Abstract
Evidence for sexual dimorphism in autonomic control of cardiovascular function is both compelling and confounding. Across healthy and disease populations sex-associated differences in neurocirculatory hemodynamics are far too complex to be entirely related to sex hormones. As an initial step toward identifying additional physiological mechanisms, we investigated whether there is a sex bias in the relative expression of low-threshold-myelinated and high-threshold-unmyelinated aortic baroreceptor afferents in rats. These two types of afferent fibers have markedly different reflexogenic effects upon heart rate and blood pressure and thus the potential impact upon baroreflex dynamics could be substantial. Our results, using a combination of a patch-clamp study of fluorescently identified aortic baroreceptor neurons (ABN) and morphometric analysis of aortic baroreceptor nerve fibers, demonstrate that females exhibit a greater percentage of myelinated baroreceptor fibers (24.8% vs. 18.7% of total baroreceptor fiber population, P < 0.01) and express a functional subtype of myelinated ABN rarely found in age-matched males (11% vs. 2.3%, n = 107, P < 0.01). Interestingly, this neuronal phenotype is more prevalent in the general population of female vagal afferent neurons (17.7% vs. 3.8%, n = 169, P < 0.01), and ovariectomy does not alter its expression but does lessen neuronal excitability. These data suggest there are fundamental neuroanatomical and electrophysiological differences between aortic baroreceptor afferents of female and male rats. Possible explanations are presented as to how such a greater prevalence of low-threshold myelinated afferents could be a contributing factor to the altered baroreflex sensitivity and vagal tone of females compared with males.
Keywords: baroreflex, vagus
sexual dimorphism in cardiovascular function is well recognized (8). The underlying mechanisms for such sex differences are proving to be multifaceted, highly integrative, and well beyond the sphere of influence that can be merely attributed to the recognized effects of sex hormones (3, 17, 26). This has presented considerable challenges to the effective management of cardiovascular health and disease in the female population (25, 32). Notable examples are replete throughout the broad spectrum of clinical measures of autonomic nervous system function, many of which have proven to be markedly sexually dimorphic (4, 6).
The cardiovagal baroreflex is critically important to the regulation of heart rate and blood pressure. As a result, baroreceptor reflex sensitivity (BRS) has been extensively studied across a wide range of cardiovascular pathologies with the prognostic value of BRS having been well demonstrated (13, 21). There is a preponderance of evidence in the literature that BRS is lower in healthy, premenopausal women compared with healthy age-matched men (1, 3, 27). Furthermore, BRS is markedly reduced in hypertensive compared with normotensive females, while no such, or a lesser, reduction occurs in hypertensive males (31). Collectively, these data are consistent with evidence that across both healthy and hypertensive subjects vagal tone is greater in premenopausal females compared with age-matched males (4). However, this issue remains controversial as some studies have shown no differences or even a greater BRS along with a reduced baseline cardiac vagal activity in females compared with males (5, 14, 33). A mechanistic explanation for these differences and controversies remains elusive and is further complicated by the stark reflexogenic disparities between myelinated and unmyelinated vagal afferents. For example, myelinated arterial baroreceptor afferents exhibit far lower thresholds for pressure-dependent discharge than unmyelinated baroreceptor afferents but require substantially higher neural discharge frequencies to elicit a comparable bradycardic effect (11, 12). While it is presently unknown whether there is a sex bias associated with the distribution of myelinated and unmyelinated baroreceptor afferents, this functional dichotomy of afferent fiber type has been shown to extend through to second-order brain stem circuitry (18).
A recent study by Christou et al. (3) concluded that the differences in baroreflex buffering between men and women are “mediated by one or more currently undetermined differences in the afferent, central regulatory, or [autonomic nervous system] efferent elements of the baroreflexes.” The current study was undertaken to definitively determine whether the electrophysiological and neuroanatomical properties of aortic baroreceptor afferents are sexually dimorphic. Our results, using age-matched female and male Sprague-Dawley rats, demonstrate marked differences in the functional characteristics and distribution of myelinated and unmyelinated baroreceptor afferent neurons and fibers between female and male Sprague-Dawley rats. Our data support the following four conclusions: 1) As a percentage of the total aortic baroreceptor fiber population, adult female Sprague-Dawley rats exhibit on average nearly 50% more myelinated baroreceptor fibers than age-matched males, 2) female Sprague-Dawley rats exhibit a functionally distinct subpopulation of low-threshold-activated myelinated baroreceptor afferent neurons that is rarely observed in male Sprague-Dawley rats, 3) this afferent neuronal phenotype is even more prevalent in the general population of vagal afferents of normal adult female Sprague-Dawley rats, and 4) that ovariectomy as a preweanling does not alter the relative expression of this functionally distinct subtype of myelinated afferent although neuronal excitability is greatly reduced. A greater percentage of low-threshold myelinated afferents may account for the presumed differences in baroreflex sensitivity and vagal tone in females compared with males (4, 17).
MATERIALS AND METHODS
The materials and methods utilized for electrophysiological study are described in our earlier publications and are only briefly described here (10, 18, 24). All animal use protocols were approved by the Institutional Animal Care and Use Committee of the School of Science, Indiana University Purdue University Indianapolis, and the School of Medical Science, Harbin Medical University. All electrophysiological and pharmacological studies were carried out at room temperatures using age-matched adult Sprague-Dawley rats (Harlan, IN). Comparative electrophysiological and pharmacological studies were also performed on a cohort of adult female Sprague-Dawley rats ovariectomized (OVX) as preweanlings by the vendor. Following dissection of the vagal ganglia, a necropsy was performed for anatomical validation of bilateral removal of the ovaries and the majority of the oviducts. Electron microscopy studies were carried out using age-matched female (n = 6, 287 ± 11 g, nonbreeder) and male (n = 6, 330 ± 15 g) Sprague-Dawley rats (Chinese Academy, Shanghai Laboratory Animal Center). Experimental data are presented as means ± SD with significance (P < 0.05) assessed using a two-way Student's t-test or ANOVA where appropriate.
Fluorescent labeling of aortic baroreceptor neurons.
Anesthesia: 10 ml ketamine (100 mg/ml) + 1.4 ml xylazine (100 mg/ml) dosed 0.1 ml/100 g ip. Under aseptic conditions, the surgical area was shaved, skin was washed with benzalkonium chloride, and a ∼2-cm incision was made along the left ventral side of the neck. Under stereomicroscopy, a blunt dissection of the underlying musculature exposed the left carotid artery and surrounding nerve fibers. Under higher magnification, the left aortic depressor nerve was identified. The aortic depressor nerve was separated from the vagus and sympathetic nerves and was placed in a 5-mm-long sterile silicon trough. A few crystals of the lipophilic fluorescent dye DiI (Molecular Probes) was placed on the aortic depressor nerve. The nerve, dye crystals, and trough were coated with ∼0.3 ml of a peripheral nerve encapsulant (Kwik-Sil; World Precision Instruments). The area was rinsed with sterile saline, and the skin was closed using vicryl suture. The animal fully recovered before returning to the vivarium. At least 2 wk passed before the animal was considered fully healed and available for experimentation.
Enzymatic isolation of vagal afferent neurons from adult rat.
