinsic cardiac nervous system are involved in regulating cardiac electrical and mechanical indices (2). It has been demonstrated that atrial tachyarrhythmias can be reproducibly induced in the normal canine heart by delivering electrical stimuli to select mediastinal nerves (5–7). Atrial fibrillation (AF) so induced can be modified by pharmacological means (21). For instance, the induction of such AF can be blunted by β-adrenoceptor blockade (5, 7) and abolished by muscarinic receptor blockade (7). Multiple lines of evidence indicate that one preferential target for exogenously administered chemicals is the intrinsic cardiac nervous system, with subsequent effects on regional cardiac function (2).
Beyond the classical recognized nicotinic mechanisms, many other receptor systems are involved in intrinsic cardiac neurotransmission (1). In particular, α- and β-adrenoceptors have been associated with the local circuit (4) and efferent neurons (29) within the intrinsic cardiac nervous system (1, 4). In accord with that, α-adrenoceptor agonists and their antagonists influence the electrical activity of such neurons (14), their synaptic transmission (27, 30), as well as their capacity to regulate cardiac electrical and mechanical function (3, 4).
The coordination of regional cardiac function is dependent upon extrinsic inputs to and interactions within the intrinsic cardiac nervous system (2, 28). We hypothesize that heterogeneous activation of the intrinsic cardiac nervous system may predispose the heart to arrhythmias. That is to say, imbalances in nerve inputs to or outputs from discrete elements of the intrinsic cardiac nervous system may create asymmetric efferent outflows to cardiac tissues, thereby eliciting regional electrical instability. As a corollary, stabilization of any imbalance that occurs within that component of the cardiac neuronal hierarchy would be expected to reduce that arrhythmic substrate. Therefore, the objective of this study was to determine whether α-adrenoceptor blockade can reduce the functional substrate for neurally induced atrial tachyarrhythmias.
MATERIALS AND METHODS
Animals.
A total of 30 adult mongrel canines (either sex), weighing 15–40 kg, were used in this study. Experiments were performed in accordance with guidelines for animal experimentation (World Medical Association-American Physiological Society, 2002) and approved by the institutional animal care committee of the University of Montreal. Animals were anesthetized with sodium thiopental (25 mg/kg iv, supplemented as required), intubated and maintained under positive-pressure ventilation. After all surgical procedures had been completed, the anesthetic agent was changed to α-chloralose (50 mg/kg iv bolus, supplemented with 25 mg/kg iv as required).
Surgical preparation.
Following a transthoracic incision, the pericardium was incised to expose the heart. Left ventricular and aortic pressures (Millar electronic pressure sensors) and a lead II ECG were recorded on a rectilinear pen recorder (Nihon Kohden, Tokyo, Japan). Atrioventricular blockade was induced by formaldehyde injection (37%; 0.1 ml) into the AV node to isolate atrial from ventricular electrical events. Right ventricular pacing (60 beats/min) was instituted to maintain adequate cardiac output.
Atrial epicardial mapping.
Multiple silicone plaques carrying 191 unipolar recording electrodes (4.6–5.9 mm spacing) were positioned on the ventral, lateral, and dorsal surfaces of the right and left atrium (7). The unipolar leads and a lead II ECG were connected to a multichannel recording system (EDI 12/256, Institut de Génie Biomédical, École Polytechnique de Montréal) controlled by custom-made software (Cardiomap III: www.crhsc.umontreal.ca/cardiomap) using a PC. The 191 unipolar electrograms (measured with reference to Wilson's central terminal derived from the four limb leads) were amplified by programmable-gain analog amplifiers (0.05–450 Hz) and converted to digital format at 1,000 samples·s−1·channel−1. Data were stored on hard disk and retrieved for detailed analysis.
Electrical stimulation of mediastinal nerves.
