Abstract
The Avi-3 antigen, which is found only in Mycobacterium avium culture sonic extracts, is species specific and results in strong skin test activity in guinea pigs sensitized with heat-killed M. avium. Its gene was cloned by using a previously developed single-probe method and was sequenced. The gene encoded a 194-amino-acid polypeptide with a molecular weight of 21,500. A recombinant Avi-3 antigen expressed in Escherichia coli reacted with monoclonal and polyclonal antibodies raised against the native Avi-3 antigen. To identify epitopes on this protein for immunodiagnostic purposes, various parts of the Avi-3 antigen were expressed as beta-galactosidase fusion proteins, using pUR and pURS expression vectors. The clones screened by both antibody reactivity and T-cell proliferative activity defined fragments with coexisting B- and T-cell epitopes. A B-cell epitope (Asn-176 to Ala-186) and two T-cell epitopes (Glu-75 to Ile-86 and Arg-155 to Leu-164) were thus defined. The synthetic polymerized peptides of the T-cell epitopes were proven to elicit a delayed cutaneous hypersensitivity reaction in guinea pigs. This mapping method would be useful in the development of a subunit vaccine consisting of an immunodominant B-cell epitope linked to a T-cell epitope in the vicinity.
Full text
PDF

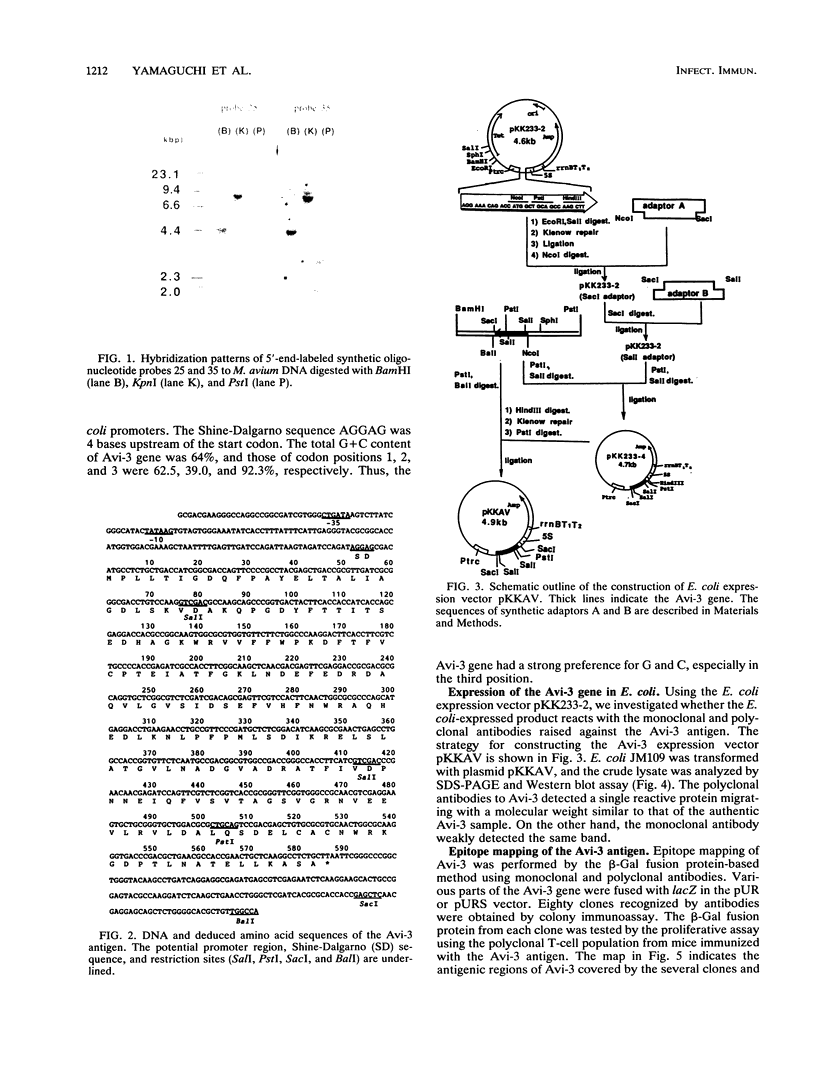
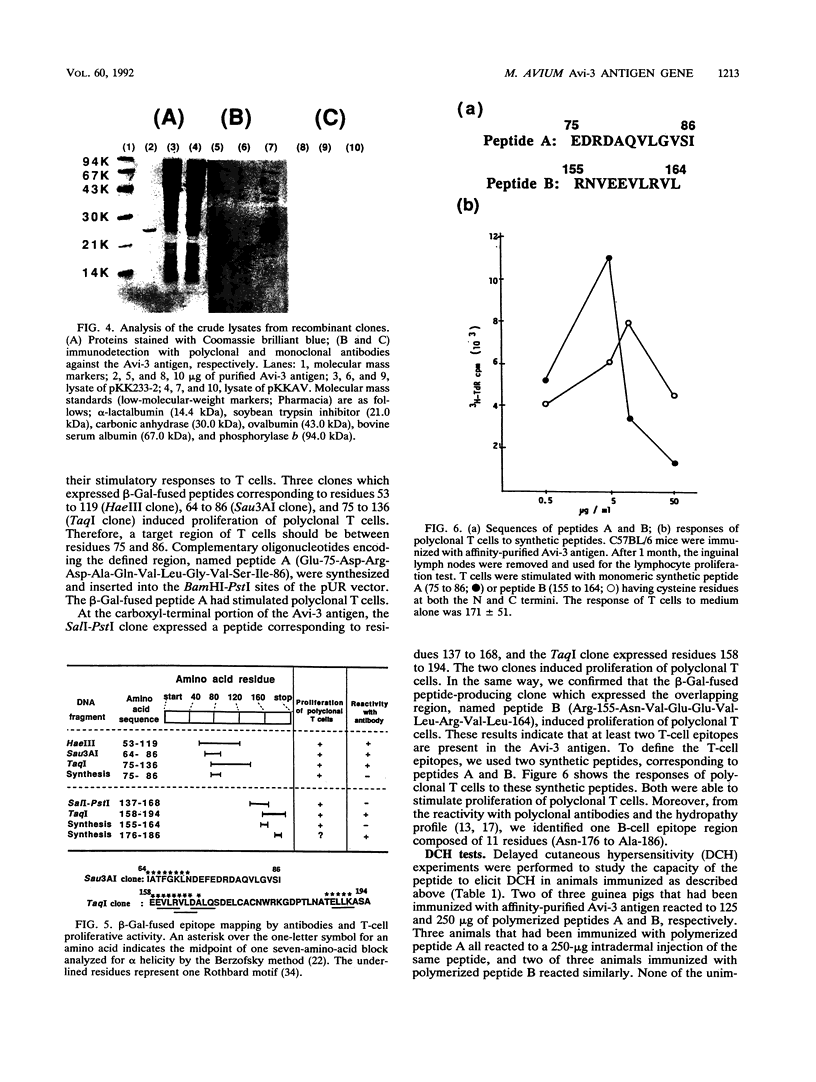


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe C., Saito H., Tomioka H., Fukasawa Y. Production of a monoclonal antibody specific for Mycobacterium avium and immunological activity of the affinity-purified antigen. Infect Immun. 1989 Apr;57(4):1095–1099. doi: 10.1128/iai.57.4.1095-1099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldovini A., Young R. A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991 Jun 6;351(6326):479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Baess I. Deoxyribonucleic acid relationships between different serovars of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):201–203. doi: 10.1111/j.1699-0463.1983.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. T-B reciprocity. An Ia-restricted epitope-specific circuit regulating T cell-B cell interaction and antibody specificity. Surv Immunol Res. 1983;2(3):223–229. doi: 10.1007/BF02918417. [DOI] [PubMed] [Google Scholar]
- Charette M. F., Henderson G. W., Markovitz A. ATP hydrolysis-dependent protease activity of the lon (capR) protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4728–4732. doi: 10.1073/pnas.78.8.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Stoker N. G., Lee S. P., Jackson M., Grant K. A., Jouy N. F., Lucas S. B., Hasan R., Hussain R., McAdam K. P. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect Immun. 