Abstract
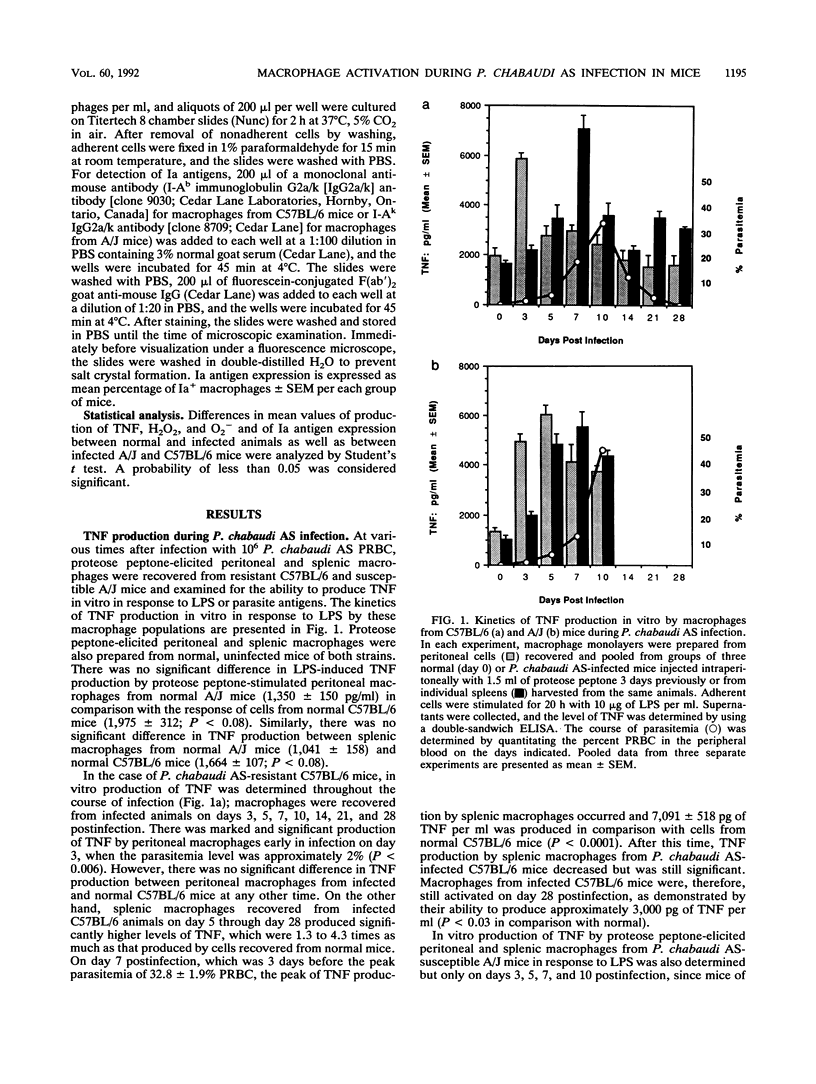
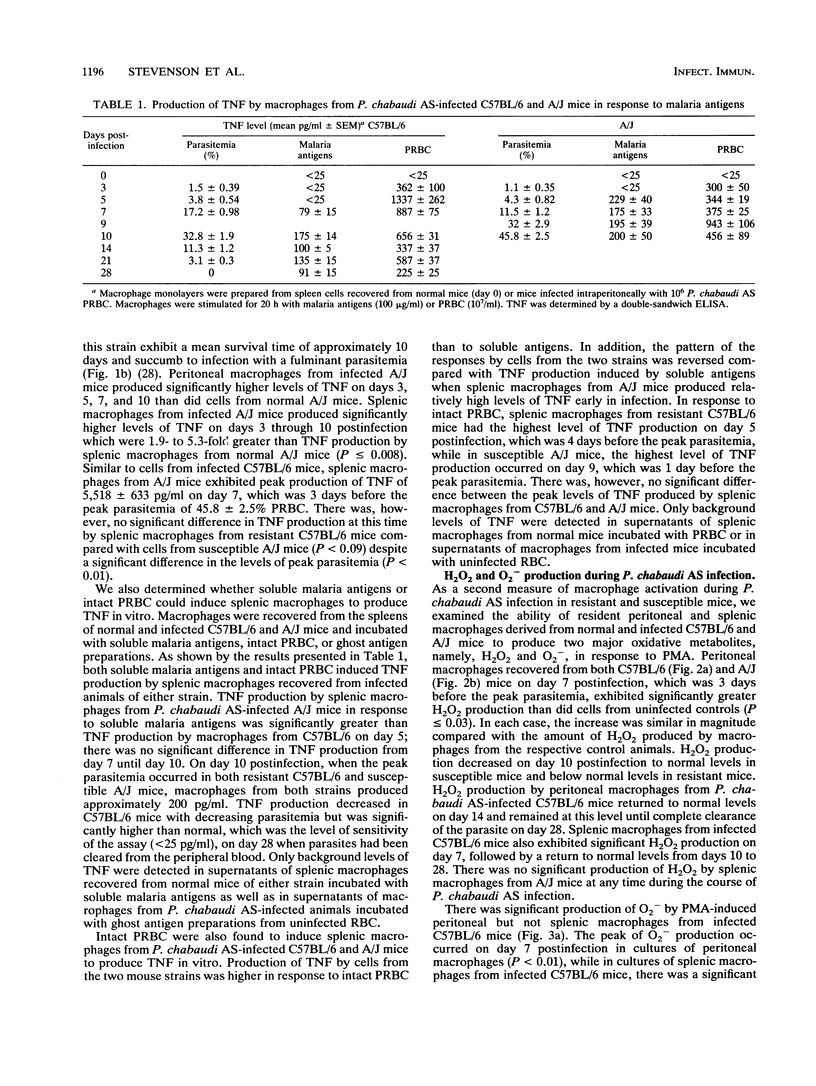
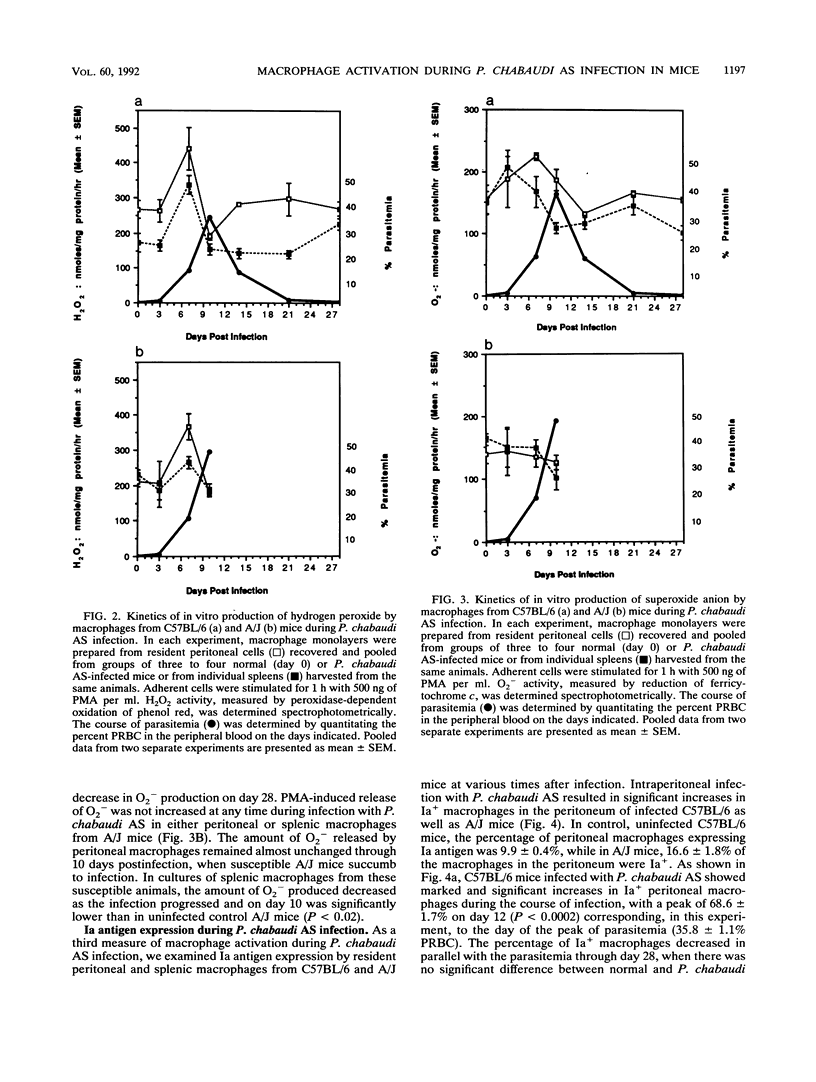
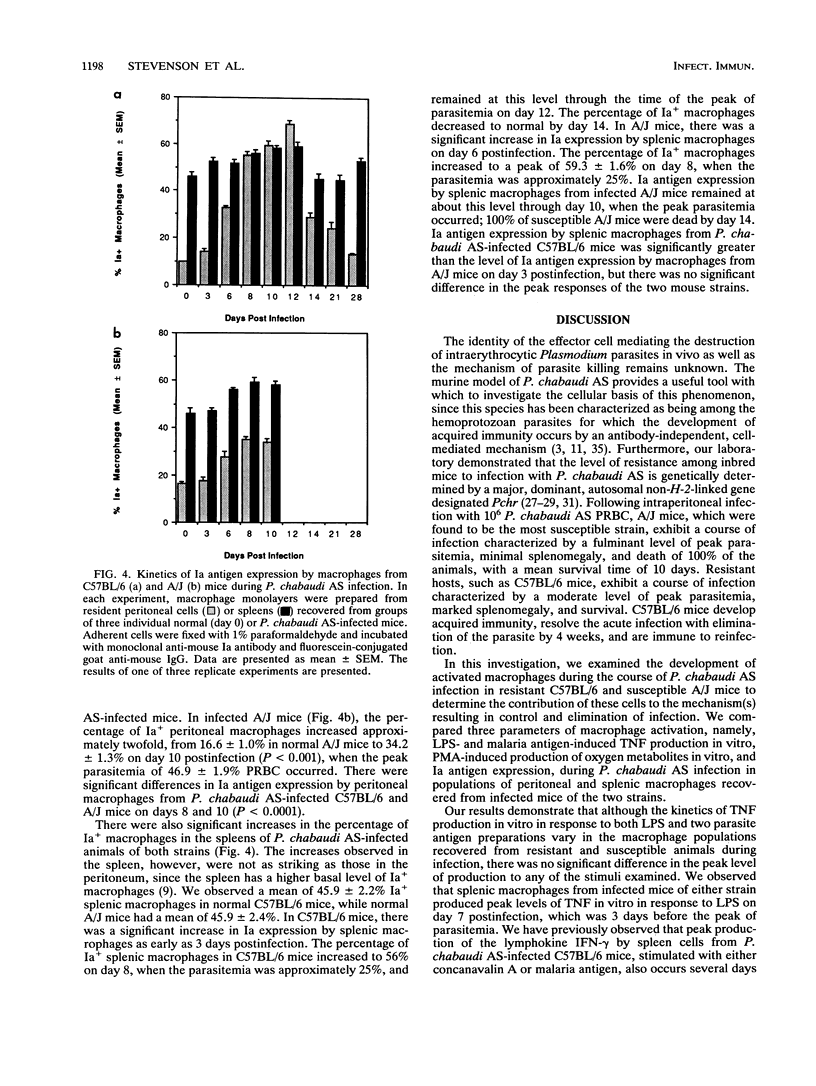
Macrophage activation was examined in resistant C57BL/6 and susceptible A/J mice during the course of blood-stage infection with Plasmodium chabaudi AS. Three parameters of macrophage activation (lipopolysaccharide [LPS]- and malaria antigen-induced tumor necrosis factor [TNF] production in vitro, phorbol myristate acetate [PMA]-induced production of oxygen metabolites in vitro, and Ia antigen expression) were assessed during infection in populations of peritoneal and splenic macrophages recovered from infected mice of the two strains. The peak level of LPS-induced TNF production in vitro by splenic macrophages from both infected C57BL/6 and infected A/J mice occurred on day 7, which was 3 days before the peak of parasitemia. Although the kinetics of TNF production in vitro in response to either LPS, soluble malaria antigen, or intact parasitized erythrocytes varied in some of the other macrophage populations during infection, there was no significant difference in the peak level of production. Peritoneal and splenic macrophages from infected C57BL/6 mice exhibited significantly increased PMA-induced production of H2O2 in vitro on day 7. Peritoneal macrophages from infected A/J mice also exhibited significant PMA-induced H2O2 production on day 7, while production by splenic macrophages from these hosts was not increased in comparison with production by cells from normal animals. Only peritoneal macrophages from infected C57BL/6 mice produced