Abstract
Changes in gene expression associated with skeletal muscle atrophy due to aging are distinct from those due to disuse, suggesting that the response of old muscle to inactivity may be altered. The goal of this study was to identify changes in muscle gene expression that may contribute to loss of adaptability of old muscle. Muscle atrophy was induced in young adult (6-mo) and old (32-mo) male Brown Norway/F344 rats by 2 wk of hindlimb suspension (HS), and soleus muscles were analyzed by cDNA microarrays. Overall, similar changes in gene expression with HS were observed in young and old muscles for genes encoding proteins involved in protein folding (heat shock proteins), muscle structure, and contraction, extracellular matrix, and nucleic acid binding. More genes encoding transport and receptor proteins were differentially expressed in the soleus muscle from young rats, while in soleus muscle from old rats more genes that encoded ribosomal proteins were upregulated. The gene encoding the cold-shock protein RNA-binding motif protein-3 (RBM3) was induced most highly with HS in muscle from old rats, verified by real-time RT-PCR, while no difference with age was observed. The cold-inducible RNA-binding protein (Cirp) gene was also overexpressed with HS, whereas cold-shock protein Y-box-binding protein-1 was not. A time course analysis of RBM3 mRNA abundance during HS showed that upregulation occurred after apoptotic nuclei and markers of protein degradation increased. We conclude that a cold-shock response may be part of a compensatory mechanism in muscles undergoing atrophy to preserve remaining muscle mass and that RBM3 may be a therapeutic target to prevent muscle loss.
Keywords: aging, microarray, Cirp, atrophy
skeletal muscle atrophy is a multifactorial process that occurs in response to decreased activity levels, reduced mechanical and/or gravitational loading, modified environmental conditions, and pathological conditions. It is characterized by a decrease in muscle mass and, consequently, reduced contractile force of the muscle. The importance of a loss in muscle mass and strength is emphasized by the fact that it is associated with an increase in mortality (65). Processes contributing to the loss of muscle mass include decreased protein synthesis, increased protein degradation, and increased apoptosis. Changes in mRNA abundance associated with muscle atrophy have been studied extensively using gene expression profiling in humans and animals. Atrophy models include food deprivation (12, 48), denervation (74, 77), hindlimb suspension (HS) (6, 21, 31, 90, 108), space flight (67), immobilization (18, 70, 88, 99), spinal cord injury (77, 97), and systemic wasting diseases (56), such as diabetes, cancer, and renal failure. Major pathways shared by most forms of atrophy have been identified, and it was postulated that there is a specific transcriptional program involved in muscle atrophy. The genes associated with this program have been termed atrogenes (48, 56), and the most strongly induced are members of the ubiquitin-proteasome pathway (6, 7, 37, 48, 56, 67, 88, 90, 97, 99), especially the E3 ubiquitin ligases muscle atrophy F-box (MAFbx)/atrogin-1 and muscle ring finger-1 (Murf-1). Interestingly, the E3 ubiquitin ligase neural precursor cells expressed developmentally downregulated-4 (Nedd4), was specifically elevated with disuse-induced atrophy and was not induced upon starvation (54). Other groups of genes that are upregulated with muscle atrophy include those encoding ribosomal proteins (6, 12, 88, 108) and proteins involved in oxidative stress, with metallothionein, an endogenous antioxidant, as an important example (56, 67, 90, 97, 99). Two major groups of genes that were downregulated with muscle atrophy include those encoding protein chaperones, such as heat shock proteins (HSPs) (6, 18, 21, 90), and those encoding extracellular matrix proteins (12, 21, 48, 56, 77, 90, 99). In addition, many forms of atrophy are associated with a switching from slow to fast fiber types and gene expression changes mostly reflect this switch (21, 90, 108).
The loss of muscle mass with age (sarcopenia) is also a multifactorial process, and the underlying mechanisms for age-related atrophy are still largely unknown. It has been suggested that a genetic program underlies the changes in muscle with age, and gene expression profiling in humans and animals have been performed to elucidate changes in gene expression with age (3, 35, 49, 51, 57, 70, 71, 104, 105). Results from these studies show conflicting results, and there is less agreement on changes in gene expression with sarcopenia than with acutely induced muscle atrophy. Welle et al. (105) showed that components of the ubiquitin-proteasome pathway were increased with age in human muscle, while in mice this pathway was decreased with age (57); however, the abundance of the 26S proteasome component TBP-1 was decreased in both mouse and human muscle (104). Most papers indicate an increase in inflammation- and oxidative stress-related genes (3, 49, 51, 57, 71), concomitant with an increase in genes for protein chaperones, such as HSPs (49, 57). However, HSP gene expression was decreased in other studies in mouse as well as human muscles (3, 104). Interestingly, a common finding between studies was the decrease in gene expression of extracellular matrix-related genes, which was also found in acute muscle atrophy induced by multiple strategies in young subjects (12, 21, 48, 56, 71, 77, 90, 99, 104). Nonetheless, these studies indicate that gene expression changes underlying sarcopenia are different and more variable than those underlying muscle atrophy induced more acutely in young subjects.
As the array of genes expressed in muscle changes as a function of age, one might expect that this will affect muscle adaptation to external stimuli in the elderly. Some studies have demonstrated that a hypertrophic response can be elicited in the elderly (10, 30, 32, 84), but other reports have shown no change in muscle size with resistance training (4, 13, 14). Moreover, the response to exercise is more variable at advanced age (59, 106), and changes in gene expression in response to exercise seem less pronounced in muscle from aged individuals compared with those in young (49, 76). With respect to muscle atrophy, one study in humans showed that immobilization caused a greater decrease in muscle mass in old individuals compared with young (98). However, the response of muscles to disuse with respect to age has mainly been investigated in animal models using immobilization and HS and the results are controversial. Some studies report that soleus muscles atrophy to a similar degree in young and old rats (91, 95, 96), whereas others suggest that soleus muscles from young animals atrophy more than those from old animals (11, 83). Pattison et al. (70) reported differences in gene expression profiles between young and old muscles recovering from immobilization, as well differences in the response to immobilization with aging (72). The goal of the present study was to identify changes in gene expression in response to a period of disuse due to simulated bed rest (HS) in young and old rats using microarray technology. The age of the old rats was taken at 50% survival. We hypothesized that gene expression changes during disuse differ between young and old animals reflecting a loss of muscle adaptability that may contribute to impaired ability to maintain or restore muscle mass in the elderly.
METHODS
Animal procedures and tissue collection.
All procedures were performed in accordance with institutional guidelines for the care and use of laboratory animals, and protocols were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. Male Fischer 344 X Brown Norway rats (6 mo and 32 mo of age) were purchased from the National Institute on Aging. This strain of rat was chosen because it has increased longevity and decreased cumulative lesion incidence compared with other strains; therefore, aging aspects can be studied in the relative absence of disease (60). The different ages were chosen to reflect a mature rat (6 mo) and an old rat at about 50% mortality (32 mo). Rats of both ages were divided into two groups (n = 6): nonsuspended control and HS for 14 days. Rats were allowed free access to food and water, and were housed in a 12:12-h light-dark cycle. HS was performed as previously described (28, 29). Briefly, a tail device containing a hook was attached with gauze and cynoacrylate glue while the animals were anesthetized with pentobarbital sodium (50 mg/kg body wt). After the animal regained consciousness, the tail device was connected via a thin cable to a pulley sliding on a vertically adjustable stainless steel bar running longitudinally above a high-sided cage with standard floor dimensions. The system was designed in such a way that the rats could not rest their hindlimbs against any side of the cage but could reach their food and water without difficulty. After 14 days of control housing or HS, rats were killed with an overdose of pentobarbital sodium (100 mg/kg body wt). The soleus muscles were dissected, weighed, and processed as follows. Soleus muscle from one leg was frozen in liquid nitrogen and stored at −80°C for RNA and protein isolation, and soleus muscle from the other leg was embedded in a freezing medium, frozen in liquid nitrogen-cooled isopentane, and stored at −80°C for determination of mean fiber cross-sectional area (CSA).
