Abstract
Giving rats 2.5% saline to drink for 3–5 days simply and reliably generates anorexia. Despite having the neurochemical and hormonal markers of negative energy balance, dehydrated anorexic rats show a marked suppression of spontaneous food intake, as well as the feeding that is usually stimulated by overnight starvation or a 2-deoxy-d-glucose (2DG) challenge. These observations are consistent with a dehydration-dependent inhibition of the core circuitry that controls feeding. We hypothesize that this inhibition is directed at those neurons in the paraventricular nucleus and lateral hypothalamic area that constitute the hypothalamic “behavior controller” for feeding rather than their afferent inputs from the arcuate nucleus or hindbrain that convey critical feeding-related sensory information. To test this hypothesis, we mapped and quantified the Fos-immunoreactive response to 2DG in control and dehydrated rats drinking 2.5% saline. Our rationale was that regions showing an attenuated Fos response to 2DG in dehydrated animals would be strong candidates as the targets of dehydration-induced suppression of 2DG feeding. We found that the Fos response to combined dehydration and 2DG was attenuated only in the lateral hypothalamic area, with dehydration alone increasing Fos in the lateral part of the paraventricular nucleus. In the arcuate nucleus and those regions of the hindbrain that provide afferent inputs critical for the feeding response to 2DG, the Fos response to 2DG was unaffected by dehydration. Therefore, dehydration appears to target the lateral hypothalamic area and possibly the lateral part of the paraventricular nucleus to suppress the feeding response to 2DG.
Keywords: feeding behavior, inhibition, 2-deoxyglucose, arcuate nucleus, hindbrain, parabrachial nucleus, area postrema, nucleus of the solitary tract
anorexia is a loss of appetite in the presence of adequate food sources and can be physiologically or pathologically generated (49). We have previously shown that animals develop anorexia after drinking hypertonic saline and becoming dehydrated (DE). These animals provide a useful model for investigating the underlying neural mechanisms of anorexia (47–49, 54).
DE-anorexic rats limit their spontaneous circadian-driven food intake despite the presence of endocrine and neuropeptidergic profiles of negative energy balance (55). In fact, DE animals share a virtually identical profile of caloric deficit markers with animals that are food restricted to match the intake of their anorexic counterparts (55). This profile includes body weight loss, undetectable levels of circulating leptin and insulin, increased plasma glucocorticoid concentrations, and elevated neuropeptide Y (NPY) gene expression in the arcuate nucleus of the hypothalamus (ARH). While these endocrine and neural components are usually very effective at stimulating food intake (1, 8, 11, 35), they are much less so in DE-anorexic rats. Moreover, not only is DE-anorexia evident as a marked reduction in spontaneous food intake, it also limits food intake following either overnight starvation or 2-deoxy-d-glucose (2DG)-induced alterations in cellular glucose metabolism (30). Importantly however, DE does not block the glucocorticoid and hyperglycemic responses to 2DG (30). Although these results collectively suggest that drinking hypertonic saline targets neural control elements that are common to spontaneous, deficit-driven, and 2DG-stimulated feeding, the mechanisms engaged by drinking hypertonic saline to generate anorexia are currently unknown.
On the basis of an exhaustive series of neuroanatomical experiments, Swanson has proposed that a column of nuclei in the medial zone of the hypothalamus are integral components of the motor control hierarchy for all motivated behaviors (39, 41). In particular, the paraventricular nucleus of the hypothalamus (PVH) contains neurons that comprise the “behavior controller” for feeding. The integrated output from the feeding behavior controller then regulates the downstream motor network that ultimately controls motor neurons in the hindbrain and spinal cord responsible for executing feeding behavior (39, 41, 54). This model further posits that different types of feeding use distinct sets of afferents from other parts of the hypothalamus (particularly the ARH), the telencephalon, and hindbrain to engage motivated feeding behavior controllers in the PVH and possibly the lateral hypothalamic area (LHA) (54). For example, 2-DG-stimulated feeding requires ascending catecholaminergic afferents from the hindbrain but not neurons in the mediobasal hypothalamus (MBH) (3, 24, 26), whereas the feeding effects of interocerebroventricular (icv) injections of leptin and ghrelin are severely compromised by lesions of MBH neurons (3).
We have recently proposed that drinking hypertonic saline produces anorexia by specifically targeting neurons in the behavioral controllers of the PVH and LHA rather than their inputs from the MBH or hindbrain that are responsible for providing critical information for stimulating particular types of feeding (54). To test this hypothesis, we now identify cell groups in which drinking hypertonic saline alters the Fos response to 200 mg/kg 2DG, which relies on ascending catecholaminergic projections to the hypothalamus (24, 26). By comparing the response patterns of Fos-immunoreactive (ir) neurons following 2DG in euhydrated (EU) rats (in which 2DG stimulates feeding) with DE animals (in which the feeding response to 2DG is markedly attenuated), we can identify potential areas where the neural networks engaged by drinking hypertonic saline act to inhibit feeding. The expression of Fos protein is well established as a functional marker of activated neurons (14, 56). Fos protein increases in the cell nucleus of activated neurons within 30–45 min following the presentation of a stimulus and has a half-life of approximately 2 h (20). Although the nonquantitative nature of Fos immunocytochemistry means that it is not possible to determine which individual neurons might receive converging inputs (56), a population-based analysis of potential convergent target areas derived from the numbers of Fos-ir neurons in an anatomically defined region is feasible.
With this limitation in mind, we predict three possible outcomes. First, a compromised Fos response to 2DG in the PVH and/or the LHA of DE animals but not in regions supplying afferent inputs would support the notion that drinking hypertonic saline targets the behavior controllers to generate anorexia. Second, alterations in the hindbrain regions that provide the afferent information required for 2DG feeding (24, 26) would be consistent with drinking hypertonic saline targeting the input neurons rather than the feeding behavior controller neurons. Finally, a compromised Fos response within many regions would suggest that drinking hypertonic saline leads to a more generalized effect on the brain and would not support the proposed model.
MATERIALS AND METHODS
Animals and Procedures
Adult male Sprague-Dawley rats weighing 240–260 g were obtained from Harlan Laboratories and singly housed in suspended Plexiglas cages with sanitized wood chips. They were maintained in a temperature-controlled room (20–22°C) on a 12:12-h light-dark schedule with lights on at 0600. Rats were provided with continuous access to food (Teklad rodent diet 8604) and water throughout the experiment except where stated. In some animals, drinking water was replaced with 2.5% saline solution for 5 days. We have previously shown that 5 days of drinking hypertonic saline increases plasma osmolality by ∼6% (46, 53). Body weights and nocturnal food intake were measured daily throughout the experiment. All procedures used were approved by the local institutional animal care and use committee.
