Abstract
Urethane is a common and often preferred anesthetic agent for urodynamic recordings in rats, but its use is often restricted to terminal procedures because of a prolonged duration of action and potentially toxic effects. When urodynamic recordings are part of survival procedures in rodent experimental models, inhalation anesthetics, such as isoflurane, are frequently used and generally well tolerated. In this study, we compared the effects of urethane and isoflurane on lower urinary tract function. For this purpose, adult female rats were anesthetized by subcutaneous administration of urethane (n = 6) or by inhalation of isoflurane (n = 5). Micturition reflexes were assessed by concurrent cystometrogram and external urethral sphincter (EUS) electromyography (EMG) recordings to determine bladder contractile properties, EUS activation patterns, and the coordination between bladder contractions and EUS activation. Compared with urethane, isoflurane reduced frequency of bursts, firing frequency, and amplitude of EUS EMG activity during voiding as well as the EUS EMG amplitude during the bladder filling phase. Isoflurane also prolonged the bladder intercontractile intervals. Other several key functional aspects of the bladder contractile properties as well as the coordination between bladder contractions were not different between the two experimental groups. We conclude that micturition reflexes were differentially affected by isoflurane and urethane. Specifically, isoflurane exhibited a significant suppression of the EUS EMG activity and prolonged the bladder intercontractile intervals compared with urethane. We suggest that these anesthetic properties be taken into consideration during the experimental design and interpretation of urodynamic recordings in rodent models.
Keywords: anesthetics, bladder, urodynamics
cystometrogram (CMG) recordings of bladder pressure and electromyography (EMG) of the external urethral sphincter (EUS) activity may be used to assess micturition reflexes and are commonly performed under general anesthesia in rodent models. For this purpose, urethane may be administered intraperitoneally or subcutaneously and in some studies has been proposed as the preferred anesthetic agent for urodynamic studies (3, 12, 16). The anesthetic effect provided by urethane is prolonged in the adult rat, usually lasting in the range of hours, and allows for comprehensive physiological recording sessions. However, the use of urethane is usually limited to terminal experimental procedures due to its potential toxic effects (9).
In contrast, inhalation anesthetics are commonly preferred for survival surgeries and nonterminal experimental procedures in rats, in part because of its relatively limited pathological properties compared with other inhalation anesthetics (8). For instance, we have previously used isoflurane, a widely used inhalation anesthetic, when combining urodynamic studies with the injection of retrogradely transported anatomical tracers as a survival procedure in the adult rat (5, 10). In the latter studies, long-term CMG and EUS EMG recordings were obtained, and the studies demonstrated functional reinnervation of the lower urinary tract (LUT) in a cauda equina injury and repair model. However, direct comparisons of urethane and isoflurane effects on urodynamic recordings are overall sparse, especially with regard to the effects of these anesthetics on EUS EMG tonic and bursting activity.
Therefore, in the present study we compared the effects of urethane and isoflurane anesthetics on urodynamic recordings in the rat. For this purpose, we performed concurrent CMG and EUS EMG recordings in neurologically intact female rats to determine bladder contractile properties, EUS activation patterns, and the coordination between bladder contractions and EUS activation. We have demonstrated that micturition reflexes are differentially affected by urethane and isoflurane anesthetics. Specifically, compared with urethane, isoflurane reduces the firing frequency and amplitude of EUS EMG bursting activity during voiding and the amplitude of EUS EMG tonic activity during bladder filling. Isoflurane also prolongs the bladder intercontractile intervals compared with urethane, whereas these anesthetic agents showed similar bladder contractile properties and coordination of reflexive bladder contractions with EUS EMG activation.
MATERIALS AND METHODS
Eleven adult female Sprague-Dawley rats (weight between 250–300 g) were used in the present study. All animal procedures were carried out according to the standards established by the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996). The experimental protocols were approved by the Chancellor's Animal Research Committee at the University of California, Los Angeles. All efforts were made to minimize any suffering and the number of animals used in the study.
