Abstract
Tachykinin neurokinin 3 receptor (NK3R) signaling has a broad role in vasopressin (VP) and oxytocin (OT) release. Hydralazine (HDZ)-induced hypotension activates NK3R expressed by magnocellular neurons, increases plasma VP and OT levels, and induces c-Fos expression in VP and OT neurons. Intraventricular pretreatment with the specific NK3R antagonist, SB-222200, eliminates the HDZ-stimulated VP and OT release. NK3R are distributed in the central pathways conveying hypotension information to the magnocellular neurons, and the NK3R antagonist could act anywhere in the pathways. Alternatively, the antagonist could act at the NK3R expressed by the magnocellular neurons. To determine whether blockade of NK3R on magnocellular neurons impairs VP and OT release to HDZ, rats were pretreated with a unilateral PVN injection of 0.15 M NaCl or SB-222200 prior to an intravenous injection of 0.15 M NaCl or HDZ. Blood samples were taken, and brains were processed for VP/c-Fos and OT/c-Fos immunohistochemistry. Intravenous injection of 0.15 M NaCl did not alter plasma hormone levels, and little c-Fos immunoreactivity was present in the PVN. Conversely, intravenous injection of HDZ increased plasma VP and OT levels and c-Fos expression in VP and OT magnocellular neurons. Intra-PVN injection of SB-222200 prior to an intravenous injection of HDZ significantly decreased c-Fos expression in both VP and OT neurons by ∼70% and attenuated plasma VP and OT levels by 33% and 35%, respectively. Therefore, NK3R signaling in magnocellular neurons has a critical role for the release of VP and OT in response to hypotension.
Keywords: tachykinin, neuroendocrine, neurokinin
the tachykinin receptor neurokinin 3 receptor (NK3R) has a broad role in the systemic vasopressin (VP) and oxytocin (OT) release from the paraventricular (PVN) and supraoptic (SON) nuclei in the hypothalamus. Recent studies show that intraventricular injection of the specific NK3R antagonist, SB-222200 (19, 52), decreases the systemic release of VP and OT in response to hypotension, hyperosmolarity (21), and peripheral CCK injections (20). NK3Rs are expressed in nuclei that innervate the magnocellular neurons (42, 57) and are expressed by VP (12, 19) and OT (17) magnocellular neurons. Intraventricular injections of the specific NK3R agonist, senktide, increase c-Fos expression in the magnocellular neurons of the PVN and SON (13) and elicit VP and OT secretion into the circulation (4, 19). Furthermore, like other G protein-coupled receptors, NK3R expressed on magnocellular neurons are internalized into the cytoplasm of the cells following the binding of a ligand, indicating receptor activation. Senktide injection activates the NK3R on magnocellular neurons (19). The senktide-induced VP release and NK3R internalization on magnocellular neurons are blocked by pretreatment of the specific NK3R antagonist SB-222200 (19).
The same pattern of c-Fos expression, VP and OT secretion, and NK3R internalization that occurs following senktide is also observed following hydralazine (HDZ)-induced hypotension (29, 46, 53). Intraventricular injection of SB-222200 eliminates systemic release of VP and OT in response to HDZ (21), which can block NK3R signaling in the central pathway relaying HDZ information to the magnocellular neurons. Blood pressure and volume information are relayed to the magnocellular neurons through the A1 and A2 cell groups (7) in the brain stem, where NK3R are expressed (8). However, several lines of evidence suggest the NK3Rs expressed on magnocellular neurons are the site of NK3R antagonist action. First, NK3Rs are heavily expressed on magnocellular neurosecretory neurons, and the NK3R antagonist could decrease VP and OT release by acting on the neurons that synthesize the hormones (12, 42, 57). Other studies indicate that intraventricular injection of an NK3R agonist acts in the PVN, as the intra-PVN injection of the NK3R agonist stimulates a greater VP release compared with intraventricular injection (40, 48). Furthermore, NK3Rs are activated, as visualized by receptor internalization, in magnocellular neurons following hypotension (29). Together, these findings suggest that intraventricular injections of SB-222200 act at the magnocellular neurons to block VP and OT response to physiological stimuli.
To test the hypothesis that the magnocellular neurons are the site of intraventricular NK3R antagonist action, a unilateral PVN injection of the specific NK3R antagonist was administered prior to an intravenous injection of HDZ. In this study, blood samples were taken and brains were processed for VP/c-Fos and OT/c-Fos immunoreactivity following an intra-PVN injection of 0.15 M NaCl or SB-222200 and an intravenous injection of 0.15 M NaCl or HDZ. Immunohistochemically labeling the c-Fos protein is an anatomical technique used to study neuronal activation via synaptic stimulation from a given stimulus (10, 11, 15, 28, 43, 56). Results from this study demonstrate that NK3R blockade in the PVN attenuates VP and OT release and decreases the c-Fos expression in magnocellular neurons following HDZ treatment.
METHODS
Animals.
All procedures were approved by the Animal Care and Use Committee at the University of Wyoming. Rats (male, 300–400 g, n = 22; Charles River Laboratory, Wilmington, MA, USA) were housed individually in standard wire mesh cages in a temperature-controlled room with a 12:12-h light-dark cycle and ad libitum access to Purina chow and water.
PVN cannula surgery.
Rats (n = 22) were anesthetized with a mixture of ketamine and acepromazine (0.07 mg/kg ip) and mounted in a stereotaxic device. A hole was drilled in the skull 1.6 mm posterior to bregma, 0.3 mm lateral to the midline, and a cannula (32 gauge; Plastics One, Roanoke, VA) was lowered 7.5 mm ventral to the skull. The cannula was secured in place with four jewelry screws and dental acrylic. All cannulas were positioned on the right side of the brain, ∼50–100 μm dorsal and lateral to the lateral magnocellular division of the PVN. This placement minimized damage to the nucleus and targeted the magnocellular neurons. Unilateral injection allowed use of the contralateral PVN as a control within each animal. Rats recovered for 24 h with ad libitum access to food and water.
Analysis of the spread of injection.
The spread of the injection was estimated by injecting 15% DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; Invitrogen Molecular Probes, Eugene OR) into the PVN cannula (n = 6). Rats fitted with an intra-PVN cannula were administered a lethal dose of ketamine and acepromazine mixture and transcardially perfused with 300 ml of cold PBS followed immediately by 300 ml of 4% paraformaldehyde. Before extraction from the skull, 250 nl of DiI was injected into the brain through the PVN cannula. Brains were postfixed in 4% paraformaldehyde for 2 h at 4°C and transferred to a 30% sucrose in PBS solution for 24 h at 4°C. Brains were blocked for the PVN, frozen in a cryostat, sectioned at 40 μm, and mounted directly onto slides. Sections were immediately visualized under the microscope.
DiI tissue sections were visualized using a Bio-Rad (Hercules, CA) confocal microscope interfaced with Laser Sharp 2000 software. Images were acquired through the PVN, rostral to caudal to determine the spread of the DiI injection. DiI labeling in single Z-section images was acquired with the 543 laser at 10× magnification.
Catheter surgery.