For the experiments involving determination of responses early after the onset of atrophy, 6-mo-old male Sprague-Dawley rats (Harlan, Indianapolis, IN) were randomly assigned to the following six groups: control and HS for 12 h, 1 day, 2 days, 4 days, or 7 days (n = 8 at each time point). HS was performed as described above, and at the designated time points, rats were euthanized by an overdose of pentobarbital sodium (100 mg/kg body wt), soleus muscles were dissected, weighed, frozen in liquid nitrogen, and stored at −80°C until RNA isolation.
Determination of muscle fiber CSA.
Cross-sections (6 μm) of soleus muscles were cut on a cryostat, air-dried, and stored at −20°C until analysis. Total muscle fiber CSA was determined on standard hematoxylin and eosin-stained sections after rehydration as described previously (29). Muscle sections were viewed and captured as digital images using a Nikon Eclipse E600 microscope, CoolSnap camera, and MetaView software. CSA was measured on a total of 150 fibers from three different areas of the midbelly region of the soleus muscle, and the mean CSA was calculated.
RNA preparation.
RNA isolation was performed as described previously (28). Briefly, total RNA was isolated from soleus muscles using the guanidinium thiocyanate-phenol-chloroform extraction method as described by Chomczynski and Sacchi (19). Total RNA was treated with one unit of DNase (Ambion, Austin, TX) using Ambion's DNA-free reagents in a 100 μl total volume. The reaction was stopped with 20 μl of inactivation reagent. The reagent had been diluted twofold using diethylpyrocarbonate-treated water containing 0.1 mM EDTA to ensure reproducible pipetting of the slurry. The integrity and abundance of the DNase-treated RNA was verified by analysis on the Agilent Bioanalyzer (Palo Alto, CA) and used for microarray analysis and real-time RT-PCR.
Microarray analysis.
In the present study, microarray analysis was used as an initial screening tool to gain preliminary insight into changes in transcription, and real-time quantitative RT-PCR was used to corroborate the findings. Rat MWG Oligos (MWG-Biotech, High Point, NC) representing 10,000 genes were printed on epoxy slides (TeleChem International, Sunnyvale, CA) using a GeneMachines Omnigrid Printer (Genomic Solutions, Ann Arbor, MI) with 16 pins (Telechem International,) in the University of Arkansas for Medical Sciences Microarray Core Facility as described (5). Two micrograms of total RNA was labeled using the Array 900 MPX kit (Genisphere, Hatfield, PA). RNA from soleus muscle from control animals was labeled with Cy3 and from HS with Cy5 to be compared on one slide. Also, reverse labeling was performed to reduce dye-specific biases in signal intensity, where control RNA was labeled with Cy5 and HS RNA with Cy3. Each array was duplicated on each slide, and, including the dye-swap slides a total of four spots for each position in the array, were analyzed. Hybridizations were performed on the Discovery Hybridization Station (Ventana Medical Systems, Tucson, AZ). Slides were removed from the hybridization station, rinsed briefly in a series of washes (reaction buffer, 1× SSC, 0.1× SSC), dried, and scanned using ScanArray 5000 (Perkin Elmer Life and Analytical Sciences, Boston, MA). Image analysis was performed using the adaptive circle method in ScanArray Express (Perkin Elmer). The raw intensities between the Cy3 and Cy5 channels were Lowess normalized to minimize systematic bias, and preprocessing steps were used to minimize the contribution of experimental artifacts. Genes whose intensities across the Cy3 and Cy5 channels were <1,000 were deemed as representative of noise floor (filtered), hence excluded from subsequent analysis. Subsequently, the intensities were log transformed. The above filtering removes genes with zero intensities ensuring the existence of the log transform. In two-dye experiments, proper flipping of the genes across the replicate arrays is essential because improper flipping may be an outcome of bias in dye binding as opposed to true biological variability. A systematic approach is described below to address flipping issues across replicate arrays. If three of the spots across the replicate arrays had changes more than 1.4-fold in one direction and the fourth was at least 1.4-fold in the opposite direction, only the disagreeing spot was set to missing. If two spots were at least 1.4-fold in opposite direction, all four spots were set to missing. Finally, the log ratio of HS over control was averaged for the nonfiltered spots for each position in the array, and a one-sample t-test was performed on these log ratios. The corresponding P values were generated. Three replicate arrays were generated for the young and six for the old animals (HS vs. control). The larger number of replicates for the old rats can be attributed to higher variability in the old rats. For young rats, any array position with a P value <0.05 in all three comparisons and in the same direction was considered significant, and for old rats at least four out of the six comparisons had to have P < 0.05 to be considered significant. Subsequently, Pathway Assist 3.0 (Ariadne Genomics, Rockville, MD) was used to annotate genes that showed a significant difference with HS, and these were grouped based on biological functions.
In our analysis, we set the cutoff for statistical significance at 1.4-fold, but reported also changes above 1.2-fold for the following reasons. The twofold cutoff used in classical array analysis can be attributed to near-normality assumption of the fold changes (i.e., 95% of the fold changes fall within 2 SDs, hence assumed to be nonsignificant). This is especially true when the sample size is large. However, often statistical significance (P value <0.05) can be observed when the gene expression distribution in control channels is well separated from that of experimental channels, whereas the mean fold change is lesser than twofold. In the present study, we found considerable variation between the groups, which prevented us from using such a rigorous cutoff in conjunction with multiple testing corrections. We therefore set the cutoff for statistical significance at 1.4-fold. Following the statistical analysis, we investigated the pathways associated with the identified genes and found that some genes in these pathways had fold changes smaller than 1.4, yet statistically significant. We therefore chose to report them if they were at least 1.2-fold different, as we felt they were biologically significant in the context of the pathway or ontology studied. Several factors may be responsible for the low fold changes in these genes including 1) variation between animals, 2) sensitivity of the arrays, and 3) probes corresponding to these genes. We used this low cutoff value for reporting since it has been shown that small changes can be validated as significantly different by other methods and that up to 90% of biologically significant changes can be missed if a twofold cutoff would be used (71).
Real-time RT-PCR.
Transcript abundance of selected genes was measured by real-time RT-PCR, as previously described (29). RNA from six animals in each group was used for corroboration of microarray results. Briefly, quantitative real-time RT-PCR was performed using the protocols, chemistries, and the amplification and detection systems of Applied Biosystems (Foster City, CA). For each sample, cDNA was synthesized from 1 μg of DNase-treated total RNA using components from the Taqman reverse transcription reagents (Applied Biosystems). The primers were allowed to anneal for 10 min at 25°C before the reaction proceeded for 1 h at 37°C followed by 5 min at 95°C. The resulting cDNA samples were aliquoted and stored at −80°C. Primer sequences were selected from the accession numbers in NCBI database using the Taqman Probe and Primer Design function of the Primer Express version 1.5 software (Applied Biosystems) and are shown in Table 1. PCR reactions were assembled using the SYBR Green PCR Master Mix that required only the addition of cDNA template and primers. Control reactions were run lacking template to check for reagent contamination and to determine the melting temperature of any primer dimer. To optimize assay efficiency, PCR standard curves were produced using a pool containing each sample cDNA. Data points were generated using fourfold serial dilutions of cDNA. Gene expression was compared in individual samples using 16 ng RNA equivalents of cDNA. The reactions were performed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems) and the instrument's universal cycling conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, and then 60°C for 1 min. An additional cycle was added in which the ramp time to 95°C was increased to 19 min and 59 s, during which time data were collected for melting curve analysis. Analysis of current and previous microarray data using the same array library indicated several candidates for use as control or housekeeping genes in normalization of RT-PCR data. The candidate list was derived from genes in the microarray results that were well expressed in all samples, however, with no detectable differential expression among any sample combination. Four genes were selected from the list of candidates based on biological appropriateness as a control: NADH subunit 1, β-2 microglobulin, 18S, and tubulin. These four potential housekeeping genes along with the genes of interest were analyzed using RT-PCR. The cycle threshold values from the RT-PCR were then formatted and input to the software program geNorm according to Vandesompele (100). The geometric mean analysis indicated the use of all four genes was optimum. Subsequently, the geometric mean of the cycle threshold values from the four housekeeping genes was used for normalization in the RT-PCR analysis (100).