Four groups of animals were maintained on ad libitum food availability and either water (EU) animals or 2.5% saline for 5 days. On the morning of the 5th day, food was removed from all cages. Two groups of DE- or EU animals were given an subscapular injection of either 2DG (200 mg·ml−1·kg−1, DE-2DG, EU-2DG) or an equal volumetric injection of physiological saline (DE-Veh, EU-Veh). 2DG is a glucose analog that is not isomerized to fructose-6-phosphate after its phosphorylation by hexokinase. Although 2DG has been used widely as tool for exploring deficit feeding and glucose-sensing mechanisms, it should be remembered that slowing the glycolytic pathway may lead to secondary effects on brain metabolism (2, 27). With that in mind, 200 mg·ml−1·kg−1 of 2DG was chosen because it generated submaximal feeding responses in a dose-response study in control animals (30) and was hypothesized to minimize any of its nonspecific effects.
Histology and Fos Immunocytochemistry
Two hours after injection, animals were anesthetized with halothane and rapidly decapitated. Brains were removed from the skull and immersion fixed for 3 days on ice-cold PBS 4% paraformaldehyde and then frozen on dry ice and stored at −70°C. We have previously shown that this fixation method is compatible with the preservation of antigens for immunocytochemistry (17). Two sets of five series of 1-in-5, 30-μm-thick frozen coronal sections were cut through the brain. The first set ran from the level of the subfornical organ to the caudal part of the ARH; the second ran from the parabrachial nucleus (PB) through the caudal hindbrain. Sections were stained for Fos immunoreactivity using previously published methods (52). Briefly, after rinsing with potassium phosphate-buffered saline, sections were incubated in Ab-5 anti-Fos antibody (1:40K, Oncogene Science, Cambridge, MA) for 48 h, with 3% normal goat serum and 0.2% Triton X-100. Sections were rinsed and incubated for 2 h with biotinylated 2° antibody, followed by incubation with Vectastain Elite ABC reagent (Vector Laboratories, Burlingame CA). Specific antibody staining was color-detected with a 1 mg/ml solution of diaminobenzidine (DAB; 3,3 diaminobenzidine tetrahydrochloride) with 0.3% hydrogen peroxide. Adjacent sections were stained with thionin. All sections were mounted, cover slipped, and examined under the microscope.
Image Analysis
To examine Fos expression in regions associated with 2DG feeding, anatomically defined regions of the hypothalamus and hindbrain were located by identifying landmarks using the Swanson Rat Brain Atlas (40) and adjacent thionin-stained sections. Because of the highly irregular shape of many of regions in which we were interested (e.g., parts of the PVH) and the fact that labeled cells were not distributed equally within some large complex regions (e.g., the LHA), we opted to use a manual counting method rather than one based on automated software counting techniques.
Images of each field were photographed and printed (Adobe Photoshop ver. 7.0; Adobe Systems, San Jose, CA). Fos-ir labeled cells were counted manually on the printed image, with additional verification of each field using microscopic examination of the original slide and its corresponding thionin-stained section. The number of Fos-ir neurons in each brain region was counted without knowledge of the experimental group. Cell counts were expressed as labeled cells-per-section (total number of cells per region/total number of sections per region) because the number of sections needed to span the entire region of interest differed from animal to animal. The mean number of cells-per-section was then compared across groups.
Paraventricular nucleus of the hypothalamus.
Neurons containing Fos-ir in the PVH were counted in a rostrocaudal series that ranged from the rostral point of the lateral zone of the posterior magnocellular part through to its forniceal part (level 26 to level 28 of [38]). Using close examination of the adjacent thionin sections, we further resolved labeled cells in the PVH into three compartments: the posterior magnocellular (lateral zone; pml), dorsal zone of the medial parvicellular part (mpd), and lateral parvicellular part (lp), which included the forniceal part.
Lateral hypothalamic area.
Neurons containing Fos-ir were counted from the caudal extent of the forniceal component of the PVH (level 27) to the rostral extent of the posterior dorsomedial nucleus (level 30). Labeled neurons were counted ventral to the dorsal limit of the third ventricle, medial from the internal capsule, lateral to the medial margin of the fornix and lateral border of the ARH, and dorsal to the supraoptic commissure/optic tract. Cells in the PVH, anterior hypothalamic nucleus, ventromedial nucleus, and dorsomedial nucleus were excluded from the LHA counts. This particular part of the LHA was chosen because it included the perifornical area of the hypothalamus (see 40), as well as those parts that contain melanin-stimulating hormone and orexin/hypocretin-producing neurons (43). These regions are strongly implicated in regulating feeding (32, 37), as well as the development of DE-anorexia (16, 49, 52, 55).
Arcuate nucleus of the hypothalamus.
Fos-ir-labeled cell counts in the ARH began at the rostral edge of the ventromedial nucleus (level 26; see Ref. 40) to the rostral extent of the posterior dorsomedial nucleus (level 29).
Parabrachial nucleus.
Labeled cells were counted beginning at the point at which the dorsal tegmental nucleus is dorsal to the medial longitudinal fasiculus (level 48) to the region where the superior cerebellar peduncle meets the ventral spinocerebellar tract (level 51). In the PB region, Fos-ir neurons were only observed in its lateral components of the PB [central, external, and ventral lateral parts (PBl)], and not in any part of the medial PB.
Area postrema.
Cells containing Fos-ir were counted throughout the entire extent of the area postrema (AP; level 69–70). Any labeled neurons seen in the nucleus gracilus and commissural part of the nucleus of the solitary tract (NTS) were excluded.
NTS/dorsal motor complex.
Cell counts began at the rostral pole of the area postrema (level 67) and continued through to the caudal limit of the AP (level 70). Cells were counted in the medial, commissural, gelatinous, and central part of the NTS, and in the dorsal motor nucleus (DMX) dorsal to the hypoglossal nucleus.
Two-way ANOVA was performed on experimental groups with statistical significance set at P < 0.05. For each region, 2DG administration and drinking hypertonic saline were the independent variables with the numbers of Fos-ir neurons the dependent variable. Post hoc comparisons were then performed using the Bonferroni pairwise multiple-comparison test to determine which groups were statistically different with a significance of P < 0.05 or greater.
RESULTS
Hypothalamus
Paraventricular nucleus.