Surgical procedures and preparations for urodynamic studies.
Six of the eleven rats in the experimental series were anesthetized by a subcutaneous injection of urethane (1.2 g/kg) 1 h before the surgery. The remaining 5 of 11 animals were placed under gas anesthesia using isoflurane (2–2.5%). The toe-pinch reflex was used to determine the level of anesthesia in the present study. At surgical planes of anesthesia, the toe-pinch reflex was abolished in both the urethane- and isoflurane-anesthetized series. All animals were maintained under anesthesia during the surgical preparations and placed on a heating pad to maintain the body temperature at 37°C. To prepare the animals for urodynamic recordings, we exposed the urinary bladder via a midline abdominal incision. The pubic symphysis and the medial portion of the pubic bones were removed to expose the midportion of the urethra. A catheter (PE-50) was inserted through the top of the bladder to measure intravesical pressure of the bladder. The bladder catheter was connected to a pressure transducer and an infusion pump for acquiring the CMG. The bladder was continuously infused with saline (0.12 ml/min) at room temperature. For recording EUS EMG activity, two 50-μm epoxy-coated platinum-iridium wire electrodes (A-M Systems, Everett, WA) were hooked at the tip of a 30-gauge needle and inserted into both sides of the EUS under direct visual inspection. The needle was withdrawn, and the electrodes were left embedded in the muscle. The abdominal incision was closed, allowing the bladder catheter and two wire electrodes to exit and connect to a personal computer. CMG and EUS EMG data were amplified and digitized (CMG at 5 samples/s and EUS EMG at 6,250 samples/s) using a 12-bit data acquisition system (DataLab 2000, model 70754; Lafayette Instruments, Lafayette, IN). The urodynamic recording sessions lasted for 3–4 h for each animal.
Urodynamic recordings.
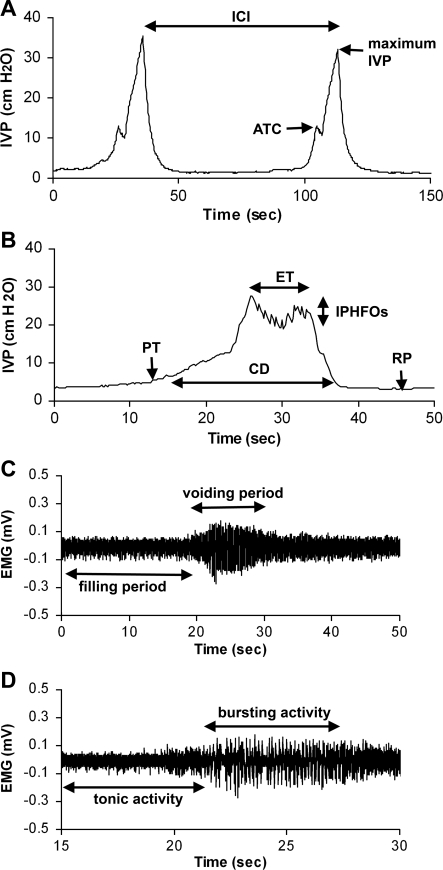
Before the start of the urodynamic recordings, rats receiving gas anesthesia were allowed to acclimate to a decreased concentration of isoflurane (1.2–1.6%). At this level of anesthesia, the rats showed no spontaneous movement during the urodynamic recordings. Also, rats showed no signs of movement in response to noise. The toe-pinch reflex was not tested at this point, however. In all animals, a minimum of 15 bladder contractions along with EUS EMG tonic and bursting activity were obtained and analyzed. For CMG recordings, the amplitude of trigger contraction (ATC), maximum intravesical pressure (IVP), and intercontraction interval (ICI) were evaluated (5, 13, 14) (Fig. 1A). The pressure threshold (PT), resting pressure (RP), expulsion time (ET), amplitude of IVP, contraction duration (CD), and amplitude of intraluminal pressure of high-frequency oscillation (IPHFO) also were determined (5, 13, 14) (Fig. 1B). The CD was defined as the time period from the PT to the return to the baseline of IVP. The EUS EMG amplitude depends in part on the placement of the thin wire electrodes, and the electrode placement may be a source of significant variation in baseline amplitude values (2). Thus the baseline fluctuations caused by a low-frequency component of the EMG (which is generally attributed to activity of the internal urethral sphincter muscle) were filtered out using a baseline zero correction at 100-ms intervals. The EUS EMG activity was rectified and analyzed using custom software written in Visual Basic (Microsoft, Redmond, WA) with Measurement Studio ActiveX components (National Instruments, Austin, TX). The amplitudes of EUS EMG tonic and bursting activity during bladder filling and voiding (Fig. 1C) were calculated (5, 10). The frequency of bursts of EUS bursting (Fig. 1D) was calculated as the number of individual bursts per second (5, 7). The firing frequency within bursts was rectified and counted as each single firing spike over the threshold at 0.02 mV during the EUS EMG bursting period.
