Abstract
NaCl absorption in the medullary thick ascending limb of the loop of Henle (THAL) is mediated by the apical Na/K/2Cl cotransporter (NKCC2). Hormones that increase cGMP, such as nitric oxide (NO) and natriuretic peptides, decrease NaCl absorption by the THAL. However, the mechanism by which cGMP decreases NaCl absorption in THALs is not known. We hypothesized that cGMP decreases surface NKCC2 levels in the THAL. We used surface biotinylation to measure surface NKCC2 levels in rat THAL suspensions. We tested the effect of the membrane-permeant cGMP analog dibutyryl-cGMP (db-cGMP) on surface NKCC2 levels. Incubating THALs with db-cGMP for 20 min decreased surface NKCC2 levels in a concentration-dependent manner (basal = 100%; db-cGMP 100 μM = 77 ± 7%; 500 μM = 54 ± 10% and 1,000 μM = 61 ± 8%). A different cGMP analog 8-bromo-cGMP (8-Br-cGMP) also decreased surface NKCC2 levels by 25%, (basal = 100%; 8-Br-cGMP = 75 ± 5%). Incubation of isolated, perfused THALs with db-cGMP decreased apical surface NKCC2 labeling levels as measured by immunofluorescence and confocal microscopy. cGMP-stimulated phosphodiesterase 2 (PDE2) mediates the inhibitory effect of NO on NaCl absorption by THALs. Thus we examined the role of PDE2 and found that PDE2 inhibitors blocked the effect of db-cGMP on surface NKCC2. Also, a nonstimulatory concentration of db-cAMP blocked the cGMP-induced decrease in surface NKCC2. Finally, db-cGMP inhibited THAL net Cl absorption by 48 ± 4%, and this effect was completely blocked by PDE2 inhibition. We conclude that cGMP decreases NKCC2 levels in the apical membrane of THALs and that this effect is mediated by PDE2. This is an important mechanism by which cGMP inhibits NaCl absorption by the THAL.
Keywords: Na-K-2Cl cotransporter, trafficking, apical, NO, epithelial
the thick ascending limb of the loop of Henle (THAL) plays an important role in the maintenance of salt and fluid homeostasis. The THAL absorbs ∼30% of NaCl filtered through the glomeruli and maintains the corticomedullary osmotic gradient necessary for concentrating urine. Absorption of NaCl by the THAL is a two-step process. First, Na+, K+, and Cl− enter the cell across the apical membrane via Na+/K+/2Cl− cotransport driven by the chemical gradient generated by basolateral Na+-K+-ATPase (13, 21). In the second step, Na+ is transported out across the basolateral membrane via Na+-K+-ATPase, while Cl− exits via K+/Cl− cotransport or Cl− channels (12). Most K+ recycles back to the lumen via the apical K+ channel (ROMK) (41). The Na+/K+/2Cl− cotransporter present in the THAL has been named NKCC2 or bumetanide-sensitive cotransporter 1 (BSC-1) (9). This cotransporter is located in the apical membrane of medullary and cortical thick ascending limbs and macula densa cells (14, 25). In rat THALs, NKCC2 is located in the apical membrane, in intracellular vesicles of the subapical cytoplasmic space, and in the Golgi apparatus (25).
cGMP, the main second messenger for nitric oxide (NO) and atrial natriuretic peptide (ANP), plays an important role in the regulation of renal hemodynamics and tubular salt transport. In the THAL, NO inhibits NaCl absorption by decreasing NKCC2-dependent Na+ entry (30). In addition to NO (28, 30, 33), other hormones such as endothelin 1 (acting through ETB receptors) (32) and ANP (2) inhibit NaCl absorption by the THAL. The signaling mechanism by which these factors inhibit NaCl absorption involves stimulation of soluble or receptor-associated guanylate cyclases, which increase cGMP levels and inhibit NKCC2-dependent Na+ entry. Consistently, membrane-permeable cGMP analogs decrease NaCl absorption in this nephron segment (24). Despite the importance of cGMP as a potent inhibitor of NaCl absorption in the THAL, the mechanism and signaling cascade by which this second messenger acts in the THAL are poorly understood.
NKCC2-dependent Na+ entry into THALs is regulated by different mechanisms. Recently, we and other investigators showed that trafficking of NKCC2 to the apical membrane and increased surface NKCC2 levels mediate cAMP-induced stimulation of NaCl absorption and NKCC2 activity in THALs (11, 19, 26). The increase in surface NKCC2 levels caused by cAMP seems to be mediated by enhanced exocytosis of NKCC2, since it was blocked by a toxin that prevents vesicle fusion with the plasma membrane (26). Together, these data indicate that NKCC2 trafficking is an important mechanism for the stimulation of NaCl absorption by cAMP. Thus we hypothesized that cGMP decreases NKCC2 levels in the apical membrane of THALs. Our results indicate that cGMP decreases surface NKCC2 levels in rat medullary THALs by activating the cGMP-stimulated phosphodiesterase PDE2. To our knowledge, this is the first evidence of regulation of transporter trafficking by cGMP in the THAL.
MATERIALS AND METHODS
Suspensions of medullary THALs.
Male Sprague-Dawley rats weighing 180–250 g (Charles River Breeding Laboratories, Wilmington, MA) were maintained on a diet containing 0.22% sodium and 1.1% potassium (Purina, Richmond, IN) with water ad libitum for at least 7 days. On the day of the experiment, rats were anesthetized with ketamine (100 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip). The abdominal cavity was opened, and the kidneys were perfused retrograde via the aorta with a physiological solution containing 0.1% collagenase (Sigma, St. Louis, MO) and 100 U/ml heparin. The inner stripe of the outer medulla was cut from coronal slices, minced, and incubated at 37°C for 30 min with 0.1% collagenase in a physiological saline solution and gassed every 5 min with 100% O2. Tissue was pelleted by gentle centrifugation at 120 g for 2 min, resuspended in chilled physiological solution, and stirred on ice for 30 min to release the tubules. The suspension was filtered through a 250-μm nylon mesh and centrifuged at 120 g for 2 min. The pellet was washed, centrifuged again, and finally resuspended in 0.4 ml chilled physiological solution. The composition of the physiological solution was (in mM) 130 NaCl, 2.5 NaH2PO4, 4.0 KCl, 1.2 MgSO4, 5 l-alanine, 1.0 Na-citrate, 5.5 glucose, 2.0 Ca-lactate, and 10 HEPES, pH 7.40. All animal protocols were approved by the institutional animal care committee.
Surface biotinylation of THAL suspensions.
