Abstract
Confocal microscopy and image analysis were used to compare driving forces, specificity, and regulation of transport of the fluorescent organic anion, Texas Red (sulforhodamine 101 free acid; TR), in lateral choroid plexus (CP) isolated from rat and an evolutionarily ancient vertebrate, dogfish shark (Squalus acanthias). CP from both species exhibited concentrative, specific, and metabolism-dependent TR transport from bath to subepithelial/vascular space; at steady state, TR accumulation in vascular/subepithelial space was substantially higher than in epithelial cells. In rat CP, steady-state TR accumulation in subepithelial/vascular spaces was reduced by Na+-replacement, but was not affected by a 10-fold increase in buffer K+. In shark CP, Na+-replacement did not alter TR accumulation in either tissue compartment; subepithelial/vascular space levels of TR were reduced in high-K+ medium. In both species, steady-state TR accumulation was not affected by p-aminohippurate or leukotriene C4, suggesting that neither organic anion transporters (SLC22A family) nor multidrug resistance-associated proteins (ABCC family) contributed. In rat CP, digoxin was without effect, indicating that organic anion transporting polypeptide isoform 2 was not involved. Several organic anions reduced cellular and subepithelial/vascular space TR accumulation in both tissues, including estrone sulfate, taurocholate, and the Mrp1 inhibitor MK571. In rat CP, TR accumulation in subepithelial/vascular spaces increased with PKA activation (forskolin), but was not affected by PKC activation (phorbol ester). In shark, neither PKA nor PKC activation specifically affected TR transport. Thus, rat and dogfish shark CP transport TR but do so using different basic mechanisms that respond to different regulatory signals.
Keywords: organic anion transport, confocal imaging, organic anion transporter, organic anion transporting polypeptide, multidrug resistance-associated protein
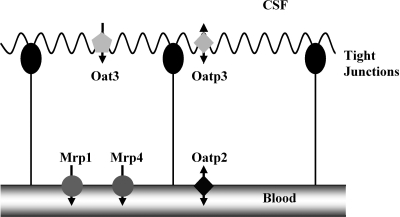
in vertebrate brain, the choroid plexus (CP) separates cerebrospinal fluid (CSF) from blood. Among the specialized functions of this epithelium is the removal from CSF of potentially toxic xenobiotics and waste products of central nervous system metabolism for eventual elimination in urine and bile. CP possesses separate transport systems for organic anions and organic cations, and recent work has begun to establish the molecular basis for such transport. In rat and mouse, five multispecific organic anion transporters have been immunolocalized to the apical or basolateral plasma membrane (Fig. 1), and each of these contributes to the transport of specific substrates, e.g., organic anion transporting isoform 3 (Oat3) for p-aminohippurate (PAH) and fluoresceine (FL) (19) and Mrp4 for topotecan (10). Nevertheless, it is clear that in crossing, the CP substrates may use more than one uptake or efflux transporter (4, 11, 19). Because of wide and overlapping substrate specificity, determining the extent to which each of these transporters contributes to the flux of a specific substrate across each membrane of the epithelium presents difficulties.
Fig. 1.
Organic anion transporters (Oats) in mammalian choroid plexus (CP). Labeled are transporters with known subcellular locations, based on specific immunostaining or transport experiments with knock-out mice (8–10). Additional transporters are known to be expressed at the message level (7). Transport studies with intact tissue have indicated additional transport pathways for which molecular correlates are lacking (4, 11, 19). CSF, cerebrospinal fluid; Oat3, Oat isoform 3; Oatp3, Oatp isoform 3; Mrp1, Mrp isoform 1.
We have been using fluorescent substrates, confocal microscopy, and quantitative image analysis to define the transport processes and transporters that contribute to the transepithelial movement of specific organic anions in isolated CP from rat, mouse, and dogfish shark, an evolutionarily ancient vertebrate. The advantage of this imaging approach is that organic anion accumulation in specific tissue compartments can be measured and the effects of inhibitors on substrate accumulation in the cells and in blood vessels can be assessed. This permits conclusions to be drawn with regard to the mechanisms contributing to both apical uptake and basolateral efflux. To date, we have applied this approach to two fluorescent organic anions in mouse, rat, and shark CP: one small, FL [325 kDa; (5, 9, 20)], and the second large, FL-methotrexate (MTX) [960 kDa; (2, 15, 18)]. In the present study, we compare the transport mechanisms in rat and shark CP for an organic anion of intermediate mass, Texas Red (sulforhodamine 101 free acid, 606 Da; TR). The overall goals were to 1) compare the driving forces, specificities, and regulation of the individual transport steps in the two species, and 2) compare these mechanisms of TR transport with those previously found for FL and FL-MTX. In both tissues, we found specific and concentrative transepithelial transport of TR. However, analysis of transport driving forces and specificity indicated species differences in the underlying mechanisms. Moreover, within a species, some of the mechanisms driving transport of FL, TR, and FL-MTX proved to be different, indicating a multiplicity of organic anion transport pathways.