Atrial tachyarrhythmias were induced in each animal by delivering electrical stimuli to individual right-sided mediastinal nerves that course over the ventral and ventrolateral surfaces of the caudal-most part of the superior vena, as identified by their accompanying vessels (6). Active sites were identified that, when stimulated electrically, immediately induced atrial arrhythmias. The most frequently identified response consisted of a bradycardia followed by an episode of spontaneous and self-terminating atrial tachyarrhythmia/fibrillation (7). Once identified, each active locus was marked with ink for repeated stimulations. Electrical stimuli were applied to neuronal elements located on the intrapericardial portion of the superior vena cava on 1) the first 1–2 cm cranial to its junction with the right atrium and 2) the first 1–2 cm caudal to that same anatomical landmark. Electrical stimuli were delivered focally via a bipolar electrode (1.5-mm spacing) mounted on a roving probe that was connected to a battery-driven current source controlled by a programmable stimulator (Bloom Associates, Philadelphia, PA). Trains of 5 electrical stimuli (1 mA, 1 ms duration; 5-ms pulse interval) were delivered during the refractory period of the closest atrial regions (beginning 30 ms after excitation of a reference electrogram). This was done to avoid atrial muscle capture. Stimulations were interrupted immediately after the onset of tachycardia prior to AF. If no sites were identified at 1 mA, the intensity of the stimulation was increased gradually to 1.5 and 2 mA such that 3–5 active neural sites could be identified prior to repetition (time-control group) or pharmacological treatment. Following the administration of drugs, if a previously identified active site no longer induced AF at the control stimulation intensity, the intensity was increased to 2 mA.
Experimental design.
After identifying 3–5 active right-sided mediastinal nerve sites, animals were randomized to one of five treatment groups. Pharmacological agents were administered within 5 min of completing active site identification. Group 1 (n = 6) evaluated the effects of time. In this group, active sites identified at baseline were restimulated 1 and 2 h later. Group 2 consisted of four dogs whose mediastinal nerves were stimulated before and after administrating the ganglionic blocker hexamethonium (1 mg/kg iv). In Group 3 (n = 8), the α1-adrenergic blocking agent naftopidil (0.2 mg/kg iv) and α2-adrenergic blocking agent yohimbine (1 mg/kg iv) were administered in combination, and the active sites restimulated with the whole procedure being completed within 1 h. In groups 4 and 5 (n = 6 each), respectively, the α1-blocking agent naftopidil or the α2-blocking agent yohimbine was administered, and previously identified active sites were restimulated. Because each agent was administered systemically, we allowed 20 min to elapse after each injection prior to nerve restimulation.
Data analysis.
Analyzing unipolar atrial electrograms with Cardiomap III software, activation times were identified as the moment in the activation complex when the negative slope of potential (−dV/dtmax) was maximal (12). This permitted identifying a QS morphology at sites of atrial origin and RS morphology at sites that were activated later. With respect to neurally induced bradycardia responses, the atrial cycle length was assessed as the maximum interval recorded from two consecutive atrial electrograms and compared with 10 consecutive basal cycle lengths obtained immediately prior to stimulation. The following response characteristics were determined during the atrial tachyarrhythmias: latency (defined as the interval from the first applied stimulus train to tachyarrhythmia initiation); tachyarrhythmia (sinus tachycardia) duration; tachyarrhythmia interbeat intervals; and duration of atrial flutter/fibrillation. For each animal, characteristics of atrial bradycardia and/or tachyarrhythmia were averaged from the multiple mediastinal nerve stimulation sites. For post hoc analysis, data were also subgrouped based on efficacy of treatment(s) to extinguish (or not) neurally induced tachyarrhythmias. Induced-rhythm types were classified based on information obtained from the biatrial epicardial recording plaques. Classes included atrial fibrillation (disorganized beats), atrial flutter (organized beats without a pause between them), atrial tachycardia (organized beat with a pause between beats), or sinus rhythm.
Data collected prior to and after drug administration were subjected to paired t-test analysis and chi-squared testing. Comparisons between experimental groups were made using univariate (for individual variables) and multivariate two-way ANOVA. The level of certainty for rejecting the null hypothesis was P ≤ 0.05. Data are presented as means ± SD.
RESULTS
Select mediastinal nerve response characteristics.