Table 1.
Primer sequences for real-time RT-PCR analysis of selected genes
| Gene Name | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| HSPb7 | AJ243193 | AGGACTTTGGCAGTTTCATGCT | CGAGAGTCTTGATGTTTCCTTGTC |
| IL1r1 | NM_013123 | CCTGTGCTGGACACTAAGGAGAA | CCCGCCTTTCCCACACTAG |
| RPL4 | NM_022510 | AAAGACTATGCGTAGGAACACCATT | CGGCTTCCAGCTTTTTCACT |
| RPS17 | NM_017152 | TCTTTTACCGAGACCCGTCAA | GGGCCGCCTTCTTCACA |
| RPS27 | NM_053597 | TGCCCAGGATGCTATAAAATCA | GGAGCAGCCAACACACAAGA |
| SLCO2B1 | AF169410 | GGAGCCTCGGGACTCACA | GGACAAAGAACTTGATGTTCTGGAA |
| MAFbx | NM_133521 | GACCTGCATGTGCTCAGTGAAG | GGATCTGCCGCTCTGAGAAGT |
| Murf-1 | AY059627 | TGCCCTGCCAGCACAAC | GGATTGGCAGCCTGGAAGAT |
| Nedd-4 | U50842 | GATCACCTCTCATACTTCAAGTTCATTG | CATCCAACAGCTTGCCATGATA |
| Rbm3 | AF355190 | TGGAGAGTCCCTGGATGGG | TGGTTCCCCTGGCAGACTT |
| Cirp | AB000362 | GCATCAGATGAAGGCAAGGT | CCAGCGCCTGCTCATTG |
| YB-1 | NM_031563 | AAGATGGCAAAGAGACAAAAGCA | CGGGAGCGGACGAATTC |
| B2M | NM_012512 | CGTGCTTGCCATTCAGAAAA | GAAGTTGGGCTTCCCATTCTC |
| NADH subunit 1 | X14848 | AGAACGGAAAATCCTAGGCTACATAC | CCATATGGGCCTTCGTTGTT |
| tubulin | NM_022298 | GGCATGGAGGAGGGAGAGTT | CCAACCTCCTCATAATCCTTCTCTAG |
| 18S | M11188 | TTCGGACGTCTGCCCTATCAA | ATGGTAGGCACGGCGACTA |
HSPb7, heat shock 27kDA protein family 7; IL1r1, interleukin-1 receptor, type 1; RPL4, ribosomal protein L4; RPS17, ribosomal protein S17; SLCO2B1, solute carrier organic transporter family member 2B; MAFbx, muscle atrophy F-box; Murf-1, muscle ring finger-1; Nedd-4, neural precursor cells-expressed developmentally downregulated-4; Rbm3, RNA binding motif protein 3; Cirp, cold-inducible RNA binding protein; YB-1, Y-Box binding protein 1; B2M, β2-microglobulin; NADH: dihydronicotinamide adenine dinucleotide.
Protein isolation and Western blot analysis.
Western blot analysis of proteins was performed as described previously with minor modifications (28). Briefly, soleus muscles were homogenized in a buffer containing 10 mM MgCl2, 10 mM KH2PO4, 1 mM EDTA, 5 mM EGTA, 1% Igepal, 50 mM βGPO4, 1 mM PMSF, 1 mM Na3VO4, 1 μg/ml leupeptin, 2 μg/ml antipain, 10 μg/ml benzamidine, 1 μg/ml aprotinin, 1 μg/ml chymostatin, and 1 μg/ml pepstatin. After homogenization, samples were centrifuged for 10 min at 1,000 g at 4°C. Protein concentration of the supernatants was determined according to Bradford (8) using the Bio-Rad (Hercules, CA) protein assay reagent. For Bcl-2 and X-linked inhibitor of apoptosis protein (XIAP; 30 μg) and for RNA-binding motif protein 3 (RBM3; 75 μg), protein was loaded onto polyacrylamide gels (10% for Bcl-2 and XIAP and 12.5% for RBM3). After electrophoretic separation, proteins were transferred to nitrocellulose membranes (Bio-Rad). Subsequently, the membranes were incubated in Ponceau S solution (Sigma, St. Louis, MO) for 5 min for visualization of the protein and assurance of equal loading in all the lanes. Membranes were blocked in Odessey blocking buffer (Licor, Lincoln, NE) for 2 h at room temperature and incubated in primary antibody overnight in Odessey blocking buffer with 0.1% Tween. Primary antibody concentrations were as follows: Bcl-2 mouse monoclonal IgG (1:1,000; Stressgen, Ann Arbor, MI), XIAP mouse monoclonal IgG (1:200; Medical and Biological Laboratories International, Woburn, MA), and RBM3 rabbit polyclonal (1:1,000; kind gift from P. Vanderklish, Scripps Research Institute, La Jolla, CA) (24, 86). After washes, membranes were incubated in the following secondary antibodies: Alexa Fluor 680 goat anti-mouse IgG highly cross-absorbed (1:30,000; Molecular Probes/Invitrogen, Carlsbad, CA), goat anti-mouse horseradish peroxidase-conjugated (1:1,000; Pierce, Rockford, IL), and IRDye 800-conjugated goat anti-rabbit IgG (1:30,000; Licor) for detection of Bcl-2, XIAP, and RNA-binding motif protein-3 (RBM3), respectively. The XIAP filter was further treated with enhanced chemiluminescence substrate (Pierce) and scanned on a Bio-Rad scanner, while the filters for Bcl-2 and RBM3 were scanned and analyzed on the Odyssey Infrared Imaging System (Licor). Densitometry was performed to quantify the bands.
Statistics.
Statistical analyses for the microarray data were described above. For other variables, to test for statistically significant differences between the groups, two-way ANOVA was used with age and group (control vs. HS) as factors; when significant F-ratios were observed, a Holm-Sidak pairwise multiple comparisons test was applied to test individual means. This test is often recommended as the first-line procedure for comparison testing. In case the normality or equal variance test failed, a one-way ANOVA on ranks was performed with a Dunn's multiple comparisons test. For the early time course experiments, a one-way ANOVA was performed with a Holm-Sidak multiple comparisons test for testing all groups vs. control. Statistical significance was assumed at P < 0.05.
RESULTS
Muscle atrophy markers.
Soleus muscle weight, muscle-to-body weight ratio and muscle fiber CSA were 24%, 35%, and 26% lower, respectively, in old compared with young control rats (Table 2), indicating a significant level of sarcopenia in these rats. In response to HS, soleus muscle size decreased significantly as indicated by a 49% and 34% decrease in muscle weight, a 38% and 22% decrease in muscle-to-body weight ratio, and a 62% and 58% decrease in muscle fiber CSA in young and old, respectively (Table 2). However, all three indicators of muscle mass were not different between young and old after 14 days of HS, indicating that the muscles atrophied to the same absolute level at both ages.
Table 2.
Soleus muscle weight, muscle-to-body weight ratio, and muscle fiber cross-sectional area in old compared with young control rats
|
6 mo |
32 mo
|
|||
|---|---|---|---|---|
| Control | HS | Control | HS | |
| Muscle weight, mg | 167.9±4.9 | 86.2±2.3* | 127.6±3.1# | 85.3±3.6* |
| Muscle weight-to-body weight ratio, mg/g | 0.430±0.008 | 0.266±0.003* | 0.279±0.008# | 0.219±0.014* |
| Cross-sectional area, μm2 | 2976.8±137.96 | 1135.3±64.6* | 2213.9±128.9# | 926.2±94.8* |
Values are means ± SE. HS, hindlimb suspension.
Significant difference from control,
significant difference from 6 mo P < 0.05.
In addition, we analyzed the mRNA abundance of atrophy-related markers to ensure that gene expression changes were indeed as expected with HS (Table 3). MAFbx (or atrogin-1), Murf-1, and Nedd4 are ubiquitin-protein ligases that have been identified as markers of skeletal muscle atrophy (7, 37, 54, 90). Transcripts encoding MAFbx, Murf-1, and Nedd4 were elevated with HS, indicating that, even at 14 days after HS, these markers are still overexpressed, consistent with previous reports (27, 90).