The day following the PVN cannula surgery, rats were reanesthetized and implanted with femoral arterial and venous catheters constructed from polyethylene (PE) tubing. Arterial catheters were constructed from 3 cm of PE-10 tubing attached to 20 cm of PE-50 tubing. Venous catheters were 25 cm in length, constructed of PE-50 tubing. One, 2-inch hind limb skin incision was made, and the femoral artery and vein were exposed using blunt dissection. Catheters were inserted into position through punctures in the vein and artery and were secured by sutures around the blood vessels. Once secured, the tubing was filled with heparinized saline (50 units/ml), and the distal ends were drawn subcutaneously to an exit wound between the scapulae and were heat sealed until testing. Rats were returned to their cages to recover for 24 to 48 h with ad libitum access to food and water.
Specificity of SB-222200.
The compound SB-222200 is specific for the NK3R, as determined by radioligand binding studies (52). Furthermore, pharmacological evidence demonstrates that SB-222200 blocks NK3R agonist actions in vitro and in vivo. Administration of SB-222200 before 125I[MePhe7] neurokinin B (NKB) blocks binding to NK3R and NKB-induced Ca2+ mobilization in three different types of cell lines (32, 52). Similarly, SB-222200 blocks senktide-induced responses in vivo. Pretreatment of SB-222200 blocks the senktide-induced NK3R receptor internalization, VP release (19), and behavioral responses (52). Moreover, SB-222200 does not block the actions of Substance P, a peptide from the tachykinin family (29). In a previous study, the 500-pmol dose of SB-222200 eliminated VP and OT release in response to HDZ (21) and, thus was chosen as the best dose of SB-222200 for this study.
Procedure.
Rats (n = 16) were acclimated to heparinized-filled syringes connected to lines of PE tubing attached to the catheters in a quiet room, as described by previous studies (20, 21). A 33-gauge injector was inserted into the cannula and connected to a 1-μl glass microsyringe with PE-50 tubing. Rats were left alone in the quiet room for 15–20 min. Once rats were acclimated, 250 nl of 500 pmol of SB-222200 (Courtesy of GlaxoSmithKline; n = 8) or 0.15 M NaCl (n = 8) was administered into the PVN. Half of the rats from each intra-PVN pretreatment group were randomly assigned to one of the intravenous groups, and each rat was only used once. Five minutes following the intra-PVN injection, rats were administered a bolus intravenous injection of 10 mg/kg of HDZ (n = 8) or equal volume of 0.15 M NaCl (n = 8).
Blood samples with replacement were taken prior to the intra-PVN injection (time 0), 30 min after the intravenous injection (time 30), and 60 min after the intravenous injection (time 60). Blood sampling procedures were identical to those used in earlier studies (19–21). Briefly, 2 ml of whole blood was taken from the arterial catheter, while a simultaneous infusion of an equal amount of donor blood was infused into the venous catheter. Donor blood, kept at 37°C, was collected earlier from an ether-anesthetized rat through a cardiac draw (1, 2, 9). Whole blood samples were spun in a refrigerated centrifuge, and plasma (1 ml/sample) was aspirated into a clean Eppendorf tube. Plasma was stored at −80°C until extraction.
Blood pressure.
One rat from each group was randomly chosen, and the arterial line was connected to a blood pressure transducer which interfaced a computer with blood pressure monitoring software (PowerLab; ADInstruments; Colorado Springs, CO). The venous line and cannula injector were attached as stated above. Rats were acclimated to the catheters, and mean arterial pressure (MAP) was continually measured through the experiment until the animal was killed (time 90).
VP and OT ELISA assay.
Hormones were extracted from plasma samples using a C-18 column extraction method. Briefly, a 1% trifluoroacetic acid (TFA) solution was used to acidify the sample, which was then filtered through an equilibrated C-18-OH column. Columns were washed with the 1% TFA solution, and proteins were eluted with a 60% acetonitrile and 1% TFA solution in dH2O. Samples were spun in a vaccufuge until a small pellet remained and stored at −80°C. The pellet was reconstituted in assay buffer 24 h prior to assay. Plasma samples were analyzed using VP and OT ELISA kits (Assay Designs, Ann Arbor, MI). Specificity of the ELISA kits was determined by applying VP standards to the OT plate and vice versa. Neither the VP nor OT ELISA plate recognized the standards from the other kit. Sensitivity of the VP ELISA kit was 1.1 pg/ml, while that of the OT ELISA kit was 5.5 pg/ml. Intra-aasay and interassay precision were determined with calculated variants, and in both assays the values were less than 4%. Extraction efficiency was ∼88% for both VP and OT. Plasma data are presented as means ± SE.
Tissue analysis.
Once the blood sampling was finished, catheters were heat sealed, and rats remained in the quiet room. Rats were administered a lethal dose (1 ml) of the ketamine/acepromazine mixture 90 min after the intravenous injection. Transcription of the c-Fos protein peaks ∼90 min after an acute stimulus (23), and previous studies have demonstrated a 10 mg/kg dose of HDZ induces c-Fos expression in the magnocellular neurons (18, 53).
Rats were transcardially perfused with 300 ml of ice-cold PBS (7.4 H; 0.1 M) followed immediately by 300 ml of 4% paraformaldehyde. Brains were removed from the skull and postfixed in 4% paraformaldehyde for 2 h. The side of the brain, in which the cannula was placed was marked with a notch in the cortex to preserve orientation of brain sections during processing. Brains were cryoprotected in a 30% sucrose in PBS solution and stored overnight. Prior to sectioning, brains were blocked for the PVN area and frozen in a cryostat. Once frozen, three serial sets of 40-μm coronal sections were cut through the PVN. One set was mounted directly to slides and stained with 0.5% cresyl violet and coverslipped. The other two sets of free-floating sections were washed 3 times in fresh PBS, one set for c-Fos/VP immunohistochemistry and one for c-Fos/OT immunohistochemistry. Sections were incubated in a 3% hydrogen peroxide solution for 20 min. After the incubation period, the sections were washed 3 times in fresh PBS. A 10% goat serum blocking solution was added to each well, and sections were incubated for 1 h. The c-Fos antibody (raised in rabbit; 0.01 μg/ml; Santa Cruz Laboratories, Santa Cruz, CA) and VP antibody (raised in guinea pig, 1 μg/ml; Peninsula Laboratories, Belmont, CA) or OT antibody (raised in guinea pig, 1 μg/ml; Peninsula Laboratories) were added to the wells, and sections were incubated for 48 h at 4°C.
Following primary antibody incubation, sections were initially processed for diaminobenzidene (DAB) labeling (c-Fos antibody) and then fluorescence (VP or OT antibodies). The sections were washed 3 times in fresh PBS and incubated in a biotinylated secondary antibody (anti-rabbit; 1 μg/ml; Jackson Laboratories) for 1 h. Sections were washed 3 times in fresh PBS and then incubated in the Avidin-Biotin complex (Vector Laboratories, Burlingame, CA), prepared 30 min prior to addition to the wells, for 1 h. The tissue was rinsed 3 times in fresh PBS and developed with Fast DAB (Sigma-Aldrich, St. Louis, MO) for 2 min. Once the detection of c-Fos was completed, the sections were rinsed 3 times in fresh PBS and then incubated in a fluorescent secondary antibody (VP, anti-guinea pig TRITC, 15 μg/ml and OT, anti-guinea pig FITC, 15 μg/ml, both from Jackson Laboratories) for 2 h. Sections were washed 3 times in PBS, mounted to slides, dehydrated, and coverslipped with DPX Mountant (Sigma-Aldrich) for viewing. For controls, primary antibodies were preadsorbed with the primary antibody antigen and processed as stated above. Tissue with the preadsorbed antibody did not show any immunohistochemical labeling, indicating antibody specificity.