MATERIALS AND METHODS
Chemicals.
TR was purchased from Sigma-Aldrich (St. Louis, MO), Mrp1 inhibitor MK571 from Biomol (Plymouth Meeting, PA). Isofluran was purchased from Abbott (Wiesbaden, Germany). All other chemicals were of the highest grade and obtained from commercial sources.
Animals.
All animal studies were approved by the Animal Care and Use Committees at the Mount Desert Island Biological Laboratory, the University of Heidelberg, and National Institutes of Health/National Institute of Environmental Health Sciences. Adult male and female spiny dogfish sharks (Squalus acanthias) were collected in the vicinity of Mount Desert Island, Maine. Sharks were held in large tanks of flowing sea water for 1–4 days before use. After decapitation, lateral and intravenous CPs were removed using fine forceps, cut into several pieces, and transferred into ice-cold, pregassed (99% O2/1% CO2) elasmobranch Ringer (ER), containing 280 mM NaCl, 6 mM KCl, 4 mM CaCl2, 3 mM MgCl2, 1 mM NaH2PO4, 0.5 mM Na2SO4, 350 mM Urea, 72 mM trimethylamine oxide, 2.5 mM glucose and 8 mM NaHCO3 at pH 7.8.
Adult male Sprague-Dawley rats were obtained from the animal facility of the University of Heidelberg. Animals were anaesthetized with isofluran and killed by cervical dislocation. Lateral CPs from each hemisphere of the brain were isolated using fine forceps. Tissue was immediately transferred to pregassed (95% O2/5% CO2) artificial CSF (aCSF) containing 103 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4 × 7 H2O, 25 mM NaHCO3, 10 mM glucose, 1 mM sodium pyruvate, and 2.5 mM CaCl2 at pH 7.4. Each CP was dissected into two pieces, each to be used for different treatments.
Transport.
All incubations were carried out in 12-well plates in sealed Ziplock bags, under 95% O2/5% CO2 (rat) or 99% O2/1% CO2 (shark). CP tissue was first incubated for 30 min in buffer (ER for shark and aCSF for rat) without (control) or with effectors of organic anion transport. Subsequently tissue was incubated to steady state (absence of time-dependent changes in fluorescence; 90 min, rat; 60 min, shark) in pregassed medium with TR or TR plus effectors. Experiments with shark tissue were carried out in a 14°C incubator, with imaging done at room temperature. Experiments with rat tissue were carried out at room temperature.
Stock solutions of substrate and inhibitors were prepared in water or DMSO. The final DMSO concentration in incubation media did not exceed 0.5%. This DMSO concentration was previously found to not affect organic anion transport in CP (7, 14). In some experiments, we used Na+-free aCSF and Na+-free ER with NaCl replaced by N-methyl-d-glucamine and NaHCO3 replaced by choline bicarbonate. High-K+ buffers had KCl increased 10-fold and NaCl reduced isoionically to compensate.
To acquire images, CP segments with incubation solution were transferred to covered Teflon chambers, with a glass coverslip bottom. Chambers were placed on an inverted confocal microscope (Leica DMIRB, Heidelberg, Germany; Olympus Fluoview) and viewed through transmitted light. For each piece of choroid plexus, we selected 5–15 areas of the tissue for imaging; each area showed undamaged epithelium, subepithelial space, and blood vessels. Confocal images (512 × 512 pixels, each the average of 4 or 8 frames) were acquired using a 543 nm HeNe laser (Leica) or the 568 nm line of an Ar-Kr laser (Olympus) and appropriate dichroic and long-pass emission filters. Objectives used were ×40 and ×63 oil immersion objectives (Leica) and a ×20 dry objective (Olympus). Images were collected at 512 × 512 pixels by 8 bits (0-255, Leica) or 12 bits (0-4095, Olympus), and the differences in bit depth is reflected in the data presented in the figures. Photomultiplier gain was adjusted so that average vascular fluorescence intensities in control tissue was about half-full scale. Laser intensity did not exceed 1% of maximum. With the settings used, tissue autofluorescence was undetectable and fluorescence photobleaching was minimal. All images of one tissue preparation were generally collected within 5 min. During microscopy, the incubation chambers were covered with a plastic cover, which was connected to an oxygen tank; thus, all samples were oxygenated during the entire observation period.
Fluorescence intensities were measured from stored images using NIH Scion Image software, as described previously (4, 5, 20). Average pixel intensity was recorded, and background fluorescence was subtracted. Experiment-to-experiment differences in control fluorescence intensities were mainly due to changes in laser strength and photomultiplier gain settings and, a to lesser extent, variations in tissue transport.
Statistics.