In Sprague-Dawley rats the proximal vagal (jugular) and distal vagal (nodose) ganglia are not well distinguished, most often presenting as one elongated structure. It was this vagal ganglion that was dissected from adult rats (200–350 g) under stereomicroscopy (×40). The vagal ganglia was immediately placed in chilled (4–8°C) nodose complete medium consisting of 90 ml of DME-F-12 medium (Sigma, St. Louis, MO), 5 ml of fetal bovine serum (Hyclone, Logan, UT), 1.0 ml of a penicillin and streptomycin solution containing 5,000 units and 5,000 μg/ml, respectively (Invitrogen, Grand Island, NY), and 100 μM of MITO + serum extender (Collaborative Biomedical Products, Bedford, MA). The ganglia were then enzymatically treated by the application of 10 U/ml of papain (Worthington, Lakewood, NJ) at 37°C for 20 min, followed by 1 mg/ml of type II collagenase and 2.5 mg/ml of dispase (Roche) at 37°C for a additional 30 min. The enzyme solution was then replaced with nodose complete medium, and the ganglia was titrated with an aspiration pipette to free the cell bodies for plating onto a poly-d-lysine (0.5 mg/ml aqueous solution)-coated coverslip. The isolated nodose neurons were maintained in an incubation chamber (5% CO2-95% air) at 37°C for at least 8 h but not more than 12 h prior to recording.
Patch-clamp electrophysiology and recording solutions.
The whole cell patch technique was used for both current clamp and voltage clamp recording protocols using an Axoclamp 700A. The borosilicate glass pipettes (Sutter) were pulled and polished down to a resistance of 1–2 MΩ. Following a giga-ohm seal, the pipette capacitance was compensated. Upon going whole cell, the total cell capacitance (30–70 pF) and electrode access resistance (3–5 MΩ) were also compensated 70–80%. All current clamp protocols consisted of a sustained, small-magnitude current injection of between 10 and 50 pA to ensure that all stimulus-evoked action potential waveforms originated from the same −60 mV resting potential. Stimulus protocols consisted of 1) short 500-μs current pulses of magnitude just sufficient to elicit an action potential waveform followed by several more pulses of increasing intensity delivered at 3-s intervals or 2) 600-ms step current injections of magnitude just sufficient to elicit discharge followed by several more steps at 3-s intervals of increasing intensity to assess capacity for repetitive discharge. All data were low-pass filtered to 10 KHz and digitized at 50 KHz. The experimental protocols, data collection, and preliminary analysis were carried out using pCLAMP 10 and the Digidata 1322A (Axon Instruments) operating on a personal computer platform.
For recording of somatic action potential waveforms, the extracellular solution consisted of (in mM): 137.0 NaCl, 5.4 KCl, 1.0 MgCl2, 2.0 CaCl2, 10.0 glucose, 10.0 N-2-hydroxyethylpiperazine-N′-2- ethanesulfonic acid; and the pipette solution consisted of (in mM): 10.0 NaCl, 50.0 KCl, 50.0 K2SO4, 5.0 MgCl2. The pH for pipette and bath solutions were adjusted to 7.25 and 7.35, respectively, using 1 N pipes/KOH and 1 N NaOH. Osmolarity of the extracellular and intracellular solutions were adjusted using d-manitol (Sigma) to 310–315 and 290–295, respectively. Just prior to recording, the pipette solution was adjusted to include 2.0 mM Mg-ATP, 2.0 mM Na-GTP, along with 4.0 mM BAPTA-K/BAPTA-Na and 0.25 mM CaCl2 for a final buffered intracellular Ca2+ concentration of 10 nM.
Pharmacological and statistical classification of afferent fiber type.
Select measures of somatic action potential wave shape were reliably used to classify an isolated neuron as being either an unmyelinated (C-type) or myelinated (Ah-type or A-type) afferent neuron. These measures are of the action potential firing threshold (APFT), the action potential upstroke velocity at 50% peak displacement (UVAPD50), and the action potential downstroke velocity at 50% peak displacement (DVAPD50). As detailed in Li and Schild (24), this classification method was validated using measures of fiber conduction velocity (CV) along with a robust differential sensitivity to 10 μM of the purinergic (P2X) receptor agonist αβ-mATP and 100 nM of the vanilloid receptor-1 agonist capsaicin (CAP). Specifically, neurons with myelinated axons were shown to be αβ-mATP positive (αβ-mATP+) and CAP negative (CAP−), while neurons with unmyelinated axons were shown to be CAP positive (CAP+) and αβ-mATP negative (αβ-mATP−). Furthermore, neurons with myelinated axons (A-type with fiber CV > 10 m/s and Ah-type with fiber CV > 3 m/s) exhibited somatic action potentials with significantly (P < 0.05) lower APFT and greater UVAPD50 than exhibited by somatic action potentials from neurons with unmyelinated axons (C-type with fiber CV < 2 m/s) (24).
Preparation of aortic depressor nerve specimens for transmission electron microscopy.
A distal (at clavicular level) segment of the aortic depressor nerve was dissected and immediately immersed in an aldehyde fixative consisting of modified Karnovsky's, (2% paraformaldehye, 2% glutaraldehyde in 0.1 M phosphate buffer) for a minimum of 1 h. The specimen was then rinsed several times with PBS followed by postfixation with 1% osmium tetroxide in phosphate buffer for 1 h. Following an additional rinse in PBS for 15 min the specimen was dehydrated through a series of graded ethyl alcohols from 70% to 100% according to the following schedule: 70% for 10 min, 95% for 10 min, and three changes of 100% for 5 min each. After dehydration, the infiltration process required steps through an intermediate solvent that consisted of two changes of 100% propylene oxide for 15 min each and finally into a 50:50 mixture of propylene oxide and the embedding resin (Embed 812; Electron Microscopy Sciences, Fort Washington, PA) for 12 to 18 h. The specimen was transferred to fresh 100% embedding media for at least 1 h. The tissue was then embedded in a fresh change of 100% embedding media. Polymerization was carried out by 12–18 h at 60°C as a final step before sectioning.
The resin blocks containing the aortic depressor nerve were first thick sectioned at 1–2 μ with glass knives using an Ultracut UCT (Leica, Bannockburn, IL) and stained with Toluidine Blue. These sections were then used as a reference to trim blocks for thin sectioning. The blocks were then thin sectioned using a diamond knife (Diatome; Electron Microscopy Sciences) at 70–90 nm (silver to pale gold using color interference), and sections were placed on either copper or nickel mesh grids. After drying on filter paper for a minimum of 1 h, the sections were stained with the heavy metals uranyl acetate and lead citrate for contrast. After drying, the grids were then viewed on a Tecnai BioTwin (FEI, Hillsboro, OR). Digital images were acquired with an charge-coupled device camera (Advanced Microscopy Techniques, Danvers, MA) and stored on a CD for later analysis.
Morphological analysis of aortic depressor nerve cross sections.
The digital images of each cross section of the aortic depressor nerve was presented in an eight-bit grayscale format. A luminosity histogram of each image was used to guide the fine adjustment of the image so that the dark myelin was in greater contrast to the surrounding tissues. Photoshop (version 7, Adobe) was used to manually identify the dark myelin surrounding each A- or Ah-type axon with the luminosity tolerance set at 40. Occasionally, a few pixels inside the myelin that were not picked up automatically by the wand tool were selected manually. All myelinated regions were transformed to black with other regions left as white. In this manner, the black-and-white pixel counts could be used to calculate the geometrical area of the myelin and encapsulated axon by using customized routines developed under MATLAB (version 7.2, MathWorks). The G ratio was then calculated as the square root of the area ratio between the axon and the sum of the axon and myelin area.