Quantification and data analysis.
Tissue sections were visualized using a Bio-Rad Confocal Microscope with Laser Sharp 2000 software. Single Z-section images from the magnocellular PVN using the 488 laser (OT), 543 laser (VP), and transmitted light (c-Fos) were acquired at ×40 magnification. The size of each image was 300 μm × 300 μm. Twelve images were acquired from each brain of both VP/c-Fos and OT/c-Fos immunohistochemically labeled sets. Magnocellular neurons were identified by comparing fluorescent sections to the cresyl violet sections and correlating photomicrographs from Paxinos and Watson's Rat Brain Atlas (plates 24, 25, and 26) (45a). Images were acquired from the injected and contralateral PVN, both the magnocellular and parvocellular divisions, in a 1-in-3 series of 40 μm. The images from each brain were taken from similar sites in the PVN to establish a representative set of VP and OT neurons within the PVN. By superimposing the red or green image with the transmitted light image, the number of VP or OT neurons that expressed c-Fos was counted. From the images, total number of VP or OT neurons was counted and compared with VP or OT neurons expressing c-Fos. Data presented as a percentage of VP or OT neurons expressing c-Fos.
c-Fos immunoreactivity was also compared between injected and contralateral magnocellular and parvocellular neurons of the PVN between the different experimental groups to determine whether NK3R drives c-Fos expression in parvocellular neurons. Tissue sections were visualized using a bright-field Olympus microscope interfaced with a digital camera and Olympus Microsuite software. At least 8 light images from each side of the PVN were taken from each brain. From the images, two areas of interest were identified for quantification, the parvocellular and magnocellular neurons of the PVN. To identify the two neuron populations, photomicrographs depicted in Paxinos and Watson's Rat Brain Atlas (3, 16, 18, 45a, 47) were used in conjunction with sections stained with cresyl violet, a technique to distinguish magnocellular neurons and parvocellular neurons within the nucleus based on neuronal size (25–27, 54). Magnocellular neurons and parvocellular neurons were mapped on the cresyl violet-stained sections and superimposed on the immunohistochemical labeled sections. Positive c-Fos immunoreactive nuclei were between 10 and 25 μm in diameter, round, and greater intensity than that of background staining (22, 59). Data are presented as the percentage of c-Fos immunoreactivity within parvocellular or magnocellular neurons of the injected side of the PVN compared with same area on the noninjected side of the PVN. These percentages were compared between the different treatment groups, which received an intra-PVN injection of 0.15 M NaCl or SB-222200, followed by intravenous injection of 0.15 M NaCl or HDZ. Images were also taken from the SON, ipsilateral and contralateral to the injection site.
Data analysis.
Data were analyzed with SPSS 13.0. Plasma VP and OT levels were analyzed with a three-factor ANOVA with repeated measures (intra-PVN injection × intravenous treatment × time [repeated measures]). Significant main effects and interactions were further analyzed with one-way ANOVAs, and Student's t-test was used for specific comparisons with critical values from Bonferroni table for multiple comparisons, and P < 0.05 was considered significant. Results are presented as group means ± SE. c-Fos expression data were analyzed with a two-way ANOVA (intra-PVN injection × intravenous injection) and P < 0.05 was required for significance. Data are presented as means ± SE.
RESULTS
Spread of PVN injection.
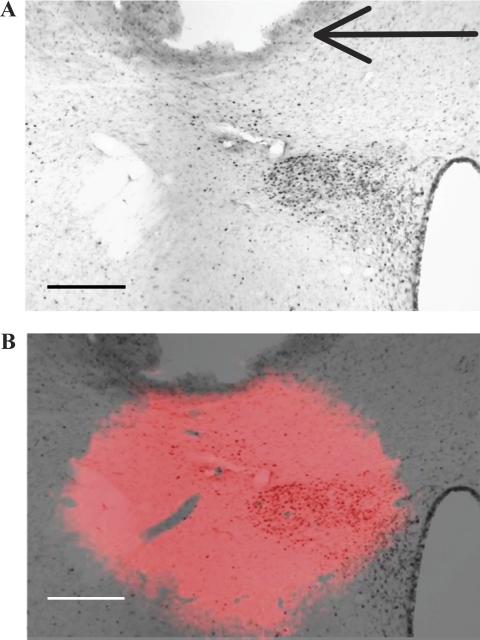
Histological analyses showed that the PVN cannulas were consistently placed dorsal and lateral to the magnocellular neurons of the PVN, with the injector 50–100 μm dorsal to the dorsal cap of the nucleus. DiI analyses suggest that the spread of the intra-PVN injection was limited to the PVN, zona incerta, posterior lateral bed nucleus of the stria terminalis, and the subparaventricular zone of the hypothalamus, without spreading into the third ventricle (Fig. 1). DiI spread was 0.75 mm lateral, 0.4 mm rostral or caudal, and 0.6 mm ventral.
Fig. 1.
Paraventricular nucleus (PVN) cannula placement and injection spread. A: photomicrograph from a rat treated with an intra-PVN injection of 0.15 M NaCl and intravenous injection of hydralazine (HDZ), in which the brain section is immunohistochemically labeled for c-Fos protein. Following HDZ, c-Fos is expressed in the PVN. The black arrow indicates the tip of the cannula. Injection site was dorsal and lateral to the magnocellular neurons of the PVN and induced little c-Fos expression around the cannula tract. B: composite image of the overlay of the fluorescent image of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) injection spread at the same coronal plane, Bregma −1.8 mm, as A. The spread of the intra-PVN injection is observed after aligning the cannula placement in the DiI image and c-Fos image. Scale bars are 175 μm.
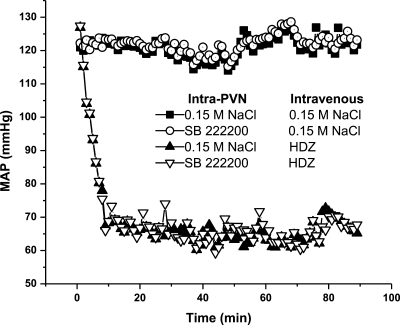
Blood pressure.
Blood pressure remained constant following intravenous injections of 0.15 M NaCl in rats administered intra-PVN injections of 0.15 M NaCl or SB-222200. Intravenous injections of 10 mg/kg HDZ in rats pretreated with intra-PVN injections of 0.15 M NaCl or SB-222200 caused blood pressure to decrease from 120 mmHg (basal) to 66 mmHg within 10 min of injection and remained at ∼66 mmHg until the animal was killed 90 min later. The effects of HDZ on blood pressure are similar to other reports (21, 29). As such, intra-PVN injection of SB-222200 did not alter the change in blood pressure from HDZ treatment (Fig. 2).
Fig. 2.
HDZ decreases mean arterial pressure (MAP), which is not altered by intra-PVN injection. MAP following intra-PVN injection of 0.15 M NaCl or SB-222200 and intravenous injection of 0.15 M NaCl or HDZ. Time 0 indicates the intravenous injection.
