Abstract
Obstructive sleep apnea is characterized by upper airway collapse, leading to intermittent hypoxia (IH). It has been postulated that IH-induced oxidative stress may contribute to several chronic diseases associated with obstructive sleep apnea. We hypothesize that IH induces systemic oxidative stress by upregulating NADPH oxidase, a superoxide-generating enzyme. NADPH oxidase is regulated by a cytosolic p47phox subunit, which becomes phosphorylated during enzyme activation. Male C57BL/6J mice were exposed to IH with an inspired O2 fraction nadir of 5% 60 times/h during the 12-h light phase (9 AM–9 PM) for 1 or 4 wk. In the aorta and heart, IH did not affect lipid peroxidation [malondialdehyde (MDA) level], nitrotyrosine level, or p47phox expression and phosphorylation. In contrast, in the liver, exposure to IH for 1 wk resulted in a trend to an increase in MDA levels, whereas IH for 4 wk resulted in a 38% increase in MDA levels accompanied by upregulation of p47phox expression and phosphorylation. Administration of an NADPH oxidase inhibitor, apocynin, during IH exposure attenuated IH-induced increases in hepatic MDA. In p47phox-deficient mice, MDA levels were higher at baseline and, unexpectedly, decreased during IH. In conclusion, oxidative stress levels and pathways under IH conditions are organ and duration specific.
Keywords: obstructive sleep apnea, NADPH oxidase
obstructive sleep apnea (OSA) results in repetitive cycles of hypoxia and reoxygenation, termed intermittent hypoxia (IH) (27). The potential role of OSA in the development of chronic diseases is becoming increasingly recognized. For example, OSA is associated with elevated risks of hypertension and atherosclerosis (24, 53, 64, 85). Endothelial dysfunction correlates with the severity of O2 desaturations in patients with OSA (24, 64, 85). A causal role of OSA in atherosclerosis is supported by evidence that continuous positive airway pressure (CPAP) improves early signs of atherosclerosis (23) and our animal data showing that chronic IH induced atherosclerosis in C57BL/6J mice fed a high-cholesterol diet (78). OSA is also associated with nonalcoholic steatohepatitis in obese individuals (1, 15, 44–46, 65, 68, 88). CPAP therapy ameliorates aminotransferase elevations (46), and experimental IH causes inflammatory liver injury in mice fed a high-fat, high-cholesterol diet (77). A mechanism has not been established for these observations.
Diseases associated with OSA are thought to be due, at least in part, to oxidative stress (51, 97). The hypothesis that IH causes oxidative stress stems from observations that ischemia-reperfusion (I/R) or anoxia-reoxygenation injury gave rise to reactive oxygen species (ROS) (3, 5, 20, 32, 48, 75, 99). Applicability of this concept to OSA has been shown in several studies, as exemplified by increased exhaled isoprostanes (9, 10), blood reactive oxygen metabolites (18), serum malondialdehyde (MDA) (6, 52), neutrophil superoxide production (80), and susceptibility of serum LDL to oxidation (80) in OSA.
Furthermore, CPAP treatment has been shown to significantly improve parameters of inflammation and oxidative stress (17, 22, 43) in patients with OSA. In rodent models of IH, oxidative stress has also been reported in association with left ventricular dysfunction (13), neurodegenerative changes (76, 96, 98), or dyslipidemia (54). However, other investigators found no increases in serum free nitrotyrosine (87), lipid peroxidation (69, 86), or LDL oxidation susceptibility (93) in OSA. Overall, there is lack of consensus regarding IH-induced oxidative stress and little investigation into a pathway by which pathological ROS might be generated.
A principal source of ROS in many tissues is NADPH oxidase (34, 63, 66). In neutrophils, two membrane-bound subunits, gp91phox and p22phox, comprise cytochrome b558. Cytosolic regulatory subunits include p47phox, p67phox, p40phox, and the small GTP-binding protein Rac (12, 25). Several isoforms of the enzyme have been described in various tissues. In most known isoforms, p47phox phosphorylation is the initial step in enzyme activation. Deletion of p47phox inhibits ROS generation in the aorta (7, 38, 47) and ameliorates atherosclerosis in apolipoprotein E-deficient mice (7), demonstrating the relevance of this enzyme to cardiovascular disease. Inhibition of NADPH oxidase with apocynin also attenuates cholesterol-induced liver injury (60).
Our study set out to answer three questions. 1) Does IH per se induce circulatory or tissue oxidative stress? 2) Does IH affect NADPH oxidase activity in tissues? 3) Does NADPH oxidase activation contribute to IH-induced oxidative stress in the aorta, heart, or liver?
METHODS
Animals.
Sixty-four 8-wk-old male, lean C57BL/6J mice and sixteen 8-wk-old male p47phox-deficient (p47phox−/−) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The study was approved by the Johns Hopkins University Animal Care and Use Committee and complied with the American Physiological Society guidelines for animal studies. Inasmuch as animals lacking functional NADPH oxidase are prone to infection, animals were housed two per cage, food and bedding were autoclaved, and cages were thoroughly cleaned with detergent and ethanol every 3–4 days. The same protocol was used with C57BL/6J mice to equalize handling. For blood sample collection, surgical procedures, and tissue collection, anesthesia was induced and maintained with 1–2% isoflurane administered through a facemask. All mice were fed a regular Purina chow diet (3.3 cal/g, 4% fat). Mice were fasted for 5 h before they were exsanguinated and killed. For biochemical assays, the heart, descending aorta, and liver were immediately frozen at −80°C for future analysis.
Intermittent hypoxia.