Cell surface biotinylation of THAL suspensions was performed as described previously by Ortiz (26). Tubule suspensions from the same animal were aliquoted in two, three, or four samples of equal volume. THALs were equilibrated for 20 min at 37°C and gassed every 5 min with 100% O2. After equilibration, THALs were treated with vehicle or membrane-permeant dibutyryl-cGMP sodium salt hydrate (db-cGMP) or 8-bromo-cGMP sodium salt (8-Br-cGMP; Sigma) for 20 min at 37°C, gassing every 5 min. In a different set of suspensions, 2-(3,4-dimethoxybenzyl)-7-[(1R)-1-[(1R)-1-hydroxyethyl]-4-phenylbutyl]-5-methylimidazo[5,1-f][1,2,4]triazin-4(3H)-one (BAY 60-7550; Axxora Platform), erythro-9-[2-hydroxy-3-nonyl] adenine (EHNA; Calbiochem), and db-cAMP were present in the bath throughout the experiment. After treatments, suspensions were rapidly cooled to 4°C, washed twice with chilled physiological solution, and centrifuged at 120 g for 2 min at 4°C. THALs were incubated with 0.75 ml chilled biotinylation solution [HEPES-Ca2+-Mg2+ buffer: (in mM) 10 HEPES, 130 NaCl, 2 MgSO4, 1 CaCl2, 5.5 and glucose, pH: 7.8–8.0], containing 1.2 mg/ml NHS-SS-biotin (Pierce Biotechnology) in a rocker platform at 4°C for 15 min. Then, 0.75 ml of freshly prepared NHS-SS-biotin (1.2 mg/ml) was added on top, and the samples were incubated for an additional 15-min period. After biotinylation, tubules were washed three times at 4°C with a physiological solution containing 100 mM glycine to remove the excess of NHS-SS-biotin. THALs were centrifuged (120 g) and lysed in buffer containing (in mM) 150 NaCl, 50 HEPES, and 5 EDTA, plus 2% Triton X-100 and 0.2% SDS, pH 7.5 and protease inhibitors [10 μg/ml aprotinin, 5 μg/ml leupeptin, 4 mmol/l benzamidine, 5 μg/ml chymostatin, and 5 μg/ml pepstatin A (Sigma)]. We previously found that this lysis buffer solubilizes 100% of NKCC2 from THAL suspensions. Total protein content in each sample was measured in duplicate by colorimetric assay, using Bradford's method (Pierce Biotechnology). Equal amounts of protein (50–75 μg) were incubated overnight at 4°C with streptavidin-coated agarose beads (10%) in lysis buffer. Beads were pulled down by centrifugation, and the supernatant was reincubated with streptavidin-coated agarose beads (10%) for 2 h at 4°C. The supernatant was saved for determination of intracellular NKCC2, whereas beads were centrifuged and pooled with the beads from the first round. Beads were then washed twice in lysis buffer, twice in high-salt buffer (500 mM NaCl, 50 mM HEPES, pH 7.4), and twice in no-salt buffer (50 mM HEPES, pH 7.4). Biotinylated proteins were extracted from the beads by boiling in 60 μl SDS-loading buffer containing 50 μM DL-dithiothreitol (DTT) and 5% β-mercaptoethanol. Proteins were resolved by SDS-PAGE (6% gels), and NKCC2 present in the membrane was detected by Western blotting. In control experiments, we found that all biotinylated NKCC2 was precipitated in two rounds of incubation with streptavidin-coated agarose beads, since no NKCC2 was detected after a third round. We also determined that all biotinylated NKCC2 is eluted from the beads in one round of boiling with loading buffer. In every experiment, a one-tenth fraction of the supernatant (7.5 μg total protein), containing intracellular nonbiotinylated proteins, was loaded on the same gels as NKCC2 samples recovered from the beads, resolved, and NKCC2 was measured by Western blotting. Optical densities from surface and intracellular NKCC2 bands were used to calculate total NKCC2 and the ratio of surface to total NKCC2. Given the small percentage of NKCC2 at the surface (3–5%), changes in the surface fraction do not result in detectable changes in the intracellular fraction. Day-to-day variation in surface biotinylation experiments in untreated samples (basals) was 15–25% across data sets.
To ensure that we could measure both decreases and increases in surface NKCC2 levels accurately, we performed concentration-response curves to increasing amounts of proteins from biotinylated THAL suspensions and measured surface and intracellular NKCC2. We found a positive linear correlation (r = 0.98) between surface NKCC2 levels and increasing amounts of total protein (from 25 to 125 μg) used to start the reaction. Thus in all experiments we used 50–75 μg of initial protein lysate to measure surface NKCC2 levels within a linear range. Control experiments showed that two intracellular proteins, GAPDH and transcription factor IIB (TF IIB), were not found in the biotinylated surface fraction but were abundant in samples from the intracellular fraction, indicating that the biotinylated fraction contains only surface proteins (data not shown).
Western blotting.
Proteins eluted from streptavidin beads or obtained from THAL lysates were centrifuged for 1 min at 10,000 g, loaded into each lane of a 6% SDS-polyacrylamide gel, separated by electrophoresis and transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blocked with 50 mM Tris, 150 mM NaCl, 5% nonfat dry milk, and 0.1% Tween 20, pH 7.4 for 60 min. Primary antibodies were incubated for 120 min at room temperature in blocking buffer at 1:1,000 dilution for rabbit anti-rat NKCC2 (directed to amino acids 859–873 in rat NKCC2 COOH terminus, Chemicon, Temecula CA) (6, 26) and 1:10,000 for chicken anti-NKCC2 (provided by J. Alvarez-Leefmans, Wright State University, Dayton, OH). The chicken anti-NKCC2 antibody was raised against a synthetic peptide corresponding to amino acids 33–55 of the NH2 terminus of NKCC2 from rat (identical sequence to l-320 antibody) (15) plus an added cysteine to facilitate attachment to solid substrates or carrier proteins. The peptide sequence was SDSTDPPHYEETSFGDEAQNRLK. This sequence is unique to NKCC2 (Slc12a1). The specific antibody (IgY) was purified using a peptide-affinity column. When the anti-NKCC2 antibody was preadsorbed with an excess of immunizing peptide, the ∼160-kDa band corresponding to mature glycosylated NKCC2 was completely ablated. No bands were observed when the secondary antibody was added in the absence of primary antibody (not shown). Probing membrane fractions from tissues other than renal (e.g., liver) gave no immunoreactive bands. In addition, this antibody reacts with NKCC2 expressed in oocytes (31). PDE2 antibodies (Fabgennix) were used at 1:1,000 dilution as reported by other investigators (23, 37). Monoclonal anti-GAPDH (Chemicon) was used at 1:10,000 dilution, and anti-TF IIB (Santa Cruz Biotechnology) was used at 1:1,000. The membrane was washed twice in a buffer containing 50 mM Tris, 150 mM NaCl, and 0.1% Tween 20, pH: 7.4 for 15 min each and incubated with a 1:1,000 dilution of a secondary antibody against the appropriate IgG conjugated to horseradish peroxidase (Amersham Pharmacia Biotech). Reaction was detected by chemiluminescence (Amersham Pharmacia Biotech) on Kodak Biomax light films and quantified by densitometry. Film was scanned at 1,200-dpi resolution, 16-bit gray scale, with an Epson 1680 Expression Pro scanner on positive film mode and saved as uncompressed TIFF files. Software on transmittance mode was used to obtain mean optical densities of the bands. Exposure time and amount of protein loaded were optimized so that optical densities were linear.
Medullary THAL isolation and perfusion.
Male Sprague-Dawley rats, weighing 120–150 g (Charles River Breeding Laboratories), were used for THAL perfusion. After anesthesia, the abdominal cavity was opened; and the left kidney was bathed in ice-cold saline and removed. Coronal slices were placed in an oxygenated physiological perfusion solution containing (in mM) 130 NaCl, 2.5 NaH2PO4, 4.0 KCl, 1.2 MgSO4, 6 l-alanine, 1.0 Na-citrate, 5.5 glucose, 2.0 Ca-lactate, and 10 HEPES, pH 7.40. Medullary THALs were dissected from the medullary rays under a stereomicroscope at 4–10°C. THALs ranging from 0.5 to 1.0 mm were transferred to a temperature-regulated chamber and perfused using concentric glass pipettes at 37 ± 1°C as described previously (30). The flow rate of the basolateral bath was 0.5 ml/min.
Immunofluorescence and confocal microscopy for detection of luminal membrane NKCC2 in isolated, perfused THALs.
To measure luminal membrane NKCC2, we generated a rabbit polyclonal antibody against a synthetic peptide corresponding to amino acids 363–376 of rat NKCC2. This amino acid sequence is located between the predicted transmembrane domains 5 and 6 of rat NKCC2 facing the extracellular (luminal) side. A cysteine was added to facilitate attachment to solid substrates or carrier proteins. The peptide sequence was CAENFGPSFTEGEGF. This sequence is specific for NKCC2, and it is 95–100% conserved in NKCC2 of various mammalian species. Preimmune serum and anti-serum with ELISA titers >1:100,000 were obtained using standard methods from immunized rabbits. The anti-serum against luminal NKCC2 was characterized in isolated, perfused rat THALs as detailed below and in results.