All experiments were repeated with tissue from 4–8 animals. Data are presented as percent of TR accumulation; values are means ± SE. Means of control and treated groups were compared using one-way ANOVA and Dunnett's post hoc test. Differences were considered to be statistically significant when P < 0.05.
RESULTS
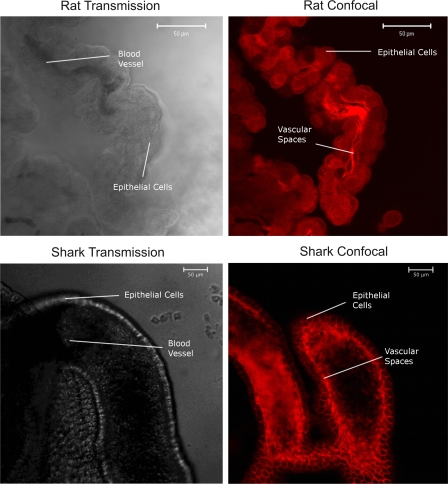
Figure 2 shows representative transmitted light and confocal images of rat and dogfish shark CP after 60-min incubation in medium containing TR. These images show both steady-state distribution of the fluorescent organic anion and the underlying tissue morphology. In the transmitted light images, the apical and basolateral plasma membranes are seen as refractile elements separating cells from medium and the subepithelial space and fenestrated capillaries, respectively. For tissue from both species, there is striking accumulation of TR within the subepithelial/vascular spaces. The difference in fluorescence intensity between incubation medium and subepithelial/vascular space is indicative of concentrative, transepithelial transport. In tissue from rat, cellular TR fluorescence clearly exceeded medium fluorescence, but the punctuate intracellular pattern suggests accumulation on or in vesicles. Confocal images of FL distribution in CP from rat and mouse show a similar punctuate appearance (5, 19). In contrast, cellular fluorescence in shark CP was clearly low, but slightly higher than the medium. Even with higher numerical aperture objectives we could not discern a punctuate distribution pattern in shark CP epithelial cells (not shown). For both species, the fluorescence distribution pattern indicates two concentrative steps arranged in series.
Fig. 2.
Transmission and confocal images of rat and dogfish shark CP at steady state, after incubation in medium with Texas Red (TR; 2 μM in rat; 1 μM in shark). In both species, highest fluorescence intensities are in the vascular/subepithelial spaces. In rat CP, epithelial cells are substantially brighter than medium; in shark CP, cells appear somewhat brighter than medium. In blood vessels, erythrocytes are seen as areas of low fluorescence.
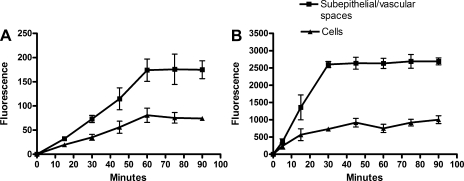
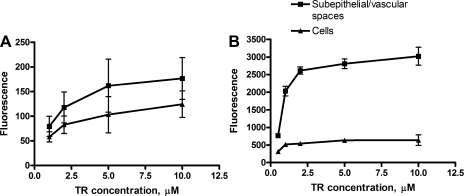
The time courses of accumulation of TR in CP tissue from rat and shark are shown in Fig. 3. For both species, TR accumulation in both tissue compartments reached steady state within 60 min. At all times studied, accumulation in the subepithelial/vascular space exceeded cellular accumulation. Steady-state TR accumulation in cells and subepithelial/vascular space was saturated with increasing medium TR concentration (Fig. 4).
Fig. 3.
Time course accumulation of 2 μM TR in rat (A) and 1 μM TR in dogfish shark (B) CP. Values are means ± SE from 4–5 measurements in 1 representative experiment.
Fig. 4.
Concentration dependence of steady-state (60 min) TR accumulation in rat (A) and dogfish shark (B) CP. Values are means ± SE from 10 measurements in 1 representative experiment.
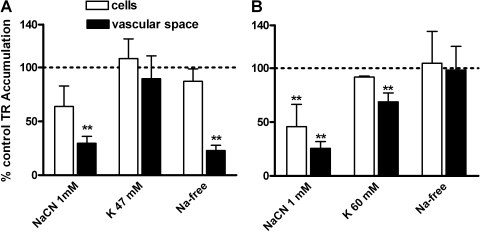
Experiments focused on understanding transport driving forces indicated fundamental species differences. In rat CP, 1 mM NaCN reduced TR accumulation in the subepithelial/vascular space but not within the epithelial cells (Fig. 5A). Increasing medium K+ 10-fold, a maneuver that depolarizes rat CP cells and reduces organic cation uptake (21), did not affect TR transport (Fig. 5A), but replacing medium Na+ with N-methylglucamine significantly reduced TR accumulation in the subepithelial/vascular space, but not the cells (Fig. 5A). In shark CP, NaCN reduced both cellular and subepithelial/vascular space TR accumulation, high K+ (10-times control) slightly reduced subepithelial/vascular space accumulation, and Na+-replacement was without effect (Fig. 5B).