significantly increased levels of O2-, and this occurred on day 7 postinfection. Ia antigen expression by both peritoneal and splenic macrophages from resistant C57BL/6 and susceptible A/J mice was significantly increased during P. chabaudi AS infection. However, the percentage of Ia+ peritoneal macrophages on days 8 and 10 postinfection and Ia+ splenic macrophages on day 3 postinfection was significantly higher in C57BL/6 than in A/J mice. Thus, these results demonstrate that macrophages from P. chabaudi AS-infected A/J mice exhibit defects in oxygen metabolism and Ia antigen expression which may contribute to the susceptibility of these hosts to this intraerythrocytic parasite. The cause-and-effect relationship between these defects and the susceptibility of A/J mice to P. chabaudi AS is unknown.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Cavacini L. A., Guidotti M., Parke L. A., Melancon-Kaplan J., Weidanz W. P. Reassessment of the role of splenic leukocyte oxidative activity and macrophage activation in expression of immunity to malaria. Infect Immun. 1989 Dec;57(12):3677–3682. doi: 10.1128/iai.57.12.3677-3682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Parke L. A., Weidanz W. P. Resolution of acute malarial infections by T cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990 Sep;58(9):2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing C., Schwartz B. D., Dickler H. B. Macrophage Ia antigens. I. macrophage populations differ in their expression of Ia antigens. J Immunol. 1978 Feb;120(2):378–384. [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- James S. L., Hibbs J. B., Jr The role of nitrogen oxides as effector molecules of parasite killing. Parasitol Today. 