RESULTS
A combination of patch-clamp electrophysiological, pharmacological, statistical, and electron microscopic techniques were utilized to test the following null hypothesis: that no measurable electrophysiological and neuroanatomical differences exist between the distributions of myelinated and unmyelinated aortic baroreceptor afferents from male and female Sprague-Dawley adult rats. The same null hypothesis was tested using a large population of vagal afferent cell bodies of unknown sensory modality of which some were presumably of cardiovascular origin. No attempt was made to control for phase of female estrus cycle beyond the inclusion of a cohort of vendor supplied OVX female rats that were subject to the same electrophysiological and pharmacological studies. Comparative analyses of these data increased the rigor of our hypothesis test by identifying any statistically significant differences between the populations of baroreceptor afferent neurons from the OVX and intact female and male populations, as well as helping to clarify the potential neurobiological, neuroanatomical, and hormonal factors contributing to population differences.
Fluorescent identification of ABN.
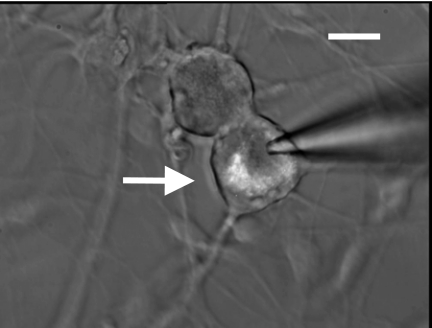
Retrograde transport of the lipophillic fluorescent indicator DiI (Molecular Probes) along the aortic depressor nerve made possible the identification of ABN (Fig. 1, arrow) from the general population of unlabeled vagal afferent neurons (VGN) of unknown sensory modality.
Fig. 1.
Di-I-labeled aortic baroreceptor (arrow) and vagal neuron from an adult Sprague-Dawley rat. Image is of enzymatically dispersed neurons taken after ∼24 h in culture. All electrophysiological data from aortic baroreceptor neurons (ABN) were collected from such fluorescently identified cells. Scale bar = 35 μm.
Functional classification of vagal neurons as either myelinated or unmyelinated afferents.
Using an intact vagal ganglion preparation suitable for measure of fiber CV, we have previously shown that a combination of unique electrophysiological and pharmacological characteristics can reliably classify isolated vagal neurons as either myelinated or unmyelinated afferents (18, 23, 24). Here, we begin our presentation of results by first classifying all isolated neurons (n = 277) as either a myelinated or an unmyelinated afferent, regardless of the sensory modality or sex of origin.
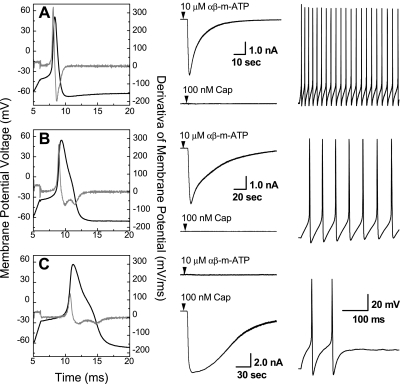
Whole cell patch-clamp electrophysiology was carried out in current clamp mode and revealed three distinct phenotypes of somatic action potential waveforms (Fig. 2). A brief current pulse (500 μs) elicited somatic action potentials that could be grouped according to collective measures of APFT, UVAPD50, and DVAPD50. Myelinated afferent neurons classified as A-type (Fig. 2A, n = 45) exhibited low-threshold (−44.0 ± 3 mV), brief-duration (0.85 ± 0.2 ms), somatic action potentials. All A-type ABN and VGN exhibited a large inward current in response to 10 μM of the purinergic (P2X) receptor agonist αβ-mATP, but no response to 100 nM of CAP. Depolarizing step current injections of 300 pA consistently evoked high-frequency action potential discharge (48.3 ± 9 Hz). All ABN and VGN myelinated afferent neurons classified as Ah-type (Fig. 2B, n = 27) exhibited somatic action potentials with significantly (P < 0.01) more depolarized thresholds (−36.3 ± 4 mV) and broader durations (1.95 ± 0.3 ms) than A-type cells but with a nearly identical differential chemosensitivity to αβ-mATP and CAP, i.e., 22 of 27 Ah-type vagal neurons were αβ-mATP+ and CAP−, where positive and negative modifiers indicate that the agonist did (+) or did not (−), respectively, elicit an inward receptor current. The chemosensitivities of the remaining Ah-type neurons were as follows: 2 of 27 were αβ-mATP+ and CAP+, 2 of 27 were αβ-mATP− and CAP−, while only 1 of 27 was αβ-mATP− and CAP+. Depolarizing step current injections of 300 pA also evoked high-frequency repetitive discharge from Ah-type neurons but at about one-third the frequency (16.28 ± 6.38 Hz, P < 0.01) of myelinated A-type neurons. Stimulus currents > 300 pA evoked higher repetitive discharge frequencies but never to the extent exhibited by A-type neurons. Unmyelinated afferent neurons classified as C-type (Fig. 2C, n = 205) exhibited somatic action potentials with significantly more depolarized thresholds (−26.3 ± 3 mV, P < 0.01) and broad duration (2.74 ± 0.5 ms, P < 0.01) along with a marked differential chemosensitivity to αβ-mATP and CAP nearly identical, albeit of opposite polarity, to that of A- and Ah-type vagal neurons, i.e., 194 of 205 C-type vagal neurons were αβ-mATP− and CAP+. The chemosensitivities of the remaining C-type vagal neurons were as follows: 5 of 204 were αβ-mATP+ and CAP+, 4 of 205 were αβ-mATP− and CAP−, while 2 of 205 were αβ-mATP+ and CAP−. Depolarizing step current injections of 600 pA evoked somatic action potential discharge frequencies from C-type vagal neurons that were significantly slower than the repetitive discharge rate of myelinated A- and Ah-type afferents (1.13 ± 0.4 Hz, P < 0.01). These data are presented in a more complete form according to sex and afferent classification as an ABN or VGN in Table 1.
Fig. 2.
Left column: somatic action potentials and time derivative of membrane voltage (gray line, right ordinate) from exemplar myelinated A-type (A), myelinated Ah-type and (B), and unmyelinated C-type (C) fluorescently identified ABN. Middle column: pharmacological validation of afferent fiber type via differential chemosensitivity of each subpopulation to 10 μM αβ-mATP and 100 nM capsaicin (see Functional classification of vagal neurons as either myelinated or unmyelinated afferents). Right column: relative comparison of cell excitability in response to a constant current step magnitude of 300 pA for both the A-type and Ah-type ABN and 600 pA for the example C-type ABN.
Table 1.