Among the 30 dogs, a total of 150 active mediastinal neuronal projection sites were identified adjacent to the superior vena cava that, when stimulated, consistently elicited atrial tachyarrhythmias. A typical response consisted of an immediate bradycardia that reached its maximum (17% change) within 1.3 ± 0.7 s of the initial stimulus application, followed by a spontaneous premature atrial depolarization initiating a tachyarrhythmia that terminated spontaneously within seconds to minutes (median duration of 8.7 s; min = 0.3 s; max = 2,158 s) of stimulus cessation (Fig. 1). The majority of the tachyarrhythmias were classified as atrial fibrillation (AF) or atrial flutter (AFl) with early epicardial breakthroughs primarily localized to the sinoatrial nodal pacemaker complex, right atrial appendage or Bachmann's Bundle. No atrial response was elicited when the electrode was moved to immediately adjacent non-neural sites, even at the highest stimulation intensity tested (2 mA).
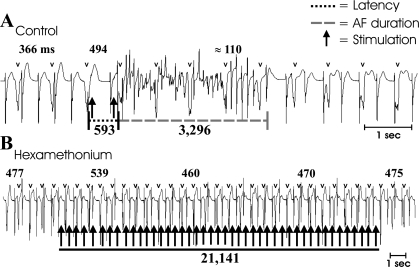
Fig. 1.
Atrial arrhythmia induction in response to electrical stimuli applied to a mediastinal nerve. Atrial recordings derived from a unipolar electrode on the ventral right atrial free wall shows dissociated atrial and ventricular (v) complexes in a canine preparation with atrioventricular block. Intermittent application of bursts of electrical stimuli (1 mAmp, 1-ms duration, 5-ms pulse interval) to a cranial right-sided mediastinal nerve (arrows) during the atrial refractory period in control states (A) typically evoked a bradycardia (cycle length prolongation from 366 to 494 ms), followed by rapid transition to atrial tachyarrhythmia (here, atrial fibrillation) that self-terminated after 3.3 s. Similar responses to repeat stimulation were induced in control states. Following nicotinic ganglionic blockade (B; hexamethonium), atrial cycle length increased to 477 ms, with a residual, but blunted, bradycardia being induced when electrical stimuli were applied to the same mediastinal nerve site. In this state, atrial tachyarrhythmia did not occur, even when the stimuli were applied for prolonged periods of time (21 s). Numbers in bold represent atrial cycle lengths.
Time control (group 1, n = 6 animals).
To assess the temporal stability of bradycardia and tachyarrhythmia induction, a total of 28 active neural sites were subjected to repeat stimulation at hourly intervals for 2 h in 6 separate animals. Throughout this period, sinus rate remained stable and bradycardias were reproducibly induced at all neural sites (Fig. 2A, left). Averaged across animals, after each subsequent hour, AF or AFl was no longer inducible from ∼20% of the previously responsive sites in spite of the fact that the magnitude of the induced bradycardias was similar (Fig. 2A, right). Increasing the intensity of the electrical stimuli at the unresponsive sites did not restore arrhythmia induction. Consequently, all subsequent treatment protocols (groups 2–5) were performed within 1 h of identifying active neural sites.
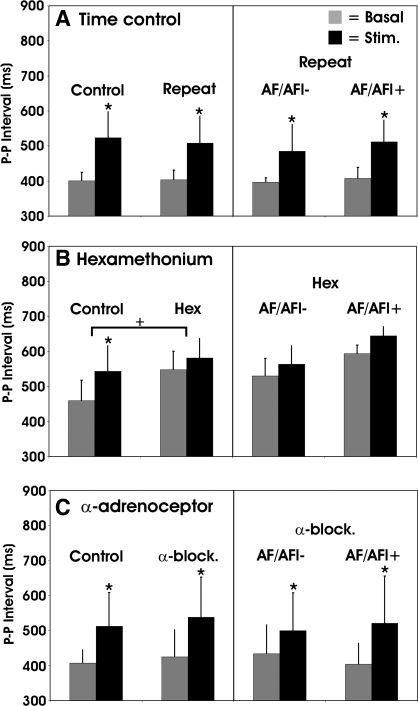
Fig. 2.