1990 Sep;6(9):303–305. doi: 10.1016/0169-4758(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Lethal Plasmodium yoelii malaria: the role of macrophages in normal and immunized mice. Bull World Health Organ. 1979;57 (Suppl 1):245–246. [PMC free article] [PubMed] [Google Scholar]
- Rockett K. A., Awburn M. M., Cowden W. B., Clark I. A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991 Sep;59(9):3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear H. L., Srinivasan R., Nolan T., Ng C. Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol. 1989 Sep 15;143(6):2038–2044. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Stevenson M. M., Ghadirian E. Human recombinant tumor necrosis factor alpha protects susceptible A/J mice against lethal Plasmodium chabaudi AS infection. Infect Immun. 1989 Dec;57(12):3936–3939. doi: 10.1128/iai.57.12.3936-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Ghadirian E., Phillips N. C., Rae D., Podoba J. E. Role of mononuclear phagocytes in elimination of Plasmodium chabaudi AS infection. Parasite Immunol. 1989 Sep;11(5):529–544. doi: 10.1111/j.1365-3024.1989.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Lyanga J. J., Skamene E. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun. 1982 Oct;38(1):80–88. doi: 10.1128/iai.38.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Nesbitt M. N., Skamene E. Chromosomal location of the gene determining resistance to Plasmodium chabaudi AS. Curr Top Microbiol Immunol. 1988;137:325–328. doi: 10.1007/978-3-642-50059-6_48. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Nowotarski M., Yap G. Review: cytokines and malaria. Clin Invest Med. 1990 Dec;13(6):353–359. [PubMed] [Google Scholar]