Somatic action potential characteristics of myelinated A-type and Ah-type and unmyelinated C-type aortic baroreceptor neurons (ABN) and vagal neurons (VGN)
|
Female ABN, n = 64 |
Male ABN, n = 44
|
|||||
|---|---|---|---|---|---|---|
| Action Potential Measures | A-Type | Ah-Type | C-Type | A-Type | Ah-Type | C-Type |
| RMP | −63.6±1.7 | −63.0±1.0 | −63.8±2.2 | −62.7±1.5 | −63 | −62.6±3 |
| APFT | −44.6±1.7 | −37.0±3.9†§ | −25.3±2.8 | −44.4±3.1 | −34.1 | −27.3±2.1 |
| APPeak | 49.0±3.1 | 55.5±3.4 | 50.1±3.5 | 50.6±2.13 | 54.9 | 51.1±5.7 |
| APD | 0.84±0.2 | 1.99±0.1†§ | 2.96±0.5 | 0.80±0.22 | 2.06 | 2.92±0.6 |
| AHPPeak | −66.9±1 | −66.6±1.2 | −64.4±1.2 | −67.4±1.9 | −65.7 | −65.6±1.4 |
| AHP80 | 20.4±10.7 | 104±3† | 93.0±27 | 12.4±2.2 | 103.02 | 82.6±24.3 |
| UVAPD50 | 202±9 | 129±9†§ | 40.1±13.4 | 243±78 | 171.7 | 90.4±25.8 |
| DVAPD50 | −138±10 | −46.6±9.4†‡ | −33.6±10.3 | −162±51 | −36.8 | −26.0±8.2 |
| N, % | 10 (15.62) | 7 (10.94) | 47 (73.43) | 7(15.9) | 1 (2.27) | 36 (81.8) |
| ΔE-mATP + | 10 | 7 | 7 | 1 | ||
| CAP+ | 45 | 34 | ||||
| + To both | 2 | 1 | ||||
| − To both | 1 | |||||
|
Female VGN, n = 90 |
Male VGN, n = 79
|
|||||
|---|---|---|---|---|---|---|
| A-Type | Ah-Type | C-Type | A-Type | Ah-Type | C-Type | |
| RMP | −63.1±2.8 | −64.1±2.8 | −62.9±3.1 | −64.6±3.4 | −63.7±2.1 | −63.1±3 |
| APFT | −43.3±2.4 | −36.6±3.9†§ | −26.1±4.1 | −43.9±2.2 | −37.3±3.4*‡ | −26.7±3.8 |
| APPeak | 48.1±4 | 52.3±5 | 47.0±5 | 49.5±3.5 | 50.2±8 | 46.7±3.9 |
| APD | 0.82±0.2 | 1.89±0.6†§ | 2.48±0.7 | 0.93±0.3 | 1.84±0.1†‡ | 2.29±0.4 |
| AHPPeak | −66.1±1.8 | −67.5±2.2 | −68.3±2.5 | −66.8±2.1 | −68.9±1.4 | −68.6±2.5 |
| AHP80 | 19.9±12.5 | 63.8±32.6† | 78.3±33.5 | 32.5±20.6 | 103±49.6† | 80.1±38.6 |
| UVAPD50 | 189±69 | 111±22†§ | 40.9±14.2 | 207±21 | 119±28†§ | 39.3±13.8 |
| DVAPD50 | −136±49 | −54.2±11.9†‡ | −41.8±16.5 | −103±20 | −53.6±17.3† | −46.9±10.6 |
| N (%) | 14 (15.55) | 16 (17.78) | 60 (66.67) | 14 (17.72) | 3 (3.79) | 62 (78.49) |
| ΔE-mATP(+) | 14 | 14 | 1 | 14 | 1 | |
| CAP+ | 56 | 1 | 59 | |||
| + To both | 1 | 1 | 2 | |||
| − To both | 1 | 2 | 1 | 1 | ||
Data are means ± SD. Inward currents were positive (+) or negative (−) elicited by 10 μM αβ-mATP or 100 nM capsaicin (CAP). RMP, resting membrane potential; APFT, action potential firing threshold; APPeak, action potential peak; APD50, AP duration at 50% deflection; AHPPeak, peak after hyperpolarization; AHP80, time for recovery to within 80% of RMP from AHPPeak; UVAPD50, upstroke velocity as measured at APD50; DVAPD50, downstroke velocity as measured at APD50.
P < 0.05,
P < 0.01 for A-type vs. Ah-type; and
P < 0.05,
P < 0.01 for Ah-type vs. C-type.
Cluster analysis of electrophysiological measures according to sensory modality and sex.
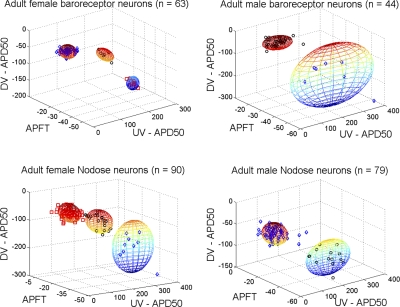
The first test of our null hypothesis was carried out using a proven method of statistical analysis for reliable classification of afferent fiber type (24) (Fig. 2) along with further segregation of the data as being either a fluorescently identified ABN or a VGN of unknown sensory modality along with further delineation according to sex (Fig. 3, Table 2). These data present two robust observations that suggest statistically significant differences in the distribution of myelinated and unmyelinated afferents across male and female populations of Sprague-Dawley rats. First, based upon a cluster analysis from measures of APFT, UVAPD50, and DVAPD50, the populations of afferent neurons from female Sprague-Dawley rats of both ABN and unidentified VGN sensory modalities could be reliably organized into three statistically distinct (P < 0.05), nonoverlapping, and functionally unique populations of myelinated (A- and Ah-type) and unmyelinated (C-type) afferents. In stark contrast, the same cluster analysis of ABN and VGN populations from male Sprague-Dawley rats could at best be only separated into two statistically distinct (P < 0.05), nonoverlapping, and functionally unique populations of myelinated (A-type) and unmyelinated (C-type) afferents. All available manipulations of the search algorithms and weighing factors failed to realize three nonoverlapping subpopulations of ABN or VGN from male Sprague-Dawley rats. The few outliers beyond the spheres that demarcate 2 SD from the cluster centroid are the few ABN and VGN myelinated afferents that closely approximated either the electrophysiological or pharmacological phenotype of Ah-type myelinated afferents in male Sprague-Dawley rats, which was quite rare, i.e., only 4 of the 123 neurons from male Sprague-Dawley rats were classified as Ah-type compared with 23 of 154 neurons from female Sprague-Dawley rats (Figs. 2 and 3). The second observation concerns data from female Sprague-Dawley rats that presented a stark difference in the separation of the clustered populations of A-, Ah-, and C-type ABN compared with the separation of the same subpopulations across the general population of VGN (Fig. 3, left). While nonoverlapping (P < 0.05) clusters of the three VGN subpopulations from female Sprague-Dawley rats could not be supported beyond spheres that demarcate 2 SD from the cluster centroid, cluster centroids of the three ABN subpopulations were separated by more than 3 SD (P < 0.01). For these and most other measures of action potential, wave-shape ABN exhibited coefficients of variation that were considerably smaller than the statistical dispersion exhibited by the general population of VGN (Table 1). Presumably this came about, at least in part, because of the functional specificity of the population of ABN compared with the population of VGN of unknown sensory modality.
Fig. 3.
Fiber classification according to cluster analyses of select measures of action potential wave shape (DVAPD50, APFT, and UVAPD50, see Cluster analysis of electrophysiological measures according to sensory modality and sex) and chemosensitivities (Fig. 2) from identified ABN (top) and vagal ganglia neurons (VGN; bottom) of unknown sensory modality. Each sphere demarcates 2 SD from the cluster centroid. Cluster analyses of neurons from female rats showed three statistically distinct (P < 0.05) subpopulations of afferents (A-, Ah-, and C-type), while the same analyses of afferent neurons from male rats could support only two statistically distinct subpopulations (P < 0.05). Interestingly, the three subpopulations of ABN (top, left) from females were separated by more than 3 SD from the centroid of the clusters (P < 0.01). DVAPD50, action potential downstroke velocity at 50% peak displacement; APFT, action potential firing threshold; UVAPD50, action potential upstroke velocity at 50% peak displacement.
Table 2.