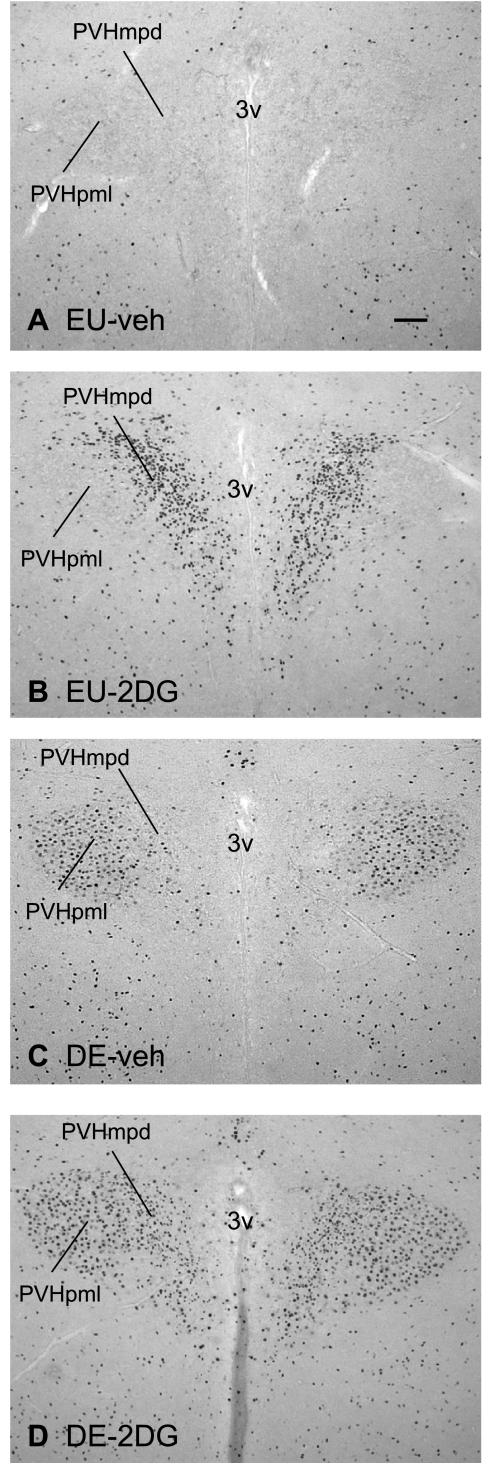
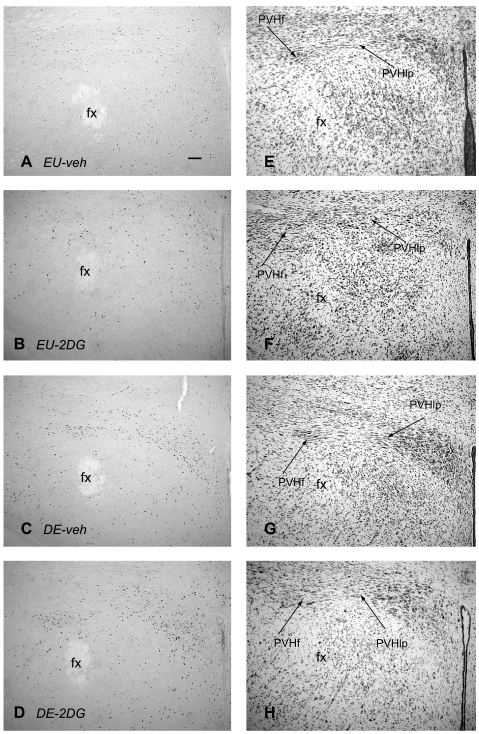
Figures 1 and 2 illustrate the distribution of Fos-ir neurons in the dorsal part of the medial parvicellular zone of the paraventricular nucleus of the hypothalamus (PVHmpd), the lateral zone of the posterior magnocellular part of the PVH (PVHpml) (both Fig. 1), and the lateral parvicellular part of the PVH (PVHlp) (Fig. 2), 2 h after beginning the various treatments.
Fig. 1.
Fos immunoreactivity in the central portion of the paraventricular nucleus of the hypothalamus. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity 2 h after injection of vehicle in a euhydrated animal (EU-veh) (A); 2 h after injection of 2DG in a euhydrated animal (EU-2DG) (B); 2 h after injection of vehicle in a 5-day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5-day dehydrated animal (DE-2DG) (D). 3v, third ventricle; PVHmpd, dorsal part of the medial parvicellular zone of the paraventricular nucleus of the hypothalamus (PVH); PVHpml, lateral zone of the posterior magnocellular part of the PVH. Scale bar in A = 100 μm.
Fig. 2.
Fos immunoreactivity in the lateral wing and forniceal component of the paraventricular nucleus of the hypothalamus. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity 2 h after injection of vehicle in an euhydrated animal (EU-veh) (A), 2 h after injection of 2DG in an euhydrated animal (EU-2DG) (B), 2 h after injection of vehicle in a 5-day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5-day dehydrated animal (DE-2DG) (D). Adjacent thionin-stained sections are shown (E–H). PVHlp, lateral parvicellular part of the PVH; PVHf, forniceal part of the PVH; fx, fornix. Scale bar in A = 100 μm.
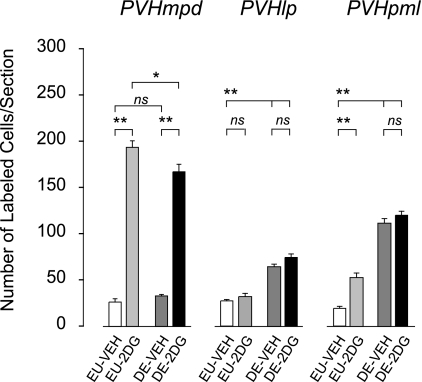
In the PVHmpd, there was a main effect of 2DG [F (3,21) = 310.832, P < 0.0001] but not drinking hypertonic saline [F (3,21) = 1.202, P = 0.288] on the number Fos-ir neurons (Fig. 3), and no significant interaction was found between them [F (3,21) = 3.435, P = 0.81]. The Fos response to 2DG was significantly lower in animals drinking hypertonic saline compared with EU-controls (P < 0.05).
Fig. 3.
Means ± SE number of Fos-immunoreactive neurons in the paraventricular nucleus of the hypothalamus 2 h after injection of vehicle in an euhydrated animal (EU-veh, white bars), 2 h after injection of 2DG in euhydrated animals (EU-2DG, light gray bars), 2 h after injection of vehicle in 5-day dehydrated animals (DE-veh, dark gray bars), and 2 h after injection of 2DG in 5-day dehydrated animals (DE-2DG, black bars). Bars indicate a significant difference of P < 0.05 (*) or P < 0.01 (**), or not significant (ns).
Drinking hypertonic saline resulted in significantly increased numbers of Fos-ir neurons in the PVHpml compared with EU-veh animals (Fig. 3). Administration of 2DG to EU animals also increased the number of Fos-ir positive neurons over that seen in EU-veh animals. When 2DG was administered to DE animals, there were more neurons in the PVHpml showing Fos activation than in EU-2DG animals, but this number was unchanged from animals just given hypertonic saline to drink (DE-veh; Figs. 1 and 3). Thus, there were main effects of both 2DG [F (3,21) = 13.869, P < 0.0025] and drinking hypertonic saline [F (3,21) = 152.071, P < 0.0001] on the number of Fos-ir neurons, but no significant interaction between them [F (3,21) = 1.908, P = 0.81]. The Fos response to 2DG was significantly greater in DE animals than EU animals (P < 0.01).