Mediastinal nerve-induced atrial bradycardia in time control animals (A) and in animals prior to and following ganglionic blockade (B; hexamethonium) or combined α1 and α2 blockade (C; naftopidil and yohimbine). Scale bars indicate P-P interval at basal rhythm (gray-shaded bar) and at the maximal stimulation induced bradycardia (black-shaded bar). Following the repeated stimulation (time control), hexamethonium or combined α1 and α2 blockade, mediastinal nerve stimulation sites were subgrouped into those with persistent atrial tachyarrhythmia (AF/AFl+), compared with those in which they were abolished (AF/AFl−). *P ≤ 0.05 baseline to stimulation. +P ≤ 0.05 control vs. post-Rx.
Hexamethonium (group 2, n = 4 animals).
Hexamethonium increased basal atrial cycle lengths (Fig. 2B, left). It also blunted the initial bradycardias elicited by mediastinal nerve stimulation (Fig. 2B, left). Comparing sites where hexamethonium eliminated atrial fibrillation/flutter induction to those in which it was maintained, there was no difference in induced changes in basal heart rate or the residual bradycardia response elicited by nerve stimulation (Fig. 2B, right).
In control states, the principal mediastinal nerve-induced arrhythmias were atrial tachyarrhythmias or flutter (Fig. 3, control) with early epicardial breakthroughs located primarily to the SA nodal pacemaker complex, right atrial appendage or Bachmann's bundle (Fig. 4, control). Following hexamethonium, mediastinal nerve-induced tachydysrhythmias were eliminated from 78% of the previously active sites (Fig. 1B; Fig. 3, hexameth.). Pacemaker activity remained localized primarily within the SA nodal pacemaker complex (Fig. 4, hexameth.). For residual tachyarrhythmia, latency (+46%) and duration (−85%) were altered after hexamethonium.
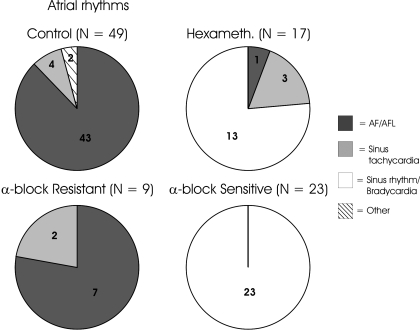
Fig. 3.
Types of atrial rhythms induced during mediastinal nerve stimulation prior to (preblock, control) and following treatment with hexamethonium or combined α1&2 blockade. Rhythm types were classified as atrial fibrillation/flutter (AF/AFL), sinus tachycardia, sinus rhythm/bradycardia, and other (3- to 4-beat salvos). Rhythm types post α1&2 blockade were subgrouped based upon those in which atrial tachyarrhythmias (including AF/AFL) were extinguished (α-block sensitive; 71.9% of sites) and those in which tachyarrhythmias were maintained (α-block resistant; 28.1% of sites). The number of stimulation sites for each group is indicated above each chart, as well as numbers for each subgrouping of induced atrial rhythms.
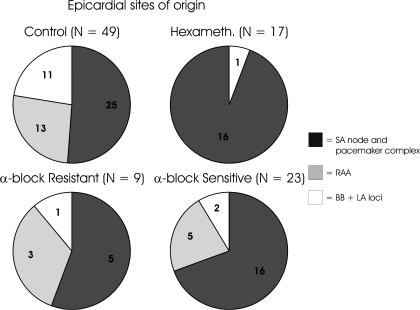
Fig. 4.
Distribution of early epicardial breakthrough during mediastinal nerve stimulation prior to (control) and following treatment with hexamethonium or combined α1&2 blockade. Epicardial breakthrough sites post α1&2 blockade were subgrouped based upon those in which atrial tachyarrhythmias (including AF/AFL) were extinguished (α-block sensitive) and those in which tachyarrhythmias were maintained (α-block resistant). RAA, right atrial appendage; BB, Bachmann's bundle; LA, left atrium.
Combined α1&2-adrenoceptor blockade (group 3, n = 8 animals).