Gender difference in morphology of aortic depressor nerve
| Female | Male | |||
|---|---|---|---|---|
| Nerve Cross Section | ||||
| Area, μm2 | 838±358* | 1678±364 | ||
| Diameter, μm | 32.1±7* | 46.0±5 | ||
| Myelinated Fibers | ||||
| Average | 72.8±11* | 103.5±20 | ||
| Total | 437 | 621 | ||
| Myelin area, μm2 | 2.76±0.3* | 3.54±0.6 | ||
| Unmyelinated Fibers | ||||
| Average | 221±36* | 451±60 | ||
| Total | 1324 | 2703 | ||
| Relative distributions, % | ||||
| Myelinated fibers | 24.8 | 18.7 | ||
| Unmyelinated fibers | 75.2 | 81.3 | ||
Data are presented as means ± SD of age-matched adult female (287 ± 11 g, n = 6) and male (330 ± 15 g, n = 6) Sprague-Dawley rats.
P < 0.01 vs. male.
Sexual dimorphism across myelinated and unmyelinated VGN.
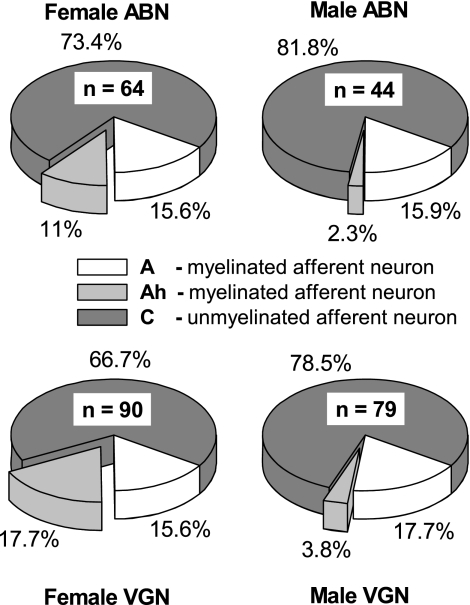
Consistent with the cluster analysis of ABN, a closer examination of the subpopulations demonstrated that myelinated Ah-type afferents were nearly five times more prevalent in female compared with male Sprague-Dawley rats (Fig. 4). Myelinated Ah-type afferents comprised 11% of the total population of ABN from females but only 2.3% from males. Because the percentages of myelinated A-type ABN were comparable for male and female Sprague-Dawley rats, a greater propensity for Ah-type myelinated afferents meant that there were nearly 1.5 times more myelinated afferents across the total population of ABN from female compared with male Sprague-Dawley rats, i.e., myelinated afferents comprised 26.6% of the total population of ABN from female compared with 18.2% in males. This prevalence for greater a percentage of myelinated and, in particular, Ah-type myelinated afferent neurons in female compared with male Sprague-Dawley rats, also extended to the general population of VGN, albeit with a slightly greater incidence of myelinated Ah-type afferents (Fig. 4). Here, one-third of VGN from female rats were myelinated afferents compared with about one-fifth in males (33.3% vs. 21.5%, P < 0.01). Again, this increase was entirely due to the presence of myelinated Ah-type VGN, which again were nearly five times more prevalent in females than in males (17.7% vs. 3.8%, P < 0.01).
Fig. 4.
Distribution summary for ABN (top) and vagal ganglia neurons of unknown sensory modality (VGN, bottom) functionally classified as either myelinated A-type or Ah-type or unmyelinated C-type afferent neurons (see Figs. 2 and 3). Female Sprague-Dawley rats consistently showed a much greater propensity for myelinated Ah-type afferent neurons than males. Across the total population of ABN and VGN in females 27% and 33%, respectively, were myelinated afferents, while in males myelinated afferent neurons comprised only about one-fifth of the total population of ABN and VGN.
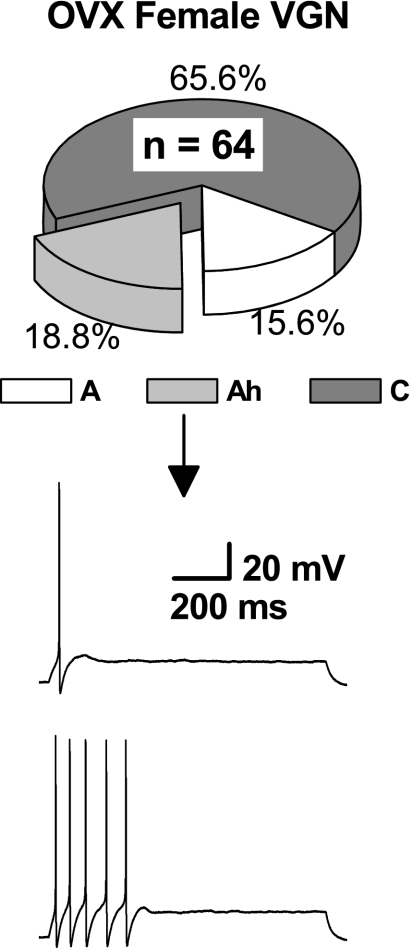
Ovariectomy lessens excitability of myelinated Ah-type vagal neurons but not prevalence.
A cohort of preweanling OVX female Sprague-Dawley rats (n = 4) were supplied by the vendor (Harlan) and allowed to grow in-house until reaching ∼150 g after ∼45 days of age. The bilateral vagal ganglia were prepared for electrophysiological study, and the same methodologies were carried out for classification of each VGN (n = 64) as either a myelinated A-type, Ah-type, or an unmyelinated C-type afferent (see Figs. 2–4). As with the population of female Sprague-Dawley rats of unknown phase in the estrus cycle, myelinated VGN afferent neurons from OVX females represented approximately one-third of the total afferent neuron population, i.e., 34.4% were either myelinated A- or Ah-type VGN. Likewise, Ah-type myelinated afferents from OVX rats comprised slightly more than one-half of the total population of myelinated VGN (Fig. 5). Although Ah-type VGN from OVX continued to exhibit all the markers of myelinated afferents, i.e., αβ-mATP+, CAP−, low APFT, and high UVAPD50, sustained repetitive discharge was no longer observed (Fig. 5, bottom traces). The 300 pA step depolarizations that consistently elicited repetitive discharge from Ah-type afferents in normal females would, at most, evoke a single spike from Ah-type VGN of OVX females, although there were no obvious changes in measures of action potential wave shape, such as APFT. Greater stimulus current intensities evoked only short bursts of action potential discharge that never lasted more than 200–300 ms. Interestingly, no such changes in excitability were observed in the population myelinated A-type and unmyelinated C-type VGN from OVX females.
Fig. 5.
From a cohort of ovariectomized (OVX) female rats, the sample population of VGN (n = 64) continued to reveal three distinct subpopulations of afferents with the distributions of the myelinated A-type and Ah-type and unmyelinated C-type afferent neurons being nearly identical to those from normal female rats (see Figs. 2–4). Waveform traces are from a representative myelinated Ah-type VGN following ovariectomy in response to step-depolarizing currents of 300 pA and 500 pA, respectively. Compare this reduced excitability with that of myelinated Ah-type neurons from normal female rats presented in Fig. 2B.
Morphological differences between aortic depressor nerves of male and female rats.