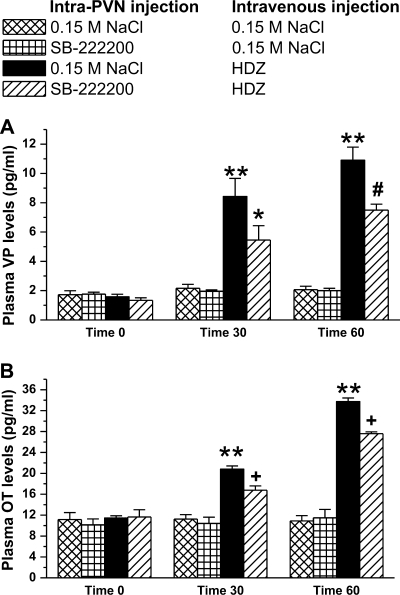
Plasma VP levels.
Following intra-PVN injection of 0.15 M NaCl and intravenous injection of 0.15 M NaCl (baseline), plasma VP levels were not significantly different between treatment groups [F (6, 24) = 16.9, P > 0.4, (Fig. 3)]. Plasma VP levels of rats treated with an intravenous injection of 0.15 M NaCl did not change with an intra-PVN pretreatment of 0.15 M NaCl or SB-222200. Intravenous injection of 10 mg/kg of HDZ significantly increased plasma VP levels from 1.6 ± 0.2 pg/ml at time 0 to 8.4 ± 1.2 pg/ml at time 30 and to 10.9 ± 0.9 pg/ml at time 60 (P < 0.001). However, intra-PVN injection of SB-222200 attenuated VP release in response to HDZ by 33%. Intravenous injection of HDZ following intra-PVN injection of 500 pmol of SB-222200 increased plasma VP levels from 1.3 ± 0.2 pg/ml at time 0 to only 5.5 ± 0.9 pg/ml at time 30. Plasma VP levels from rats treated with intra-PVN injection of SB-2222200 and intravenous injection of HDZ were significantly higher than baseline levels at time 60, 7.5 ± 0.4 pg/ml (P < 0.01), and significantly less than plasma VP levels of rats treated with 0.15 M NaCl intra-PVN injections and intravenous injection of HDZ (P < 0.003).
Fig. 3.
Intra-PVN injection of SB-222200 attenuates vasopressin (VP) and oxytocin (OT) release in response to HDZ. Plasma VP levels (A) and plasma OT levels (B) following an intra-PVN injection of either 0.15 M NaCl or 500 pmol of SB-222200 and an intravenous injection of 0.15 M NaCl or HDZ. Intravenous injection of HDZ significantly increased plasma VP and OT levels, and pretreatment of SB-222200 attenuated VP and OT release. (**P < 0.001 compared with baseline VP or OT levels; +P < 0.003, *P < 0.01, #P < 0.04 compared with 0.15 M NaCl intra-PVN injection and intravenous injection of HDZ).
Plasma OT levels.
Baseline plasma OT levels were not significantly different between treatment groups [F (6, 24) = 47.5, P > 0.7; see Fig. 3]. There was no change in plasma OT levels in rats treated with an intravenous injection of 0.15 M NaCl with either intra-PVN injection. A significant increase in plasma OT levels was measured after intravenous injection of HDZ. Plasma OT levels were 11.4 ± 0.4 pg/ml at time 0, 20.8 ± 0.6 pg/ml at time 30, and 33.7 ± 0.7 pg/ml at time 60 (P < 0.001). Intra-PVN injection of SB-222200 attenuated OT release in response to HDZ by 35%. Intravenous injection of HDZ following intra-PVN injection of SB-222200 increased plasma OT levels from baseline, 11.6 ± 1.4 pg/ml at time 0 to only 16.8 ± 0.8 pg/ml at time 30 and only 27.6 ± 0.4 pg/ml at time 60 (P < 0.001). Plasma OT levels following intra-PVN injection of SB-222200 and intravenous injection of HDZ were significantly less than plasma OT levels after intra-PVN injection of 0.15 M NaCl and intravenous injection of HDZ at time 30 and time 60 (P < 0.04).
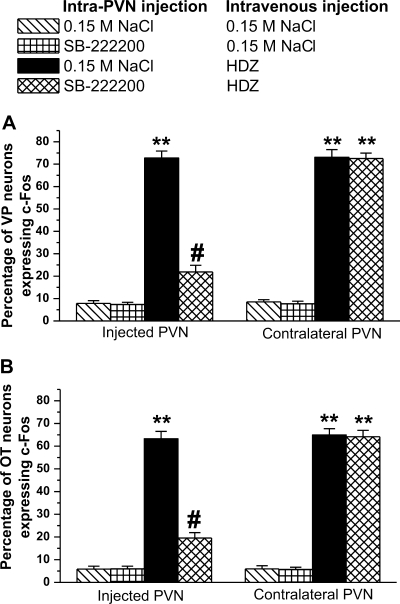
VP and c-Fos Histology.
Following intravenous injection of 0.15 M NaCl, little c-Fos expression was found within the PVN. Only 10 ± 5 of 129 ± 18 VP-positive cells expressed c-Fos in magnocellular VP neurons. Following intravenous injection of 0.15 M NaCl, 7.7% ± 1.1% of VP neurons expressed c-Fos, regardless of the type of intra-PVN injection. No difference in c-Fos expression in VP neurons was observed between injected and contralateral PVN (Figs. 4 and 5). Following intravenous injection of HDZ, 88 ± 8 of 121 ± 10 VP neurons expressed c-Fos. The percentage of VP neurons expressing c-Fos was 72.7% ± 3.1% (P < 0.001, Figs. 4 and 6) on both injected and contralateral PVN. However, intra-PVN injection of 500 pmol of SB-222200 prior to intravenous HDZ significantly decreased the number of VP neurons expressing c-Fos by 71% (P < 0.001). Following intra-PVN injection of SB-222200 and intravenous injection of HDZ, only 27 ± 9 of 123 ± 8 VP-positive cells had c-Fos-positive nuclei in the injected PVN (Figs. 4 and 6). The contralateral side of the SB-222200 intra-PVN injection following intravenous HDZ was not significantly different from the intra-PVN injection of 0.15 M NaCl and intravenous injection of HDZ.
Fig. 4.
Intra-PVN injection of SB-222200 decreases c-Fos expression in VP and OT magnocellular neurons. Percent of VP (A) or OT (B) neurons expressing c-Fos in the injected and contralateral PVN following intra-PVN injection of 0.15 M NaCl or SB-222200 and intravenous injection of 0.15 M NaCl or HDZ. HDZ increased c-Fos expression in VP and OT neurons. Pretreatment of SB-222200 decreased c-Fos expression in VP and OT neurons. (**P < 0.001, compared with intravenous injection of 0.15 M NaCl; #P < 0.001, compared with intra-PVN injection of 0.15 M NaCl and intravenous injection of HDZ).
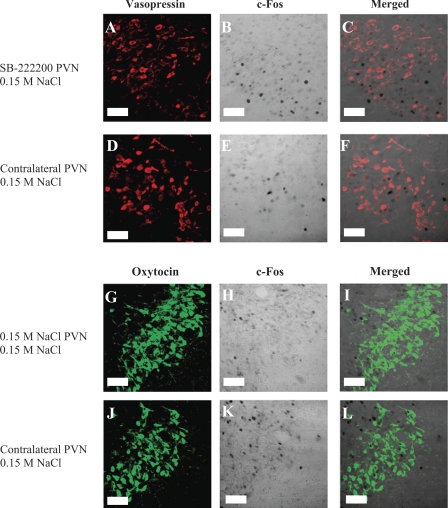
Fig. 5.