A gas control delivery system was designed to regulate the flow of room air, N2, and O2 into customized cages housing the mice, as previously described (72). During each period of IH, the inspired O2 fraction was reduced from 20.9% to 4.9 ± 0.1% over a 30-s period and then rapidly restored to room air levels in the subsequent 30-s period. Animals were kept in a controlled environment (22–24°C with a 12:12-h light-dark cycle; lights on at 0900) with free access to food and water. Control animals were exposed to intermittent air (IA), with a flow pattern similar to that of the IH group, but at a fixed 21% inspired O2 fraction. IH and IA states were induced during the 12-h light phase and alternated with 12 h of constant room air during the dark phase.
Apocynin injections.
Mice were given daily injections of apocynin (2 mg·kg−1·day−1 ip) dissolved in 0.9% saline according to the method used by Hart et al. (35). A matched control group (n = 8) received 0.9% saline placebo (2 mg·kg−1·day−1 ip).
Lipid peroxidation.
Tissues were isolated and homogenized in 10 μl/mg ice-cold PBS containing 5 mM butylated hydroxytoluene to inhibit ex vivo oxidation. Serum and tissue thiobarbituric acid-reactive substance was determined using commercially available kits (Zeptometrix, Buffalo, NY). For determination of oxidized LDL (oxLDL), lipids were first isolated from mouse serum by manganese citrate-heparin precipitation (29). As a positive oxidized control, a separate aliquot of serum was incubated with 50 μM CuSO4 for 300 min according to method described by Cominacini et al. (19). oxLDL was assessed by measurement of thiobarbituric acid-reactive substance in the solubilized LDL pellet.
3-Nitrotyrosine.
3-Nitrotyrosine (3-NT) levels, a marker of peroxynitrite formation, were assessed with a commercially available ELISA (Oxis Research, Portland, OR). Tissues were first homogenized and diluted to a concentration of 1 mg/ml protein in dilution buffer supplied by the manufacturer. Serum samples were diluted 1:10 in dilution buffer. Antigen captured by a solid-phase monoclonal antibody was detected with a biotin-labeled goat polyclonal anti-nitrotyrosine. Streptavidin peroxidase was allowed to bind to the biotinylated antibody. Addition of a tetramethylbenzidine substrate yielded a yellow product, which was measured at 450 nm. All unknowns were run in triplicate, and log 3-NT levels were interpolated by nonlinear regression using Prism software (GraphPad, San Diego, CA). 3-NT levels were normalized to milligrams of protein as determined by Bradford assay. Nitrated albumin was used as a competitive blocking control to verify specificity of observed interactions. Liver from mice treated for 4 wk with acetaminophen (200 mg·kg−1·day−1 ip) was used as a positive control and demonstrated elevated hepatic and serum 3-NT levels (data not shown).
GSH and GSH-to-GSSG ratio.
GSH and GSH-to-GSSG ratio (GSH/GSSG) were determined by use of commercially available colorimetric assays (Oxis Research). For GSSG measurement in erythrocytes, an aliquot of 100 μl of freshly drawn whole blood was immediately mixed with 10 μl of 1-methyl-2-vinyl-pyridium trifluoromethane sulfonate to scavenge reduced glutathione before freeze-thaw lysis. Frozen tissues were placed in PBS containing 10% 1-methyl-2-vinyl-pyridium trifluoromethane sulfonate before homogenization. Samples were further diluted in metaphosphoric acid and assayed according to the manufacturer's protocol. GSH/GSSG was calculated as follows: {[GSH] − (2*[GSSG])}/[GSSG], where [GSH] and [GSSG] are GSH and GSSG concentrations.
SOD activity.
Liver or heart tissue was homogenized and diluted to a concentration of 5 mg/ml protein per sample. Homogenate (40 μl) was then used in a commercially available kinetic assay (Oxis Research). For SOD activity in red blood cells, whole blood was centrifuged to obtain the erythrocyte pellet that was diluted in 4 vol of ice-cold water for hypotonic lysis. Before assay, the lysate was diluted another fourfold in assay buffer supplied by the manufacturer. The assay measures the SOD-mediated increase in the rate of autooxidation of 5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzo[c]fluorene in aqueous alkaline solution to yield a chromophore with maximum absorbance at 525 nm. Absorbance of the chromophore was measured over 3 min, and the slope of the inflection point (Vs) was divided by the slope of a blank autooxidation sample (Vc). The Vs-to-Vc ratio was used to calculate the SOD units, where 1 unit of SOD activity is defined as the activity that doubles the autooxidation rate of the control blank (Vs/Vc = 2).
Abundance of p47phox protein and serine phosphorylation.
Descending aorta was homogenized in 150 mM NaCl, 20 mM Tris (pH 7.2), 1% Triton X-100, and 1 mM DTT with complete protease inhibitor (Roche Diagnostics, Mannheim, Germany). Homogenate was electrophoresed in 4–15% Tris-glycine SDS polyacrylamide with a Bio-Rad precast system. Proteins were transferred onto polyvinyl diethyl fluoride membranes and probed with anti-p47phox (catalog no. C20, Santa Cruz Biotechnology, Santa Cruz, CA; 1:100 dilution) followed by goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology; 1:5,000 dilution). Immune complexes were visualized with an enhanced chemiluminescence detection system. After immunoblotting with anti-p47phox, we assessed serine phosphorylation by stripping the membrane and reprobing with phosphorylated (Ser/Thr) Akt substrate antibody (Cell Signaling Technologies; 1:1,000 dilution). Actin was used as a loading control for all samples.
Statistical analysis.
Values are means ± SE. Statistical comparisons between groups of mice were performed by a general linear model ANOVA across two independent variables, hypoxia and time course, followed by Tukey's post hoc test. In experiments involving apocynin, ANOVA was performed independently for the 1-wk and the 4-wk data sets. The error of the ratios was estimated as follows: (standard errorIH/mean MDAIH) + (standard errorIA/mean MDAIA).