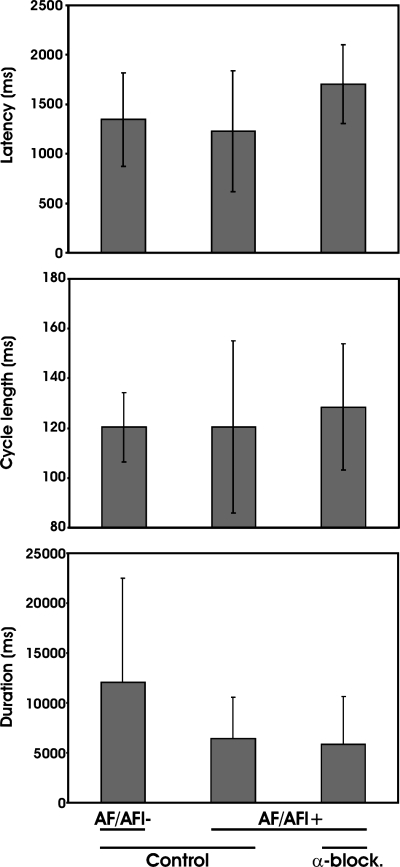
Averaged across animals, the number of mediastinal nerve sites at which tachyarrhythmias were induced by electrical stimulation was reduced by 72 ± 15% following the combined administration of naftopidil and yohimbine (Fig. 5 and Fig. 3, bottom). This was significantly greater than that identified in time controls (19 ± 11%). The elimination of mediastinal-induced tachyarrhythmias by combined adrenergic blockade was accompanied by less dispersion of the primary pacemaker location compared with control conditions (Fig. 4, α-block sensitive). For the stimulation sites that maintained tachyarrhythmias postcombined alpha blockade (Fig. 3, α-block resistant), the rhythm type and early epicardial breakthrough points remained similar to control (Fig. 4, α-block resistant). For all sites, there was no significant difference in the magnitude of mediastinal nerve induced bradycardia when comparing rhythms before to after combined alpha blockade (Fig. 2, bottom). Moreover, there was no difference in tachyarrhythmia latencies, cycle lengths, or durations under control conditions, among the AF/AFl- and AF/AFl+ subgroups, nor between responses for those sites with residual tachyarrhythmias postblockade (Fig. 6). Arterial blood pressure was reduced by combined alpha blockade (systolic/diastolic pressure, mmHg; control 138 ± 30 / 93 ± 21; α1&2-adrenoceptor blockade 111 ± 22/76 ± 8; P < 0.05). Heart rate was unaffected by combined alpha blockade (151 ± 23 beats/min, control; 142 ± 25 beat/min, α1&2-adrenoceptor blockade; P = 0.48).
Fig. 5.
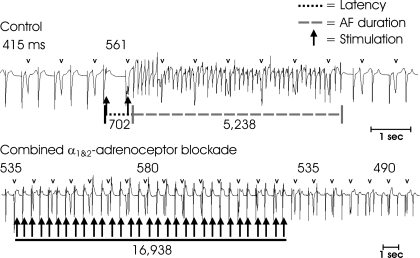
Combined alpha blockade modifies neurally induced atrial arrhythmias. A: in control states, mediastinal nerve stimulation induced a bradycardia immediately followed by a tachyarrhythmia (atrial flutter), which persisted for 5.2 s. B: after drug treatment, the tachyarrhythmia induction was abolished even with repeated stimulus applications over periods of 15–20 s. Labels are the same as in Fig. 1.
Fig. 6.
Persistence of tachyarrhythmia characteristics in AF-alpha block-resistant stimulation sites. Shown are latency (ms), cycle length (ms), and duration (ms) of atrial fibrillation/flutter prior to and after treatment with combined α1&2 blockade. Mediastinal nerve stimulation sites are subgrouped into those with persistent atrial tachyarrhythmia (AF/AFl+), compared with those that were abolished (AF/AFl−) post-treatment.
Single α-adrenoceptor subtype blockade (naftopidil, group 4, n = 6 animals, or yohimbine, group 5, n = 6 animals).