Fig. 5.
Steady-state accumulation of 2 μM TR in rat (A) and 1 μM TR in shark (B) CP. Values are means ± SE for tissue from 4–8 animals. *Significantly different from control, P < 0.05; **significantly different from control, P < 0.01.
Our previous imaging-based studies of organic anion transport in rat and shark CP demonstrated three patterns of inhibition by nonfluorescent organic anions (2, 4, 5, 20). First, parallel reductions in cellular and subepithelial/vascular space accumulation indicate inhibition of uptake at the apical membrane, but does not provide information about possible effects at the basolateral membrane. Second, reduced subepithelial/vascular space accumulation with no change in cellular accumulation indicates inhibition of efflux at the basolateral membrane but no effect of the inhibitor on apical transporters. Third, reduced subepithelial/vascular space accumulation accompanied by increased cellular accumulation also indicates inhibition of efflux at the basolateral membrane. Here, however, increased cellular accumulation results from a situation where steady-state cellular accumulation is dominated by potent efflux.
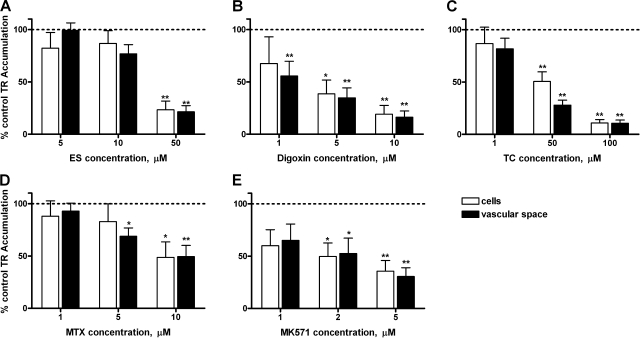
We examined the effects of a panel of transport inhibitors, chosen based on their known abilities to interact with mammalian Oats expressed in CP (9, 10, 15, 17). These include PAH, cimetidine, and 2,4-dichlorophenoxyacetic acid (2,4-D; apical Oat3), digoxin (basolateral Oatp2), azidothymidine (AZT; basolateral Mrp4), MK571 (basolateral Mrp1, possibly apical Oatp3), taurocholate (TC), and estrone sulfate (ES; Oat3, Oatps, and Mrps), and MTX and leukotriene C4 (LTC4; Mrps and possibly Oatps).
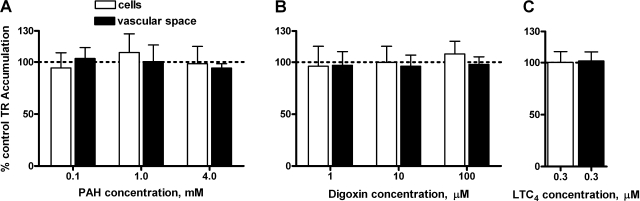
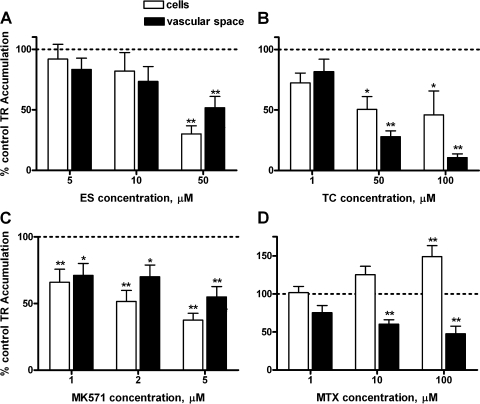
In rat CP, PAH, digoxin, and LTC4 were without effect (Fig. 6). In initial experiments, AZT, 2,4-D, and cimetidine also did not inhibit (results from 2–3 animals showed cellular and vascular accumulation of TR, averaged 104% and 108% of controls, respectively; in tissue exposed to 50 μM AZT, 103% and 101% of controls, respectively; in tissue exposed to 500 μM 2,4-D and 87% and 103% of controls for tissue exposed to 1 mM cimetidine). These results eliminate Oat3, Oatp2, and Mrp4 from the list of possible contributors to TR transport in rat CP. In contrast, ES, TC, and MK571 reduced both cellular and subepithelial/vascular space TR accumulation (Fig. 7, A–C). MTX reduced TR accumulation only in the subepithelial/vascular space; at the highest concentration tested it also significantly increased cellular TR accumulation (Fig. 7D).
Fig. 6.
Lack of effect of p-aminohippurate (PAH; A), digoxin (B), and leukotriene C4 (LTC4; C) on TR (2 μM) accumulation at steady state in rat CP. Values are means ± SE for tissue from 4–8 animals.
Fig. 7.