- Stevenson M. M., Skamene E. Murine malaria: resistance of AXB/BXA recombinant inbred mice to Plasmodium chabaudi. Infect Immun. 1985 Feb;47(2):452–456. doi: 10.1128/iai.47.2.452-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Belosevic M., van der Meide P. H., Podoba J. E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990 Oct;58(10):3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Nowotarski M. Role of interferon-gamma and tumor necrosis factor in host resistance to Plasmodium chabaudi AS. Immunol Lett. 1990 Aug;25(1-3):115–121. doi: 10.1016/0165-2478(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Rae D. Dependence on cell-mediated mechanisms for the appearance of crisis forms during Plasmodium chabaudi AS infection in C57BL/6 mice. Microb Pathog. 1990 Nov;9(5):303–314. doi: 10.1016/0882-4010(90)90065-x. [DOI] [PubMed] [Google Scholar]
- Stevenson M., Lemieux S., Skamene E. Genetic control of resistance to murine malaria. J Cell Biochem. 1984;24(1):91–102. doi: 10.1002/jcb.240240108. [DOI] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Playfair J. H. Malaria exoantigens induce TNF, are toxic and are blocked by T-independent antibody. Immunol Lett. 1990 Aug;25(1-3):207–212. doi: 10.1016/0165-2478(90)90116-8. [DOI] [PubMed] [Google Scholar]
- Taverne J., Tavernier J., Fiers W., Playfair J. H. Recombinant tumour necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987 Jan;67(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]