Fig. 1.
Parameters of cystometrogram (CMG; A and B) and external urethral sphincter (EUS) electromyography (EMG) activity (C and D). ATC, amplitude of trigger contraction; IVP, intravesical pressure; ICI, intercontraction interval; PT, pressure threshold; RP, resting pressure; ET, expulsion time; CD, contraction duration; IPHFO, intraluminal pressure high-frequency oscillation.
Statistical analyses.
Quantitative data are expressed as means ± SE. Means for each variable in all animals were calculated before a mean was created for each experimental group. The data obtained in urethane- and isoflurane-anesthetized groups were compared statistically using Student's t-test. We regarded P < 0.05 to indicate a statistically significant difference between the experimental groups.
RESULTS
EUS EMG activity.
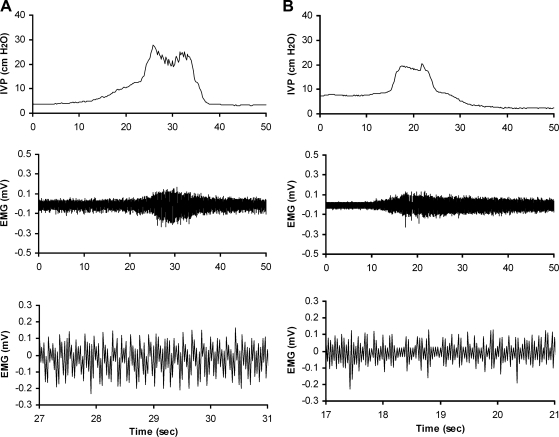
Quantitative analysis of EUS EMG activity was performed in rats of the urethane- and isoflurane-anesthetized series (Fig. 2). The frequency of bursts of EUS EMG bursting activity during voiding in isoflurane-anesthetized rats (3.1 ± 0.2 Hz, n = 5) was significantly lower compared with that in urethane-anesthetized rats (6.3 ± 0.2 Hz, n = 6; P < 0.05) (Fig. 2). The firing frequency during EUS bursting in isoflurane-anesthetized rats (55.3 ± 5.1 Hz, n = 5) was significantly lower than the corresponding firing frequency in urethane-anesthetized rats (81.9 ± 4.1 Hz, n = 6; P < 0.05). The amplitude of the EUS EMG bursting activity obtained during bladder voiding in isoflurane-anesthetized rats (0.087 ± 0.016 mV, n = 5) was significantly smaller compared with that in urethane-anesthetized rats (0.159 ± 0.009 mV, n = 6; P < 0.05) (Fig. 2). The amplitude of the EUS EMG tonic activity obtained during bladder filling in isoflurane-anesthetized rats (0.033 ± 0.006 mV, n = 5) was significantly lower compared with that in urethane-anesthetized rats (0.075 ± 0.005 mV, n = 6; P < 0.05) (Fig. 2).
Fig. 2.