Intra-PVN injections did not alter c-Fos expression in magnocellular neurons following intravenous injection of 0.15 M NaCl. Left: VP immunoreactivity (red; A and D) and OT immunoreactivity (green; G and J). Middle: c-Fos immunoreactivity (transmitted light; B, E, H, and K). Right: composite images of the VP/OT and c-Fos panels (C, F, I, and L). A–F: rat treated with an intra-PVN injection of SB-222200 and intravenous injection of 0.15 M NaCl. G–L: rat treated with an intra-PVN injection of 0.15 M NaCl and an intravenous injection of 0.15 M NaCl. Images correspond to plate 25 in Paxinos and Watson Rat Brain Atlas (45a). Scale bars are 50 μm.
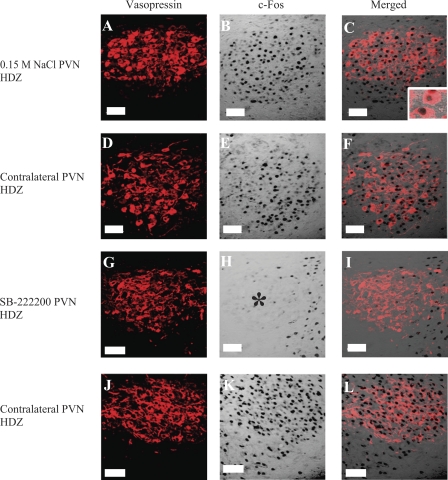
Fig. 6.
Intra-PVN injection of SB-222200 decreases c-Fos expression in magnocellular VP neurons in the PVN following HDZ injection. Left: VP immunoreactivity (red). Middle: c-Fos immunoreactivity (transmitted light). Right: composite images of the VP and c-Fos panels. A–F: images from a rat treated with an intra-PVN injection of 0.15 M NaCl and an intravenous injection of HDZ, whereas panels G–L are from a rat treated with an intra-PVN injection of SB-222200 and an intravenous injection of HDZ. C: higher magnification of VP neurons, one of which expresses c-Fos. H: asterisk indicates decrease in c-Fos expression in response to HDZ due to intra-PVN injection of SB-222200. Images correspond to plate 25 in Paxinos and Watson Rat Brain Atlas (45a). Scale bars are 50 μm.
OT and c-Fos histology.
Little c-Fos expression in magnocellular OT neurons was identified in the PVN following intravenous injection of 0.15 M NaCl. Of the 136 ± 19 OT neurons, only 8 ± 6 positive c-Fos nuclei were identified. The percentage of OT neurons expressing c-Fos following intravenous injection of 0.15 M NaCl, 5.8% ± 1.2% was not different between injected and contralateral PVN (Figs. 4 and 7). Intravenous injection of HDZ following intra-PVN pretreatment of 0.15 M NaCl significantly increased the percentage of OT neurons expressing c-Fos to 63.3% ± 3.2%. A total of 95 ± 7 positive c-Fos nuclei in OT neurons were counted among 150 ± 20 OT neurons (P < 0.001; Fig. 4 and 7). No difference in c-Fos expression was observed between the injected side and the noninjected side of the PVN (P = 1.0). However, intra-PVN injection of SB-222200 significantly decreased the percentage of OT neurons expressing c-Fos by 69%. In the SB-222200 injected side, only 29 ± 8 c-Fos-positive nuclei were identified in 148 ± 15 OT neurons (Fig. 5). The contralateral side of the PVN was not significantly different from the intra-PVN injection of 0.15 M NaCl following HDZ. Intravenous injection of HDZ also induced c-Fos expression in 80% of parvocellular OT neurons. Of 11 ± 4 parvocellular OT neurons, 9 ± 3 neurons had c-Fos-positive nuclei. Intra-PVN injection of SB-222200 decreased the number of c-Fos-positive nuclei in OT neurons, and only 4 ± 2 neurons had c-Fos-positive nuclei out of 9 ± 4 parvocellular OT neurons.
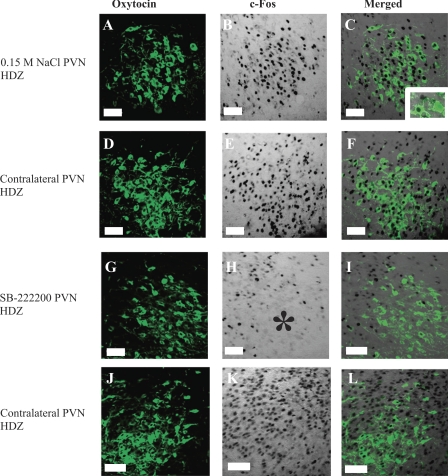
Fig. 7.
Intra-PVN injection of SB-222200 decreases c-Fos expression in magnocellular OT neurons in the PVN following HDZ injection. Left: OT immunoreactivity (green). Middle: c-Fos immunoreactivity (transmitted light). Right: composite images of the OT and c-Fos panels. A–F: images from a rat treated with an intra-PVN injection of 0.15 M NaCl and an intravenous injection of HDZ, whereas panels G–L are from a rat treated with an intra-PVN injection of SB-222200 and an intravenous injection of HDZ. C: higher magnification of OT neurons, one of which expresses c-Fos. Asterisk indicates a decrease in c-Fos expression in response to HDZ due to intra-PVN injection of SB-222200. Images correspond to plate 25 in Paxinos and Watson Rat Brain Atlas (45a). Scale bars are 50 μm.
Magnocellular and parvocellular c-Fos histology.
Intravenous injection of 0.15 M NaCl induced little c-Fos in the magnocellular and parvocellular neurons of the PVN. No difference in c-Fos expression was observed between injected and contralateral PVN with an intra-PVN injection of 0.15 M NaCl or SB-222200 and 0.15 M NaCl intravenous injection (P = 1.0). Following intravenous injection of 0.15 M NaCl, 25 ± 6 c-Fos-positive nuclei were identified in the magnocellular PVN and 10 ± 6 c-Fos-positive nuclei were identified in the parvocellular PVN (data not shown). c-Fos expression within the PVN was significantly increased following intravenous injection of HDZ. Intra-PVN injection of 0.15 M NaCl did not alter the c-Fos expression following HDZ in either magnocellular (186 ± 15 c-Fos positive nuclei) or parvocellular neurons (55 ± 12 c-Fos positive nuclei; Fig. 8) compared with the same area on the noninjected side. However, intra-PVN injection of SB-222200 significantly decreased c-Fos expression in the injected side of the PVN within the magnocellular neurons by 70% (P < 0.001) and the parvocellular neurons by 18% (P < 0.01). Following intra-PVN injection of SB-222200 and HDZ treatment, 55 ± 13 c-Fos-positive nuclei were identified in magnocellular neurons and 45 ± 7 c-Fos-positive nuclei were identified in the parvocellular neurons on the injected side of the PVN. No difference was observed between the contralateral side of the PVN with pretreatment of SB-222200 and intravenous HDZ, indicating our intra-PVN injection did not spread into the third ventricle to alter NK3R signaling in other nuclei.