Table 3.
mRNA abundance of atrophy-related genes
| Gene |
6 mo |
32 mo
|
||
|---|---|---|---|---|
| Control | HS | Control | HS | |
| MAFbx | 1.12±0.12 | 2.06±0.22a* | 1.17±0.09 | 1.78±0.27a* |
| Murf-1 | 1.33±0.13 | 1.78±0.16a | 1.43±0.19 | 2.10±0.37a |
| Nedd4 | 0.71±0.05 | 2.14±0.30a* | 1.18±0.15 | 1.78±0.27a |
Values are means ± SE.
Significant difference from control within age;
main effect for hindlimb suspension, P = 0.02.
Microarray analysis.
As multiple studies have compared gene expression profiles between young and old muscles (3, 35, 49, 51, 57, 70, 71, 104, 105), we focused our analyses on the effect of age on response to disuse. Gene expression patterns in soleus muscles from control and HS rats of each age were directly compared using two-color spotted microarrays. Of the 10,000 genes represented on the array, 200 were differentially expressed in response to HS. Thirty-eight genes were differentially expressed with HS in both young and old, of which 15 were downregulated and 23 were upregulated. Genes that changed in both age groups were altered in the same direction with the exception of one gene (ionotropic glutamate receptor, Table 3). The expression of 70 genes changed with HS exclusively in old muscle, with 33 decreased and 37 increased. In muscle of young rats, 55 unique genes decreased expression with HS and 37 genes increased, totaling 92 genes.
Genes that were differentially expressed with HS in young and old were categorized into functional classes using Pathway Assist 3.0 and the results are shown in Table 4. Genes encoding chaperones (mainly HSPs) were downregulated with HS in both young and old muscles to approximately the same extent, although HSP70 was specifically downregulated in old muscles. Downregulation of protein chaperones has previously been noted in atrophying skeletal muscles (6, 18, 21, 90). Changes in expression of genes encoding extracellular matrix proteins, another class of proteins associated with muscle atrophy (12, 21, 48, 56, 77, 90, 99), were similar in muscles from both young and old rats. In addition, genes encoding proteins involved in muscle structure and contraction changed similarly in young and old soleus muscles, suggesting that the change in fiber type observed with HS is not affected by age, at least at the transcriptional level. Finally, the expression of genes encoding proteins involved in nucleic acid binding, including transcription factors and repair enzymes, generally showed a similar response to HS, regardless of age, with a few notable exceptions. The expression of the genes encoding the repair enzyme 8-oxoguanine DNA glycosylase, two RNA-binding proteins, the transcription factor immunoglobulin μ-binding protein-2 increased, while the gene expression of one GTP binding protein decreased in both young and old muscle after HS; however, thymine DNA-glycosylase, Kruppel-like factor-15, and transcription factor 12 (HTF4) gene expression decreased in young muscle only.
Table 4.
Changes in gene expression as measured by microarray analysis
| Accession No. | 6 mo | 32 mo | |
|---|---|---|---|
| Structural constituent ribosome | |||
| Ribosomal protein S27 | NM_053597 | 1.69 | |
| Ribosomal protein S17 | NM_017152 | 1.43 | |
| Ribosomal protein L4 | NM_022510 | 1.92 | 1.60 |
| Ribosomal protein L28 | NM_022697 | 1.34 | |
| Ribosomal protein L8 | X62145 | 1.23 | |
| Ribosomal protein L12 | X53504 | 1.42 | |
| Ribosomal protein L34 | X14401 | 1.59 | |
| Ribosomal protein L23 | X65228 | 1.46 | |
| Ribosomal protein S3 | X51536 | 1.92 | |
| Protein folding | |||
| Heat shock 27kDA protein family, member-7 | AJ243193 | −2.60 | −1.67 |
| Crystallin, αB | NM_012935 | −6.64 | −3.94 |
| αB-crystallin-related protein | D29960 | −3.36 | −2.06 |
| Heat shock protein 70 kDA (Hspa4) | AF077354 | −1.44 | |
| Heat shock protein 70 kDA (Hspa8) | NM_024351 | −1.55 | |
| Low-density lipoprotein receptor-related protein | M31051 | −1.84 | |
| Muscle structural and contraction-related proteins | |||
| Troponin I (skeletal slow) | NM_017184 | −3.35 | −2.04 |
| Calsequestrin-2 | NM_017131 | −2.41 | |
| Tropomyosin | AF053360 | −4.43 | |
| ATPase, calcium transporting, fast twitch | NM_058213 | 1.35 | 1.99 |
| Parvalbumin | NM_022499 | −5.52 | |
| Transcription factor-12 (HTF4) | NM_013176 | −2.61 | |
| Myosin light chain-3, skeletal fast | NM_020104 | 1.82 | |
| Myosin light chain-2, heart | X07314 | −16.97 | −4.28 |
| Actin, alpha, cardiac muscle | X00306 | −4.76 | |
| Ryanodine receptor-1 | AF011788 | 1.90 | |
| αActin, vascular | X06801 | −3.28 | −1.78 |
| Muscle LIM protein, cysteine and glycine-rich protein 3 | NM_057144 | −1.87 | |
| Transport | |||
| Solute carrier family-22 (organic anion transporter) | NM_053537 | 1.94 | |
| Solute carrier family protein-5 (sodium/glucose cotransporter) | NM_013033 | −2.87 | |
| Solute carrier family 21, member 9 (organic anion transporter) | AF169410 | 1.94 | |
| Solute carrier family-16 (monocarboxylic acid transporters) | NM_0.0834 | 1.81 | |
| Solute carrier family-7 (cationic amino acid transporter) | NM_017353 | 1.95 | |
| Ryanodine receptor-1 | AF011788 | 1.90 | |
| Receptor (calcitonin) activity modifying protein-1 | NM_031645 | 1.90 | |
| Purinergic receptor P2X, ligand-gated ion channel-1 | NM_012997 | −4.07 | |
| Calcium channel, voltage dependent, αG1-subunit | AF290212 | −1.73 | |
| ATPase, calcium transporting, fast twitch | NM_012916 | 1.35 | 1.99 |
| Fatty acid binding protein-3, muscle, and heart | NM_024162 | −12.14 | |
| Inositol, 1,4,5,-triphosphate receptor, type 3 | NM_013138 | −1.41 | |
| Nucleic acid binding | |||
| 8-oxoguanine DNA glycosylase | NM_0.0870 | 1.87 | 1.50 |
| Ribosomal protein S3 | X51536 | 1.92 | |
| Ribosomal protein S17 | NM_017152 | 1.43 | |
| Ribosomal protein S27 | NM_053597 | 1.69 | |
| Ribosomal protein L4 | NM_022510 | 1.92 | 1.60 |
| H1 histone family, member 0 | NM_012578 | 1.73 | 1.28 |
| immunoglobulin mu binding protein-2 | NM_031586 | 1.73 | 1.75 |
| RNA binding motif (RNP1, RRM) protein-3 | AF355190 | 1.91 | 2.76 |
| Ribosomal protein L28 | NM_022697 | 1.34 | |
| Ribosomal protein L8 | X62145 | 1.23 | |
| Poly (A) binding protein, cytoplasmic-1 | AJ298278 | 1.99 | 1.37 |
| Thymine DNA-glycosylase | NM_053729 | −1.71 | |
| Telomerase-associated protein-1 | NM_022591 | −4.40 | |
| Kruppel-like factor-15 | NM_053536 | −1.60 | |
| Transcription factor-12 (HTF4) | NM_013176 | −2.61 | |
| GNAS complex locus | NM_019132 | −1.57 | −1.32 |
| Receptors | |||
| Glutamate receptor, metabotropic-6 | AJ245718 | −2.45 | |
| Adenylate cyclase activating polypeptide-1 receptor | Z23272 | 1.31 | 1.62 |
| Glutamate receptor, ionotropic | AF061945 | −1.67 | 1.31 |
| CD24 antigen (small cell lung carcinoma cluster-4 antigen) | NM_012752 | 1.42 | |
| Ryanodine receptor-1 (skeletal) | AF011788 | 1.90 | |
| Interleukin-1 receptor, type 1 | NM_013123 | −2.46 | |
| Bradykinin receptor B2 | L26173 | −2.24 | |
| Cholinergic receptor, muscarinic-3 extracellular matrix structural constituents | NM_012527 | −3.95 | |
| Collagen, type I, α1 | M27208 | −8.05 | −3.44 |
| Proplatelet basic protein (chemokine C-X-C motif) ligand | AF349115 | 1.93 | 1.39 |
| Collagen, type IV, α3 (Goodpasture antigen) | L47281 | −1.69 |
Many genes encoding structural components of the ribosome were changed with HS in muscle from old but not young rats, and all of these ribosomal genes increased in expression, possibly suggesting a compensatory response to the loss of muscle protein already present in old rats. In contrast, genes encoding proteins involved in transport, such as solute carriers (SLCs), and those encoding receptors were changed exclusively in muscles from young rats, potentially indicating that adaptive changes to metabolic demands are dampened with age.