When administered alone, naftopidil or yohimbine induced a 31% (11/36) or 34% (11/32), respectively, reduction in the number of active neural sites at which atrial tachyarrhythmias were induced by electrical stimulation. Such reductions were not significantly different from the 19 ± 11% average reduction identified in the time control group (group 1).
DISCUSSION
Previous findings indicated that neuronal imbalances within discrete elements of the intrinsic cardiac nervous system augments the atrial arrhythmogenic substrate (2, 7). They also indicate the possibility of effectively targeting select intrinsic cardiac neuronal populations, in this instance, one possessing alpha-adrenoceptors, to stabilize such imbalance in the suppression of atrial arrhythmia formation. The latter is in accord with the fact that atrial tachyarrhythmias of neural origin can be suppressed by hexamethonium (7).
There are multiple targets of alpha blockade that could potentially impact upon atrial electrical stability, including cardiac-related neurons, cardiomyocytes, and vascular smooth muscle. In that regard, the stabilization of atrial electrical activity occurred, even though heart rate and blood pressure remained close to control values, indicating that overall autonomic neural status and vascular tone was largely unaltered by the blocking doses used herein. With respect to cardiomyocytes, the electrophysiological properties of atrial muscle, including the sinus node, can be directly affected by alpha adrenoceptor blockade, albeit, such effects are minor in nature (9, 26). Because of these considerations and the location of neural elements activated, we propose that any tachyarrhythmia suppression initiated by α1- and α2-adrenoceptor blockade involves alpha-adrenergic-mediated neurotransmission within the intrinsic cardiac nervous system. While the effects of single α1 or α2 alpha blockade by themselves may impact to some degree on the atrial arrhythmogenic potential, the data presented herein suggest that the effects of combined blockade may act in a synergistic manner to exert their influence.
In the past, we have indicated that electrical stimulation of select mediastinal nerve inputs to discrete elements of the intrinsic cardiac nervous system can lead to excessive and heterogeneous activation of the intrinsic cardiac nervous system that increases the arrhythmogenic substrate for atrial tachyarrhythmias (7, 19). The suppression of such events by combined α1- and α2-adrenoceptor blockade identified in this work may reside in its stabilizing effects on select neuronal elements of the intrinsic cardiac nervous system.
α1 and α2-adrenoceptors and autonomic neurons.
α-adrenoceptors have been identified on intrinsic cardiac parasympathetic efferent neurons (29). Activation of α1-adrenoceptors associated with intrinsic cardiac parasympathetic neurons induces a two-step response (16), an initial inhibitory response (18) followed by a longer lasting excitatory phase, in which parasympathetic intrinsic cardiac neurons fire repetitively (15). Although in vitro studies have failed to demonstrate modulation of sympathetic efferent neurons by α1-adrenoceptor agonists (24, 25), select populations of intrinsic cardiac neurons can be activated in situ by locally applied α1- or α2-adrenergic agonists (4). In fact, alpha adrenergic activation of intrinsic cardiac local circuit neurons enhances regional cardiac, electrical, and mechanical function (4). Furthermore, it is known that α2-adrenoceptors on parasympathetic and sympathetic efferent nerve terminals (29) act to inhibit neurotransmitter release (1). Taken together, these data suggest the potential for α-adrenoceptor blockade to modify intrinsic cardiac local circuit and efferent neuronal function.
Hexamethonium vs. combined α1- and α2-adrenoceptor blockade.
Although targeting neural elements within the intrinsic cardiac nervous system with either hexamethonium or α1,2-adrenergic blockade was effective in suppressing tachyarrhythmia formation, bradycardia responses were blunted by hexamethonium but not by α1,2-adrenergic blockade. In fact, combined α1- and α2-adrenoceptor blockade minimally affected the initial bradycardia induced by mediastinal nerve stimulation, regardless of whether or not subsequent atrial tachyarrhythmias were suppressed (Fig. 2). These data indicate that the primary throughput of parasympathetic efferent neuronal projections was maintained after α-adrenoceptor blockade.