Representative examples of EUS EMG bursting activity in urethane (A)- and isoflurane-anesthetized series (B). Top, CMG; middle, EUS EMG activity during filling and voiding; bottom, EUS bursting activity during voiding in faster time phase.
CMG recordings.
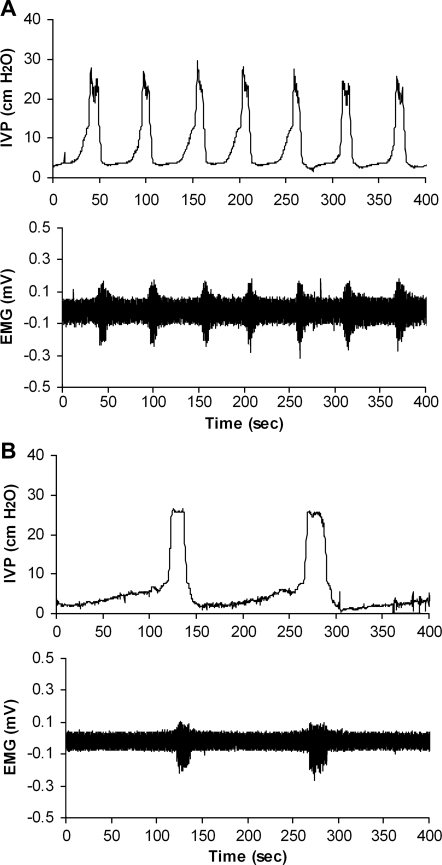
In all animals of the urethane- and isoflurane-anesthetized series, CMG recordings demonstrated bladder contractions and functional micturition reflexes. The ICI was significantly longer in the isoflurane-anesthetized series (Fig. 3) than in the urethane-anesthetized series (Table 1, P < 0.05). The quantitative analysis of the CMG recordings, including ET, RP, CD, PT, ET/CD, maximum IVP, ATC, and IPHFO, exhibited no significant differences between the urethane- and isoflurane-anesthetized series (Table 1).
Fig. 3.
Representative examples of ICI in urethane (A)- and isoflurane-anesthetized series (B). Top, CMG; bottom, EUS EMG activity during filling and voiding.
Table 1.
Parameters of cystometry during filling and voiding in urethane- and isoflurane-anesthetized rats
| Urethane-Anesthetized Rats | Isoflurane-Anesthetized Rats | |
|---|---|---|
| n | 6 | 5 |
| ICI, s | 58.87±6.18 | 157.80±37.63* |
| ET, s | 8.31±1.05 | 12.70±3.19 |
| CD, s | 26.46±3.98 | 30.87±4.16 |
| ET/CD | 0.33±0.04 | 0.39±0.07 |
| ATC, cmH2O | 27.46±2.37 | 23.0±1.67 |
| Maximum IVP, cmH2O | 29.51±1.80 | 26.99±4.68 |
| PT, cmH2O | 7.70±0.87 | 7.29±1.19 |
| RP, cmH2O | 2.49±0.40 | 1.96±0.19 |
| IPHFO, cmH2O | 3.77±0.68 | 3.59±1.56 |
Values are means ± SE. ICI, intercontraction intervals; ET, expulsion time; CD, contraction duration; IVP, intravesical pressure; ATC, amplitude of trigger contraction; PT, pressure threshold; RP, resting pressure; IPHFO, amplitude of intraluminal pressure high-frequency oscillations.
P < 0.05.
Coordination between EUS activation and CMG activity.