Microdissected medullary THALs were perfused and equilibrated for 20 min at 37°C; and then either vehicle (control conditions) or 500 μM db-cGMP was added to the bath and incubated for another 20 min. Then, the flowing bath was rapidly exchanged (3 s) with chilled perfusion solution (4°C), and the chamber temperature was maintained at 6°C to stop protein trafficking for a total period of 45 min. After the initial cooling, luminal perfusion solution was replaced by a perfusion solution containing 2.5% BSA, pH 7.6, and incubated for 5 min to block nonspecific antigenic sites. Then, either preimmune or immune serum (1:100 dilution in 2.5% BSA, pH 7.6) was perfused into the THAL lumen for 30 min while the bath temperature was maintained at 6°C. In a different set of THALs, we observed that this temperature condition completely blocked apical endocytosis, as measured by uptake of the fluid-phase marker FITC-dextran (10,000 Da, Molecular Probes, Eugene OR) added to the THAL lumen (data not shown). After labeling of intact THALs, the lumen was washed at 6°C for 10 min by exchanging the luminal solution with physiological saline. THALs were immediately fixed at 6°C with 4% paraformaldehyde in PBS, pH 7.4, for 10 min and then incubated with fixative for an additional 20 min at room temperature. Fixed tubules were blocked for 5 min with 2.5% BSA in a perfusion solution added into the lumen and bath, followed by 30-min luminal perfusion with Alexa Fluor 488 goat anti-rabbit IgG secondary antibody cross-adsorbed against IgGs from other species (Molecular Probes) diluted 1:200 in 2.5% BSA-containing perfusion solution. THALs were washed for 10 min with the perfusion solution in the lumen and bath. Control experiments showed undetectable background staining when the secondary antibody was incubated in the absence of antiserum. Fluorescent images were acquired using a laser-scanning confocal system (Visitech International) mounted on a Nikon TE2000-eclipse microscope. Secondary labeling was visualized by exciting at 488 nm, and fluorescence was measured and recorded with 500-nm LP filter. Identical laser, slit, and acquisition settings were used to obtain cross-sectional (z-axis) images of labeled THALs. To diminish day-to-day variation, fluorescence intensity was regularly calibrated to a linear range using Focal-check slides (Molecular Probes) containing a serial dilution of fluorescent-labeled beads. Two-dimensional image analysis (Visitech Acquisition software) of original images was used to measure mean fluorescent intensity in regions of interest encompassing the apical membrane of labeled THALs. Several cross-sectional images at different depths (z-axis) were obtained from individual THAL cells along the tubule to obtain the best possible focus of apical membranes. For quantification, regions of interest were generated encompassing the apical membranes of several cells in each THAL image (generally 4–8 cells at each side of the tubule wall). Mean fluorescence intensity of apical labeling was obtained and averaged for each tubule. One control and one db-cGMP treatment experiment were performed each day. Image files were converted to TIFF and pseudocolored using Adobe Photoshop software.
Measurement of Cl absorption.
THALs were mounted on concentric glass pipettes and perfused. Luminal perfusion rate was set at 5–10 nl·min−1·mm−1. A perfusion solution gassed with air (pH = 7.40) was used for the bath and perfusate. The composition of the solution was (in mM) 130 NaCl, 2.5 NaH2PO4, 4.0 KCl, 1.2 MgSO4, 6 l-alanine, 1.0 Na-citrate, 5.5 glucose, 2.0 Ca-lactate, and 10 HEPES. After initial perfusion, THALs were equilibrated for 20 min, and four measurements were made to calculate the basal Cl absorption rate. Then, compounds of interest were added to the bath as indicated in results. After a 20-min reequilibration period, four additional collections were made. Chloride concentration in the perfusate and collected fluid was measured by microfluorometry. All data were recorded and stored using data-acquisition software (DATAQ Instruments, Akron, OH). Data analysis was performed with software for voltage-spike analysis. Because water is not reabsorbed by the THAL, chloride absorption (J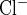 ) was calculated as follows: J
) was calculated as follows: J = CR (C
= CR (C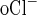 − C
− C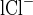 ), where CR is the collection rate normalized per tubule length, C
), where CR is the collection rate normalized per tubule length, C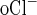 is the Cl concentration in the perfusion solution, and C
is the Cl concentration in the perfusion solution, and C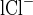 is the Cl concentration in the collected fluid.
is the Cl concentration in the collected fluid.
Intracellular cAMP measurements.
cAMP was determined by RIA after sample acetylation (Biomedical Technologies). Briefly, THAL suspensions were aliquoted and equilibrated in physiological perfusion solution (same as above) at 37°C for 10 min in the absence of PDE inhibitors. Then, vehicle or db-cGMP (final concentration 500 μM) was added to the bath and incubated for 4 min. The reaction was stopped by adding 1.5 ml of chilled perfusion solution containing the general PDE inhibitor 3-isobutyl-1-methylxanthine (1 mM). To prevent endogenous NO production, which could decrease basal cAMP levels and mask the effect of cGMP, the NO synthase inhibitor Nω-l-arginine methyl ester (l-NAME) (1 mM) was included in the solution. Tubules were then washed by centrifugation (160 g) at 4°C, lysed in chilled solution containing 50% methanol/distilled water, and stored at −80°C for 1 h. Lysates were spun at 16,000 g, and the supernatant was recovered and stored at −80°C. The pellet was used for determination of total protein (in duplicate) by colorimetric assay using Bradford's method (Pierce Biotechnology). Samples were dried overnight on a Savant centrifuge dryer, the dried pellet was reconstituted in 240 μl of assay buffer, and cAMP was determined by RIA. Internal standards and recoveries were run with each experiment. Intracellular cAMP content was expressed as femtomoles per microgram of protein.
Statistics.
Data are expressed as means ± SE. One-way ANOVA was used to determine statistical differences between means in different treatment groups when surface and total NKCC2 were measured by Western blotting and by fluorescent imaging. A paired t-test was used determine changes in net Cl absorption experiments. P < 0.05 was considered significant.
RESULTS
Effect of cGMP on surface NKCC2 levels.