Preganglionic and postganglionic neuronal elements within the intrinsic cardiac nervous system play a major role in the induction of atrial tachyarrhythmias when excessively and heterogeneously activated. Targeting of neural elements within the intrinsic cardiac nervous system with systemic hexamethonium or α1,2-adrenergic blockade stabilized atrial pacemaker function and location in response discrete stimulation of efferent inputs to its ganglia. In the case of alpha blockade, this occurred without interfering with local autonomic control of chronotropic function (Figs. 1 and 4, bottom). In addition to sympathetic and parasympathetic efferent postganglionic neurons, the intrinsic cardiac nervous system contains local circuit neurons (2). It has been proposed that this population functions as interneurons coordinating intraganglionic and interganglionic neuronal interactions (11). As such, this system represents an interactive network coordinating sympathetic and parasympathetic efferent neuronal outflows at the level of the target organ (4). Some of these local circuit neurons possess α-adrenoceptors (4). As such, α-adrenoceptors may be involved not only as feedback mediators at the terminals of efferent postganglionic neurons (30) but also as processors of information analogous to what is observed in the central nervous system (13). Our knowledge of the role that local circuit neurons play in cardiac control remains limited. Data obtained from this study indicate that combined α1,2-adrenoceptor blockade may not only target such neurons (4), but also modifies/stabilizes information processing within the intrinsic cardiac nervous system to reduce the neuronal component of the arrhythmogenic substrate. In contrast, hexamethonium exerts a more global suppression, impacting not only local circuit neurons but also neurotransmission in the sympathetic and parasympathetic efferent limbs of the cardiac nervous system.
Perspectives and Significance
Current pharmacological or physical (i.e., ablation) management of atrial fibrillation targets atrial myocytes, as well as regional cardiac neural tissue that is concentrated around the pulmonary vein orifices (22, 23). The results of this study indicate that intrinsic cardiac local circuit neurons may also be regarded as a potential therapeutic target in arrhythmia suppression. Notably, pharmacological therapy would act to spare atrial tissue, something that ablation of regional intrinsic cardiac neural tissue does not accomplish (23). Such targeted drug therapy has the potential to stabilize the multiple components within the intrinsic cardiac nervous system, rather than destroying critical elements of a system that are essential for coordinating regional cardiac indices (2, 4). Taken together, these data support the concept that stabilization of the intrinsic cardiac nervous system in the presence of excessive inputs may affect the latter's involvement in atrial arrhythmia formation.
Acknowledgments
The authors gratefully acknowledge the support of the Canadian Institutes of Health Research, the National Institutes of Health (JLA, HL71380), and the Quebec Heart Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Basic and Clinical Neurocardiology, edited byArmour, J. A., and J. L. Ardell. New York: Oxford University Press, 2004, pp. 1–60.
- 2.Ardell JL Intrathoracic neuronal regulation of cardiac function. In: Basic and Clinical Neurocardiology, edited by Armour, J. A. and J. L. Ardell. New York: Oxford University Press, 2004, pp. 118–152.