The number of Fos-ir neurons in the PVHlp significantly increased over EU-veh animals after drinking hypertonic saline, both with and without 2DG administration [F (3,21) = 112.237, P < 0.0001]. This was particularly noticeable in its forniceal part (Fig. 2). However, there was no main effect of 2DG [F (3,21) =3.567, P = 0.076], and no significant interaction between either independent variable on Fos responses in the PVHlp [F (3,21) = 0.172, P = 0.684].
Lateral hypothalamic area.
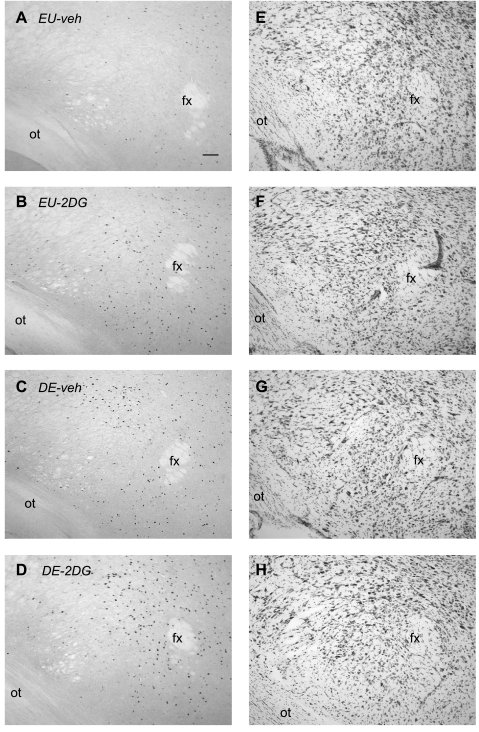
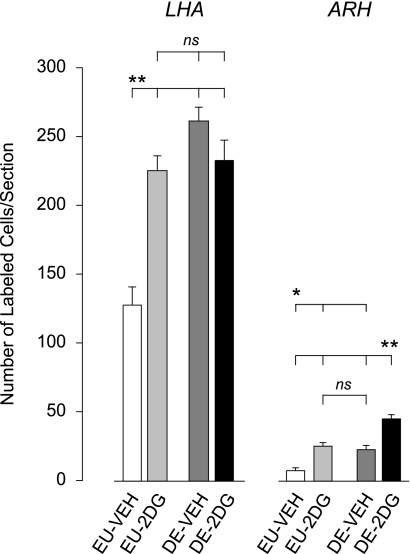
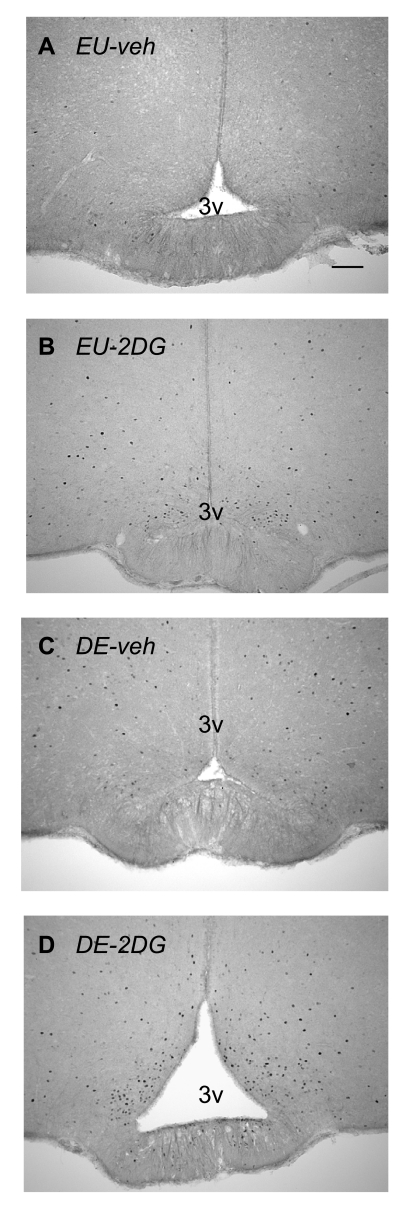
Figures 4 and 5 show increased numbers of Fos-ir neurons in the LHA after 2DG and after 5 days of drinking hypertonic saline compared with control animals [F (3,21) = 30.718, P < 0.0001]. The was also a main effect of 2DG [F (3,21) = 7.238, P < 0.02], and a significant interaction [F (3,21) = 24.894, P < 0.0001].
Fig. 4.
Fos immunoreactivity in the lateral hypothalamic area. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity, 2 h after injection of vehicle in an euhydrated animal (EU-veh) (A), 2 h after injection of 2DG in an euhydrated animal (EU-2DG) (B), 2 h after injection of vehicle in a 5 day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5-day dehydrated animal (DE-2DG) (D). Adjacent thionin-stained sections are shown (E–H). ot, optic tract. Scale bar in A = 100μm.
Fig. 5.
Means ± SE number of Fos-positive neurons in the lateral hypothalamic area (LHA) and arcuate nucleus (ARH) 2 h after injection of vehicle in euhydrated animals (EU-veh, white bars), 2 h after injection of 2DG in euhydrated animals (EU-2DG, light gray bars), 2 h after injection of vehicle in 5-day dehydrated animals (DE-veh, dark gray bars), and 2 h after injection of 2DG in 5-day dehydrated animals (DE-2DG, black bars). Bars indicate a significant difference of P < 0.05 (*) or P < 0.01 (**), or not significant (ns).
Arcuate nucleus.
Figures 5 and 6 show that there was a main effect of both 2DG [F (3,18) = 38.589, P < 0.0001] and drinking hypertonic saline [F (3,18) = 29.193, P < 0.0001] in the ARH, but no significant interaction was found [F (3,18) = 0.4, P = 0.537]. The Fos response to 2DG was significantly greater in the ARH of DE animals than in EU animals (P < 0.01).
Fig. 6.
Fos immunoreactivity in the arcuate nucleus of the hypothalamus. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity, 2 h after injection of vehicle in an euhydrated animal (EU-veh) (A), 2 h after injection of 2DG in an euhydrated animal (EU-2DG) (B), 2 h after injection of vehicle in a 5-day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5-day dehydrated animal (DE-2DG) (D). Scale bar in A = 100 μm.
Parabrachial nucleus.