RESULTS
Body weight and food intake.
In the 1- and 4-wk exposure groups, mice exposed to IA gained weight while mice exposed to IH lost weight. Most of the weight was lost by the end of the 1st wk, after which food intake increased (Table 1). Thus, chronic IH resulted in weight loss and decreased food intake, consistent with our previous observations (54).
Table 1.
Mouse weight at the start and end of IA or IH exposures and average daily food consumption
|
Group |
P | ||||
|---|---|---|---|---|---|
| IA | IH | ||||
| 1 wk | |||||
| Body wt, g | |||||
| Start | 23.6±0.4 | 21.2±0.8 | NS | ||
| End | 25.0±0.7 | 20.1±0.7 | <0.001 | ||
| Δ | 1.4 | −1.1 | <0001 | ||
| Daily food intake, g | 3.4±0.2 | 2.9±0.4 | <0.001 | ||
| 4 wk | |||||
| Body wt, g | |||||
| Start | 26.5±0.7 | 26.9±0.9 | NS | ||
| End | 28.7±0.5 | 25.4±0.7 | <0.005 | ||
| Δ | 2.1 | −1.4 | <0.001 | ||
| Daily food intake, g | 3.8±0.2 | 3.3±0.1 | <0.001 | ||
Values are means ± SE. IA, intermittent air (control); IH, intermittent hypoxia; NS, not significant.
Serum and blood markers of oxidative stress and antioxidant status.
IH exposure for 1 or 4 wk did not produce detectable changes in serum MDA or 3-NT, erythrocyte SOD activity, or GSH/GSSG (Table 2). There was a trend toward decreased erythrocyte GSH after 1 wk (P = 0.17), but not after 4 wk. To determine the effect of IH on oxLDL, a recognized precursor to atheroma formation (16, 40, 83, 84, 95), we isolated LDL and VLDL fractions from serum and measured lipid peroxidation in the solubilized lipid pellet. We were surprised to find a decrease in oxLDL in the serum after 1 wk of IH (Table 2). At 4 wk, there was no difference in oxLDL between controls and experimental animals.
Table 2.
Biomarkers of oxidative stress and antioxidant status in serum and blood of mice exposed to IA or IH
|
Group |
||
|---|---|---|
| IA | IH | |
| Serum MDA, μM/ml | ||
| 1 wk | 0.36±0.01 | 0.34±0.01 |
| 4 wk | 0.32±0.04 | 0.37±0.04 |
| Serum ox-LDL, μM/ml | ||
| 1 wk | 92.9±5.2 | 66.9±4.2* |
| 4 wk | 93.3±6.7 | 88.1±6.7 |
| Serum 3-NT, nM/ml | ||
| 1 wk | 4.1±1.0 | 3.5±0.9 |
| 4 wk | 3.2±0.6 | 3.4±0.8 |
| RBC SOD, U/100 μl | ||
| 1 wk | 7.86±1.24 | 7.38±1.84 |
| 4 wk | 7.57±0.98 | 7.64±1.61 |
| RBC GSH, μM/100 μl | ||
| 1 wk | 200.9±4.36 | 185.2±9.72† |
| 4 wk | 206.6±4.61 | 202.4±5.15 |
| GSH/GSSG | ||
| 1 wk | 142±33.7 | 156±56.8 |
| 4 wk | 138±31.4 | 164±38.4 |
Values are means ± SE; n = 32 (8 mice per group). MDA, malondialdehyde; oxLDL, oxidized LDL; 3-NT, 3-nitrotyrosine.
P < 0.001;
P = 0.17 vs. IA.
Cardiovascular oxidative stress, NADPH oxidase activity, and antioxidant status.
Biomarkers of oxidative stress and antioxidant status in the heart and aorta are shown in Table 3. In the heart, IH did not lead to increases in MDA or 3-NT at 1 or 4 wk. There was also no apparent change in p47phox expression or serine phosphorylation, suggesting unchanged NADPH oxidase activity (Figs. 1 and 2). SOD activity, GSH, and GSH/GSSG in the heart were unchanged across all groups (Table 4). In aortic tissue, there was a trend toward increased MDA after 1 wk of IH (Table 3), but not after 4 wk. There were no IH-induced increases in MDA, 3-NT, or p47phox expression and serine phosphorylation.
Table 3.
Biomarkers of oxidative stress in heart and aorta of mice exposed to IA or IH
|
Heart |
Aorta
|
|||
|---|---|---|---|---|
| IA | IH | IA | IH | |
| MDA, μM/mg | ||||
| 1 wk | 3.56±0.42 | 3.18±0.24 | 4.47±0.96 | 6.87±1.08* |
| 4 wk | 3.38±0.30 | 2.76±0.49 | 4.28±1.04 | 4.13±0.35 |
| 3-NT, nM/mg | ||||
| 1 wk | 1.63±0.51 | 1.71±0.65 | 11.01±1.43 | 11.40±2.26 |
| 4 wk | 1.34±0.37 | 1.16±0.23 | 16.07±1.76 | 16.25±1.38 |
Values are means ± SE; n = 32 (8 mice per group).
P = 0.14 vs. IA.
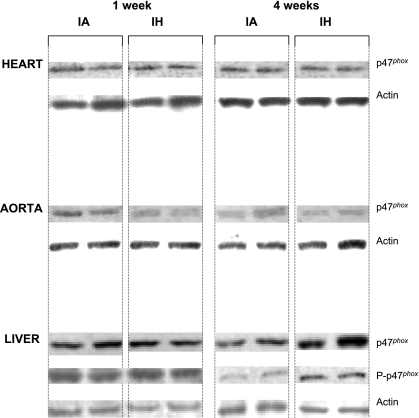
Fig. 1.