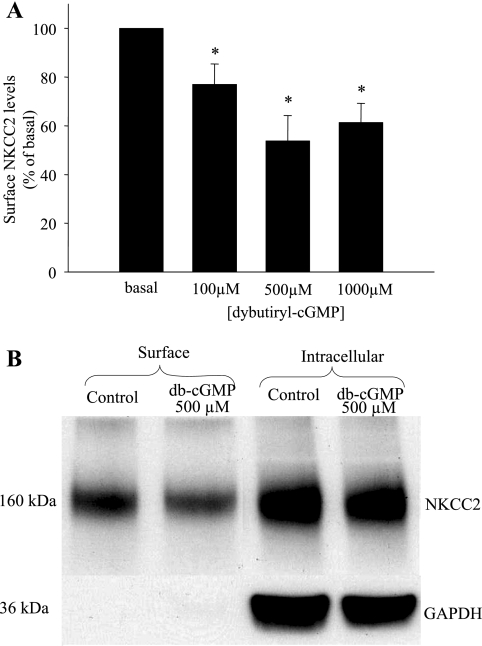
The mechanism by which cGMP decreases NaCl absorption by the THAL is not known. We hypothesized that cGMP decreases NKCC2 levels in the plasma membrane of THALs. To begin studying the effect of cGMP on NKCC2 levels in the apical membrane of THALs, we used surface biotinylation of THAL suspensions and Western blotting. To test whether cGMP decreases surface NKCC2 levels in the THAL, we first studied the effect of the membrane-permeant cGMP analog db-cGMP on surface NKCC2 levels in the THAL. Suspensions of THALs were equilibrated for 20 min at 37°C and then treated for 20 min with vehicle or increasing concentrations of db-cGMP (100, 500, and 1,000 μM). We found a maximal decrease in surface NKCC2 levels with a concentration of 500 μM, whereas a higher concentration did not decrease surface NKCC2 further (basal = 100%; 100 μM db-cGMP = 77 ± 7%; 500 μM db-cGMP = 54 ± 10%; and 1,000 μM db-cGMP = 61 ± 8%; P < 0.001 vs. basal; n = 5 for each concentration) (Fig. 1A). The total expression of NKCC2 was not changed after treatment with db-cGMP [basal = 100%, db-cGMP 500 μM = 114 ± 14%, not significant (NS)], and the intracellular protein GAPDH was not detected in the surface protein fraction (Fig. 1B). To ensure that the decrease in surface NKCC2 was not due to decreased reactivity of the antibody caused by conformational changes in NKCC2, we performed the same experiments but measured NKCC2 with an antibody directed to the NH2 terminus. With this antibody we found that db-cGMP decreased surface NKCC2 levels by 26% (basal = 100%, 500 μM db-cGMP = 74 ± 4%; P < 0.01 vs. basal; n = 4, data not shown).
Fig. 1.
Dibutyryl cGMP (db-cGMP) decreases surface apical Na/K/2Cl cotransporter (NKCC2) levels in thick ascending limb of the loop of Henle (THALs). A: dose-response curve showing maximum inhibition by db-cGMP at 500 μM (n = 5 for each concentration, *P < 0.05 vs. basal). B: representative Western blotting showing surface NKCC2 levels in THALs treated with vehicle (basal, lane 1) and db-cGMP 500 μM (lane 2) and intracellular NKCC2 in the same samples vehicle, (basal, lane 3) and db-cGMP 500 μM (lane 4). The intracellular protein GAPDH is absent from the surface fraction (lanes 1 and 2) but strongly expressed in the intracellular fraction (lanes 3 and 4).
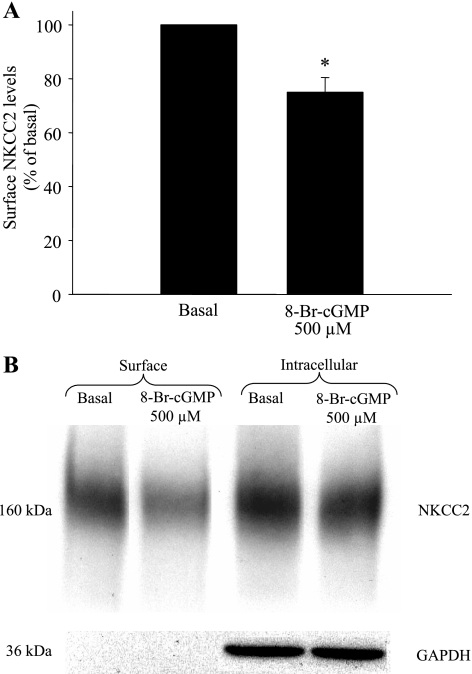
To ensure that the decrease in surface NKCC2 levels caused by db-cGMP was not unique to this analog, we studied whether a different membrane-permeant cGMP analog (8-Br-cGMP) could decrease surface NKCC2. THAL suspensions were equilibrated for 20 min at 37°C and then treated for 20 min with vehicle or 8-Br-cGMP (500 μM). We found that 8-Br-cGMP also decreased surface NKCC2 levels (basal = 100%, 500 μM 8-Br-cGMP = 75 ± 5%, P < 0.001; n = 6) (Fig. 2, A and B). Total NKCC2 expression was not significantly changed after treatment with 8-Br-cGMP (basal = 100%, 500 μM 8-Br-cGMP = 95 ± 9%, NS), and GAPDH was not detected in the surface protein fraction. Taken together, these data indicate that cGMP decreases surface NKCC2 expression in THALs. Our data suggest that a decrease in surface NKCC2 is caused by regulation of NKCC2 trafficking into or out of the plasma membrane rather than a decrease in NKCC2 expression during the 20 min-treatment.
Fig. 2.
8-Br-cGMP decreases surface NKCC2 levels in THALs. A: 20-min incubation with 8-Br-cGMP (500 μM) decreased surface NKCC2 by 25% (n = 5, *P < 0.02 vs. basal). B: representative Western blot showing surface NKCC2 levels in THALs treated with vehicle (basal; lane 1) and 8-Br-GMP (500 μM; lane 2) and intracellular NKCC2 in the same samples [vehicle, basal (lane 3) and 8-Br-GMP (500 μM; lane 4)]. The intracellular protein GAPDH is absent from the surface fraction (lanes 1 and 2) but strongly expressed in the intracellular proteins (lanes 3 and 4).
Effect of cGMP on surface NKCC2 levels in isolated, perfused THALs.
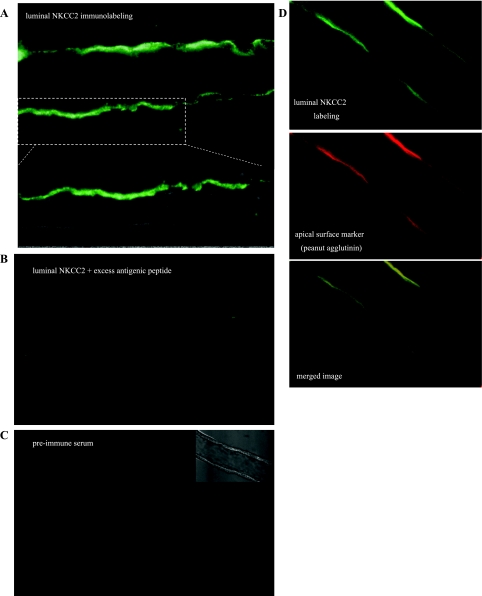
To directly image the effect of cGMP on apical membrane NKCC2 levels in isolated, perfused medullary THALs, we used immunofluorescence and confocal microscopy. For this, we generated rabbit polyclonal antibodies against an amino acid sequence located between the predicted transmembrane domains 5 and 6 of rat NKCC2 facing the extracellular (luminal) side (9). Intact, nonfixed THALs incubated at 6°C with luminal NKCC2 antibody showed strong labeling of the apical surface of all THAL cells along perfused tubules after detection with fluorescent-labeled secondary antibodies (Fig. 3A), whereas no intracellular labeling was detected. In contrast, preadsorption of the luminal NKCC2 antibody with excess antigenic peptide (4 μg/ml) completely eliminated apical labeling (Fig. 3B). Similarly, incubation of THALs with preimmune serum from the same rabbit under identical conditions resulted in undetectable fluorescent labeling (Fig. 3C). In different experiments, we observed colocalization of luminal NKCC2 labeling with the apical surface marker peanut agglutinin (7, 34, 40), indicative of antibody binding to a surface (extracellular) epitope (Fig. 3D). Although all cells along a THAL were labeled with the luminal NKCC2 antibody, we observed some cell-to-cell heterogeneity on the intensity of apical NKCC2 labeling that was independent of the focal plane. It is possible this is due to different amounts of apical NKCC2 in the rough- and smooth-surfaced cells normally found in the THAL.
Fig. 3.