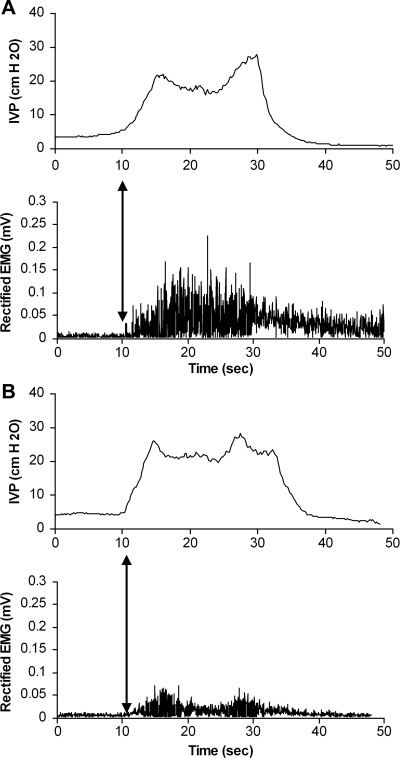
To assess the coordination between bladder contractions and EUS activation during reflexive micturition, we examined the time interval separating the start of a bladder voiding phase by CMG and the onset of EUS bursting activity by EMG within the same voiding cycle. The onset of EUS EMG activity was slightly delayed in relation to the onset of the bladder voiding phase in rats anesthetized with urethane (0.50 ± 0.09 s, n = 6) and isoflurane (0.49 ± 0.05 s, n = 5). Statistically, there was no difference in this aspect of coordination between the two groups (Fig. 4).
Fig. 4.
Coordination between CMG (top) and EUS activation (bottom) in urethane (A)- and isoflurane-anesthetized series (B). Arrows indicate the onset of EUS activation coordinated in relationship to the PT of the bladder contraction.
DISCUSSION
In the present study, we investigated the effects of isoflurane and urethane anesthetics on the micturition reflexes in rats. For this purpose, we performed urodynamic studies in adult, neurologically intact female rats. Our outcome measures included quantitative assessments of EUS EMG activity, CMG recordings, and a determination of the coordination between bladder contractions and EUS activation during functional micturition. We have demonstrated that compared with the effects of urethane, isoflurane markedly suppressed the EUS EMG activity and was associated with a much prolonged bladder filling phase between bladder contractions.
Urodynamic investigations in rodents are sensitive to many general anesthetic agents. The micturition reflex, in response to bladder filling, may be suppressed in rats by a variety of sedating agents, including pentobarbital, ketamine-diazepam, isoflurane, propofol, and buprenorphine (16). CMG studies in the rat also have detected altered bladder capacity or contractile properties in response to the administration of a variety of anesthetics, including halothane, urethane, and ketamine-xylazine (3, 18, 22, 23). In the present study, the ICI was significantly longer in the isoflurane-anesthetized rats compared with the urethane-anesthetized rats. Such prolongation of the ICI may imply an increased bladder capacity. However, we cannot rule out the possibility that an increased postvoiding residual volume may contribute to the ICI findings in the isoflurane-anesthetized group, since direct measurements of such residual volumes were not performed in this study. However, other CMG outcome measures did not exhibit significant changes in the isoflurane-anesthetized rats compared with the urethane-anesthetized rats. In contrast, previous studies using isoflurane for rat CMG assessments have reported suppressed micturition (16). A possible explanation for this discrepancy between the studies may be depth of anesthesia. A concentration of 2% isoflurane was used in the investigation by Matsuura and Downie (16), whereas we maintained the level of anesthesia at a dose of 1.2–1.6% isoflurane in the present study. However, without comparing the present data to those of urodynamic studies in conscious animals, it does not appear possible to determine whether isoflurane may increase ICI or urethane may shorten ICI. However, since we performed the placement of the bladder catheter and EUS EMG electrodes under direct visual guidance and as part of a surgical procedure, our research design limits our studies to anesthetized animals.