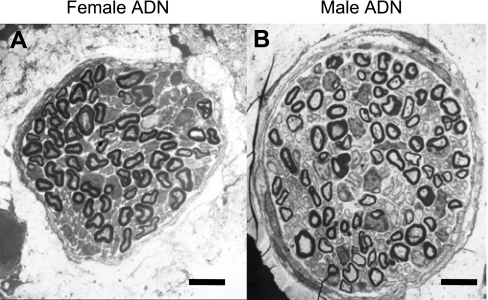
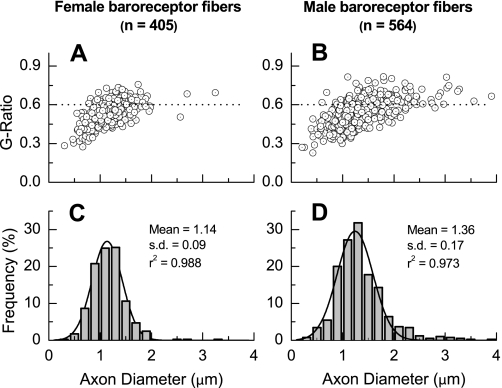
Electron microscopic examination and morphological analysis of aortic depressor nerve from age-matched female (n = 6, 287 ± 11 g) and male (n = 6, 330 ± 15 g) Sprague-Dawley rats revealed a number of neuroanatomical differences (Fig. 6, Table 2). The most visually striking being the significantly smaller aortic depressor nerve diameter from female compared with male rats (32.1 ± 7 μm vs. 46.0 ± 5 μm, P < 0.01). The average numbers of both myelinated and unmyelinated aortic baroreceptor fibers, consistent with a smaller cross-sectional area, were markedly lower in females compared with males, i.e., 72.8 ± 11 vs. 103.5 ± 20 myelinated fibers and 221 ± 36 vs. 450 ± 60 unmyelinated fibers, both P < 0.01. An examination of myelination and axon diameter for baroreceptor fibers from female (n = 405 fibers) and male (n = 564 fibers) rats exhibited a slightly smaller, albeit significant (P < 0.05), average G ratio of 0.52 ± 0.08 vs. 0.54 ± 0.09, respectively (Fig. 7). The distribution frequency of myelinated axon diameters was centered at a significantly lower mean value for females compared with males; 1.14 ± 0.09 μm vs. 1.36 ± 0.17 μm, respectively with P < 0.05. Both distributions of fiber diameters were decidedly normal (r2 > 0.95). Interestingly, the coefficient of variation, i.e., ratio of the SD to the mean, for male axon diameters was nearly 60% larger than that for female axon diameters as evidenced by the spread of the two frequency histograms (Figs. 7, C and D).
Fig. 6.
Electron microscopic image of the cross section of a left aortic depressor nerve from age-matched adult female (A) and male (B) Sprague-Dawley rats. The aortic depressor nerve (ADN) diameter was significantly smaller (P < 0.01) across the population of age-matched female (n = 6) compared with male (n = 6) Sprague-Dawley rats. See (Morphological differences between aortic depressor nerves of male and female rats) for summary and comparison of morphometric measures. Magnification, ×1500; scale bar, 5.0 μm for both images.
Fig. 7.
Scatter diagrams of G ratio as a function of axon diameter (A and B) and distribution frequency (C and D) of myelinated baroreceptor axon diameters from age-matched female (A and C) and male (C and D) Sprague-Dawley rats. Myelinated axon diameters binned in increments of 0.2 μm exhibited normal distributions (black lines represent an automated fit to the fiber diameter distributions with r2 values >0.95 for both graphs.) Axon diameter is the difference between fiber diameter (axon + myelin) and 2× average myelin thickness.
DISCUSSION
In this study, we have shown that across the total population of aortic baroreceptor afferents, female Sprague-Dawley rats (n = 6, Figs. 6 and 7, Table 2) exhibit a significantly greater percentage of myelinated baroreceptor fibers than age-matched males (24.8% vs. 18.7%, P < 0.01). An examination of fluorescently identified ABN (Fig. 1) from age-matched female (n = 64 ABN) and male (n = 44 ABN) Sprague-Dawley rats showed a markedly similar sex bias toward myelinated afferents (26.6% vs. 18.2%, P < 0.01). Electrophysiological and pharmacological examination of all these cells revealed that the myelinated ABN from females was comprised of two functionally distinct populations of A- and Ah-type ABN (Fig. 2), while males essentially exhibited only A-type ABN, i.e., only one of forty-four ABN from males was functionally classified as a myelinated Ah-type (Figs. 3 and 4). Interestingly, A-type ABN presented with nearly the same relative frequency in females and males; 15.6% and 15.9%, respectively, of the total neuron populations. All A-type ABN discharged repetitively at ∼48 Hz in response to 300 pA depolarizing step currents. Between sexes, no statistical difference was found across a broad range of action potential wave shape characteristics and differential sensitivity to αβ-mATP and CAP (Table 1). The Ah-type ABN in females represented 11% of the total neuron population. As with the A-type ABN, this unique subtype of myelinated afferent also discharged repetitively but at a lower frequency of ∼15–20 Hz at 300 pA. Greater magnitude step currents could elicit higher discharge frequencies but never to the extent observed in A-type ABN. Interestingly, Ah-type ABN exhibited many wave shape characteristics typical of unmyelinated C-type ABN, e.g., broad duration action potentials, an inflection along the action potential downstroke and prominent afterhyperpolarization (compare Fig. 2, B and C). However, unlike the low-threshold, repetitively discharging, Ah-type ABN, unmyelinated C-type ABN exhibited very low discharge frequencies of just a few hertz and often responded to step current magnitudes as high as 600 pA with short bursts of action potential discharge. As with the A-type myelinated ABN, there was no statistical difference between the female and male populations of unmyelinated C-type ABN (Table 1). However, on account of a greater percentage of myelinated ABN in females compared with males, the relative population of unmyelinated ABN in females was proportionately less than in males (73.4% vs. 81.8%) as was the case for unmyelinated fibers in the aortic depressor nerve (75.2% vs. 81.3% of the total population of baroreceptor fibers).
Morphological evidence for sex bias toward low-threshold myelinated baroreceptors.
Our morphological data from male Sprague-Dawley rats, such as fiber number, myelinated axon diameter, and G ratio are quite consistent with a detailed quantitative study from Fazan et al. (12a), which also showed that myelinated axons in the aortic depressor nerve of male rats comprise no more than 17–19% of the total fiber population (Table 2, Fig. 7). As the aortic depressor nerve samples from age-matched male and female rats were processed and analyzed in an identical manner, it is unlikely that the morphological differences between aortic depressor nerve of female and male rats are the result of methodological bias. To date, there has been only a single published report quantifying the histological characteristics of the aortic depressor nerve in adult female Sprague-Dawley rats (37). Although this study by Yamasaki et al. (37) centered around the effects of microgravity upon nerve histology, the control group showed morphological characteristics such as fiber number, diameter, and G ratio that were similar to our data, e.g., the control group exhibited percentages of myelinated (∼26%) and unmyelinated (∼74%) fibers similar to our own (Table 2, Fig. 7). In all cases, a distal nerve segment was dissected near the clavicular level, and yet the aortic depressor nerve cross sections from female Sprague-Dawley rats were consistently of smaller diameter and on average contained far fewer total baroreceptor fibers than age-matched males (Figs. 6 and 7, Table 2). Furthermore, it is notable that the population of fluorescently identified ABN (Figs. 1 and 4) from females compared with males exhibited a quantitatively similar bias toward myelinated afferents (26.6% vs. 18.2%). Collectively, these data are highly suggestive of an elevated afferent drive in the aortic depressor nerve of female compared with male rats. It remains to be determined how such an increased afferent baroreceptor drive may impact the efferent autonomic function of the aortic baroreceptor reflex and whether this may present with a sex bias.
Sex bias toward low-threshold myelinated baroreceptor afferents: implications concerning baroreflex function.