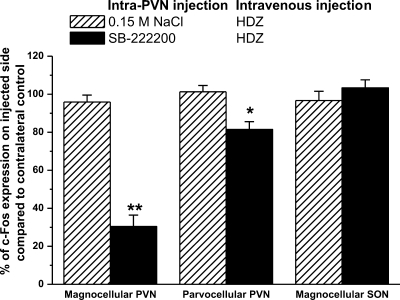
Fig. 8.
Intra-PVN injection of SB-222200 decreases c-Fos expression following intravenous injection of HDZ in magnocellular neurons of the PVN. C-Fos expression in the magnocellular PVN, parvocellular neurons of the PVN, and magnocellular SON following an intra-PVN injection of 0.15 M NaCl or SB-222200 and an intravenous injection of HDZ expressed as a percentage of the contralateral control. (**P < 0.001, *P < 0.01, compared with the 0.15 M NaCl intra-PVN injection and intravenous injection of HDZ).
To determine whether NK3R blockade in the PVN alters synaptically driven c-Fos expression in the ipsilateral SON, c-Fos expression was compared in the SON ipsilateral and contralateral to the PVN injection. No difference in c-Fos expression was observed in SON following 0.15 M NaCl or SB-222200 injection and intravenous HDZ injection. In fact, the same number of c-Fos-positive nuclei was identified in ipsilateral SON (121 ± 8) compared with the number of c-Fos-positive nuclei in the contralateral SON (125 ± 9; Fig. 8). As such, the intra-PVN injection of SB-222200 only alters c-Fos expression locally in the PVN and does not alter synaptic stimulation of c-Fos.
DISCUSSION
Hypotension is a potent stimulus for the release of VP and OT (46, 53, 55). Hemodynamic information is relayed to the magnocellular neurons in afferent pathways arising from the area of the caudal nucleus of solitary tract (NST) (8). NK3R are present in the brain stem pathways but are also highly expressed on magnocellular neurons themselves (14, 42). Intraventricular injection of the specific NK3R antagonist, SB-222200, eliminates the HDZ-stimulated systemic VP and OT release (21), without identifying the site of the NK3R antagonist action within the brain. Because intra-PVN injection of NK3R agonist elicits a greater VP release than intraventricular injection (40, 48), NK3R are highly expressed by the magnocellular neurons (12, 57) and NK3R are internalized on magnocellular neurons after HDZ (29), we hypothesized that the NK3R antagonist was acting at the magnocellular neurons themselves.
To test our hypothesis, the NK3R antagonist injection was administered into the PVN. Histological analyses of the spread of the injection show that the intra-PVN injection encompassed the target area, the magnocellular neurons of the PVN, and did not spread into the third ventricle (3V). Results that c-Fos expression decreased only in the injected PVN and not the contralateral PVN or the SON, where NK3R is highly expressed (12, 14, 42), further shows that the antagonist did not spread into the 3V. Although the injection spread to areas adjacent to the PVN, most of the neurons in the extra-PVN tissue do not express NK3R. However, the only nucleus included in the intra-PVN injection spread in which the neurons express NK3R is the zona incerta (14, 42).
Intravenous injections of 0.15 M NaCl did not alter plasma hormone levels regardless of the intra-PVN injection. This result is consistent with previous experiments showing that baseline plasma VP and OT levels did not change when intraventricular injection of the NK3R antagonist was administered prior to an intravenous injection of 0.15 M NaCl (20, 21). Therefore, NK3R signaling does not alter the basal release of the two hormones. However, intra-PVN injection of SB-222200 attenuated VP and OT release in response to HDZ by 33% and 35%, respectively. Intra-PVN injection of SB-222200 had no effect on the blood pressure response to HDZ (or 0.15 M NaCl). As such, the decrease in VP and OT release in SB-222200-treated rats is not secondary to an attenuation in the blood pressure response to HDZ.
In this study, the intra-PVN injection of the NK3R antagonist was unilateral. The neurosecretory neurons in the SON and the contralateral PVN are the putative source of the VP and OT release in response to HDZ with unilateral PVN pretreatment of SB-222200. Previous reports indicate that intraventricular pretreatment of SB-222200, presumably acting on NK3R in both PVN and SON, eliminates VP and OT release in response to HDZ (21). Unilateral blockade of NK3R in the PVN attenuated the release of both hormones in response to HDZ. Blockade of NK3R at additional sites, including the contralateral PVN and SON, would be expected to have a cumulative effect on VP and OT release, resulting in a greater reduction in the hormone release and mimic the effect of an intraventricular injection of SB-222200. Therefore, the combined NK3R signaling in magnocellular neurons of the PVN and SON is critical for the systemic VP and OT release in response to HDZ. Furthermore, previous reports demonstrate intraventricular injection of SB-222200 attenuates VP and OT release in response to 2 M NaCl (21). We would expect a decrease in VP and OT release following a unilateral, intra-PVN injection of SB-222200 prior to 2 M NaCl.
Intravenous injection of 0.15 M NaCl did not induce c-Fos expression in the magnocellular neurons. HDZ induces c-Fos expression in magnocellular and parvocellular neurons of the PVN (18, 33, 46, 53). However, intra-PVN injection of SB-222200 decreased c-Fos expression in response to HDZ in magnocellular neurons by 70% and parvocellular neurons by only 18%. NK3R are expressed in high concentration on magnocellular neurons (14) and in lower concentration on parvocellular neurons of the PVN (42). Therefore, the decrease in c-Fos expression in response to HDZ by SB-222200 reflects the distribution of NK3R in magnocellular and parvocellular neurons.
The protein product of the immediate early gene c-fos, c-Fos, is a marker of neuronal activation by synaptic stimulation (10, 11, 43). c-Fos protein is measured after an acute stimulus, with the protein expression peaking ∼90 min after the stimulus (23) and has been used to study neuroendocrine function (18, 50, 53). Unilateral intra-PVN injection of SB-222200 significantly decreased c-Fos expression to HDZ treatment in both VP and OT magnocellular neurons by ∼70%, indicating a role for NK3R in c-Fos expression. NK3R activation triggers an intracellular cascade that results in c-Fos expression. The NK3R stimulates G-protein-mediated PKC, PKD, phosphotidylinositol hydrolysis, and cAMP pathways (44, 49). Induction of the c-Fos protein in the nucleus is mediated by MAP kinase pathways (31), and G protein receptor cascades, including the pathways activated by NK3R, increase MAP kinase activity (35, 36). As such, the specific NK3R antagonist blocks second messenger cascades, which activate MAP kinase pathways, resulting in a decrease in c-Fos expression.
As previously mentioned, NK3R are expressed in afferent pathways relaying hemodynamic changes to the magnocellular neurons (8). NK3R signaling in afferent pathways could synaptically induce c-Fos expression in the magnocellular neurons in response to HDZ. Alternatively, the NK3R expressed by the magnocellular neurons could induce c-Fos expression in the magnocellular neurons in response to HDZ. This study demonstrates that blockade of NK3R in PVN decreases c-Fos expression in magnocellular VP and OT neurons in response to HDZ. To our knowledge, this is the first study to demonstrate a decrease in c-Fos expression in response to HDZ in magnocellular neurons of the PVN by local actions of a receptor antagonist and not from a loss of receptor signaling in afferent pathways.