Inhibition of TR (2 μM) accumulation in rat CP at steady state by estrone sulfate (ES; A), taurocholate (TC; B), MK571 (C), and methotrexate (MTX; D). Values are means ± SE for tissue from 4–8 animals. *Significantly different from control, P < 0.05; **significantly different from control, P < 0.01. MK571, Mrp1 inhibitor.
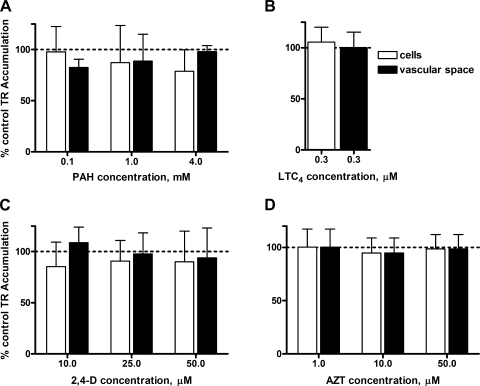
In shark CP, incubation with cimetidine (1 mM), resulted in fluorescence intensities of 91% in cells and 102% in vascular spaces. As in rat, also PAH, 2,4-D, AZT, and LTC4 were without effect on TR transport (Fig. 8). ES, digoxin, TC, and MK571 reduced both cellular and subepithelial/vascular space TR accumulation largely in a concentration-dependent manner (Fig. 9, A–C, E). MTX, at 5 μM, reduced only accumulation in the subepithelial/vascular space, at 10 μM cellular fluorescence was also affected (Fig. 9D). Importantly, we found no evidence for decreased subepithelial/vascular space accumulation accompanied by increased cellular TR accumulation (case 3, above; seen in rat CP with 100 μM MTX) in dogfish shark CP.
Fig. 8.
Lack of effect of PAH (A), LTC4 (B), 2,4-dichlorophenoxyacetic acid (2,4-D; C), and azidothymidine (AZT; D) on TR (1 μM) accumulation in shark CP. Values are means ± SE for tissue from 4 animals.
Fig. 9.
Inhibition of TR (1 μM) accumulation in shark CP by ES (A), digoxin (B), TC (C), MTX (D), and MK571 (E). Values are means ± SE for tissue from 4–6 animals. *Significantly different from control, P < 0.05; **Significantly different from control, P < 0.01.
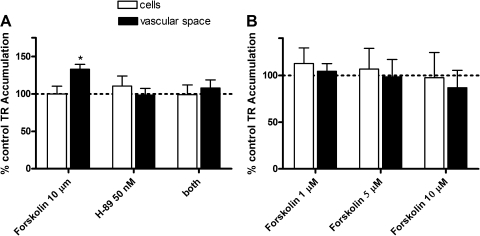
Our recent experiments have shown that transport of FL-MTX in shark CP is under control of protein kinases, increasing when PKA is activated and decreasing when PKC is activated (2). Figure 10 shows differential effects of PKA activator on TR transport in rat and shark CP. In rat tissue, the phosphodiesterase inhibitor forskolin significantly increased accumulation of TR in the subepithelial/vascular space, but not in the cells (Fig. 10A). This increase was abolished by the PKA inhibitor H-89, which by itself did not affect TR transport. In shark CP, forskolin did not affect TR transport (Fig. 10B). The concentration of forskolin used here was previously found to increase FL-MTX transport in shark CP (2). Thus, PKA appears to increase TR transport in rat CP but not in shark CP.
Fig. 10.
Effects of PKA activation by forskolin on TR transport in rat (A) and dogfish shark (B) CP. Medium concentration of TR was 2 μM in rat and 1 μM in shark. After preincubation (30 min) with effectors, tissue was incubated to steady state (90 min in rats and 60 min in sharks) in pregassed medium with TR or TR plus effectors. H-89, an PKA inhibitor. Values are means ± SE for tissue from 4–6 animals. *Significantly different from control, P < 0.05; **significantly different from control, P < 0.01.
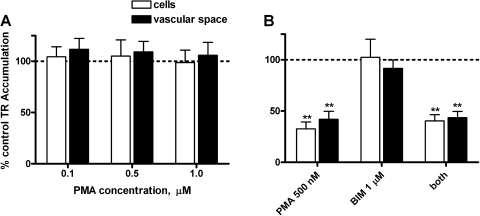
PMA, a phorbol ester that activates PKC did not change TR transport in rat CP (Fig. 11A). In shark CP, PMA reduced both cellular and subepithelial/vascular space TR accumulation. However, that reduction was not attenuated by the PKC inhibitor BIM (Fig. 11B) at a concentration that previously abolished the effects of PMA on FL-MTX transport in shark CP (2), suggesting a nonspecific effect.
Fig. 11.
Effects of PKC activation on TR transport in rat (A) and dogfish shark (B) CP. Medium concentration of TR was 2 μM in rat and 1 μM in shark. PMA, a phorbol ester that activates PKC; BIM, PKC inhibitor. Values are means ± SE for tissue from 4–6 animals. **Significantly different from control, P < 0.01.