The mRNA abundance of selected genes was verified with real-time RT-PCR (Table 5) using RNA from soleus muscle of six individual rats in each group for the validation. Genes previously shown to change with HS, such as extracellular matrix proteins or those involved in the fiber-type switching, were not included in the verification process. Normalization of the RT-PCR was performed with a geometric mean calculation among four housekeeping genes (NADH subunit 1, β-2 microglobulin, 18S, and tubulin) using GeNorm, which optimized normalization (100). In general, the changes in gene expression measured with real-time RT-PCR were very similar in direction and magnitude to the microarray results. We had two false-negative and two false-positive results, indicating that our statistical analysis did not favor one error type.
Table 5.
Verification of mRNA abundance for selected genes
| Gene |
6 mo |
32 mo
|
||
|---|---|---|---|---|
| Array | RT-PCR | Array | RT-PCR | |
| Hspb7 | −2.60 | −2.13 | −1.67 | NCD |
| SLC02B1 | 1.94 | 1.59 | NCD | 2.06 |
| RPS27 | NCD | 1.77 | 1.69 | 1.60 |
| RPL4 | 1.92 | 1.67 | 1.60 | 1.57 |
| RPS17 | NCD | NCD | 1.43 | 1.39 |
| Rbm3 | 1.91 | 2.54 | 2.76 | 4.32 |
| Il1r1 | −2.46 | NCD | NCD | NCD |
Numbers indicate fold changes with hindlimb suspension. NCD, no change detected (P > 0.05).
RBM3 analysis.
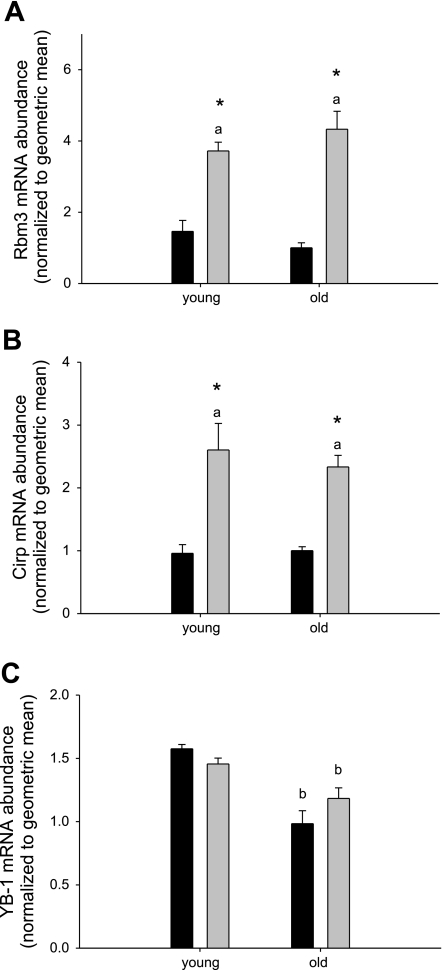
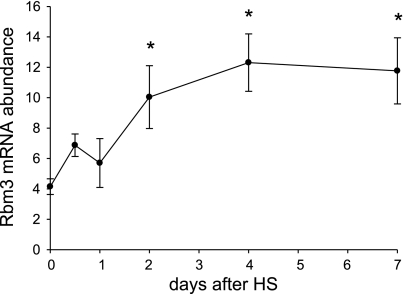
The gene that was most highly induced with HS in soleus muscle from old rats encoded RBM3 (Table 4). Quantification of gene expression changes with real-time RT-PCR verified that the RBM3 gene was more highly induced in old (4.3-fold) compared with young (2.5-fold) muscle with disuse (Fig. 1A). A time course of HS showed that RBM3 mRNA abundance first increased after 2 days of HS, which coincides with the first measure of atrophy but is after the increase in apoptosis (29) and the increase in expression of mRNAs for the ubiquitin ligases MAFbx and Murf-1 (Fig. 3) (7).
Fig. 1.
Gene expression of cold-shock protein RNA-binding motif protein 3 (RBM3) and cold-inducible RNA-binding protein (Cirp), but not Y-box binding protein 1 (YB-1), is elevated with hindlimb suspension (HS) in soleus muscle from both young and old rats. mRNA abundance of RBM3 (A), Cirp (B), and YB-1 (C) normalized to the geometric mean is shown for soleus muscle from control (black bars) and HS (gray bars) rats. Values are means ± SE. aSignificant main effect for HS, bsignificant main effect for age, *significant difference from control within the age group, P < 0.05.
Fig. 3.
RBM3 gene expression increases at the onset of muscle atrophy. mRNA abundance of RBM3 is shown for soleus muscle at early time points after the onset of HS (0.5, 1, 2, 4, and 7 days). Values are means ± SE. *Significant difference from control at time 0, P < 0.05.
RBM3.
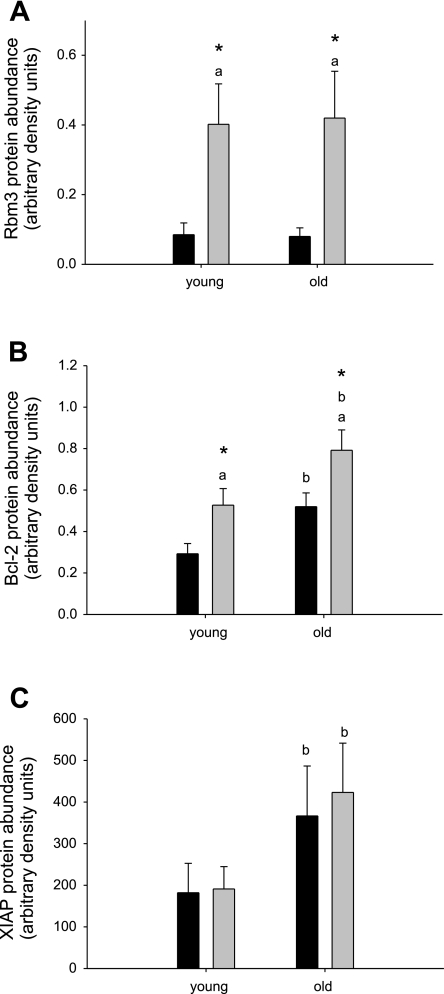
RBM3 was first identified as a RNA-binding protein (23), but also belongs to the cold-shock protein family. Therefore, we determined whether other cold-shock genes were upregulated with HS. Y-box-binding protein-1 (YB-1) and cold-inducible RNA binding protein (Cirp) are part of the cold-shock domain protein superfamily of RNA binding proteins (22, 38, 53), with Cirp most closely related to RBM3, since both contain only one RNA binding motif adjacent to a COOH-terminal arginine- and glycine-rich domain (22, 68) in contrast to YB-1, which contains basic/aromatic islands in its COOH-terminus (38). mRNA abundance for Cirp increased with HS in soleus muscles of both young and old rats, comparable to RBM3 (Fig. 1B). YB-1 mRNA did not change with HS, but instead showed a decrease with age regardless of suspension status (Fig. 1C), suggesting that the response to HS may be specific to small cold-shock domain proteins with a glycine-rich region. We further found that protein abundance of RBM3 increased 4.7- and 5.3-fold in soleus muscle from young and old rats, respectively (Fig. 2A), indicating that this protein is effectively translated. This is likely due to the use of internal ribosome entry sites (IRES) in the RBM3 mRNA (17) that mediates cap-independent translation (41) in the face of decreased total protein synthesis (94). Two additional proteins, Bcl-2 and XIAP, containing functional IRES were also measured. Bcl-2 protein abundance increased with HS in soleus muscle of both young and old rats, but XIAP protein abundance did not change, suggesting that increased cap-independent translation is not a general feature of HS.