Although research on magnocellular neuron function has primarily focused on two excitatory neurotransmitters, norepinephrine and glutamate, neither neurotransmitter system has reported to have a broad role like that of the NK3R. For example, NMDA receptor antagonists decrease the systemic VP and OT release in response to hypertonic saline, but not to CCK-8 injection (45). Intraventricular injection of α- and β- adrenergic receptors do not block the VP release in response to intraventricular norepinephrine injection (5). However, intraventricular injection of β-adrenergic antagonists, but not α1- or α2-adrenergic receptor antagonists, decrease VP response to hemorrhage (6). In contrast, intraventricular injection of the NK3R antagonist decreases VP and OT release in response to three different stimuli, hyperosmolarity, hypotension (21), and peripheral CCK-8 injections (20). Therefore, NK3R has a broad role in the systemic VP and OT release from the magnocellular neurons, whereas other neurotransmitter systems demonstrate more specific roles.
Intra-PVN injection of the NK3R antagonist prevents the endogenous ligand for the NK3R, presumably neurokinin B (NKB) (34, 51), from binding to the receptor. The decrease in c-Fos expression within the magnocellular neurons and decrease in VP and OT release suggest that NKB binding to the NK3R in the PVN contributes to the c-Fos protein expression and hormone release. The afferent source of NKB to the PVN is unclear. Although the majority of afferent input to the PVN does not contain NKB, a few areas, including the ipsilateral SON, NST, preoptic nucleus, and the amygdala project to the PVN and contain NKB (39, 41, 58, 60). However, the number of projections is small considering the high expression of NK3R on magnocellular neurons. Alternatively, studies have suggested that the source of NKB is the magnocellular neurons of the PVN themselves. Hatae and colleagues (24) reported that a large number of vasopressin neurons in both the PVN and SON coexpress NKB and suggested that, like the soma-dendritic release of VP and OT from neuroendocrine cells (38), NKB may be similarly released (41). Thus, NK3R expressed on VP neurons may be stimulated by the intranuclear release of NKB and could act as an autocrine or paracrine factor for the neurons. Although the intranuclear release of NKB is a possibility, other reports fail to detect NKB or NKB mRNA cell bodies of the PVN (36) or identify just a few immunoreactive cells in the magnocellular portions of the PVN (37). Ultimately, the activation of NK3R may rely on a combination of NKB released from afferent projections and from magnocellular neurons themselves.
Perspectives and Significance
The novel finding in this study is that NK3R signaling in magnocellular neurons mediates neurohormone release and c-Fos expression in the neurons. Other neurotransmitter systems carry particular information, such as osmolarity or blood volume, and alter magnocellular neuron function under specific physiological conditions; however, NK3R appears to have a broad or general role in VP and OT release. The NK3R is a unique receptor on these neurons, as it translocates to the nucleus following activation by specific receptor agonists and physiological challenges (19, 29, 30). To date, no other G protein-coupled receptor has been shown to translocate to the nucleus of cells in vivo. Therefore, NK3R could alter the genomic regulation of the cells, including the induction of c-Fos expression, as well as systemic VP and OT release. Furthermore, many central pathways converge on the magnocellular neurons to elicit systemic VP and OT release. The broad role of the NK3R in VP and OT release from the magnocellular neurons indicates that the receptor could be a common path of activation for the release of VP and OT under multiple physiological conditions.
GRANTS
This publication was made possible by National Institutes of Health (NIH) Grants DK-50586, NS-57823, and P20 RR-15640 from the National Center of Research Resources, part of the NIH.
Acknowledgments
We thank GlaxoSmithKline for their generous gift of SB-222200. Also, we would like to thank Shawna McBride and Donald Pratt for their assistance in preparing this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akins VF, Bealer SL. Central nervous system histamine regulates peripheral sympathetic activity. Am J Physiol Heart Circ Physiol 260: H218–H224, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Akins VF, Bealer SL. Hypothalamic histamine release, neuroendocrine, and cardiovascular responses during tuberomammillary nucleus stimulation in the conscious rat. Neuroendocrinology 57: 849–855, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Arnhold MM, Wotus C, Engeland WC. Differential regulation of parvocellular neuronal activity in the paraventricular nucleus of the hypothalamus following single vs. repeated episodes of water restriction-induced drinking. Exp Neurol 206: 126–136, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bealer SL, Flynn FW. Central neurokinin 3 receptors increase systemic oxytocin release: interaction with norepinephrine. Exp Neurol 184: 1027–1033, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DP, Share L, Crofton JT. Central adrenergic control of vasopressin release. Neuroendocrinology 42: 416–420, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DP, Share L, Crofton JT. Central adrenergic mechanisms in hemorrhage-induced vasopressin secretion. Am J Physiol Heart Circ Physiol 251: H1158–H1162, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Buller KM, Smith DW, Day TA. Differential recruitment of hypothalamic neuroendocrine and ventrolateral medulla catecholamine cells by non-hypotensive and hypotensive hemorrhages. Brain Res 834: 42–54, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier C, Baude A. Immunocytochemical localization of NK3 receptors in the dorsal vagal complex of rat. Brain Res 736: 327–331, 1996. [PubMed] [Google Scholar]
- 9.Crofton JT, Share L. Osmotic control of vasopressin in male and female rats. Am J Physiol Regul Integr Comp Physiol 257: R738–R743, 1989. [DOI] [PubMed] [Google Scholar]
- 10.Curran T, Miller AD, Zokas L, Verma IM. Viral and cellular fos proteins: a comparative analysis. Cell 36: 259–268, 1984. [DOI] [PubMed] [Google Scholar]
- 11.Curran T, Morgan JI. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science 229: 1265–1268, 1985. [DOI] [PubMed] [Google Scholar]
- 12.Ding YQ, Lu BZ, Guan ZL, Wang DS, Xu JQ, Li J. Neurokinin B receptor (NK3)-containing neurons in the paraventricular and supraoptic nuclei of the rat hypothalamus synthesize vasopressin and express Fos following intravenous injection of hypertonic saline. Neuroscience 91: 1077–1085, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Ding YQ, Shi J, Su LY, Xu JQ, Su CJ, Guo XE, Ju G. Intracerebroventricular injection of senktide-induced Fos expression in vasopressin-containing hypothalamic neurons in the rat. Brain Res 882: 95–102, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol 364: 290–310, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 281: R738–R746, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Flynn FW, Haley GE, Adams DJ, Bealer SL. Magnocellular oxytocin neurons express the tachykinin NK3 receptor. Society for Neuroscience Abstract, Abstract 260.24 Atlanta, GA, 2006.