Expression of p47phox protein in heart, aorta, and liver of mice exposed to 1 or 4 wk of intermittent air (control, IA) or intermittent hypoxia (IH). Each immunoblot represents results from 4 observations. In heart and aorta, IH did not induce changes in p47phox expression or in serine phosphorylation (not shown) at 1 or 4 wk. In liver, IH induced an increase in p47phox expression and serine phosphorylation after 4 wk. P-p47phox, phosphorylated p47phox.
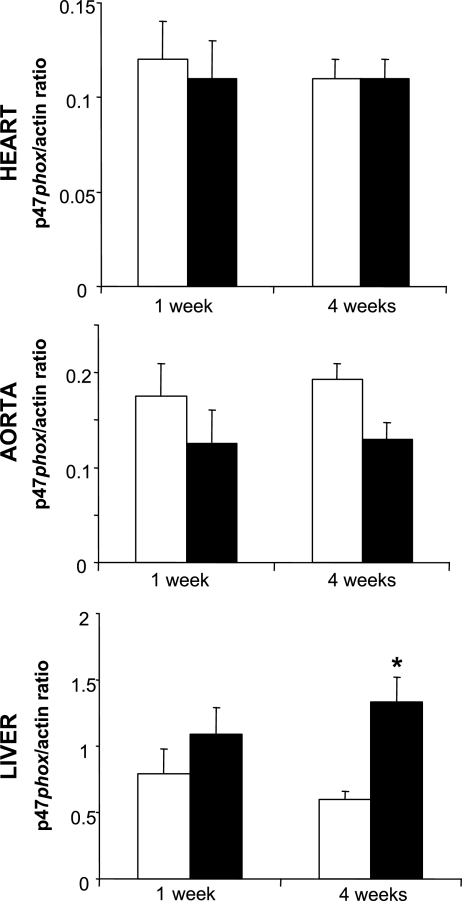
Fig. 2.
Quantification by optical densitometry of immunoblots in Fig. 1. Open bars, IA; solid bars, IH. IH induced a 2-fold increase in hepatic p47phox protein expression and phosphorylation. *P < 0.05 vs. IA.
Table 4.
SOD activity, GSH, and GSH-to-GSSG ratio in heart and liver following exposure to IA or IH
|
Heart |
Liver
|
|||
|---|---|---|---|---|
| IA | IH | IA | IH | |
| SOD, U/mg | ||||
| 1 wk | 11.6±1.1 | 10.9±1.4 | 24.7±1.4 | 25.6±1.2 |
| 4 wk | 10.0±1.2 | 10.8±1.2 | 22.5±0.1 | 25.1±0.7 |
| GSH, μM/mg | ||||
| 1 wk | 17.3±1.8 | 14.7±1.4 | 69.1±4.8 | 56.7±3.1* |
| 4 wk | 18.5±1.7 | 21.1±2.0 | 79.1±6.2 | 79.3±10.5 |
| GSH/GSSG | ||||
| 1 wk | 176±23.1 | 206±36.2 | 167±31 | 132±13 |
| 4 wk | 191±29.9 | 188±35.0 | 195±33 | 176±28 |
Values are means ± SE; n = 32 (8 mice per group).
P = 0.058 vs. IA.
Liver oxidative stress, NADPH oxidase activity, and antioxidant status.
IH led to increases in lipid peroxidation in the liver (Fig. 3). The effect was significant after 4 wk, leading to a 38% increase in hepatic MDA levels. There was a concurrent increase in p47phox expression and serine phosphorylation after 4 wk of IH exposure (Figs. 1 and 2). GSH trended toward a decrease (P = 0.058) after 1 wk but was restored at 4 wk, and GSH/GSSG was unaffected at 1 and 4 wk (Table 4). There was no effect of IH on liver 3-NT (not shown) or SOD activity (Table 4). IH did not induce overt liver injury, as indicated by serum aminotransferase levels or organ weight (Table 5). Serial sections of liver showed no evidence of tissue injury.
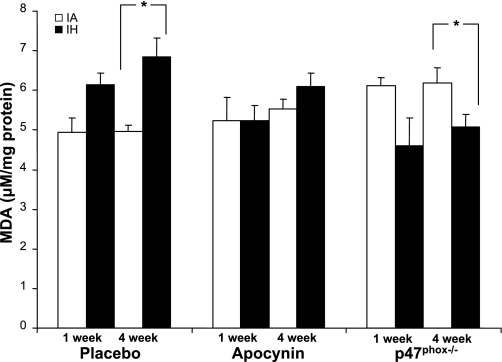
Fig. 3.
Liver malondialdehyde (MDA) after exposure to 1 or 4 wk of IA or IH in mice treated with placebo or apocynin (2 mg·kg−1·day−1) or mice lacking functional p47phox (p47phox−/−). At 1 wk, there was a trend toward increased MDA with IH (P = 0.18). At 4 wk, IH induced a 38% increase in MDA. Apocynin attenuated the rise in MDA (*P < 0.005). In p47phox−/− mice, increased MDA levels normalized during IH. Values are means ± SE; n = 96 (8 animals per group).
Table 5.
Liver weight and function following exposure to IA or IH
|
Group |
P | ||
|---|---|---|---|
| IA | IH | ||
| Liver wt, mg | |||
| 1 wk | 1,291±70.3 | 1,165±43.4 | NS |
| 4 wk | 1,355±32.6 | 1,149±45.2 | NS |
| Liver wt/body wt | |||
| 1 wk | 0.052 | 0.058 | NS |
| 4 wk | 0.047 | 0.045 | NS |
| AST, U/l | |||
| 1 wk | 50.5±2.63 | 48.0±2.80 | NS |
| 4 wk | 41.4±3.0 | 47.3±5.64 | NS |
| ALT, U/l | |||
| 1 wk | 23.4±1.15 | 18.7±1.61 | NS |
| 4 wk | 25.0±1.0 | 24.8±5.83 | NS |
| Total protein, g/dl | |||
| 1 wk | 4.6±0.07 | 4.4±0.07 | NS |
| 4 wk | 4.9±0.05 | 5.0±0.04 | NS |
| Albumin, g/dl | |||
| 1 wk | 3.5±0.02 | 3.2±0.06 | NS |
| 4 wk | 3.3±0.05 | 3.5±0.04 | NS |
Values are means ± SE; n = 32 (8 mice per group). ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Effect of altered NADPH oxidase function on liver lipid peroxidation.