In rat, the aortic depressor nerve contains baroreceptor afferent fibers that solely elicit the baroreflex responses of hypotension and respiratory suppression with no functional evidence for chemoreflexive responses (19). To date, the most definitive physiological explanation of the afferent aspects of the aortic baroreflex comes about through classification of baroreceptors as having either myelinated or unmyelinated axons. These two types of baroreceptor fibers have markedly dissimilar pressure-discharge characteristics and frequency-dependent reflex responses (11, 12). Myelinated baroreceptors are generally active at normal arterial pressures and exhibit stable discharge frequencies that robustly correlate with arterial hemodynamics. In stark contrast, unmyelinated baroreceptors generally require more elevated arterial pressures for activation and exhibit irregular discharge frequencies not well correlated with arterial hemodynamics (20).
Regardless of fiber type, the net effect of baroreceptor activation is an elevation of the parasympathetic and inhibition of the sympathetic autonomic pathways for neurocirculatory control. Our electrophysiological data shows that females have a greater percentage of low-threshold myelinated ABN than males. This increase comes almost entirely from the population of myelinated Ah-type afferents that exhibit far lower discharge thresholds and a much greater propensity for repetitive discharge than unmyelinated C-type ABN (Figs. 2–4). Likewise, our morphological data from aortic depressor nerve demonstrates a significant bias toward a greater percentage of myelinated baroreceptor fibers in females compared with age-matched males (Table 2). Although as yet unproven, it is reasonable to speculate from these in vitro data that female Sprague-Dawley rats may have a greater percentage of low-pressure-threshold myelinated aortic baroreceptor functioning in vivo than male Sprague-Dawley rats. This would imply that for comparable arterial pressures, females may have a greater percentage of repetitively discharging aortic depressor nerve fibers driving centrally mediated baroreflex pathways than males. We postulate that such an elevated barosensory drive may account for, at least in part, the observed lower mean arterial pressure and reduced tonic sympathoadrenal activity-related autonomic support of blood pressure in females compared with males (16, 33).
There are a multitude of anatomical, physiological, and biochemical factors that contribute to the well-recognized differences in short- and long-term regulation of arterial blood pressure between premenopausal women and men (7). However, there are compelling data in the literature consistent with a role for elevated baroreceptor drive underlying, at least in part, the sex differences in autonomic control of cardiovascular function. It has been reported that BRS is lower in healthy, premenopausal women compared with healthy, age-matched men (1, 3). Furthermore, parasympathetic markers for heart rate variability are greater and BRS is even further reduced in hypertensive compared with normotensive females, while such changes in hypertensive males did not achieve statistical significance (31). A baroreceptor-mediated elevation in parasympathetic drive and concomitant reduction in activity of sympathetic pathways could be an important factor contributing to the blunted BRS of females compared with males. Likewise, a myelinated baroreceptor-mediated bias toward lower tonic autonomic support of blood pressure could contribute to the lower resting blood pressure levels of premenopausal women (3, 15). It is important to note, however, that some studies have shown similar and even a greater BRS along with a reduced baseline cardiac vagal activity in females compared with males (5, 14, 33). Such observations require an alternative interpretation of our data concerning baroreflex function, perhaps implicating sex-associated differences in baroreceptor resetting as a result of the increased prevalence of myelinated afferent fibers in females.
During elevations in arterial pressure, arterial baroreceptors and the baroreflex reset by way of peripheral and central mechanisms, respectively, such that the set point for pressure control shifts toward the higher arterial pressure (2, 9). Common to both mechanisms is an initial elevation in baroreceptor discharge. In peripheral resetting, the pressure threshold for baroreceptor discharge adapts in a manner that reduces net afferent activity so as to retain the capacity for dynamic encoding of changing arterial pressure (35). Evidence from animal studies indicate that myelinated baroreceptor afferents reset to a greater degree than unmyelinated (30, 34). Our data from female rats would suggest that with peripheral resetting, a greater percentage of low pressure threshold myelinated afferent fibers would still be available to respond to increases in arterial pressure. Such increased capacity may account for observations of increased BRS in females compared with males. In central resetting, adjustments to elevated afferent discharge comes about through neuromodulation of the responsiveness of barosensitive neurons in the central nervous system (9). Our data demonstrating a greater percentage of low-pressure-threshold myelinated baroreceptor fibers and ABN in females therefore may potentially offer an afferent-mediated explanation for those studies indicating greater resetting of the arterial baroreflex in hypertensive females compared with age-matched males (27, 31). More low-threshold myelinated afferent fibers in female rats may also suggest an increased capacity to compensate for both transient and sustained increases in arterial pressure compared with males. Which would be consistent with data from some laboratories suggesting increased BRS in females (5, 14).
Myelinated Ah-type VGN from intact and OVX female rats: physiological implications.
Interestingly, the bias toward a greater percentage of myelinated baroreceptor afferents in females compared with males was present to an even greater extent in a sampled population of VGN of unknown sensory modality (33.3 vs. 21.5%, P < 0.01, see Figs. 3 and 4). While these percentages are likely somewhat influenced by differences in visceral anatomy, the data strongly demonstrate that Ah-type-myelinated VGN are again more prevalent in females compared with males (17.7% vs. 3.8%, P < 0.01, see Figs. 3 and 4). Furthermore, OVX female rats as preweanlings presented myelinated A-type (15.6%) and Ah-type (18.8%) and unmyelinated C-type (65.6%) VGN with nearly the same frequency as the sample population of VGN from female rats of unknown phase of estrus. These data from OVX females suggest there are fundamental neuroanatomical and electrophysiological differences between myelinated vagal afferents of female and male rats. At present, it is unknown whether the aortic depressor nerve in OVX females exhibits any significant morphological changes. The similarity in the distributions of myelinated A-type and Ah-type and unmyelinated C-type VGN between normal and OVX female populations (Figs. 4 and 5) and our inability to discriminate any bimodal distribution in diameter and G ratio of myelinated aortic depressor nerve fibers suggest that any potential neuroanatomical changes may require a more rigorous histological examination. However, a lower G ratio combined with a lower mean axon diameter suggests there exists a greater percentage of myelin per ADN fiber in females than males (Fig. 7). A range of neuroactive steroids, including estrogen, have been shown to impart a protective effect upon peripheral nerve integrity and in particular Schwann cells (22). Therefore, sustained loss of female sex hormones, as a result of OVX, could potentially have an impact on baroreceptor fiber function. Perhaps presenting as a reduction in membrane excitability (Fig. 5) or potentially a reduction in fiber CV. Interestingly, it has been shown that acute injection of 17β-estradiol enhances cardiovascular reflexes and autonomic tone in OVX female rats.(29) While it was unclear whether the effect was peripherally or centrally mediated, further investigation of the potential effects this estrogen hormone receptor agonist may have upon the excitability of Ah-type baroreceptor afferents in OVX females is warranted.