Characterization of luminal (extracellular) NKCC2-directed antiserum. A: representative confocal image showing apical immunofluorescent labeling in medullary THAL cells after incubation of intact nonfixed perfused tubules with antiserum targeting an extracellular (luminal) sequence between transmembrane domains 5 and 6 of NKCC2. B: representative confocal image showing complete absence of immunofluorescent labeling after preadsorption of the luminal NKCC2 antiserum with excess immunizing peptide under the same conditions as in A. C: representative confocal image showing complete absence of immunofluorescent labeling after incubation of intact nonfixed THALs with preimmune serum from the same rabbit. D: representative confocal images showing dual fluorescent labeling in a medullary THAL with luminal NKCC2 antiserum (top; green) and with the apical surface marker peanut agglutinin (middle; red). The merged image (bottom) shows colocalization of staining in the apical surface of THAL cells.
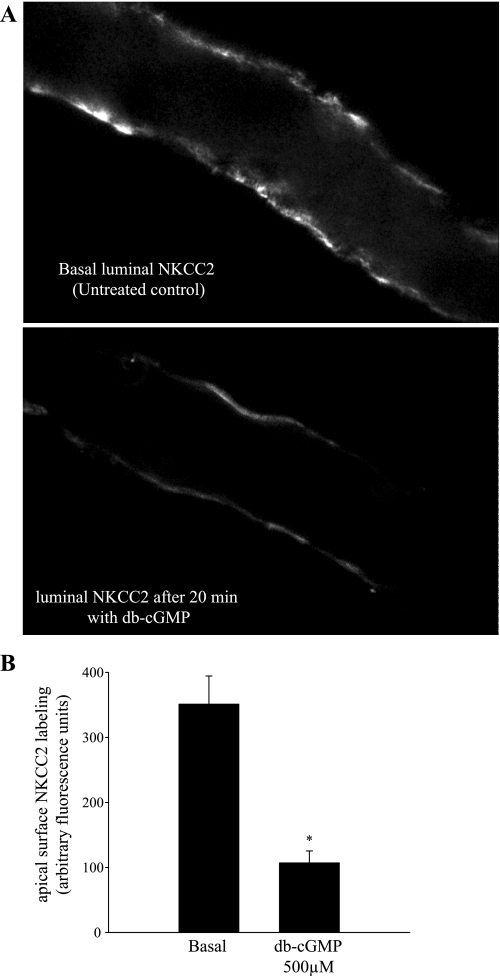
We then studied the effect of db-cGMP on luminal membrane NKCC2 labeling in isolated THALs. THALs were equilibrated for 20 min, and then either vehicle (basal conditions) or 500 μM db-cGMP was added to the bath and incubated for another 20 min. Then, luminal NKCC2 was labeled and imaged as described in materials and methods. We found that surface NKCC2 staining was lower in THALs treated with db-cGMP (Fig. 4A). Mean fluorescent intensity of apical membrane NKCC2 under basal conditions averaged 351 ± 43 arbitrary fluorescent units (n = 5), whereas luminal NKCC2 staining in THALs treated with db-cGMP was decreased to 107 ± 18 arbitrary fluorescent units (n = 6, P < 0.05) (Fig. 4B). Taken together, these data indicate that db-cGMP decreases surface NKCC2 levels in the THAL.
Fig. 4.
db-cGMP decreases apical surface NKCC2 staining in perfused medullary THALs. A: representative confocal micrographs showing apical surface NKCC2 staining in isolated, perfused medullary THALs under basal (unstimulated) conditions (top) and in a THAL treated with db-cGMP (500 μM) for 20 min (bottom). B: cumulative data for apical NKCC2 immunofluorescence staining under basal conditions (n = 5) and in THALs treated with db-cGMP (500 μM) (n = 6). The mean fluorescent intensity in the apical membrane was measured in 15–20 THAL cells in each tubule and then averaged.
Role of PDE2 in cGMP-induced decrease in surface NKCC2.
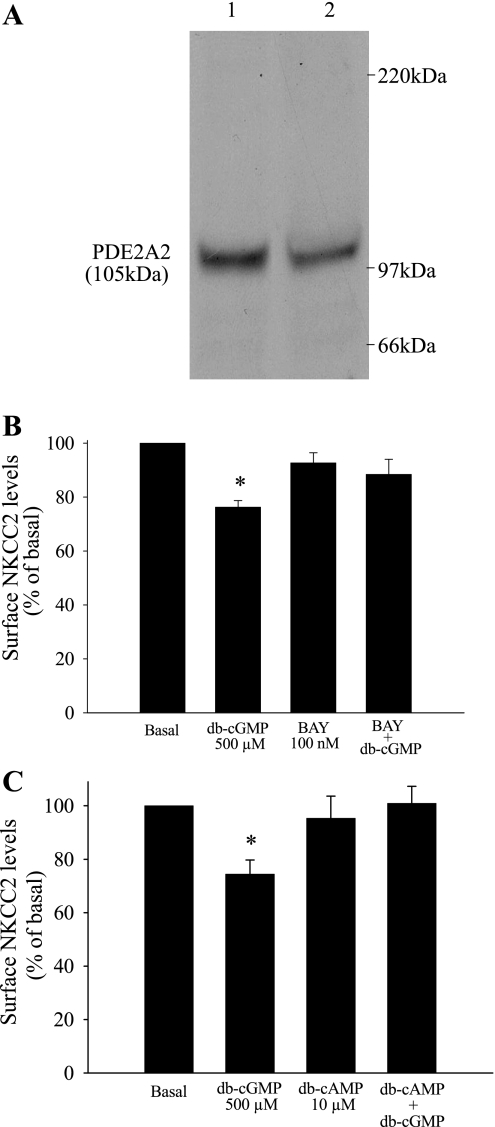
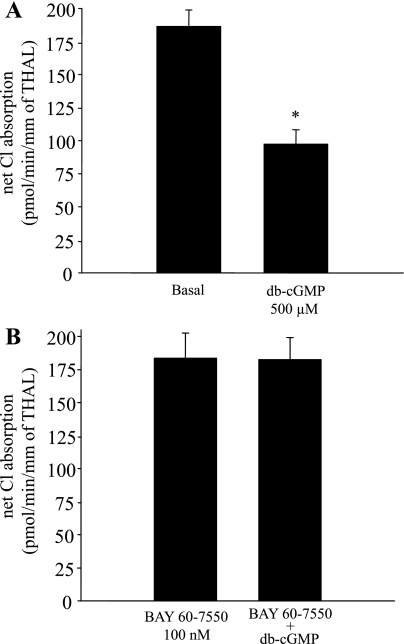
The second messenger cascade by which cGMP inhibits NaCl absorption by the THAL is not clear. In most cells, cGMP may signal through cGMP-dependent protein kinase (PKG) (17, 39), cGMP-stimulated phosphodiesterase (PDE2), or cGMP-inhibited phosphodiesterase (PDE3) (3, 8). In the THAL, NO decreases NaCl absorption by activating the cGMP-stimulated phosphodiesterase PDE2, which in turn may decrease cAMP levels (28). Therefore, we tested whether cGMP decreases surface NKCC2 levels via cGMP-stimulated PDE2. First, we studied the expression of PDE2 in THALs by Western blotting. THAL suspensions were obtained from four rats, and an equal amount of total protein (25 μg) from each suspension was loaded, resolved by SDS-PAGE, and PDE2 was detected with specific antibodies, as reported by others (23, 37). A single band at the expected molecular weight of PDE2A (105 kDa) was observed (n = 4) (Fig. 5A). Then, we examined the role of PDE2 in mediating the inhibitory effect of cGMP on surface NKCC2. THAL suspensions were equilibrated for 20 min at 37°C in the presence or absence of the PDE2 inhibitor BAY 60-7550 (100 nM) (4) and then treated for 20 min with vehicle or db-cGMP (500 μM). We found that in the presence of BAY 60-7550, db-cGMP did not decrease surface NKCC2 levels (basal = 100%, db-cGMP = 76 ± 7%, BAY 60-7550 = 93 ± 4%, BAY 60-7550+db-cGMP = 88 ± 6%, NS; n = 7) (Fig. 5B). BAY 60-7550 alone did not change surface NKCC2 levels from basal. Total NKCC2 levels were not changed after treatment with BAY 60-7550 or BAY 60-7550 plus db-cGMP. These data indicate that cGMP decreases surface NKCC2 by activating PDE2. To provide further evidence for PDE2 as a mediator of cGMP signaling, we tested a different PDE2 inhibitor, EHNA (20). THAL suspensions were equilibrated for 20 min in the absence or presence of EHNA (50 μM) and then treated with vehicle or db-cGMP for 20 min. In the presence of EHNA, addition of db-cGMP did not decrease surface NKCC2 levels (basal = 100%, db-cGMP = 78 ± 5%, EHNA = 102 ± 4%, EHNA+db-cGMP = 97 ± 8%, NS; n = 8) (data not shown). EHNA alone did not change surface NKCC2 levels. Taken together, these data indicate that PDE2 mediates the inhibitory effect of cGMP on surface NKCC2 levels in the THAL.