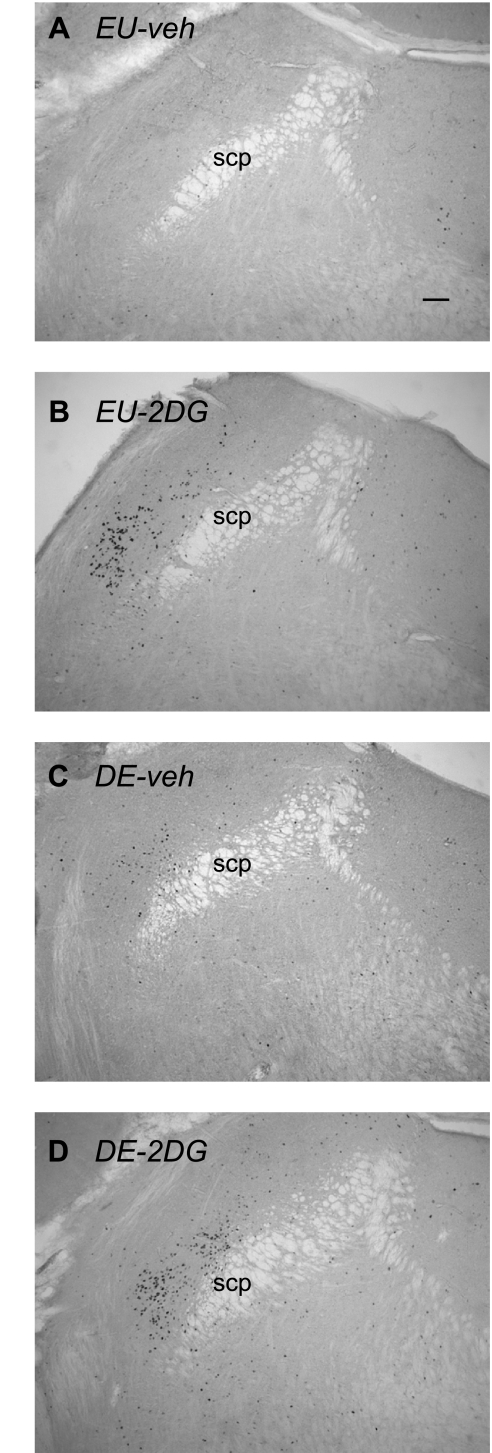
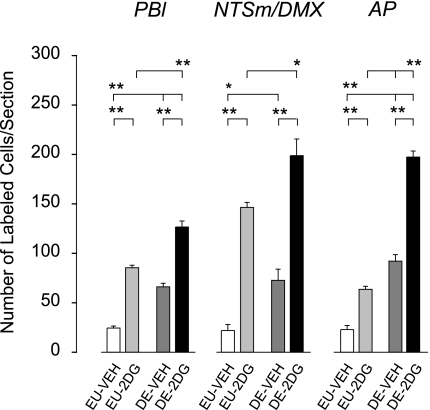
The was a main effect of both 2DG [F (3,19) = 130.627, P < 0.0001] and drinking hypertonic saline [F (3,19) = 74.039, P < 0.0001] on Fos responses in the PBl. However, there was no significant interaction [F (3,19) = 0.003, P = 0.857]. Figures 7 and 8 shows that the number of neurons in the PBl containing Fos-ir nuclei was significantly higher following 2DG administration (P < 0.01) to DE animals compared with 2DG administration in EU animals.
Fig. 7.
Fos immunoreactivity in the parabrachial nucleus of the hypothalamus. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity 2 h after injection of vehicle in an euhydrated animal (EU-veh) (A), 2 h after injection of 2DG in an euhydrated animal (EU-2DG) (B), 2 h after injection of vehicle in a 5-day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5-day dehydrated animal (DE-2DG) (D). scp, superior cerebellar peduncle. Scale bar in A = 100 μm.
Fig. 8.
Means ± SE number of Fos-positive neurons in the mid- and hindbrain 2 h after injection of vehicle in euhydrated animals (EU-veh; white bars), 2 h after injection of 2DG in euhydrated animals (EU-2DG, light gray bars), 2 h after injection of vehicle in 5-day dehydrated animals (DE-veh, dark gray bars), and 2 h after injection of 2DG in 5-day dehydrated animals (DE-2DG, black bars). Bars indicate a significant difference of P < 0.05 (*) or P < 0.01 (**), or not significant (ns).
In all cases, most of the Fos-ir neurons were seen in the external lateral, dorsal lateral, and ventral lateral parts of the PBl (Fig. 7). However, Fos-ir neurons were located primarily in the external lateral PB in EU-2DG animals, whereas labeled neurons were located more medially in the lateral part of the ventral lateral PB in DE-veh animals (Fig. 7). Interestingly, the distribution pattern of Fos-ir neurons in DE-2DG animals was a combination of that seen in EU-2DG and DE-veh animals (Fig. 7).
Nucleus of the solitary tract and dorsal motor nucleus of the vagus.
Figure 8 shows that there was a main effect of both 2DG [F (3,19) = 94.3, P < 0.0001] and drinking hypertonic saline [F (3,19) = 18.078, P < 0.0001] on the number of Fos-ir neurons in the NTS and DMX, but no significant interaction was found [F (3,19) = 0.018, P = 0.896]. Figures 8 and 9 show that Fos response to 2DG was significantly greater in DE animals than EU animals (P < 0.05).
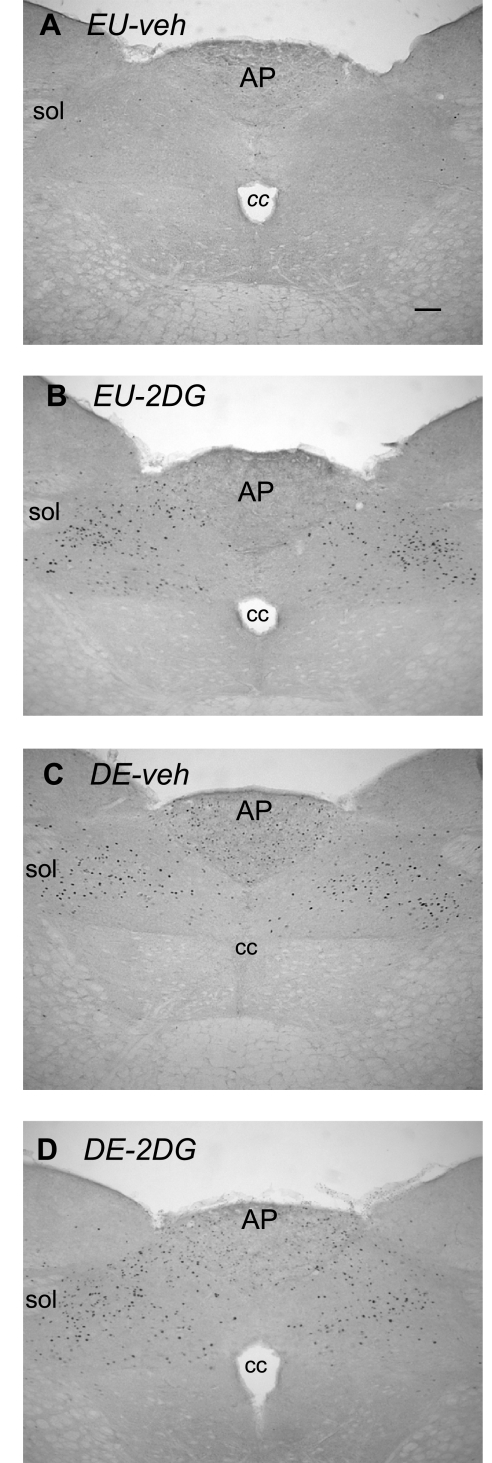
Fig. 9.