- 18.Graham JC, Hoffman GE, Sved AF. c-Fos expression in brain in response to hypotension and hypertension in conscious rats. J Auton Nerv Syst 55: 92–104, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 290: R1242–R1250, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Haley GE, Flynn FW. Tachykinin neurokinin 3 receptor (NK3R) signaling in cholecystokinin-elicited release of oxytocin and vasopressin. Am J Physiol Regul Integr Comp Physiol 294: R1760–R1767, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Haley GE, Flynn FW. Tachykinin NK3 receptor contribution to systemic release of vasopressin and oxytocin in response to osmotic and hypotensive challenge. Am J Physiol Regul Integr Comp Physiol 293: R931–R937, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hammond DL, Presley R, Gogas KR, Basbaum AL. Morphine or U-50488 suppresses Fos protein-like immunoreactivity in the spinal cord and nucleus tractus solitarii evoked by a noxious visceral stimulus in the rat. J Comp Neurol 315: 244–253, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Harris JA Using c-fos as a neural marker of pain. Brain Res Bull 45: 1–8, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hatae T, Kawano H, Karpitskiy V, Krause JE, Masuko S. Arginine-vasopressin neurons in the rat hypothalamus produce neurokinin B and co-express the tachykinin NK-3 receptor and angiotensin II type 1 receptor. Arch Histol Cytol 64: 37–44, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 9: 3072–3082, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JP, Thomas GJ, Wiegand SJ, Gash DM. Lesions of parvocellular subdivisions of the hypothalamic paraventricular nucleus alter open field behavior and acquisition of sensory and spatial discrimination. Brain Res 550: 291–297, 1991. [DOI] [PubMed] [Google Scholar]
- 27.Hiscock JJ, MacKenzie L, Willoughby JO. Fos induction in subtypes of cerebrocortical neurons following single picrotoxin-induced seizures. Brain Res 738: 301–312, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman GE, Smith MS, Fitzsimmons MD. Detecting steroidal effects on immediate gene expression in the hypothalamus. Neuroprotocols 1: 52–66, 1992. [Google Scholar]
- 29.Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci 24: 10103–10110, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen D, Zhang Z, Flynn FW. Trafficking of tachykinin NK3 receptor to nuclei of neurons in the paraventricular nucleus of the hypothalamus following osmotic challenge. Neuroscience 155: 308–316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansen C, Kragballe K, Henningsen J, Westergaard M, Kristiansen K. 1α,25-dihydroxyvitamin D3 stimulates activator protein 1 DNA binding activity by a phosphatidylinositol 3-kinase/Ras/MEK/extracelluar signal-regulated kinase 1/2 and c-Jun N-terminal kinase 1-dependent increase in c-Fos, Fra1, and c-jun expression in human keratinocytes. J Invest Dermatol 120: 561–570, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Kojima S, Ikeda M, Kamikawa Y. Loperamide inhibits tachykinin NK3-receptor-triggered serotonin release without affecting NK2-receptor-triggered serotonin release from guinea pig colonic mucosa. J Pharmacol Sci 98: 175–180, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Krukoff TL, Mactavish D, Jhamandas JH. Activation by hypotension of neurons in the hypothalamic paraventricular nucleus that project to the brainstem. J Comp Neurol 385: 285–296, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Lavielle S, Chassaing G, Ploux O, Loeuillet D, Besseyre J, Julien S, Marquet A, Convert O, Beaujouan JC, Torrens Y, Bergstrom L, Saffroy M, Glowinski J. Analysis of tachykinin binding site interactions using constrained analogues of tachykinins. Biochem Pharmacol 37: 41–49, 1988. [DOI] [PubMed] [Google Scholar]
- 35.Liebmann C Regulation of MAP kinase activity by peptide receptor signalling pathway: paradigms of multiplicity. Cell Signal 13: 777–785, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science 275: 394–397, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Lucas LR, Hurley DL, Krause JE, Harlan RE. Localization of the tachykinin neurokinin B precursor peptide in rat brain by immunocytochemistry and in situ hybridization. Neuroscience 51: 317–345, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci 26: 255–261, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. J Comp Neurol 317: 341–356, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Massi M, Saija A, Polidori C, Perfumi M, Gentili L, Costa G, de Caro G. The hypothalamic paraventricular nucleus is a site of action for the central effect of tachykinins on plasma vasopressin. Brain Res Bull 26: 149–154, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Merchenthaler I, Maderdrut JL, O'Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides 13: 815–829, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience 89: 1269–1290, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14: 421–451, 1991. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima Y, Tsuchida K, Negishi M, Ito S, Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem 267: 2437–2442, 1992. [PubMed] [Google Scholar]
- 45.Onaka T, Yagi K. Involvement of N-methyl-d-aspartic acid receptor activation in oxytocin and vasopressin release after osmotic stimuli in rats. J Neuroendocrinol 13: 166–174, 2001. [DOI] [PubMed] [Google Scholar]
- 45a.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press, 1997.
- 46.Petrov T, Harris KH, MacTavish D, Krukoff TL, Jhamadas JH. Hypotension induces Fos immunoreactivity in NADPH-diaphorase positive neurons in paraventricular and supraoptic hypothalamic nuclei of the rat. Neuropharmacology 34: 509–514, 1995. [DOI] [PubMed] [Google Scholar]
- 47.Pirnik Z, Kiss A. Fos expression variances in mouse hypothalamus upon physical and osmotic stimuli: co-staining with vasopressin, oxytocin, and tyrosine hydroxylase. Brain Res Bull 65: 423–431, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Polidori C, Saija A, Perfumi M, Gentili L, de Caro G, Costa G, Massi M. The paraventricular nucleus as a site of action for the vasopressin releasing effect of tachykinins. Pharmacol Res 21: 141–142, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Poole DP, Amadesi S, Rozengurt E, Thacker M, Bunnett NW, Furness JB. Stimulation of the neurokinin 3 receptor activates protein kinase Cɛ and protein kinase D in enteric neurons. Am J Physiol Gastrointest Liver Physiol 294: G1245–G1256, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Roberts MM, Robinson AG, Fitzsimmons MD, Grant F, Lee WS, Hoffman GE. c-fos expression in vasopressin and oxytocin neurons reveals functional heterogeneity within magnocellular neurons. Neuroendocrinology 57: 388–400, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Sadowski S, Huang RR, Fong TM, Marko O, Cascieri MA. Characterization of the binding of [125-I-Iodo-histidyl, Methyl-Phe7] neurokinin B to the neurokinin-3 receptor. Neuropeptides 24: 317–319, 1993. [DOI] [PubMed] [Google Scholar]
- 52.Sarau HM, Griswold DE, Bush B, Potts W, Sandhu P, Lundberg D, Foley JJ, Schmidt DB, Webb EF, Martin LD, Legos JJ, Whitmore RG, Barone FC, Medhurst AD, Luttmann MA, Giardina GA, Hay DW. Nonpeptide tachykinin receptor antagonists. II. Pharmacological and pharmacokinetic profile of SB-222200, a central nervous system penetrant, potent and selective NK3 receptor antagonist. J Pharmacol Exp Ther 295: 373–381, 2000. [PubMed] [Google Scholar]
- 53.Schiltz JC, Hoffman GE, Stricker EM, Sved AF. Decreases in arterial pressure activate oxytocin neurons in conscious rats. Am J Physiol Regul Integr Comp Physiol 273: R1474–R1483, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz MD, Nunez AA, Smale L. Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience 127: 13–23, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Shen E, Dun SL, Ren C, Bennett-Clarke C, Dun NJ. Hypotension preferentially induces c-fos immunoreactivity in supraoptic vasopressin neurons. Brain Res 593: 136–139, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4: 477–485, 1990. [DOI] [PubMed] [Google Scholar]
- 57.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol 372: 395–414, 1996. [DOI] [PubMed] [Google Scholar]
- 58.Silverman AJ, Hoffman DL, Zimmerman EA. The descending afferent connections of the paraventricular nucleus of the hypothalamus (PVN). Brain Res Bull 6: 47–61, 1981. [DOI] [PubMed] [Google Scholar]
- 59.Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK3 receptor agonist, senktide. J Comp Neurol 426: 413–428, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Swanson LW, Sawchenko PE. Hypothalamic Integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 324, 1983. [DOI] [PubMed] [Google Scholar]