Fig. 5.
A–C: PDE2 inhibition blocks the cGMP-induced decrease in surface NKCC2 levels in THALs. A: representative Western blots showing the expression of PDE2 in medullary THALs from 2 different rats (25 μg of proteins THAL lysate/lane; n = 4). B: cumulative data showing the effect of db-cGMP and the PDE2 inhibitor BAY 60-7550 (BAY), alone and combined, on surface NKCC2 levels in the THAL (n = 6, *P < 0.05 vs. basal). C: clamping cAMP levels blocks the cGMP-induced decrease in surface NKCC2 levels in THALs. Cumulative data showing the effect of db-cGMP and a nonstimulatory concentration of db-cAMP (10 μM), alone and combined, on surface NKCC2 levels in the THAL (n = 6, *P < 0.05 vs. basal).
In most cells, stimulation of PDE2 activity by cGMP enhances the hydrolysis of cAMP, thereby decreasing cAMP levels (23). Therefore, we studied the effect of cGMP on basal unstimulated intracellular cAMP levels. THAL suspensions were aliquoted and treated with either vehicle or db-cGMP (500 μM), and intracellular cAMP was measured by RIA. We found that db-cGMP decreased basal cAMP from 14.9 ± 0.8 to 12.7 ± 0.9 fmol/μg of protein (n = 13, P < 0.05) (Data not shown). Next, we tested whether a decrease in cAMP is required for cGMP to diminish surface NKCC2 levels. For this, we tested whether we could block the effect of db-cGMP by clamping cAMP levels at a concentration that does not stimulate NKCC2, using a cAMP analog (db-cAMP) poorly hydrolyzed by PDEs (18). THAL suspensions were equilibrated for 20 min at 37°C in the presence of a nonstimulatory concentration of db-cAMP (10 μM) (28) and then treated for 20 min with vehicle or db-cGMP (500 μM). In the presence of db-cAMP (10 μM), db-cGMP did not change surface NKCC2 levels (basal = 100%, db-cGMP = 74 ± 5%, db-cAMP = 95 ± 8%, db-cAMP+db-cGMP = 101 ± 6%; n = 6) (Fig. 5C). db-cAMP at this concentration (10 μM) did not significantly affect surface NKCC2 levels. These data suggest that preventing a decrease in intracellular cAMP concentration blocked the cGMP-induced decrease in surface NKCC2 levels. Our data indicate that cGMP decreases surface NKCC2 levels in the THAL by stimulating PDE2 activity.
Role of PDE2 on cGMP-induced decrease in THAL net Cl absorption.
cGMP decreases NaCl absorption by the THAL. Since PDE2 inhibition blocked the cGMP-induced decrease in surface NKCC2, we tested whether PDE2 inhibition blocks the effect of db-cGMP on THAL NaCl transport. For this, we measured net Cl absorption by isolated, perfused THALs. We found that addition of db-cGMP (500 μM) to the bath decreased net Cl absorption by 48 ± 4% (from 187 ± 12 to 98 ± 10 pmol·min−1·mm−1 of THAL; n = 6, P < 0.05) (Fig. 6A). A different set of tubules was pretreated with the PDE2 inhibitor BAY 60-7550 (100 nM) throughout the experimental period. In the presence of the PDE2 inhibitor, addition of db-cGMP (500 μM) to the bath did not significantly affect net Cl absorption (from 184 ± 19 to 183 ± 16 pmol·min−1·mm−1 of THAL; n = 6) (Fig. 6B). In control experiments, addition of BAY 60-7550 to the bath did not significantly affect net Cl absorption (from 174 ± 17 to 176 ± 21 pmol·min−1·mm−1 of THAL; n = 6). These data indicate that blockade of PDE2 prevents cGMP-induced decreases in surface NKCC2 and NaCl absorption in THALs.
Fig. 6.
PDE2 inhibition blocks cGMP-induced decrease in net Cl absorption by isolated, perfused THALs. A: cumulative data showing the effect of db-cGMP (500 μM) on THAL net Cl absorption (n = 6, *P < 0.05). B: cumulative data showing the effect of db-cGMP (500 μM) on THAL net Cl absorption in tubules preincubated with the PDE2 inhibitor BAY 60-7550 (100 nM) (n = 6). Addition of the PDE2 inhibitor alone to the bath did not affect net Cl absorption (see results).
DISCUSSION
cGMP, the second messenger for NO and ANP, decreases NaCl absorption by the THAL by inhibiting NKCC2-dependent Na+ entry (30). Recently, we and other investigators reported that NKCC2-dependent Na+ entry and NaCl absorption are modulated by changes in NKCC2 levels in the plasma membrane (11, 19, 26). Thus we tested the hypothesis that cGMP decreases NKCC2 levels in the plasma membrane of THALs. We found that membrane-permeant cGMP analogs decreased surface NKCC2 levels and apical membrane staining for NKCC2 without affecting the total pool of NKCC2 in rat THALs. We also found that 1) PDE2 inhibitors completely blocked the cGMP-induced decrease in surface NKCC2 levels and 2) preventing a decrease in cAMP levels with a nonstimulatory concentration of db-cAMP blocked the cGMP-induced decrease in surface NKCC2 levels. Finally, PDE2 inhibition blocked the cGMP-induced decrease in net Cl absorption by THALs. From these data, we conclude that cGMP decreases NKCC2 levels in the apical membrane by activating PDE2. To our knowledge, these are the first data to show that an inhibitor of THAL NaCl absorption decreases apical surface expression of NKCC2 and that there is an interaction between cGMP and cAMP signaling in the regulation of surface NKCC2 levels.
The second messenger cGMP exerts an inhibitory effect on NaCl absorption along the nephron (10, 38, 42). In the THAL, cGMP either added exogenously or produced by stimulation with NO or ANP consistently decreases net NaCl absorption (24, 27, 28). NaCl absorption by the THAL is primarily mediated by apical NKCC2. Since NO inhibits NKCC2-dependent Na+ entry in THALs, the mechanism by which cGMP decreases net NaCl absorption most likely involves direct inhibition of NKCC2-dependent Na+ entry (30). We previously found that the cAMP-induced increase in NaCl absorption is mediated by an increase in surface NKCC2 levels (26). Thus we examined whether membrane-permeable cGMP analogs could decrease NKCC2 levels in the plasma membrane. In surface biotinylation experiments, we found that db-cGMP for 20 min decreased surface NKCC2 by 25 to 45%. A different membrane-permeable cGMP analog, 8-Br-cGMP (500 μM), also decreased surface NKCC2 levels by 25%. The reason for the enhanced inhibitory effect of db-cGMP over 8-Br-cGMP might be related to enhanced membrane permeability of db-cGMP, which is 25 times more lipophilic than 8-Br-cGMP (36). Our experiments showed that neither db-cGMP nor 8-Br-cGMP affected the total pool of NKCC2. Taken together, these data suggest that cGMP decreases surface NKCC2 levels by regulating some membrane-trafficking step rather than inhibiting the transcription, or stimulating degradation of total NKCC2.