Apocynin prevented a rise in MDA at 1 wk and attenuated IH-induced increases in liver MDA after 4 wk (Figs. 3 and 4). Baseline liver MDA levels were ∼20% higher in p47phox−/− than in wild-type C57BL/6J mice. In contrast to wild-type mice, IH resulted in a significant decrease of the hepatic MDA levels in p47phox−/− mice (Figs. 3 and 4). Neither apocynin nor p47phox deletion had a detectable effect on mouse weight, liver phenotype, noninvasively measured blood pressure, 3-NT, GSH, GSH/GSSG, or SOD activity (data not shown). Despite stringent hygiene for all animals, several p47phox−/− mice developed lung granulomas at the time of death. After 1 wk of exposure, two of eight control p47phox−/− mice and three of eight hypoxic p47phox−/− mice had evidence of pulmonary infection; after 4 wk of exposure, pulmonary infection was present in five of eight control p47phox−/− mice and four of eight p47phox−/− hypoxic animals. There was no evidence of infection in any of the placebo- or apocynin-treated animals.
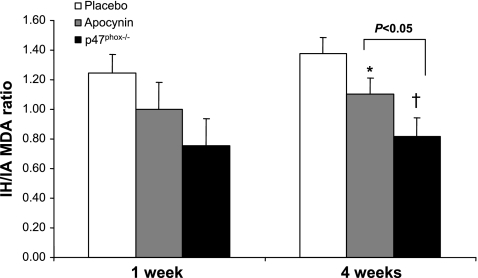
Fig. 4.
IH-to-IA MDA ratios in mice treated with placebo or apocynin (2 mg·kg−1·day−1) or p47phox−/− mice exposed to 1 or 4 wk of IA or IH. At 4 wk, the time point at which IH resulted in significant increases in MDA, apocynin significantly attenuated the rise in liver MDA (*P < 0.01 vs. placebo). p47phox−/− mice exhibited decreased MDA after IH as a result of increased oxidative stress during IA (†P < 0.005 vs. placebo). Decrease in MDA was greater in p47phox than in apocynin-treated mice (P < 0.05). Error bars represent the sum of the standard errors of both IA and IH groups as a percentage of MDA total.
DISCUSSION
We found that IH had variable effects, depending on exposure length and the organ examined. IH did not affect markers of oxidative stress in the circulation or cardiovascular system; NADPH oxidase expression in the heart and aorta did not change. In contrast, in the liver, IH increased lipid peroxidation, NADPH oxidase p47phox subunit protein levels, and phosphorylation. The IH-induced hepatic lipid peroxidation was attenuated by apocynin and abolished by knockout of p47phox. All these effects became significant after 4 wk. Taken together, our data demonstrate a spectrum of tissue susceptibilities to oxidative stress from IH, and this susceptibility appears related to activation of NADPH oxidases.
We did not detect significant oxidative stress in the circulation and cardiovascular organs during IH. It is clear from the literature that myocardial I/R induces an injurious cascade of ROS (2, 26, 36, 92). Our model of hypoxia-reoxygenation does not lead to a similar phenomenon and suggests that the nature of the IH insult does not closely parallel I/R physiology. This could be related to the short duration of hypoxia cycles, incomplete anoxia, or absence of other flow-related I/R events, such as altered ion gradients, capillary damage, and neutrophil influx. However, our group and other researchers employing similar, if not milder, IH exposures in mice have shown upregulation of ROS in the brain, carotid bodies, adrenal glands, and liver (50, 54, 71, 76, 79, 98). IH in the rat led to progressive cardiac lipid peroxidation (13). It is readily apparent that IH-induced oxidative stress is a phenomenon that is species, organ, and time dependent.
Among the organs considered in the present study, the liver was uniquely susceptible to IH. We found an increase in lipid peroxidation over time with upregulation of liver NADPH oxidases. A trend toward decreased GSH stores was seen at 1 wk, and levels were restored after 4 wk. Because GSH/GSSG was unaffected, we infer that GSH synthesis may have been impaired, but the pool of available GSH was not oxidized by IH. Decreased overall food intake with a relative cysteine deficiency can rapidly deplete hepatic GSH stores (62, 89).
Two lines of evidence point to NADPH oxidase as a source of IH-induced ROS in our model. 1) There was an increase in liver p47phox expression and phosphorylation in the liver coinciding with the increase in MDA. 2) MDA levels in wild-type mice treated with apocynin and in p47phox−/− animals were blunted or even decreased after IH. Our finding of increased MDA during IA in p47phox−/− mice is consistent with other reports showing paradoxically increased ROS in the absence of the p47phox subunit (56). It has been postulated that p47phox actually inhibits NADPH oxidase in unstimulated cells (57). In the absence of functional NADPH oxidase, other ROS-generating systems may also become activated in a compensatory manner, as has been described in the context of acute lung injury (49). The reason for the decrease in MDA to control levels during IH is less clear. An otherwise competitive antioxidant effect, such as decreased food intake and weight loss induced by IH, may have become unmasked under p47phox−/− conditions. Thus, the inhibitory effects of apocynin and p47phox deletion demonstrate that functional NAPDH oxidase in the liver is necessary and sufficient for lipid peroxidation during IH.