DISCUSSION
Recent studies have implicated transporters from three families as contributors to organic anion transport in mammalian CP; these include Oat3, Oatp2, Oatp3, Mrp1, and Mrp4 (Fig. 1). All of these transporters are multispecific, in that they can handle a wide range of organic anions. As a consequence, several organic anions, e.g., ES and TC, are substrates for at least four of the five transporters (15, 17). Expression of multiple Oats with overlapping specificities confounds attempts to evaluate the contributions of individual transporters to the flux of a given substrate. Three approaches to this problem have been partially successful: 1) use of tissue from transporter knockout animals e.g., Oat3-null mouse (18, 19); 2) use of substrates and inhibitors whose interactions are limited to single transporters to eliminate or block transport mediated by that transporter, e.g., PAH for Oat3 in CP (11, 19); and 3) comparison of the transport characteristics of the substrate (driving forces, kinetics, inhibitor profile) in intact CP with those found in cells overexpressing each of the candidate transporters (11, 13, 14).
For mammals, the first approach is largely limited to mouse, although some rat strains are available that provide natural transporter knockouts, e.g., TR-/ESAI which are null for Mrp2. For fish, genome sequencing, bioinformatic tools, and improved RNA silencing technology hold the promise of generation of gene knockdowns that will be of value in dissecting transport pathways in epithelia. But such approaches are currently restricted to a few species of teleost fish, e.g., zebrafish. Thus, at least for the present, a molecular understanding of transport in tissues from many species of comparative/evolutionary interest, e.g., dogfish shark, will have to come from studies employing the second and third approaches combined with comparisons to data in species where transport has been functionally mapped.
The present data for both rat and shark show transport from aCSF/ER to subepithelial/vascular space to be saturable, concentrative, metabolism dependent (inhibited by NaCN), and specific (inhibited by other organic anions). In both species, apical TR uptake and basolateral efflux could be differentially inhibited by organic anions, indicating two-step, transepithelial transport. In neither species did we find membrane potential (PD) dependence (high K+) of uptake, although high-K+ medium did reduce basolateral efflux in the shark. In rat CP, basolateral TR efflux was Na+-dependent, but in shark CP we found no such Na+-dependence. In both species, apical uptake was reduced by the organic anions ES, TC, and MK571; MTX appeared to inhibit primarily basolateral efflux. TR transport was insensitive to inhibition by PAH and LTC4. Digoxin inhibition of transport was found in shark CP, but not rat CP. These results indicate that the mechanisms mediating TR transport are clearly different in rat and shark CP.
Apical organic anion uptake.
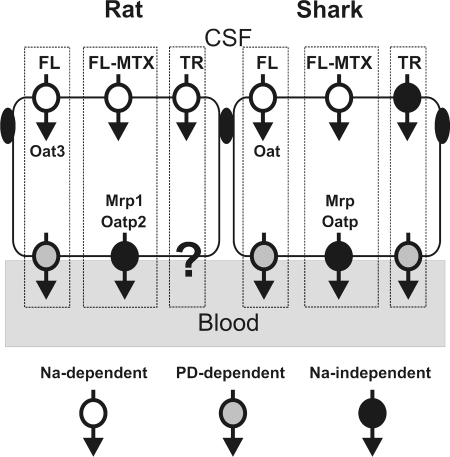
Previous confocal imaging studies in rat, mouse, and shark have focused on FL (∼300 kDa) and FL-MTX (∼900 kDa). The present study with TR considers transport of an organic anion of intermediate size (∼600 kDa). Available information for mechanisms responsible for FL, FL-MTX, and TR transport in rat and shark CP are summarized in Fig. 12, which provides a guide to the comparative aspects of transport with regard to both species and substrate differences. FL uptake in rat, mouse, and shark CP is Na+-dependent and sensitive to inhibition by PAH or 2,4-D. Experiments with tissue from Oat3-null mice indicate that transporter is responsible for FL uptake in mouse CP (18, 19); this also appears to be the case for rat CP [(5) and Miller DS, unpublished data]. Although no Oat family member has been cloned from elasmobranchs, teleost fish express one or more Oats that are capable of indirectly coupling organic anion uptake to Na+ and that are inhibited by PAH and ES (1, 12, 23). Given the similarities in Na+-dependence and inhibitor sensitivities, an elasmobranch, Oat, likely mediates FL uptake in shark CP (20).
Fig. 12.
Pathways involved in the transport of fluorescent organic anions by rat and shark CP. Findings shown are compiled from previous experiments (2, 4, 5, 18–20) and the present study. FL-MTX, fluoresceine-methotrexate; PD, membrane potential.