Fig. 2.
Protein abundance of RBM3, Bcl-2, and X-linked inhibitor of apoptosis protein (XIAP), proteins known to contain a functional internal ribosome entry site, are not consistently correlated with HS or age. Protein abundance of RBM3 (A), Bcl-2 (B), and XIAP (C) is shown for soleus muscles from control (black bars) and HS (gray bars) rats. Values are means ± SE. aSignificant main effect for HS, bsignificant main effect for age, *significant difference from control within the age group, P < 0.05.
DISCUSSION
The capacity of skeletal muscle to recover from muscle atrophy induced by disuse is diminished at old age (15, 33, 111), and thus it is important to find ways to prevent atrophy during a period of disuse, particularly in the elderly. Understanding age-related differences in the response to muscle atrophy will aid in developing interventions for atrophy prevention. Therefore, we performed a microarray analysis to identify differences in gene expression in muscles from young and old rats during atrophy due to disuse. We found that the number of genes differentially expressed with atrophy in some functional categories was similar between young and old, but there were also changes indicative of a differential response with age. Functional groups whose gene expression profile changes were similar between young and old were: protein folding, muscle structural and contraction-related proteins, nucleic acid binding, and extracellular matrix constituents. By contrast, in old animals, there were more genes encoding ribosomal proteins changed with HS, while in young animals more genes encoding transport proteins and receptors were changed after disuse.
Transport proteins, including SLCs, control the uptake and efflux of compounds into and out of cells, and thereby influence cellular metabolism and protein homeostasis. SLCs have previously been shown to be changed in response to atrophy-inducing conditions (56, 77, 90), likely in response to the change in metabolic demands during disuse. SLC7 functions as a major entry path for cationic amino acids that feed into the protein synthetic pathway (20), while SLC5 (Na+/glucose cotransporter), SLC16 (pyruvate/lactate transporter), and SLC22 (organic anion transporter) appear to be more involved in the metabolic regulation of cellular homeostasis (39, 75, 109). Interestingly, very few changes with HS were observed in this category in muscles from old rats, suggesting that the adaptive response to disuse may be lost at old age. The same is true for the category of genes encoding receptor proteins, which primarily changed in young rats. On the other hand, the response of genes encoding ribosomal proteins was greatest in muscles from old rats. The increase in ribosomal protein mRNA abundance with disuse appears to be in conflict with the fact that protein synthesis is decreased during HS (94) but has been observed previously in different models of muscle atrophy (6, 12, 77, 88, 108). We suggest that gene expression of ribosomal proteins may be increased as a compensatory response to the loss of muscle protein in an attempt to minimize disuse-induced muscle atrophy in already sarcopenic muscles.
In contrast to the failed compensatory response, muscles from old rats still exhibit adaptability to disuse, since altered expression of many classes of genes with HS was similar in young and old. These include protein folding proteins (mainly HSPs) that function as molecular chaperones for proteins and play a role in maintaining cellular homeostasis (61). Genes encoding HSPs decrease with muscle atrophy due to sciatic nerve transection (46), tenotomy (46), denervation (69), and HS (55, 69, 78), and this decrease may contribute to the elevation in protein degradation. Indeed, when hyperthermia is applied prior to the induction of disuse atrophy to induce HSPs, the loss of muscle mass was attenuated (66, 81). Old animals are known to have an attenuated response to exercise-induced HSP expression (101, 102) and life-long overexpression of HSP70 decreased age-associated oxidative stress parameters (9), indicating that protein chaperone activity may be decreased with advancing age. Also, since most HSPs, and αB-crystallin in particular, are negative regulators of apoptosis in muscle cells (34, 45, 50, 103), they may contribute to the loss of nuclei that occurs with disuse atrophy, especially in old muscle (58).
Genes encoding proteins involved in muscle structure and contraction also changed in response to HS independent of age. Disuse is generally associated with a switch in fiber types from slow to fast (47, 64, 89), and this was observed in our study where expression of genes encoding slow proteins, such as slow troponin I and myosin light chain-2, decreased, while that of fast proteins, such as fast-twitch calcium ATPase and myosin light chain-3, increased. Thus, it seems that the switch in fiber types with HS occurs in soleus muscle from old animals to the same degree as it does in young animals, at least at the level of transcription. Similarly, genes encoding extracellular matrix (ECM) proteins changed in response to HS in both young and old muscles. ECM gene expression decreases in atrophying muscles of animals and humans (21, 56, 67, 79, 98) and even though ECM gene expression, collagens in particular, are decreased with age in skeletal muscle (36, 71), expression declines further with disuse.
The expression of genes encoding nucleic acid binding proteins was also relatively unaffected by age, with RBM3 (23), showing the greatest induction of expression in old muscle. RBM3 belongs to the glycine-rich RNA-binding protein family, comprised of proteins that contain one amino-terminal RNA recognition motif and a COOH-terminal glycine-rich domain (22). Proteins from this family have been found in cyanobacterium (80), plants (42), and mammalian cells (1), and are induced by various environmental stresses, but particularly by cold stress (1, 22, 87). They are proposed to function as RNA chaperones that facilitate translation, and to protect and restore native RNA conformations during stress (1, 38, 73, 87). RBM3, in particular, was shown to enhance global protein synthesis, to promote translation, and to decrease miRNA levels in neurons (24, 86). Interestingly, RBM3 was identified as the only protein to be highly induced during hibernation in liver, brain, and cardiac tissue in the golden-mantled ground squirrel (107) and to be elevated in all tissues studied, including skeletal muscle, of the hibernating arctic ground squirrel (110) whose body temperature drops dramatically during torpor. During hibernation, muscle mass in mammals does not decrease to the extent expected by the level of inactivity (82), and there is evidence that black bears actually retain protein content and are in protein balance during hibernation (40, 62, 63). We show here that both RBM3 and Cirp (also a glycine-rich RNA binding protein) are elevated 2 wk after HS, indicating that hibernation and disuse atrophy may have common responses as was also suggested by Yan et al. (110). Gene expression of a cold-shock protein from the Y-box family (YB-1) was not elevated with HS, as we showed previously for gastrocnemius muscle (43), suggesting that the response is likely specific to the glycine-rich RNA binding protein family. In addition to its proposed function in protein translation, RBM3 also has been shown to decrease apoptosis (52, 92), which is known to be elevated with both disuse- and age-associated muscle atrophy (2, 25, 26). We therefore hypothesize that the elevation of RBM3 might function as a compensatory response to the increase in apoptosis and the decrease in muscle protein with HS. Siu et al. (85) proposed a similar response for antiapoptotic proteins which are increased in old muscle undergoing atrophy. Indeed, the increase in RBM3 mRNA abundance occurred at a point in time that apoptosis is already elevated and protein degradation markers are detected (29). Furthermore, the increase in RBM3 gene expression also resulted in an increase in RBM3 protein abundance, even though it is known that total protein synthesis is decreased under HS-induced muscle atrophy (44, 93) as is also the case during cold shock (1). We suggest that translation of RBM3 may have occurred through cap-independent translation using an IRES site contained in the 5′ leader of RBM3 (16, 17). Similarly, Bcl-2, an anti-apoptotic protein known to contain a functional IRES site, was also elevated during HS, as has been shown previously in atrophying old muscle (85). However, the anti-apoptotic protein XIAP, which also contains a functional IRES site, was not elevated with HS, indicating that the response is likely not a general phenomenon with HS.