What predisposed the liver, an organ with high antioxidant reserves, to IH-mediated oxidative stress? 1) The liver likely faced a greater hypoxic insult than the cardiovascular system. Under hypoxic and stressful conditions, blood flow and oxygen delivery are diverted to the cardiovascular and cerebral tissue beds at the expense of visceral organs. Lactate generated from anaerobic glycolysis is readily metabolized by the heart and skeletal muscle (30, 73) but must be salvaged for gluconeogenesis in the liver. The liver was thus effectively hypoxic for longer periods of time. 2) Responses to the hypoxic insult may differ between liver and heart. Liver I/R injury was attenuated in apocynin-treated (58) or gp91phox-deficient (33) mice. On the contrary, p47phox−/− mice were not protected from myocardial I/R injury (37). The liver is also host to phagocytic Kupffer cell NADPH oxidases, which release superoxide during ischemia (90) and mediate oxidative injury from toxins (21, 67). 3) Liver oxidative stress may have occurred via an indirect pathway. Chronic IH elevates serum and hepatic cholesterol levels (54, 55). In rats fed a high-cholesterol diet, liver injury was attenuated by apocynin with concurrent reversal of oxidative stress (60).
MDA, a marker of lipid peroxidation, and 3-NT, a marker of tyrosine oxidation and peroxynitrite formation, are widely used biomarkers of oxidative stress chosen in the present study for their documented relevance to atherosclerosis (16, 70, 82–84, 95) and hepatic oxidative stress (41, 42, 59, 91). In our study, MDA, but not 3-NT, changed with IH exposure. One possibility for the discrepancy is that superoxide was generated during reoxygenation and quickly reacted with regional lipids, rather than with nitric oxide (39). Furthermore, protein nitration may occur in subcellular compartments (8, 28) without detectable increases during assessment of total tissue homogenates.
We must acknowledge several limitations of the present study. Our investigation was designed primarily to examine oxidative stress, rather than functional or anatomic tissue injury. Therefore, we conducted our experiments on relatively healthy C57BL/6J mice fed regular diets, with the knowledge that mice of the same genetic background developed atherosclerosis only if exposed to IH and a high-cholesterol diet (78) and suffered liver injury only if exposed to IH and another insult such as a high-fat diet or acetaminophen (77, 79). In this “single-hit” study of IH, we were not surprised to find modest oxidative stress without a visible effect on organ structure or function. It is therefore beyond the scope of the present study to conclude that the observed oxidative stress causes a detrimental phenotype.
With regard to detection of oxidative stress, it is conceivable that our methods lacked sensitivity. Antioxidant defenses and tissue repair mechanisms may have obscured oxidative stress generated by IH. Lack of change in antioxidant levels might be interpreted as an absence of an oxidizing insult, or they could also reflect ample, unaltered antioxidant reserve in the face of a mild insult. We also should be careful to distinguish oxidative stress from ROS in general. Low, signal-level ROS can have physiological roles or induce downstream pathological changes without overt tissue modification (74). Another potential confounder was the effect of IH on diet and body weight. Mice exposed to IH lost weight and consumed less food than IA controls. Although reasons for the weight loss and decreased food intake are not the focus of the present study, caloric restriction is protective against oxidative stress (4, 11, 14, 31, 81), potentially negating the injury of IH. Indeed, dietary changes provide a logical explanation for several of our observations. Early decreases in liver and erythrocyte GSH, serum oxLDL, and MDA levels in p47phox−/− mice coincide with early reduced food intake, and some of these parameters normalized when food intake improved later in the time course. A weight-matched experiment or one with a food-restricted control group might enable us to answer this question.
We must also acknowledge limitations with the use of apocynin and p47phox−/− mice. Apocynin is a drug with nonspecific effects and antioxidant properties potentially unrelated to NADPH oxidase inhibition (94). Nevertheless, it has been widely used in vivo and with more specificity than other pharmacological agents such as diphenylene iodonium (94). p47phox−/− mice are susceptible to infections, and there was evidence of lung granulomas in several mice in our experiment at the time of death. It is difficult to know the impact of this finding on our results, even though a similar number of mice in IH and control groups was affected. We also did not see a significant difference in liver MDA levels between p47phox−/− mice with granulomas and those without. Despite these limitations, we believe that our collective data suggest a role for NADPH oxidase during IH.
Perspective and Significance
To our knowledge, this is the first investigation of oxidative stress induced by chronic IH in multiple organ systems. There were strikingly heterogeneous responses to IH across different organs over time. Over the 4-wk time course of IH, there was no evidence of oxidative stress in the circulation, heart, or aorta, whereas the liver appeared to be affected. We also capture the differential role of NADPH oxidase in different tissues during IH and provide evidence that IH-induced liver lipid peroxidation is mediated via NADPH oxidases. Our data show that oxidative stress in the circulation during IH does not necessarily reflect end-organ effects and that serum markers may be of limited use in assessing oxidative stress in the OSA patient.
GRANTS
Financial support was provided by National Heart, Lung, and Blood Institute Grants HL-68715 (V. Y. Polotsky), HL-80105 (V. Y. Polotsky), and HL-79554 (P. L. Smith); American Heart Association Mid-Atlantic Affiliate Beginning Grant-in-Aid 0765293U (V. Y. Polotsky) and Postdoctoral Fellowship 0625514U (J. Li); and National Institute of Diabetes and Digestive and Kidney Diseases Clinical Nutrition Research Unit Pilot and Feasibility Grant DK-72488 (V. Savransky).