1989 Jul;57(7):1979–1983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis M. J., Hastings G. Z., Clarke B. E., Brown A. L., Beddell C. R., Rowlands D. J., Brown F. Neutralizing antibodies to all seven serotypes of foot-and-mouth disease virus elicited by synthetic peptides. Immunology. 1990 Feb;69(2):171–176. [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K., Onodera O., Miyatake T., Tsuji S. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction (PCR). Neurology. 1990 Oct;40(10):1617–1618. doi: 10.1212/wnl.40.10.1617. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc C., Deriaud E., Mimic V., van der Werf S. Identification of a T-cell epitope adjacent to neutralization antigenic site 1 of poliovirus type 1. J Virol. 1991 Feb;65(2):711–718. doi: 10.1128/jvi.65.2.711-718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Terasaka K., Totsuka M., Kobayashi K., Yukitake H., Yamada T. Establishment of a foreign antigen secretion system in mycobacteria. Infect Immun. 1990 Dec;58(12):4049–4054. doi: 10.1128/iai.58.12.4049-4054.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Terasaka K., Yamada T. Cloning and expression of the gene for the cross-reactive alpha antigen of Mycobacterium kansasii. Infect Immun. 1990 Feb;58(2):550–556. doi: 10.1128/iai.58.2.550-556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Yamaguchi R., Yamazaki A., Tasaka H., Yamada T. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J Bacteriol. 1988 Sep;170(9):3847–3854. doi: 10.1128/jb.170.9.3847-3854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., McLachlan A., Bitter G., Moriarty A., Hughes J. L. Importance of subtype in the immune response to the pre-S(2) region of the hepatitis B surface antigen. II. Synthetic Pre-S(2) immunogen. J Immunol. 1990 May 1;144(9):3544–3551. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Chisari F. V., Thornton G. B. Nonoverlapping T and B cell determinants on an hepatitis B surface antigen pre-S(2) region synthetic peptide. J Exp Med. 1986 Aug 1;164(2):532–547. doi: 10.1084/jem.164.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., Houghten R. A., Spear J. R., Shinnick T. M. A chemically synthesized peptide which elicits humoral and cellular immune responses to mycobacterial antigens. Infect Immun. 1986 Sep;53(3):560–564. doi: 10.1128/iai.53.3.560-564.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. L., Rouse D. A., Hussong D., Chaparas S. D. Isolation and characterization of recombinant lambda gt11 bacteriophages expressing four different Mycobacterium intracellulare antigens. Infect Immun. 1990 Jan;58(1):17–20. doi: 10.1128/iai.58.1.17-20.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Shinnick T. M., Mustafa A. S., Lundin K. E., Godal T., Nerland A. H. Heterogeneity among human T cell clones recognizing an HLA-DR4,Dw4-restricted epitope from the 18-kDa antigen of Mycobacterium leprae defined by synthetic peptides. J Immunol. 