In contrast, FL-MTX uptake in mouse and rat CP is Na+-dependent but not mediated by Oat3 (4, 18). This also appears to be the case for shark CP, since uptake was not inhibited by high concentrations of PAH or ES (2). In rat CP, TR uptake was Na+-independent and insensitive to inhibition by high concentrations of PAH, 2,4-D, and cimetidine (present study). These results eliminate an Oat3 homologue as a TR uptake transporter. Potent inhibition of TR uptake by ES and TC suggests involvement of Oatp3, but for this transporter it is not clear how uptake is coupled to metabolism to drive cellular accumulation of organic anions (6, 22). In shark CP, where TR uptake is also Na+-independent (present study), an Oatp family member could be responsible for TR uptake. As in rat, this shark uptake transporter is clearly different from those responsible for FL and FL-MTX uptake, which are Na+-dependent (2, 20).
Mdr1 appears to be expressed in rat CP (8), and the question arises, whether Mdr1 is able to establish a barrier against MDR1 substrates used, e.g., MTX. However, our own previous studies (3) have shown that the Mdr1 gene product is predominantly expressed in subapical vesicles and only to a minor extent in the apical plasma membrane. Consequently, we were not able to demonstrate any Mdr1-related transport in intact rat CP but also not in shark or porcine CP (data not shown). Therefore, it is unlikely that Mdr1 plays a role in the CP disposition of these compounds in rat CP.
Basolateral organic anion efflux.
In all three species FL efflux into the vascular/subepithelial space is PD driven (5, 18, 20). Based on inhibitor specificity, FL-MTX efflux into the vascular/subepithelial space of rat CP appears to be mediated by Mrp1 (MK571 sensitivity) and Oatp2 [digoxin sensitivity (4)]. Similar findings for FL-MTX efflux in shark CP suggest involvement of elsmobranch Oatp and Mrp homologues (2).
The mechanisms driving TR efflux are not so clear-cut. In rat CP, TR efflux into the subepithelial/vascular space was concentrative and Na+-dependent. The lack of effect of high-K+ medium suggests that Na+-dependence is not secondary to depolarization, which could reduce facilitated organic anion efflux. At present, there is no known transporter that supports organic anion/Na+-exchange. However, Oatps have been suggested to mediate organic anion/hydroxyl or organic anion/bicarbonate exchange (16). Thus, it is possible that through inhibition of Na+/H+ exchange, medium Na+-depletion acidified the cell interior and reduced either TR/hydroxyl exchange or TR/bicarbonate exchange. It can be assumed that efflux of TR by a TR/H+ cotransport system would be accelerated by a decrease of intracellular pH. However, the decreased efflux of TR in shark and the lack of effect in rat points against the contribution of TR/H+ cotransport in TR efflux at the basolateral side. Evaluating these possibilities requires further experiments.
Note that in the rat the lack of effect of digoxin and LTC4 on TR efflux at concentrations that clearly block FL-MTX efflux in rat and shark CP eliminates Oatp2 and Mrp1 as candidates. Similarly, the lack of effect of millimolar AZT appears to eliminate Mrp4 as an efflux pathway in rat CP. At present it is not clear which type of transporter mediates TR efflux from rat CP.
In shark CP, basolateral TR efflux was PD sensitive, suggesting that transport required net movement of negative charge. Previous studies showed that FL efflux in shark CP was also PD-sensitive (20). However, FL efflux was inhibited by 2,4-D, and TR efflux was not. Thus, FL and TR do not share a common PD-driven, basolateral efflux pathway (Fig. 12).
Regulation of transport.
Little is known about signals that regulate organic anion transport in CP. We recently showed that FL-MTX transport in shark CP increased when PKA was activated and decreased when PKC was activated (2). However, preliminary experiments indicate that neither PKA activation nor PKC activation specifically altered FL-MTX transport in rat CP (Miller DS, unpublished data). Here we show specific activation of TR transport from cell to subepithelial/vascular space in rat CP by PKA. In contrast, neither step of TR transport in rat CP was affected by PKC activation and neither PKA nor PKC activation altered TR transport in shark CP. These results are consistent with the conclusion (above) that in both species TR and FL-MTX are handled by different transporters.
Perspectives and Significance
Several general conclusions can be drawn from the functional map of organic anion transport in rat and shark CP presented in Fig. 12. First, in both species, the map is complex with multiple apical and basolateral transporters available. However, based on transport driving forces and inhibitor studies, within a species, all three fluorescent organic anions tested appear to utilize different transporters to cross the epithelium. This means that a minimum of six transporters are required. Certainly, in rat, a species for which several Oats have been localized to one side of the epithelial cell or the other, this functional map shows processes for which there are no readily identifiable molecular correlates. These include the PD-dependent efflux steps for FL and TR and the Na+-dependent uptake step for FL-MTX. Second, comparison of transport pathways across species shows similarities for FL and FL-MTX and for TR uptake but distinct differences for TR efflux (Fig. 12). Finally, Fig. 12 suggests that for organic anion transport in CP the level of functional map complexity is the same in tissue from shark and rat. Thus, for CP, evolutionary age (older vertebrate) does not necessarily indicate reduced complexity with regard to organic anion transport.