Acknowledgments
We thank Susheel Patil for assistance with statistical analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Acarturk G, Unlu M, Yuksel S, Albayrak R, Koken T, Peker Y. Obstructive sleep apnoea, glucose tolerance and liver steatosis in obese women. J Int Med Res 35: 458–466, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosio G, Chiariello M. Myocardial reperfusion injury: mechanisms and management—a review. Am J Med 91: 86S–88S, 1991. [DOI] [PubMed] [Google Scholar]
- 3.Ambrus CM, Lajos TZ, Stadler I, Stadler A, Alfano J, Tulumello JA, Ambrus JL Jr. Myocardial release of non-transferrin-bound iron during cardio-pulmonary bypass surgery. J Med 30: 157–167, 1999. [PubMed] [Google Scholar]
- 4.Antebi A Ageing: when less is more. Nature 447: 536–537, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Baker JE, Felix CC, Olinger GN, Kalyanaraman B. Myocardial ischemia and reperfusion: direct evidence for free radical generation by electron spin resonance spectroscopy. Proc Natl Acad Sci USA 85: 2786–2789, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG. Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J 16: 644–647, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J Clin Invest 108: 1513–1522, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolan EA, Gracy KN, Chan J, Trifiletti RR, Pickel VM. Ultrastructural localization of nitrotyrosine within the caudate-putamen nucleus and the globus pallidus of normal rat brain. J Neurosci 20: 4798–4808, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 122: 1162–1167, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 124: 1386–1392, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age-related rat aorta sclerosis. FASEB J 19: 1863–1865, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Cathcart MK Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24: 23–28, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Chiarpotto E, Bergamini E, Poli G. Molecular mechanisms of calorie restriction's protection against age-related sclerosis. IUBMB Life 58: 695–702, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Chin K, Nakamura T, Takahashi K, Sumi K, Ogawa Y, Masuzaki H, Muro S, Hattori N, Matsumoto H, Niimi A, Chiba T, Nakao K, Mishima M, Ohi M, Nakamura T. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med 114: 370–376, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med 28: 1815–1826, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Christou K, Kostikas K, Pastaka C, Tanou K, Antoniadou I, Gourgoulianis KI. Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Med. In press. [DOI] [PubMed]
- 18.Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI. Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 7: 105–110, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Cominacini L, Garbin U, Davoli A, Micciolo R, Bosello O, Gaviraghi G, Scuro LA, Pastorino AM. A simple test for predisposition to LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J Lipid Res 32: 349–358, 1991. [PubMed] [Google Scholar]
- 20.Cuong DV, Warda M, Kim N, Park WS, Ko JH, Kim E, Han J. Dynamic changes in nitric oxide and mitochondrial oxidative stress with site-dependent differential tissue response during anoxic preconditioning in rat heart. Am J Physiol Heart Circ Physiol 293: H1457–H1465, 2007. [DOI] [PubMed] [Google Scholar]
- 21.De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol 23 Suppl 1: S98–S103, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of CPAP on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest. In press. [DOI] [PubMed]
- 23.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of CPAP on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 176: 706–712, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 172: 613–618, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Dworakowski R, Anilkumar N, Zhang M, Shah AM. Redox signalling involving NADPH oxidase-derived reactive oxygen species. Biochem Soc Trans 34: 960–964, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Forman MB, Virmani R, Puett DW. Mechanisms and therapy of myocardial reperfusion injury. Circulation 81: IV69–IV78, 1990. [PubMed] [Google Scholar]
- 27.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res 1: 167–186, 1966. [DOI] [PubMed] [Google Scholar]
- 28.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290: 985–989, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA. Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res 23: 1206–1223, 1982. [PubMed] [Google Scholar]
- 30.Gladden LB A lactatic perspective on metabolism. Med Sci Sports Exerc 40: 477–485, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Goto S, Takahashi R, Radak Z, Sharma R. Beneficial biochemical outcomes of late-onset dietary restriction in rodents. Ann NY Acad Sci 1100: 431–441, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Griffioen KJ, Kamendi HW, Gorini CJ, Bouairi E, Mendelowitz D. Reactive oxygen species mediate central cardiorespiratory network responses to acute intermittent hypoxia. J Neurophysiol 97: 2059–2066, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys 423: 103–108, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol 91: 7A–11A, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Hart BA, Elferink JG, Nibbering PH. Effect of apocynin on the induction of ulcerative lesions in rat skin injected with tubercle bacteria. Int J Immunopharmacol 14: 953–961, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Hearse DJ, Bolli R. Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res 26: 101–108, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmeyer MR, Jones SP, Ross CR, Sharp B, Grisham MB, Laroux FS, Stalker TJ, Scalia R, Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res 87: 812–817, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T. Vascular effects following homozygous disruption of p47phox: an essential component of NADPH oxidase. Circulation 101: 1234–1236, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Ischiropoulos H Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun 305: 776–783, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Ishigaki Y, Katagiri H, Gao J, Yamada T, Imai J, Uno K, Hasegawa Y, Kaneko K, Ogihara T, Ishihara H, Sato Y, Takikawa K, Nishimichi N, Matsuda H, Sawamura T, Oka Y. Impact of plasma oxidized low-density lipoprotein removal on atherosclerosis. Circulation 118: 75–83, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Jaeschke H Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 1: 389–397, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89: 31–41, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117: 2270–2278, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jouet P, Sabate JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, Coffin B. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg 17: 478–485, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol 41: 918–921, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Kheirandish-Gozal L, Sans CO, Kheirandish E, Gozal D. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest 133: 92–99, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20: 1529–1535, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Kloner RA, Przyklenk K, Whittaker P. Deleterious effects of oxygen radicals in ischemia/reperfusion. Resolved and unresolved issues. Circulation 80: 1115–1127, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Kubo H, Morgenstern D, Quinian WM, Ward PA, Dinauer MC, Doerschuk CM. Preservation of complement-induced lung injury in mice with deficiency of NADPH oxidase. J Clin Invest 97: 2680–2684, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavie L Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27: 123–128, 2004. [PubMed] [Google Scholar]