To directly measure the effect of cGMP on surface NKCC2 in isolated, perfused THALs, we used immunofluorescence and confocal microscopy. For this, we raised polyclonal antibodies toward an NKCC2-specific sequence located between predicted transmembrane domains 5 and 6 that faces the extracellular luminal space in THAL cells. As expected, this antibody labeled the luminal membrane of THAL cells and colocalized with an apical surface marker (peanut agglutinin) (7, 34, 40) but resulted in undetectable intracellular labeling. These results were expected because in intact cells maintained at a low temperature, antibodies are not likely to access the intracellular space whereas they can readily bind surface antigens. We observed that in THALs treated with db-cGMP for 20 min, there was a significant decrease in apical NKCC2 labeling as measured after analysis of confocal images. Therefore, data obtained with this method correlated with the inhibitory effect of db-cGMP on surface NKCC2 measured by surface biotinylation. To our knowledge, these are the first data showing that cGMP regulates apical surface levels of a transporter in THALs.
The membrane-trafficking mechanism by which cGMP decreases surface NKCC2 levels is not known. In other cell types, cGMP inhibits protein trafficking to the plasma membrane. In the proximal tubule, cGMP decreased the activity of the Na/Pi cotransporter (NaPi2) by inducing its redistribution from the brush-border membrane to a subapical compartment; however, the trafficking mechanism affected by cGMP was not examined (1). In cardiac myocytes, cGMP inhibits glucose uptake by reducing the translocation of GLUT-4 transporters to the plasma membrane by an unknown mechanism (16). In the THAL, cGMP could decrease surface NKCC2 levels by inhibiting exocytic insertion or by stimulating endocytosis of NKCC2 from the apical membrane. We previously found that cAMP increases surface NKCC2 levels in THALs and that this increase was blocked by tetanus toxin, an agent that inhibits vesicle insertion into the plasma membrane (26). Because we found that cGMP decreased surface NKCC2 by reducing cAMP signaling, it is possible to speculate that cGMP decreases exocytic insertion of NKCC2 into the plasma membrane rather than stimulating endocytosis. However, this remains a hypothesis and the trafficking mechanism affected by cGMP needs to be further studied.
We found that cGMP analogs decreased surface NKCC2 levels by a range of 25–45%. The magnitude of this decrease is consistent with the reported effect of cGMP and cGMP-elevating hormones in net NaCl absorption by the THAL. We previously found that NO decreased NKCC2-dependent Na+ entry by 45% (30) and decreased net Cl absorption in rat medullary THALs by 30–45% (28, 29, 33). In this study, we found that db-cGMP decreased net Cl absorption by 45%. Neant and Bailly (2, 14) studied the effect of 8-Br-cGMP and ANP on THAL Cl absorption in mice. In agreement with our data, they found that 8-Br-cGMP decreased Cl absorption by 30% and ANP decreased it by 28% (2, 24). Taken together, our data indicate that a decrease in surface NKCC2 is involved in cGMP-induced inhibition of NaCl absorption. Several factors, such as membrane permeability and the activity of intracellular and extracellular esterases, affect the active intracellular concentration obtained with membrane-permeant cGMP analogs. Thus it is difficult to compare whether the intracellular concentration of cGMP obtained with db-cGMP is similar to that obtained with physiological agonists. However, we found that the inhibitory effect of db-cGMP in surface NKCC2 was dose dependent and saturated at 500 μM. At this concentration, db-cGMP inhibited THAL Cl absorption by 45%, which is comparable to the effect of NO and ANP previously reported. Thus it is likely that regulation of NKCC2 trafficking by cGMP is a physiologically relevant mechanism for the natriuretic action of NO and natriuretic peptides in the THAL. However, we cannot rule out that mechanisms other than membrane trafficking, such as changes in the affinity of transported ions, the phosphorylation state of NKCC2, or protein-protein interactions are also involved in the inhibition of NKCC2 by cGMP.
The signaling cascade by which cGMP regulates surface NKCC2 levels is not known. In general, cGMP may signal through cGMP-stimulated phosphodiesterase (PDE2) (23), cGMP-inhibited phosphodiesterase (PDE 3) (22), or cGMP-activated protein kinase (PKG) (17). We previously found that a PDE2 inhibitor (EHNA) completely blocked the NO-induced decrease in Cl absorption, whereas a specific PKG inhibitor (KT-5823) did not (28), indicating a role for PDE2 rather than PKG in mediating the effects of NO and cGMP in the THAL. PDE3 is inhibited by cGMP, resulting in an increase in cAMP levels (3, 22), an effect that would tend to increase surface NKCC2 and stimulate NaCl absorption; thus we focused on PDE2 as a mediator of cGMP's effects on surface NKCC2 levels. We examined PDE2 expression in rat THALs by Western blotting and detected a single band having the predicted molecular mass of ≈105 kDa, consistent with PDE2A2 (rat PDE2) expression (35). Given the different molecular weights between PDE2 (105 kDa) and all other PDEs detected in the kidney (PDE1: 50–70 kDa, PDE3: 130–145 kDa, PDE4: 55–90 kDa, PDE5: 95 KDa, PDE10: 85 kDa, PDE11: 55 kDa), it is unlikely that the 105-kDa band observed was caused by cross-reaction of the PDE2 antibody with other PDEs. We also found that the PDE2 inhibitor (BAY 60-7550) completely blocked the inhibitory effect of db-cGMP on surface NKCC2 levels and net Cl absorption without affecting basal transport rates. BAY 60-7550 inhibits PDE2 with an IC50 ≈ 5 nM and exhibits at least 50–100 times greater selectivity compared with other PDEs. We used this inhibitor at 100 nM (20 times the IC50), a concentration previously shown to inhibit PDE2 activity in cardiac myocytes and neurons (4, 5). In addition, a different PDE2 inhibitor, EHNA was used at a concentration previously shown to inhibit the effect of NO on Cl absorption without affecting basal transport rates (20). This antagonist also blocked the cGMP-induced decrease in surface NKCC2 levels. Addition of PDE2 inhibitors alone did not significantly change surface NKCC2 levels nor affected net Cl absorption, consistent with low basal hydrolytic activity of PDE2 reported in most cells (18). Taken together, our data indicate that PDE2 mediates the inhibitory effect of cGMP on surface NKCC2 levels in THALs. Because PDE2 inhibition also completely blocked the effect of db-cGMP on net Cl absorption, our data suggest that PDE2-induced decrease in surface NKCC2 mediates in part the inhibitory effect of cGMP on NaCl reabsorption by the THAL.