- 3.Armour JA Synaptic transmission in thoracic autonomic ganglia of the dog. Can J Physiol Pharmacol 61: 793–801, 1983. [DOI] [PubMed] [Google Scholar]
- 4.Armour JA Intrinsic cardiac neurons involved in cardiac regulation possess alpha 1-, alpha 2-, beta 1- and beta 2-adrenoceptors. Can J Cardiol 13: 277–284, 1997. [PubMed] [Google Scholar]
- 5.Armour JA, Hageman GR, Randall WC. Arrhythmias induced by local cardiac nerve stimulation. Am J Physiol 223: 1068–1075, 1972. [DOI] [PubMed] [Google Scholar]
- 6.Armour JA, Randall WC, Sinha S. Localized myocardial responses to stimulation of small cardiac branches of the vagus. Am J Physiol 228: 141–148, 1975. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA, Richer LP, Page P, Vinet A, Kus T, Vermeulen M, Nadeau R, Cardinal R. Origin and pharmacological response of atrial tachyarrhythmias induced by activation of mediastinal nerves in canines. Auton Neurosci 118: 68–78, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brandys JC, Randall WC, Armour JA. Functional anatomy of the canine mediastinal cardiac nerves located at the base of the heart. Can J Physiol Pharmacol 64: 152–162, 1986. [DOI] [PubMed] [Google Scholar]
- 9.Brown RA, Carpentier RG. Alpha-adrenoceptor-mediated effects of norepinephrine on the guinea pig sinus node. J Electrocardiol 21: 213–217, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal R, Page P, Vermeulen M, Bouchard C, Ardell JL, Foreman RD, Armour JA. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol 291: R1369–R1375, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gray AL, Johnson TA, Ardell JL, Massari VJ. Parasympathetic control of the heart. II. A novel interganglionic intrinsic cardiac circuit mediates neural control of heart rate. J Appl Physiol 96: 2273–2278, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Helie F, Vinet A, Cardinal R. Cycle length dynamics at the onset of postinfarction ventricular tachycardias induced in canines: dependence on interval-dependent excitation properties of the reentrant substrate. J Cardiovasc Electrophysiol 11: 531–544, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hirono M, Obata K. Alpha-adrenoceptive dual modulation of inhibitory GABAergic inputs to Purkinje cells in the mouse cerebellum. J Neurophysiol 95: 700–708, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Horackova M, Armour JA. Role of peripheral autonomic neurones in maintaining adequate cardiac function. Cardiovasc Res 30: 326–335, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda SR Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380: 255–258, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi H, Umezu M, Jang IS, Ito Y, Akaike N. Alpha 1-adrenoceptor-activated cation currents in neurones acutely isolated from rat cardiac parasympathetic ganglia. J Physiol 548: 111–120, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janes RD, Johnstone DE, Brandys JC, Armour JA. Functional and anatomical variability of canine cardiac sympathetic efferent pathways: implications for regional denervation of the left ventricle. Can J Physiol Pharmacol 64: 958–969, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Jeong SW, Ikeda SR, Wurster RD. Activation of various G-protein coupled receptors modulates Ca2+ channel currents via PTX-sensitive and voltage-dependent pathways in rat intracardiac neurons. J Auton Nerv Syst 76: 68–74, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Page P, Andrew Armour J, Yin Y, Vermeulen M, Nadeau R, Cardinal R. Differential effects of cervical vagosympathetic and mediastinal nerve activation on atrial arrhythmia formation in dogs. Auton Neurosci 128: 9–18, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, Geng N, Lazzara R, Jackman WM. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm 3: 201–208, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Randall WC, Armour JA. Complex cardiovascular responses to vagosympathetic stimulation. Proc Soc Exp Biol Med 145: 493–499, 1974. [DOI] [PubMed] [Google Scholar]
- 22.Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, Lazzara R, Jackman WM. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 12: 592–599, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol 13 Suppl 1: 37–42, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Schofield GG Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an alpha 2-adrenoceptor. Eur J Pharmacol 180: 37–47, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Song SY, Saito K, Noguchi K, Konishi S. Adrenergic and cholinergic inhibition of Ca2+ channels mediated by different GTP-binding proteins in rat sympathetic neurones. Pflügers Arch 418: 592–600, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Talajic M, Villemaire C, Nattel S. Electrophysiological effects of alpha-adrenergic stimulation. Pacing Clin Electrophysiol 13: 578–82, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Thompson GW, Horackova M, Armour JA. Ion channel modifying agents influence the electrical activity generated by canine intrinsic cardiac neurons in situ. Can J Physiol Pharmacol 78: 293–300, 2000. [PubMed] [Google Scholar]
- 28.Waldmann M, Thompson GW, Kember GC, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101: 413–419, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Xu ZJ, Adams DJ. Alpha-adrenergic modulation of ionic currents in cultured parasympathetic neurons from rat intracardiac ganglia. J Neurophysiol 69: 1060–70, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi N, de Champlain J, Nadeau RA. Regulation of norepinephrine release from cardiac sympathetic fibers in the dog by presynaptic alpha- and beta-receptors. Circ Res 41: 108–117, 1977. [DOI] [PubMed] [Google Scholar]