Fos immunoreactivity in the area postrema (AP) and nucleus of the solitary tract. Brightfield photomicrographs of representative coronal sections showing Fos immunoreactivity 2 h after injection of vehicle in an euhydrated animal (EU-veh) (A), 2 h after injection of 2DG in an euhydrated animal (EU-2DG) (B), 2 h after injection of vehicle in a 5-day dehydrated animal (DE-veh) (C), and 2 h after injection of 2DG in a a 5 day dehydrated animal (DE-2DG) (D). cc, central canal; sol, solitary tract. Scale bar in A = 100 μm.
Area postrema.
The number of Fos-ir cells in the AP (Fig. 8) was similar to those seen in the NTS across each of the experimental treatments, although the staining intensity of Fos-ir neurons within the AP appeared somewhat lighter than in the NTS (Fig. 9). There was a main effect of both 2DG [F (3,19) = 161.75, P < 0.0001] and drinking hypertonic saline [F (3,19) = 312.63, P < 0.0001] on the number of Fos-ir neurons in the NTS and DMX, as well as a significant interaction [F (3,19) = 31.52, P < 0.0001]. Figures 8 and 9 show that Fos response to 2DG was significantly greater in DE animals than in EU animals (P < 0.01).
DISCUSSION
Previous studies have shown that when presented by themselves, drinking hypertonic saline and 2DG are each followed by extensive Fos expression in the hypothalamus and hindbrain, including regions closely associated with feeding and metabolic regulation (9, 23, 25, 52). Fos expression after 2DG has been used to implicate particular brain regions in the control of feeding (18, 23), while regions showing increased Fos-ir only after drinking hypertonic saline may be associated with increased thirst. Our data now reveal regions where these two stimuli might interact to suppress 2DG feeding in DE animals.
On the basis of the behavior control column model (39), the PVH and LHA are likely sites of action for dehydration's effects on the suppression of the feeding response to 2DG (54). If this hypothesis is correct, then either the PVH or LHA or both should show no further increase in Fos expression when drinking hypertonic saline and 2DG are combined, compared with when they are presented separately. On the other hand, regions that do not contain sites of convergence should show Fos responses that are approximately equivalent to the sum of the responses to each stimulus when they are presented separately. Furthermore, if drinking hypertonic saline does not target the afferent sets that provide critical inputs to control column neurons (54), then Fos responses in hindbrain regions where the afferents engaged by 2DG originate should also be unaffected and show approximately additive Fos responses to the two stimuli. When we quantified Fos expression patterns in those hypothalamic and hindbrain regions most closely associated with 2DG-stimulated feeding, we found a number of response categories in DE animals.
The first group was consistent with the hypothesis that drinking hypertonic saline targets neurons in the LHA to suppress 2DG feeding. We found that drinking hypertonic saline alone elicited significant Fos expression in LHA neurons, particularly in those parts lateral and dorsolateral to the fornix. These observations are consistent with our previous findings (52), which showed that after DE, Fos-ir was found in neurons expressing CRH mRNA but not those with orexin or melanocyte-stimulating hormone mRNAs. 2DG also increased Fos activation in these same parts of the LHA. But when 2DG was administered to DE animals, the number of Fos-ir neurons did not increase beyond that seen with drinking hypertonic saline alone, consistent with the notion that both stimuli target the same LHA neurons. We do not believe this is a maximal response because reversing DE anorexia by returning drinking water generates significantly greater numbers of Fos-ir neurons than DE alone, primarily in orexin mRNA-containing neurons (52).
We have previously shown that parts of the LHA receive afferents that are sensitive to plasma osmolality (16). The LHA also receives NPY-containing projections from the ARH and hindbrain that are important for stimulating feeding after energy deficit and 2DG administration (3, 10). Thus, the LHA may house neuronal populations that can integrate information about hydration and energy status. Our data suggest that the attenuated Fos response to 2DG in the LHA of DE animals reflects a DE-imposed alteration of this integrative process. Thus, information encoded by NPY-containing circuits, which normally elicit food intake in response to caloric deficits, is no longer effective in DE-anorexia. This notion is further supported by our data showing that the LHA is progressively desensitized to the feeding stimulatory effects of exogenous NPY during drinking hypertonic saline (31, 54).
Excitotoxic lesions of the LHA reveal a major role for some LHA neurons within a hypothalamic ingestive behavior controller (4–6, 57). These LHA neurons appear to integrate information from a variety of interosensory and exterosensory modalities and then initiate appropriate ingestive behavioral responses, probably by way of their extensive projections to the PVH, telencephalon, and hindbrain (55, 57). Thus, LHA-lesioned animals do not eat after 2DG, nor do they drink appropriately after hypertonic saline injections (5, 57). However, LHA-lesioned animals continue to regulate ingestive behaviors appropriately after food or water deprivation (4, 5). These data demonstrate that parts of the LHA are essential for appropriate compensatory responses when the deficit is signaled entirely through interosensory signals (glucoprivation and elevated plasma osmolality) but not when the deficit also involves more complex exterosensory signals, such as those arising from deprivation (57).
The second response pattern was seen in some regions when drinking hypertonic saline and 2DG were combined. Here, Fos-ir neurons were more numerous than when either stimulus was presented separately and often approximated the sum of the numbers seen when the two stimuli were presented separately. This pattern is predicted by separate populations responding to either 2DG or DE, rather than a single population receiving convergent inputs.
We found that 2DG administration increased Fos-ir neurons in the NTS, DMX, AP, PBl, and the ARH. Similarly, drinking hypertonic saline alone also increased Fos expression in these same regions. But when 2DG was given to DE animals the number of Fos-ir neurons in each region increased to values that were approximately the sum of those seen following separate administration. Thus, there was no suppression of the 2DG response by DE. This result is consistent with the existence of separate populations of neurons within each region that respond to only one of the two stimuli. This pattern was clearest in the PBl, where 2-DG-responsive neurons were seen more laterally than those responding to DE. These results strongly suggest that drinking hypertonic saline and 2DG each target distinct sets of neurons in the NTS, DMX, AP, PB, and the ARH, rather than converging on a single population.