Perspectives and Significance
We propose that sarcopenic muscles induce an age-dependent compensatory response to maintain a minimal size. The increase in ribosomal protein gene expression, one of the few classes of genes differentially expressed with age after HS, may be part of this response to maintain protein synthesis. Similarly, elevated RBM3 may act to stabilize RNA to maintain the translatable pool in the face of nuclear loss during disuse-induced muscle atrophy. The significant increase in RBM3 gene expression in old muscle suggests the intriguing possibility that disuse-associated changes in muscle, particularly from old animals, resembles the cold-shock response, preserving remaining muscle tissue, similar to what occurs during hibernation in mammals. Therefore, RBM3, and potentially other glycine-rich RNA binding proteins, may be targets for prevention of muscle wasting during prolonged periods of disuse where significant muscle loss is expected.
GRANTS
This work was supported by National Institutes of Health Grant AR-47577 (to C. A. Peterson), and Grants AG-20407 and AG-028925 (to E. E. Dupont-Versteegden). The Microarray Core Facility was supported through The Arkansas Tobacco Settlement Proceeds Act of 2000, and by National Institutes of Health Grant P20-RR-16460 from the Biomedical Research Infrastructure Network Program of the National Center for Research Resources.
Acknowledgments
We thank Micheal Knox, Nicole Turesky, and Amy Ferry for technical assistance. We thank P. Vanderklish, Scripps Research Institute, La Jolla, CA, for the RBM3 antibody.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Al-Fageeh MB, Smales CM. Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J 397: 247–259, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, Norstedt G, Kessler BM, Ulfhake B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 36: 223–233, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bassey EJ, Fiatarone MA, O'Neill EA, Kelley M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci 82: 321–327, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Bearden ED, Simpson PM, Peterson CA, Beggs ML. Assessing the reliability of amplified RNA used in microarrays: a DUMB table approach. Appl Bioinformatics 5: 67–76, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bey L, Akunuri N, Zhao P, Hoffman EP, Hamilton DG, Hamilton MT. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol Genomics 13: 157–167, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 9.Broome CS, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J 20: 1549–1551, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Brown A, McCartney N, Sale D. Positive adaptations to weight-lifting training in the elderly. J Appl Physiol 69: 1725–1733, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging 8: 99–105, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Byrne KA, Wang YH, Lehnert SA, Harper GS, McWilliam SM, Bruce HL, Reverter A. Gene expression profiling of muscle tissue in Brahman steers during nutritional restriction. J Anim Sci 83: 1–12, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr 60: 167–175, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol Regul Integr Comp Physiol 268: R208–R213, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Chakravarthy MV, Davis BS, Booth FW. IGF-1 restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89: 1365–1379, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Chappell SA, Mauro VP. The internal ribosome entry site (IRES) contained within the RNA-binding motif protein 3 (Rbm3) mRNA is composed of functionally distinct elements. J Biol Chem 278: 33793–33800, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Chappell SA, Owens GC, Mauro VP. A 5′ Leader of Rbm3, a cold stress-induced mRNA, mediates internal initiation of translation with increased efficiency under conditions of mild hypothermia. J Biol Chem 276: 36917–36922, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics, 2007. [DOI] [PubMed]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Closs EI, Boissel JP, Habermeier A, Rotmann A. Structure and function of cationic amino acid transporters (CATs). J Membr Biol 213: 67–77, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Cros N, Tkatchenko AV, Pisani DF, Leclerc L, Leger JJ, Marini JF, Dechesne CA. Analysis of altered gene expression in rat soleus muscle atrophied by disuse. J Cell Biochem 83: 508–519, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, Fujita J. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 236: 804–807, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Derry JM, Kerns JA, Francke U. RBM3, a novel human gene in Xp11.23 with a putative RNA-binding domain. Hum Mol Genet 4: 2307–2311, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 102: 1865–1870, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dupont-Versteegden EE Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 40: 473–481, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Dupont-Versteegden EE Apoptosis in skeletal muscle and its relevance to atrophy. World J Gastroenterol 12: 7463–7466, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupont-Versteegden EE, Fluckey JD, Knox M, Gaddy D, Peterson CA. Effect of flywheel-based resistance exercise on processes contributing to muscle atrophy during unloading in adult rats. J Appl Physiol 101: 202–212, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Dupont-Versteegden EE, Knox M, Gurley CM, Houlé JD, Peterson CA. Maintenance of muscle mass is not dependent on the calcineurin-NFAT pathway. Am J Physiol Cell Physiol 282: C1387–C1395, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol 291: R1730–R1740, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 263: 3029–3034, 1990. [PubMed] [Google Scholar]
- 31.Fluck M, Schmutz S, Wittwer M, Hoppeler H, Desplanches D. Transcriptional reprogramming during reloading of atrophied rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 289: R4–R14, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol 64: 1038–1044, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol 97: 1082–1090, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 286: 433–442, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, Kandarian SC. Identification of a molecular signature of sarcopenia. Physiol Genomics 21: 253–263, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4: 183–191, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graumann PL, Marahiel MA. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci 23: 286–290, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: 619–628, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature 409: 997, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 15: 1593–1612, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Hirose T, Sugita M, Sugiura M. cDNA structure, expression and nucleic acid-binding properties of three RNA-binding proteins in tobacco: occurrence of tissue-specific alternative splicing. Nucleic Acids Res 21: 3981–3987, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol 43: 563–570, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howard G, Steffen JM, Geoghegan TE. Transcriptional regulation of decreased protein synthesis during skeletal muscle unloading. J Appl Physiol 66: 1093–1098, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda R, Yoshida K, Ushiyama M, Yamaguchi T, Iwashita K, Futagawa T, Shibayama Y, Oiso S, Takeda Y, Kariyazono H, Furukawa T, Nakamura K, Akiyama S, Inoue I, Yamada K. The small heat shock protein αB-crystallin inhibits differentiation-induced caspase 3 activation and myogenic differentiation. Biol Pharm Bull 29: 1815–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Inaguma Y, Goto S, Shinohara H, Hasegawa K, Ohshima K, Kato K. Physiological and pathological changes in levels of the two small stress proteins, HSP27 and α-B crystallin, in rat hindlimb muscles. J Biochem (Tokyo) 114: 378–384, 1993. [DOI] [PubMed] [Google Scholar]
- 47.Ishihara A, Oishi Y, Roy RR, Edgerton VR. Influence of two weeks of non-weight bearing on rat soleus motoneurons and muscle fibers. Aviat Space Environ Med 68: 421–425, 1997. [PubMed] [Google Scholar]
- 48.Jagoe RT, Lecker SH, Gomes M, Goldberg AL. Patterns of gene expression in atrophying skeletal muscles: response to food deprivation. FASEB J 16: 1697–1712, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Jozsi AC, Dupont-Versteegden EE, Taylor-Jones JM, Evans WJ, Trappe TA, Campbell WW, Peterson CA. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev 120: 45–56, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem 277: 38731–38736, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci USA 98: 5093–5098, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kita H, Carmichael J, Swartz J, Muro S, Wyttenbach A, Matsubara K, Rubinsztein DC, Kato K. Modulation of polyglutamine-induced cell death by genes identified by expression profiling. Hum Mol Genet 11: 2279–2287, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 25: 691–698, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Koncarevic A, Jackman RW, Kandarian SC. The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J 21: 427–437, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Lawler JM, Song W, Kwak HB. Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve 33: 200–207, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18: 39–51, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science 285: 1390–1393, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1288–R1296, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Lexell J, Downham D, Larsson Y, Bruhn E, Morsing B. Heavy resistance training in older Scandinavian men and women: short-and long-term effects on arm and leg muscles. J Med Sci Sports 5: 329–341, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Lipman R, Chrisp E, Hazzard D, Bronson R. Pathologic characterization of Brown Norway, Brown Norway X Fischer 344, and Fischer 344 X Brown Norway rats with relation to age. J Gerontol Biol Sci 51A: B54–B59, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6: D12–D25, 2001. [DOI] [PubMed] [Google Scholar]