- 53.Leineweber C, Kecklund G, Janszky I, Akerstedt T, Orth-Gomer K. Snoring and progression of coronary artery disease: The Stockholm Female Coronary Angiography Study. Sleep 27: 1344–1349, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Li J, Savransky V, Nanayakkara A, Smith PL, O'Donnell CP, Polotsky VY. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J Appl Physiol 102: 557–563, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47phox in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-α. Circ Res 90: 143–150, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Li JM, Wheatcroft S, Fan LM, Kearney MT, Shah AM. Opposing roles of p47phox in basal versus angiotensin II-stimulated alterations in vascular O2− production, vascular tone, and mitogen-activated protein kinase activation. Circulation 109: 1307–1313, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol 14: 2832–2837, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med 34: 1–10, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Lu LS, Wu CC, Hung LM, Chiang MT, Lin CT, Lin CW, Su MJ. Apocynin alleviated hepatic oxidative burden and reduced liver injury in hypercholesterolaemia. Liver Int 27: 529–537, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Lu SC Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 13: 1169–1183, 1999. [PubMed] [Google Scholar]
- 63.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172: 625–630, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Misra VL, Chalasani N. Obstructive sleep apnea and nonalcoholic fatty liver disease: causal association or just a coincidence? Gastroenterology 134: 2178–2179, 2008. [DOI] [PubMed] [Google Scholar]
- 66.Mohazzab KM, Kaminski PM, Wolin MS. NADH oxidoreductase is a major source of superoxide anion in bovine coronary artery endothelium. Am J Physiol Heart Circ Physiol 266: H2568–H2572, 1994. [DOI] [PubMed] [Google Scholar]
- 67.Muriel P, Escobar Y. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J Appl Toxicol 23: 103–108, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Norman D, Bardwell WA, Arosemena F, Nelesen R, Mills PJ, Loredo JS, Lavine JE, Dimsdale JE. Serum aminotransferase levels are associated with markers of hypoxia in patients with obstructive sleep apnea. Sleep 31: 121–126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozturk L, Mansour B, Yuksel M, Yalcin AS, Celikoglu F, Gokhan N. Lipid peroxidation and osmotic fragility of red blood cells in sleep-apnea patients. Clin Chim Acta 332: 83–88, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res 75: 291–302, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576: 289–295, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol 552: 253–264, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poortmans JR Principles of exercise biochemistry. Med Sport Sci 46: 153–196, 2004. [Google Scholar]
- 74.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal 9: 1397–1403, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Przyklenk K, Kloner RA. “Reperfusion injury” by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circ Res 64: 86–96, 1989. [DOI] [PubMed] [Google Scholar]
- 76.Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Savransky V, Bevans S, Nanayakkara A, Li J, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia causes hepatitis in a mouse model of diet-induced fatty liver. Am J Physiol Gastrointest Liver Physiol 293: G871–G877, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology 45: 1007–1013, 2007. [DOI] [PubMed] [Google Scholar]
- 80.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162: 566–570, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Sharma S, Kaur G. Intermittent dietary restriction as a practical intervention in aging. Ann NY Acad Sci 1114: 419–427, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 289: 1675–1680, 2003. [DOI] [PubMed] [Google Scholar]
- 83.Steinberg D Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 272: 20963–20966, 1997. [DOI] [PubMed] [Google Scholar]
- 84.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320: 915–924, 1989. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki T, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Yamauchi M, Kimura H. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep 27: 129–133, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, Somers VK. Oxidative stress in obstructive sleep apnoea. Eur Heart J 26: 2435–2439, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Svatikova A, Wolk R, Wang HH, Otto ME, Bybee KA, Singh RJ, Somers VK. Circulating free nitrotyrosine in obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol 287: R284–R287, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Tanne F, Gagnadoux F, Chazouilleres O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology 41: 1290–1296, 2005. [DOI] [PubMed] [Google Scholar]
- 89.Tateishi N, Higashi T, Shinya S, Naruse A, Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J Biochem (Tokyo) 75: 93–103, 1974. [DOI] [PubMed] [Google Scholar]
- 90.Tejima K, Arai M, Ikeda H, Tomiya T, Yanase M, Inoue Y, Nishikawa T, Watanabe N, Ohtomo N, Omata M, Fujiwara K. Induction of ischemic tolerance in rat liver via reduced nicotinamide adenine dinucleotide phosphate oxidase in Kupffer cells. World J Gastroenterol 13: 5071–5078, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thong-Ngam D, Samuhasaneeto S, Kulaputana O, Klaikeaw N. N-acetylcysteine attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. World J Gastroenterol 13: 5127–5132, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem 14: 1539–1549, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Wali SO, Bahammam AS, Massaeli H, Pierce GN, Iliskovic N, Singal PK, Kryger MH. Susceptibility of LDL to oxidative stress in obstructive sleep apnea. Sleep 21: 290–296, 1998. [PubMed] [Google Scholar]
- 94.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50: 9–16, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans? Trends Cardiovasc Med 11: 93–102, 2001. [DOI] [PubMed] [Google Scholar]
- 96.Xu W, Chi L, Row BW, Xu R, Ke Y, Xu B, Luo C, Kheirandish L, Gozal D, Liu R. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apnea. Neuroscience 126: 313–323, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antioxid Redox Signal 10: 765–768, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 172: 921–929, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao G, al-Mehdi AB, Fisher AB. Anoxia-reoxygenation versus ischemia in isolated rat lungs. Am J Physiol Lung Cell Mol Physiol 273: L1112–L1117, 1997. [DOI] [PubMed] [Google Scholar]