1990 Feb 15;144(4):1478–1483. [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D. A., Morris S. L., Karpas A. B., Probst P. G., Chaparas S. D. Production, characterization, and species specificity of monoclonal antibodies to Mycobacterium avium complex protein antigens. Infect Immun. 1990 May;58(5):1445–1449. doi: 10.1128/iai.58.5.1445-1449.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safley S. A., Cluff C. W., Marshall N. E., Ziegler H. K. Role of listeriolysin-O (LLO) in the T lymphocyte response to infection with Listeria monocytogenes. Identification of T cell epitopes of LLO. J Immunol. 1991 May 15;146(10):3604–3616. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshinaga K., Ono Y., Nagata A., Yamada T. Organization of rRNA genes in Mycobacterium bovis BCG. J Bacteriol. 1987 Feb;169(2):839–843. doi: 10.1128/jb.169.2.839-843.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K., Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Yamada T. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol Lett. 1989 Apr;49(2-3):273–276. doi: 10.1016/0378-1097(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toida I., Yamamoto S., Takuma S., Suzuki T., Hirata M. Lack of tuberculin activity of synthetic peptides. Infect Immun. 1985 Dec;50(3):614–619. doi: 10.1128/iai.50.3.614-619.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. M., Hannah J. B. Mycobacterium avium complex infection in patients with the acquired immunodeficiency syndrome. A clinicopathologic study. Chest. 1988 May;93(5):926–932. doi: 10.1378/chest.93.5.926. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Abe C., Nagai S., Terasaka K., Yamada T. Cloning and characterization of the gene for immunogenic protein MPB64 of Mycobacterium bovis BCG. Infect Immun. 1989 Jan;57(1):283–288. doi: 10.1128/iai.57.1.283-288.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Kagawa H., Nagai S., Yamada T. Fusion protein based epitope mapping of the MPB57 protein from Mycobacterium bovis BCG and its epitope insertion into the native protein. Can J Microbiol. 1991 Jan;37(1):7–13. doi: 10.1139/m91-002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Matsuo K., Yamazaki A., Nagai S., Terasaka K., Yamada T. Immunogenic protein MPB57 from Mycobacterium bovis BCG: molecular cloning, nucleotide sequence and expression. FEBS Lett. 1988 Nov 21;240(1-2):115–117. doi: 10.1016/0014-5793(88)80350-8. [DOI] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakowski P., Fligiel S., Berlin G. W., Johnson L., Jr Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA. 1982 Dec 10;248(22):2980–2982. doi: 10.1001/jama.1982.03330220024029. [DOI] [PubMed] [Google Scholar]