- 62.Lohuis TD, Harlow HJ, Beck TD. Hibernating black bears (Ursus americanus) experience skeletal muscle protein balance during winter anorexia. Comp Biochem Physiol B Biochem Mol Biol 147: 20–28, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Lohuis TD, Harlow HJ, Beck TD, Iaizzo PA. Hibernating bears conserve muscle strength and maintain fatigue resistance. Physiol Biochem Zool 80: 257–269, 2007. [DOI] [PubMed] [Google Scholar]
- 64.McCarthy JJ, Fox AM, Tsika GL, Gao L, Tsika RW. β-MHC transgene expression in suspended and mechanically overloaded/suspended soleus muscle of transgenic mice. Am J Physiol Regul Integr Comp Physiol 272: R1552–R1561, 1997. [DOI] [PubMed] [Google Scholar]
- 65.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Naito H, Powers SK, Demirel HA, Suguira T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol 88: 359–363, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, Kominami E, Nonaka I, Ogawa T, Adams GR, Baldwin KM, Yasui N, Kishi K, Takeda S. Skeletal muscle gene expression in space-flown rats. FASEB J 18: 522–524, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Nishiyama H, Higashitsuji H, Yokoi H, Itoh K, Danno S, Matsuda T, Fujita J. Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) cDNA and chromosomal assignment of the gene. Gene 204: 115–120, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Oishi Y, Ishihara A, Talmadge RJ, Ohira Y, Taniguchi K, Matsumoto H, Roy RR, Edgerton VR. Expression of heat shock protein 72 in atrophied rat skeletal muscles. Acta Physiol Scand 172: 123–130, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Pattison JS, Folk LC, Madsen RW, Booth FW. Identification of differentially expressed genes between young and old rat soleus muscle during recovery from immobilization-induced atrophy. J Appl Physiol 95: 2171–2179, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Pattison JS, Folk LC, Madsen RW, Childs TE, Booth FW. Transcriptional profiling identifies extensive downregulation of extracellular matrix gene expression in sarcopenic rat soleus muscle. Physiol Genomics 15: 34–43, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Pattison JS, Folk LC, Madsen RW, Childs TE, Spangenburg EE, Booth FW. Expression profiling identifies dysregulation of myosin heavy chains IIb and IIx during limb immobilization in the soleus muscles of old rats. J Physiol 553: 357–368, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phadtare S, Alsina J, Inouye M. Cold-shock response and cold-shock proteins. Curr Opin Microbiol 2: 175–180, 1999. [DOI] [PubMed] [Google Scholar]
- 74.Raffaello A, Laveder P, Romualdi C, Bean C, Toniolo L, Germinario E, Megighian A, Danieli-Betto D, Reggiani C, Lanfranchi G. Denervation in murine fast-twitch muscle: short-term physiological changes and temporal expression profiling. Physiol Genomics 25: 60–74, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res 24: 450–470, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Roth SM, Ferrell RE, Peters DG, Metter EJ, Hurley BF, Rogers MA. Influence of age, sex, and strength training on human muscle gene expression determined by microarray. Physiol Genomics 10: 181–190, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21: 140–155, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Sakurai T, Fujita Y, Ohto E, Oguro A, Atomi Y. The decrease of the cytoskeleton tubulin follows the decrease of the associating molecular chaperone αB-crystallin in unloaded soleus muscle atrophy without stretch. FASEB J 19: 1199–1201, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Salem M, Kenney PB, Rexroad CE, 3rd, Yao J. Microarray gene expression analysis in atrophying rainbow trout muscle: a unique nonmammalian muscle degradation model. Physiol Genomics 28: 33–45, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Sato N A family of cold-regulated RNA-binding protein genes in the cyanobacterium Anabaena variabilis M3. Nucleic Acids Res 23: 2161–2167, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289: R134–R139, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Shavlakadze T, Grounds M. Of bears, frogs, meat, mice and men: complexity of factors affecting skeletal muscle mass and fat. Bioessays 28: 994–1009, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Simard C, Lacaille M, Vallieres J. Effects of hypokinesia/hypodynamia on contractile and histochemical properties of young and old rat soleus muscle. Exp Neurol 97: 106–114, 1987. [DOI] [PubMed] [Google Scholar]
- 84.Singh MA, Ding W, Manferdi TJ, Solares GR, O'Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol Endocrinol Metab 277: E135–E143, 1999. [DOI] [PubMed] [Google Scholar]
- 85.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences the cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol 288: C338–C349, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. J Neurochem 101: 1367–1379, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Sonna LA, Fujita J, Gaffin SL, Lilly CM. Invited review: effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92: 1725–1742, 2002. [DOI] [PubMed] [Google Scholar]
- 88.St-Amand J, Okamura K, Matsumoto K, Shimizu S, Sogawa Y. Characterization of control and immobilized skeletal muscle: an overview from genetic engineering. FASEB J 15: 684–692, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Stevens L, Sultan KR, Peuker H, Gohlsch B, Mounier Y, Pette D. Time-dependent changes in myosin heavy chain mRNA and protein isoforms in unloaded soleus muscle of rat. Am J Physiol Cell Physiol 277: C1044–C1049, 1999. [DOI] [PubMed] [Google Scholar]
- 90.Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551: 33–48, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stump CS, Tipton CM, Henriksen EJ. Muscle adaptations to hindlimb suspension in mature and old Fisher 344 rats. J Appl Physiol 82: 1875–1881, 1997. [DOI] [PubMed] [Google Scholar]
- 92.Sutherland LC, Rintala-Maki ND, White RD, Morin CD. RNA binding motif (RBM) proteins: a novel family of apoptosis modulators? J Cell Biochem 94: 5–24, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Thomason DB, Biggs RB, Booth FW. Protein metabolism and β-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol 257: R300–R305, 1989. [DOI] [PubMed] [Google Scholar]
- 94.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol 68: 1–12, 1990. [DOI] [PubMed] [Google Scholar]
- 95.Thompson LV, Johnson SA, Shoeman JA. Single soleus muscle fiber function after hindlimb unweighting in adult and aged rats. J Appl Physiol 84: 1937–1942, 1998. [DOI] [PubMed] [Google Scholar]
- 96.Thompson LV, Shoeman JA. Contractile function of single muscle fibers after hindlimb unweighting in aged rats. J Appl Physiol 84: 229–235, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol 579: 877–892, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Urso ML, Clarkson PM, Price TB. Immobilization effects in young and older adults. Eur J Appl Physiol 96: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol 101: 1136–1148, 2006. [DOI] [PubMed] [Google Scholar]
- 100.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: research 0034.1–0034.11, 2002. [DOI] [PMC free article] [PubMed]
- 101.Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 25: 902–905, 2002. [DOI] [PubMed] [Google Scholar]
- 102.Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev 127: 830–839, 2006. [DOI] [PubMed] [Google Scholar]
- 103.Webster KA Serine phosphorylation and suppression of apoptosis by the small heat shock protein αB-crystallin. Circ Res 92: 130–132, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Welle S, Brooks A, Thornton CA. Senescence-related changes in gene expression in muscle: similarities and differences between mice and men. Physiol Genomics 5: 67–73, 2001. [DOI] [PubMed] [Google Scholar]
- 105.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics 14: 149–159, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol 51A: M270–M275, 1996. [DOI] [PubMed] [Google Scholar]
- 107.Williams DR, Epperson LE, Li W, Hughes MA, Taylor R, Rogers J, Martin SL, Cossins AR, Gracey AY. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics 24: 13–22, 2005. [DOI] [PubMed] [Google Scholar]
- 108.Wittwer M, Fluck M, Hoppeler H, Muller S, Desplanches D, Billeter R. Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB J 16: 884–886, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflügers Arch 447: 510–518, 2004. [DOI] [PubMed] [Google Scholar]
- 110.Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Zarzhevsky N, Carmeli E, Fuchs D, Coleman R, Stein H, Reznick AZ. Recovery of muscles of old rats after hindlimb immobilisation by external fixation is impaired compared with those of young rats. Exp Gerontol 36: 125–140, 2001. [DOI] [PubMed] [Google Scholar]