PDE2 activation by cGMP increases cAMP degradation by this enzyme, thereby reducing cAMP levels (23). We found that db-cGMP caused a modest but significant decrease in unstimulated intracellular cAMP. Thus we tested whether we could block the effect of cGMP on surface NKCC2 levels by preventing the decrease in cAMP levels. For this purpose, we clamped intracellular cAMP concentration with db-cAMP, which is poorly hydrolyzed by PDE2 (18) at a concentration that does not stimulate NaCl absorption (28). db-cAMP (10 μM) did not affect basal surface NKCC2 levels, but blocked the cGMP-induced decrease in surface NKCC2. These data suggest that a decrease in whole cell cAMP concentration or in a specific compartment where PDE2 is located is required for cGMP to inhibit NKCC2 trafficking to the apical membrane. In agreement with these data, we previously found that clamping cAMP levels with db-cAMP blocked the inhibitory effect of NO on NaCl absorption by THALs (28). In addition, in other cell types PDE2 is known to mediate the inhibitory interaction between the cGMP and cAMP pathways. In cardiac myocytes, PDE2 mediates the inhibitory effect of NO-induced cGMP on cardiac contractility by decreasing compartmentalized cAMP levels during stimulation of β3-adrenergic receptors (23). In adrenal zone glomerulosa cells, PDE2 mediates cGMP-induced inhibition of aldosterone production by decreasing cAMP levels (18). Thus, similar to other cells, our data support an important role for PDE2 in cGMP regulation of surface NKCC2 in the THAL. While we and others have found that cGMP decreases basal cAMP levels in PDE2-expressing cells, to our knowledge there is no evidence that the opposite occurs. We think it is unlikely that cAMP decreases basal cGMP by activating a PDEs because in the absence of stimuli, intracellular cGMP concentration is well below the Km of most cGMP-specific PDEs. However, it is possible that cAMP decreases cGMP after stimulation of cGMP levels with NO or natriuretic peptides, perhaps by activating one of the cGMP-specific PDEs. This is an interesting possibility that requires further study.
We conclude that cGMP decreases surface NKCC2 levels in rat THALs and that PDE2 mediates the inhibitory effect of cGMP on surface NKCC2 levels and net Cl absorption. Our data indicate that PDE2-induced decrease in surface NKCC2 levels is involved in the inhibitory effect of cGMP on NaCl transport by the THAL. The regulation of NKCC2 trafficking into or out of the plasma membrane by cGMP may be an important part of the mechanism by which NO and other natriuretic hormones that elevate cGMP inhibit THAL NaCl absorption. Our data also show that PDE2 is an important mediator for the interaction between the cGMP and cAMP signaling pathways in the THAL.
GRANTS
This work was supported in part by National Institutes of Health (NIH) Grant RO-1 HL-080409, American Heart Association Grant 0850126Z to P. Ortiz, and NINDS-NIH Grant NS29227 to F. J. Alvarez-Leefmans. G. Ares is supported in part by a predoctoral fellowship grant from the American Heart Association.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bacic D, Hernando N, Traebert M, Lederer E, Volkl H, Biber J, Kaissling B, Murer H. Regulation of the renal type IIa Na/Pi cotransporter by cGMP. Pflügers Arch 443: 306–313, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bailly C Effect of luminal atrial natriuretic peptide on chloride reabsorption in mouse cortical thick ascending limb: inhibition by endothelin. J Am Soc Nephrol 11: 1791–1797, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, De Vente J, Prickaerts J, Blokland A, Koenig G. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology 47: 1081–1092, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113: 2221–2228, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YC, Cadnapaphornchai MA, Summer SN, Falk S, Li C, Wang W, Schrier RW. Molecular mechanisms of impaired urinary concentrating ability in glucocorticoid-deficient rats. J Am Soc Nephrol 16: 2864–2871, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Constantinescu A, Silver RB, Satlin LM. H-K-ATPase activity in PNA-binding intercalated cells of newborn rabbit cortical collecting duct. Am J Physiol Renal Physiol 272: F167–F177, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Dousa TP Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int 55: 29–62, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 10.Garvin JL Inhibition of Jv by ANF in rat proximal straight tubules requires angiotensin. Am J Physiol Renal Fluid Electrolyte Physiol 257: F907–F911, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Gimenez I, Forbush B. Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Greger R Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int 49: 40–47, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 276: F96–F103, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lei B, Matsuo K, Labinskyy V, Sharma N, Chandler MP, Ahn A, Hintze TH, Stanley WC, Recchia FA. Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo. Proc Natl Acad Sci USA 102: 6966–6971, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 18.MacFarland RT, Zelus BD, Beavo JA. High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem 266: 136–142, 1991. [PubMed] [Google Scholar]
- 19.Meade P, Hoover RS, Plata C, Vazquez N, Bobadilla NA, Gamba G, Hebert SC. cAMP-dependent activation of the renal-specific Na+-K+-2Cl− cotransporter is mediated by regulation of cotransporter trafficking. Am J Physiol Renal Physiol 284: F1145–F1154, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Mery PF, Pavoine C, Pecker F, Fischmeister R. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic GMP-stimulated phosphodiesterase in isolated cardiac myocytes. Mol Pharmacol 48: 121–130, 1995. [PubMed] [Google Scholar]
- 21.Molony DA, Reeves WB, Andreoli TE. Na+:K+:2Cl− cotransport and the thick ascending limb. Kidney Int 36: 418–426, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Mongillo M, McSorley T, Evellin S, Sood A, Lissandron V, Terrin A, Huston E, Hannawacker A, Lohse MJ, Pozzan T, Houslay MD, Zaccolo M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ Res 95: 67–75, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res 98: 226–234, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Neant F, Imbert-Teboul M, Bailly C. Cyclic guanosine monophosphate is the mediator of platelet-activating factor inhibition on transport by the mouse kidney thick ascending limb. J Clin Invest 94: 1156–1162, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol 275: F885–F893, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz PA cAMP increases surface expression of NKCC2 in rat thick ascending limbs: role of VAMP. Am J Physiol Renal Physiol 290: F608–F616, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz PA, Garvin JL. Autocrine effects of nitric oxide on HCO3− transport by rat thick ascending limb. Kidney Int 58: 2069–2074, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz PA, Garvin JL. NO Inhibits NaCl absorption by rat thick ascending limb through activation of cGMP-stimulated phosphodiesterase. Hypertension 37: 467–471, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz PA, Garvin JL. Interaction of O2− and NO in the thick ascending limb. Hypertension 39: 591–596, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Paredes A, Plata C, Rivera M, Moreno E, Vazquez N, Munoz-Clares R, Hebert SC, Gamba G. Activity of the renal Na+-K+-2Cl− cotransporter is reduced by mutagenesis of N-glycosylation sites: role for protein surface charge in Cl−1 transport. Am J Physiol Renal Physiol 290: F1094–F1102, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Plato CF, Pollock DM, Garvin JL. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol Renal Physiol 279: F326–F333, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Praetorius J, Backlund P, Yergey AL, Spring KR. Specific lectin binding to beta1 integrin and fibronectin on the apical membrane of Madin-Darby canine kidney cells. J Membr Biol 184: 273–281, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Rosman GJ, Martins TJ, Sonnenburg WK, Beavo JA, Ferguson K, Loughney K. Isolation and characterization of human cDNAs encoding a cGMP-stimulated 3′,5′-cyclic nucleotide phosphodiesterase. Gene 20;191: 89–95, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Schwede F, Maronde E, Genieser H, Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther 87: 199–226, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krull M, Schutte H, Hippenstiel S, Suttorp N. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood 105: 3569–3576, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Stoos BA, Carretero OA, Farhy RD, Scicli G, Garvin JL. Endothelium-derived relaxing factor inhibits transport and increases cGMP content in cultured mouse cortical collecting duct cells. J Clin Invest 89: 761–765, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaandrager AB, Hogema BM, De Jonge HR. Molecular properties and biological functions of cGMP-dependent protein kinase II. Front Biosci 10: 2150–2164, 2005. [DOI] [PubMed] [Google Scholar]
- 40.van Adelsberg JS, Edwards JC, Al Awqati Q. The apical Cl/HCO3 exchanger of beta intercalated cells. J Biol Chem 268: 11283–11289, 1993. [PubMed] [Google Scholar]
- 41.Wang WH Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am J Physiol Renal Physiol 290: F14–F19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeidel ML, Silva P, Brenner BM, Seifter JL. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 252: F551–F559, 1987. [DOI] [PubMed] [Google Scholar]