Ascending catecholaminergic projections to the PVH—many of which originate in the NTSm (7, 33)—are critical components of the feeding response to 2DG (24). In this way, they provide information about metabolic state to the hypothalamic behavior control column (54). The fact that drinking hypertonic saline did not attenuate the Fos response to 2DG in the NTSm supports our hypothesis that drinking hypertonic saline targets the behavior controller neurons in the PVH and LHA, rather than those neurons that provide afferent input to the PVH and LHA.
The ARH is critical to the behavioral, endocrine, and autonomic motor responses to alterations in energy status, particularly those signaled by changes in circulating leptin, insulin, or ghrelin (3, 10, 21). A hypocaloric state is characterized by sharply lower circulating levels of insulin and leptin, which reduce Pomc expression and increase Npy and Agrp expression in ARH neurons (19, 29, 31, 34). Rats that chronically drink hypertonic saline are also severely hypocaloric and show the same changes in ARH gene expression as food-restricted animals (22, 55).
In agreement with previous studies (18), we found that 2DG administration increased Fos-ir in ARH cells of EU animals, although the number of labeled neurons was small. Similarly, drinking hypertonic saline also increased Fos-ir in the ARH (36, 52). However, when 2DG was administered to DE animals, the numbers of Fos-ir neurons were approximately double those seen when each treatment was administered separately. Although more Fos-ir neurons were present in the medial component of the ARH after 2DG than after drinking hypertonic saline, the neuropeptidergic content of the activated neurons in response to each stimulus is not known. Regardless, drinking hypertonic saline does not appear to inhibit the Fos response of the 2DG-sensitive population. This conclusion is consistent with our previous findings showing that ARH neurons in DE animals remain capable of responding appropriately to signals of diminished energy status, at least in terms of gene expression (55). Furthermore, the fact that 2DG-stimulated feeding is normal after complete chemical ablation of mediobasal hypothalamic neurons that express NPY receptors (3) supports the notion that the inhibition of 2DG feeding by drinking hypertonic saline is not initiated at the level of the ARH. Rather, our more recent findings suggest that one reason DE animals remain anorexic is the significantly reduced sensitivity of some of their NPY target sites, particularly the PVH and LHA (30, 49, 54).
The PVH is a critical part of the hypothalamic behavior control column (39). It contains sets of parvicellular neurons that are heavily implicated in autonomic and behavioral control, including those in the PVHlp that project to the midbrain, hindbrain, and spinal cord (12, 13, 42). The PVH also has two neuroendocrine compartments: the PVHpml, which contains vasopressin and oxytocin magnocellular neurons; and the PVHmp, which contains neuroendocrine CRH neurons responsible for increased glucocorticoid secretion following 2DG (26). Because the Fos responses in the various parts of the PVH following DE, 2DG or both stimuli together were more complex than elsewhere, we now discuss our results for each PVH compartment.
We found significant Fos-ir in the PVHlp after drinking hypertonic saline, but not 2DG in either EU or DE animals. These results, together with others (38), support the notion that PVHlp neurons are chronically activated by DE. Because of their location in a part of the PVH that by virtue of its connections (42) is well placed to regulate feeding behavior, these neurons are potential contributors to DE anorexia. Of course, they may also be critical to the increased thirst apparent in these animals.
Consistent with other published reports (18, 23, 26), 2DG substantially increased the numbers of Fos-ir neurons in the PVHmpd. However, drinking hypertonic saline generated few Fos-ir neurons in this part of the PVH, which again has been previously reported (51). However, when 2DG was given to DE animals, the number of Fos-ir neurons actually decreased from that seen in EU animals. This finding is consistent with the diminished responsiveness of CRH neurons in DE animals that we have previously reported with other stressors (45, 51).
We found significant numbers of Fos-ir neurons in the PVHpml following drinking hypertonic saline. Although 2DG administration also increased Fos-ir in these neurons, their number was significantly less than after drinking hypertonic saline alone. However, when DE animals were given 2DG, there was little change in the number of Fos-ir neurons, most likely because they were already fully activated by 5 days of drinking hypertonic saline. Why 2DG alone increased activation in these magnocellular neurons is unclear but may result from osmotic stimuli associated with the sustained hyperglycemia (15) that occurs after 2DG administration.
Although the Fos-ir response profiles in the two PVH neuroendocrine compartments is consistent with one that might generate anorexia (i.e., the Fos response to 2DG is attenuated by drinking hypertonic saline), three observations make it unlikely that these neurons can be directly implicated in this manner. First, these neurons are neuroendocrine and projections to regions other than to the neurohypophysis have not been reported. Second, DE anorexia does not result from either elevated corticosterone or reduced CRH mRNA levels in the PVHmp because adrenalectomy abolishes the reduction in CRH mRNA following drinking hypertonic saline but not the development of anorexia (50, 55). Third, 2DG-stimulated corticosterone secretion remains intact, while feeding is markedly attenuated in DE animals (30).
Perspectives and Significance
A useful model for the neural control of motivated behaviors is the behavioral control column proposed by Swanson (39). As yet unidentified neurons in the PVH and possibly the LHA comprise the behavioral controllers for ingestive behaviors. Two hypotheses for understanding how DE anorexia is generated derive from this model: first, DE targets the afferent inputs to the behavioral controllers; second, DE targets the behavioral controllers themselves. The present data provide evidence that DE inhibits feeding by targeting neurons in the LHA and possibly PVHlp that are critical for feeding, rather than the afferents themselves. One way that anorexia develops under these circumstances is a dehydration-induced desensitization of appropriate neurons in the LHA and PVH to afferent inputs that would ordinarily stimulate feeding.
Considered in the larger perspective of understanding why animals develop anorexia, our results support the notion that some types of anorexia develop, in part, because of an active inhibition of control neurons common to all types of feeding. This notion would be consistent with our observations that all types of feeding so far examined are suppressed by DE, despite a diversity of stimuli that contribute to their expression (30, 31, 46, 49, 54).
GRANTS
This study was supported by National Institutes of Health Grants R0-1 MH066168 (to A. G. Watts) and F31 MH067392 (to D. Salter-Venzon).