It is well recognized that myelinated mechanoreceptors innervating the heart and other visceral organs exhibit lower thresholds for physiological activation than unmyelinated mechanosensitive afferents (see Ref. 36 for a review). Our experimental observations are consistent with evidence that across both healthy and hypertensive subjects vagal tone is greater in premenopausal females compared with age-matched males (4). Furthermore, OVX female rats exhibit enhanced sympathetic activation and attenuated vagal tone (7), which would be consistent with our observed reduction in excitability of myelinated Ah-type VGN from OVX females (Fig. 5). Interestingly, both fluorescently identified ABN and VGN were in culture media that lacked such hormones for at least 10 to 12 h prior to study, and yet, low stimulus current magnitudes would still evoke repetitive discharge from myelinated Ah-type afferent neurons, albeit at lower frequencies than for myelinated A-type afferent neurons (Fig. 2). Certainly, arterial blood pressure does not abruptly increase with loss of estrogen at the menopause and nonhormonal mechanisms (e.g., diminished aortic distensibility) are also contributing factors in the development of hypertension in women (28). However, the molecular and cellular basis of cardiovascular sex differences in both health and disease span a broad spectrum of genomic and nongenomic mechanisms (26). We contend that, collectively, our electrophysiological and neuroanatomical data are consistent with a hypothesis that elevated baroreceptor and vagal drive via low-threshold myelinated afferent fibers are a contributing factor to the sexual dimorphism in cardiovascular function.
Perspectives and Significance
In the aortic depressor nerve of Sprague-Dawley rats, myelinated baroreceptor afferent fibers are more prevalent in females than in males. In female rats, the neuronal cell bodies that give rise to these myelinated axons are comprised of two functionally distinct afferent phenotypes. Myelinated A-type ABN from male and female rats exhibit discharge characteristics and relative percentages that are quite similar between males and females (Figs. 2–4), whereas myelinated Ah-type ABN are rarely found in males and represent nearly the entirety of the increase in the population of myelinated afferents in females. This sex bias toward lower threshold Ah-type-myelinated afferents also extends to VGN of unknown sensory modality. Loss of sex hormones through OVX does not alter the relative expression of myelinated A-type and Ah-type and unmyelinated C-type VGN but apparently does bring about a reduction in the excitability of myelinated Ah-type VGN without measurably effecting the excitability of the myelinated A-type and unmyelinated C-type afferent VGN populations. Elucidation of the potential ionic channel mechanisms that may contribute to the observed reduction in myelinated Ah-type afferent excitability in OVX females will require further electrophysiological study. Likewise, correlative studies of baroreflex control of heart rate and blood pressure using intact and OVX female rats could help clarify the integrated physiological significance of this unique sex-specific subtype of myelinated baroreceptor afferent.
GRANTS
This work was supported by an American Heart Association Scientist Development Grant 9630277N, a National Heart, Lung, and Blood Institute Grant HL-072012 (to J. H. Schild), and a National Natural Science Foundation of China Award (to G.-F. Qiao and B.-Y. Li).
Acknowledgments
The authors acknowledge the critical review of these data from members of the sensory neuron research group of the Medical Neurosciences Program, Indiana University School of Medicine. The authors also gratefully acknowledge the thorough critiques of the reviewers who markedly improved the writing and scientific balance of our manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beske SD, Alvarez GE, Ballard TP, Davy KP. Gender difference in cardiovagal baroreflex gain in humans. J Appl Physiol 91: 2088–2092, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Chapleau MW, Hajduczok G, Abboud FM. Mechanisms of resetting of arterial baroreceptors: an overview. Am J Med Sci 295: 327–334, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation 111: 494–498, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Conte MR Gender differences in the neurohumoral control of the cardiovascular system. Ital Heart J 4: 367–370, 2003. [PubMed] [Google Scholar]
- 5.Convertino VA Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol Regul Integr Comp Physiol 275: R1909–R1920, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Curtis BM, O'Keefe JH Jr. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 77: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Dart AM, Du XJ, Kingwell BA. Gender, sex hormones and autonomic nervous control of the cardiovascular system. Cardiovasc Res 53: 678–687, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Denton K, Baylis C. Physiological and molecular mechanisms governing sexual dimorphism of kidney, cardiac, and vascular function. Am J Physiol Regul Integr Comp Physiol 292: R697–R699, 2007. [DOI] [PubMed] [Google Scholar]
- 9.DiCarlo SE, Bishop VS. Central baroreflex resetting as a means of increasing and decreasing sympathetic outflow and arterial pressure. Ann NY Acad Sci 940: 324–337, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods 137: 37–48, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632–H640, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol Regul Integr Comp Physiol 277: R748–R756, 1999. [DOI] [PubMed] [Google Scholar]
- 12a.Fazan VP, Salgado HC, Barreira AA. A descriptive and quantitative light and electron microscopy study of the aortic depressor nerve in normotensive rats. Hypertension 30: 693–698, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Frenneaux MP Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart 90: 1248–1255, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol 26: 122–126, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Hogarth AJ, Burns J, Mackintosh AF, Mary DA. Sympathetic nerve hyperactivity of essential hypertension is lower in postmenopausal women than men. J Hum Hypertens 2008. [DOI] [PubMed]
- 16.Hogarth AJ, Mackintosh AF, Mary DA. Gender-related differences in the sympathetic vasoconstrictor drive of normal subjects. Clin Sci (Lond) 112: 353–361, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Huxley VH Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ 31: 17–22, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Cheng ZB, Tanaka K, Nosaka S. Is the aortic depressor nerve involved in arterial chemoreflexes in rats? J Auton Nerv Syst 78: 38–48, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Kunze DL, Andresen MC. Arterial baroreceptors: excitation and modulation. In: Reflex Control of the Circulation, edited by Zucker IH and Gilmore JP. Boca Raton, FL: CRC, 1991, p. 141–166.
- 21.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Leonelli E, Ballabio M, Consoli A, Roglio I, Magnaghi V, Melcangi RC. Neuroactive steroids: a therapeutic approach to maintain peripheral nerve integrity during neurodegenerative events. J Mol Neurosci 28: 65–76, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rat. J Neurosci Methods 115: 157–167, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Li BY, Schild JH. Electrophysiological and pharmacological validation of vagal afferent fiber type of neurons enzymatically isolated from rat nodose ganglia. J Neurosci Methods 164: 75–85, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McBride SM, Flynn FW, Ren J. Cardiovascular alteration and treatment of hypertension: do men and women differ? Endocrine 28: 199–207, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 308: 1583–1587, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Peckerman A, Hurwitz BE, Nagel JH, Leitten C, Agatston AS, Schneiderman N. Effects of gender and age on the cardiac baroreceptor reflex in hypertension. Clin Exp Hypertens 23: 645–656, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Safar ME, Smulyan H. Hypertension in women. Am J Hypertens 17: 82–87, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17β-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci 84: 78–88, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Seagard JL, Gallenberg LA, Hopp FA, Dean C. Acute resetting in two functionally different types of carotid baroreceptors. Circ Res 70: 559–565, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Sevre K, Lefrandt JD, Nordby G, Os I, Mulder M, Gans RO, Rostrup M, Smit AJ. Autonomic function in hypertensive and normotensive subjects: the importance of gender. Hypertension 37: 1351–1356, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 47, Suppl 3: S4–S20, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 45: 1159–1164, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Thoren P, Andresen MC, Brown AM. Resetting of aortic baroreceptors with non-myelinated afferent fibers in spontaneously hypertensive rats. Acta Physiol Scand 117: 91–97, 1983. [DOI] [PubMed] [Google Scholar]
- 35.Thrasher TN Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol 288: R819–R827, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Undem BJ, Weinreich D. Advances in Vagal Afferent Neurobiology. Boca Raton, FL: CRC, 2005.
- 37.Yamasaki M, Shimizu T, Miyake M, Miyamoto Y, Katsuda S, Ishi H, Nagayama T, Waki H, Katahira K, Wago H, Okouchi T, Nagaoka S, Mukai C. Effects of space flight on the histological characteristics of the aortic depressor nerve in the adult rat: electron microscopic analysis. Biol Sci Space 18: 45–51, 2004. [DOI] [PubMed] [Google Scholar]