GRANTS
This research was supported, in part, by Grants DFG FR1211/12-1 and FR1211/13-1 from the German Research Foundation, by 3R-Foundation (Switzerland) Grant 91-04, the Boehringer Ingelheim Fonds, National Institute of Environmental Health Sciences Grant ES-03828, and by the Division of Intramural Research of the National Institute of Environmental Health Sciences.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aslamkhan AG, Thompson DM, Perry JL, Bleasby K, Wolff NA, Barros S, Miller DS, Pritchard JB. The flounder organic anion transporter fOat has sequence, function, and substrate specificity similarity to both mammalian Oat1 and Oat3. Am J Physiol Regul Integr Comp Physiol 291: R1773–R1780, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baehr CH, Fricker G, Miller DS. Fluorescein-methotrexate transport in dogfish shark (Squalus acanthias) choroid plexus. Am J Physiol Regul Integr Comp Physiol 291: R464–R472, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baehr C, Reichel V, Fricker G. Choroid plexus epithelial monolayers-a cell culture model from porcine brain (Abstract). Cerebrospinal Fluid Res 3: 13, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breen CM, Sykes DB, Baehr C, Fricker G, Miller DS. Fluorescein-methotrexate transport in rat choroid plexus analyzed using confocal microscopy. Am J Physiol Renal Physiol 287: F562–F569, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Breen CM, Sykes DB, Fricker G, Miller DS. Confocal imaging of organic anion transport in intact rat choroid plexus. Am J Physiol Renal Physiol 282: F877–F885, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Cattori V, van Montfoort JE, Stieger B, Landmann L, Meijer DK, Winterhalter KH, Meier PJ, Hagenbuch B. Localization of organic anion transporting polypeptide 4 (Oatp4) in rat liver and comparison of its substrate specificity with Oatp1, Oatp2 and Oatp3. Pflügers Arch 443: 188–195, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos 31: 1337–1345, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Gao B, Meier PJ. Organic anion transport across the choroid plexus. Microsc Res Tech 52: 60–64, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Kusuhara H, Sugiyama Y. Efflux transport systems for organic anions and cations at the blood-CSF barrier. Adv Drug Delivery Res 56: 1741–1763, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24: 7612–7621, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowes S, Sykes D, Breen CM, Ragone LJ, Miller DS. Multiple components of 2,4-dichlorophenoxyacetic acid uptake by rat choroid plexus. J Pharmacol Exp Ther 315: 136–143, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Miller DS, Pritchard JB. Indirect coupling of organic anion secretion to sodium in teleost (Paralichthys lethostigma) renal tubules. Am J Physiol Regul Integr Comp Physiol 261: R1470–R1477, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Nagata Y, Kusuhara H, Hirono S, Endou H, Sugiyama Y. Carrier-mediated uptake of H2-receptor antagonists by the rat choroid plexus: involvement of rat organic anion transporter 3. Drug Metab Dispos 32: 1040–1047, 2004. [PubMed] [Google Scholar]
- 14.Nagata Y, Kusuhara H, Imaoka T, Endou H, Sugiyama Y. Involvement of rat organic anion transporter 3 in the uptake of an organic herbicide, 2,4-dichlorophenoxyacetate, by the isolated rat choroid plexus. J Pharm Sci 93: 2724–2732, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Russel FG, Masereeuw R, Van Aubel RA. Molecular aspects of renal anionic drug transport. Annu Rev Physiol 64: 563–594, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Satlin LM, Amin V, Wolkoff AW. Organic anion transporting polypeptide mediates organic anion/HCO3− exchange. J Biol Chem 272: 26340–26345, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol 290: F251–F261, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 [Oat3 (Slc22a8)] knockout mice. J Biol Chem 277: 26934–26943, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Sykes D, Sweet DH, Lowes S, Nigam SK, Pritchard JB, Miller DS. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (Slc22a8)-null mice. Am J Physiol Renal Physiol 286: F972–F978, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Villalobos AR, Miller DS, Renfro JL. Transepithelial organic anion transport by shark choroid plexus. Am J Physiol Regul Integr Comp Physiol 282: R1308–R1316, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Villalobos AR, Parmelee JT, Pritchard JB. Functional characterization of choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther 282: 1109–1116, 1997. [PubMed] [Google Scholar]
- 22.Walters HC, Craddock AL, Fusegawa H, Willingham MC, Dawson PA. Expression, transport properties, and chromosomal location of organic anion transporter subtype 3. Am J Physiol Gastrointest Liver Physiol 279: G1188–G1200, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Wolff NA, Werner A, Burkhardt S, Burckhardt G. Expression cloning and characterization of a renal organic anion transporter from winter flounder. FEBS Lett 417: 287–291, 1997. [DOI] [PubMed] [Google Scholar]