Acknowledgments
Present address for D. Salter-Venzon: Keck School of Medicine, Institute of Preventive Medicine, University of Southern California, Alhambra, CA 91803.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res 848: 114–123, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR, Mogenson GJ. Ingestive behavior after intracerebral and intracerebroventricular infusions of glucose and 2-deoxy-d-glucose. Am J Physiol Regul Integr Comp Physiol 233: R127–R133, 1977. [DOI] [PubMed] [Google Scholar]
- 3.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146: 1179–1191, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Clark AJ, Clark JM, Winn P. NMDA lesions of rat lateral hypothalamus: effects of dietary and physiological challenges. Neuroreport 1: 263–266, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Clark AJ, Bartle A, Winn P. The regulation of feeding and drinking in rats with lesions of the lateral hypothalamus made by N-methyl-d-aspartate. Neuroscience 45: 631–640, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM, Clark AJ, Warne D, Rugg EL, Lightman SL, Winn P. Neuroendocrine and behavioural responses to hyperosmolality in rats with lesions of the lateral hypothalamus made by N-methyl-d-aspartate. Neuroscience 45: 625–629, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham ET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 273: 60–76, 1988. [DOI] [PubMed] [Google Scholar]
- 8.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, sequelae. Endocrinology 140: 4015–4023, 1999. [DOI] [PubMed] [Google Scholar]
- 9.De Luca LA, Xu Z, Schoorlemmer GH, Thunhorst RL, Beltz TG, Menani JV, Johnson AK. Water deprivation-induced sodium appetite: humoral and cardiovascular mediators and immediate early genes. Am J Physiol Regul Integr Comp Physiol 282: R552–R559, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci 1: 445–450, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol 433: 222–238, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Hallbeck M, Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J Comp Neurol 411: 201–211, 1999. [PubMed] [Google Scholar]
- 14.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14: 173–213, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki Y, Kondo K, Murase T, Hasegawa H, Oiso Y. Osmoregulation of plasma vasopressin in diabetes mellitus with sustained hyperglycemia. J Neuroendocrinol 8: 755–760, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Kelly AB, Watts AG. Mediation of dehydration-induced peptidergic gene expression in the rat lateral hypothalamic area by forebrain afferent projections. J Comp Neurol 370: 231–246, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Khan AM, Watts AG. Intravenous 2-deoxy-d-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology 145: 351–359, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by administration of 2-deoxy-d-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Brain Res Mol Brain Res 33: 305–310, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140: 814–817, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci 12: 459–462, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea RD, Gundlach AL. NPY mRNA and peptide immunoreactivity in the arcuate nucleus are increased by osmotic stimuli: correlation with dehydration anorexia. Peptides 16: 1117–1125, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 641: 111–20, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 432: 197–216, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-d-glucose induced metabolic challenge. Brain Res 805: 41–54, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357–1367, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Russell PJ, Mogenson GJ. Drinking and feeding induced by jugular and portal infusions of 2-deoxy-d-glucose. Am J Physiol 229: 1014–1018, 1975. [DOI] [PubMed] [Google Scholar]
- 28.Sahu A Evidence suggesting that galanin (GAL), melanin-concentrating hormone (MCH), neurotensin (NT), proopiomelanocortin (POMC) and neuropeptide Y (NPY) are targets of leptin signaling in the hypothalamus. Endocrinology 139: 795–798, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL), and neuropeptide Y (NPY) in the rat. Endocrinology 139: 4739–4742, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Salter D, Watts AG. Differential suppression of hyperglycemic, feeding, and neuroendocrine responses in anorexia. Am J Physiol Regul Integr Comp Physiol 284: R174–R182, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Salter D, Watts AG. Feeding response to hypothalamic injections of NPY are attenuated in dehydration anorexia. Soc Neurosci Abst 29: 615, 2003. [Google Scholar]
- 32.Saper CB, Chou TC, Elmquist JK. The need to feed. Homeostatic and hedonic control of eating. Neuron 36: 199–211, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 257: 275–325, 1982. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46: 2119–2123, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Solano JM, Jacobson L. Glucocorticoids reverse leptin effects on food intake and body fat in mice without increasing NPY mRNA. Am J Physiol Endocrinol Metab 277: E708–E716, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. C-fos expression in arcuate nucleus following intracerebroventricular hypertonic saline injections. Neurosci Lett 164: 217–220, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res 604: 304–317, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Swanson LW Cerebral hemisphere regulation of motivated behavior. Brain Res 886: 113–164, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW Brain Maps: Structure of the Rat Brain. A Laboratory Guide With Printed and Electronic Templates for Data, Models and Schematics. 3rd ed. Amsterdam: Elsevier, 2004.
- 41.Swanson LW Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol 493: 122–131, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 43.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett 387: 80–84, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Ueta Y, Yamashita H, Kawata M, Koizumi K. Water deprivation induces regional expression of c-fos protein in the brain of inbred polydipsic mice. Brain Res 677: 221–228, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Watts AG The impact of physiological stimuli on the expression of corticotropin- releasing hormone (CRH) and other neuropeptide genes. Front Neuroendocrinol 17: 281–326, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Watts AG Dehydration-associated anorexia: development and rapid reversal. Physiol Behav 65: 871–878, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Watts AG Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav 37: 261–283, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Watts AG Neuropeptides and the integration of motor responses to dehydration. Annu Rev Neurosci 24: 357–384, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Watts AG, Salter D. Neural Mechanisms of Anorexia. In: Handbook of Behavioral Neurobioogy: Neurobiology of Food and Fluid Intake, edited by Stricker E, Woods S., 2nd ed. New York, NY: Kluwer Academic/Plenum Publishers, 2004, vol 14, p. 383–420. [Google Scholar]
- 50.Watts AG, Sanchez-Watts G. A cell-specific role for the adrenal gland in regulating CRH mRNA levels in rat hypothalamic neurosecretory neurones after cellular dehydration. Brain Res 687: 63–70, 1995. [DOI] [PubMed] [Google Scholar]
- 51.Watts AG, Sanchez-Watts G. Interactions between heterotypic stressors and corticosterone reveal integrative mechanisms for controlling corticotropin-releasing hormone gene expression in the rat paraventricular nucleus. J Neurosci 22: 6282–6289, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watts AG, Sanchez-Watts G. Rapid and preferential activation of Fos protein in hypocretin/orexin neurons following the reversal of dehydration-anorexia. J Comp Neurol 502: 768–782, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Watts AG, Kelly AB, Sanchez-Watts G. Neuropeptides and thirst: the temporal response of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci 109: 1146–1157, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Watts AG, Salter DS, Neuner CM. Neural network interactions and ingestive behavior control during anorexia. Physiol Behav 91: 389–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci 19: 6111–6121, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts AG, Khan AM, Sanchez-Watts G, Salter D, Neuner CM. Activation in neural networks controlling ingestive behaviors: what does it mean and how do we map and measure it? Physiol Behav 89: 501–520, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Winn P The lateral hypothalamus and motivated behavior: An old syndrome reassessed and a new perspective gained. Curr Dir Psychol Sci 4: 182–187, 1995. [Google Scholar]