Main findings of the present study include the demonstration of markedly decreased amplitude, frequency of bursts, and firing frequency of EUS EMG bursting activity during the voiding phase and decreased amplitude of EUS EMG tonic activity during the bladder filling phase in rats anesthetized with isoflurane compared with the urethane-anesthetized rats. There is strong support in the literature for an important role of bladder high-frequency oscillations and EUS EMG bursting during micturition in rats (4, 7, 14, 20). Lack of EUS EMG bursting activity also has been shown to reduce voiding efficiency (20). Thus, because we have demonstrated that the EUS EMG bursting activity was reduced in the isoflurane-anesthetized group compared with the urethane-anesthetized rats, there may be a significant difference in voiding efficiency between the isoflurane- and urethane-anesthetized groups of our study. Previous studies in both humans and animal models also have suggested that isoflurane may suppress neuronal responses. For instance, isoflurane anesthesia may result in a dose-dependent suppression of somatosensory-evoked responses and detectable transcranial motor-evoked responses in only a subset of patients undergoing intraoperative electrophysiological monitoring during spinal surgery (6). In human electrodiagnostic studies on the excitability of spinal motoneurons, it also has been demonstrated that isoflurane may suppress the H-reflex and F-wave responses induced by electrical stimulation of the posterior tibial nerve (24). In rats, isoflurane anesthesia induced a reduction in the amplitude of F-waves, which were induced by electrical stimulation of the tibial nerve and recorded electromyographically from the intrinsic muscles of the foot (11). A similar isoflurane-induced suppression of the F-wave response also was obtained during sciatic nerve stimulation and EMG recordings obtained from the gastrocnemius muscle in goats (1). The latter reports are of interest for the interpretation of the collected data in the present study, since spinal motoneurons provide the final common pathway for the activation of the EUS and the generation of EMG responses we recorded. Specifically, somatic motoneurons, which innervate the EUS, are located in the dorsolateral nucleus of primarily the L6 spinal cord segment in the rat (15, 17, 21). Although the bladder filling phase was prolonged by isoflurane compared with urethane, as indicated by an increased duration of the ICI, the bladder contractile properties were identical between the isoflurane- and urethane-anesthetized groups. The efferent projection from the spinal cord responsible for innervation of pelvic ganglia and bladder detrusor contractions is provided by the autonomic preganglionic parasympathetic neurons (PPNs) (19). Thus our findings raise the possibility that isoflurane exerts a differential effect on the excitability of motoneurons and PPNs compared with urethane.
Furthermore, in our previous studies in the rat on the repair of cauda equina injuries using a surgical root reimplantation strategy in attempts to reverse the effects of lumbosacral ventral root avulsion injuries (5, 10), we used isoflurane as an anesthetic/sedating agent for the bladder catheter and EUS EMG electrode placements as well as during the urodynamic recordings. In these studies, we demonstrated axonal regeneration with spinal cord neurons, extending axons into the implanted roots as well as successful reinnervation of the LUT with the return of reflexive micturition (5, 10). However, in light of the above-demonstrated suppressive effects of isoflurane on motoneuron function, including EUS EMG activity, our reports on successful functional reinnervation of the LUT in our cauda equina injury and repair model may represent a conservative assessment and an underestimation of the actual degree of functional reinnervation of the LUT.
We conclude that isoflurane and urethane exhibit different effects on the EUS EMG and CMG recordings in rats, with a particularly prominent suppressive effect provided by isoflurane on the EUS EMG activity compared with urethane. The choice of anesthetic agents is often influenced by many factors in experimental studies, including the species, the nature of the surgery and physiological recordings, and whether a terminal or survival procedure is planned. We encourage investigators to take the above features into account when designing and interpreting urodynamic studies in rodent models.
GRANTS
H.-Y. Chang was supported by a postdoctoral fellowship from the Neural Repair Training Program at UCLA, funded by National Institute of Neurological Disorders and Stroke (NINDS) Grant T32 NS07449. L. A. Havton was supported by NINDS Grant NS042719 and The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Antognini JF, Carstens E, Buzin V. Isoflurane depresses motoneuron excitability by a direct spinal action: an F-wave study. Anesth Analg 88: 681–685, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Callsen-Cencic P, Mense S. Abolition of cystitis-induced bladder instability by local spinal cord cooling. J Urol 160: 236–241, 1998. [PubMed] [Google Scholar]
- 3.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 69: 1193–1202, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Chang HY, Cheng CL, Chen JJ, de Groat WC. Serotonergic drugs and spinal cord transections at different levels indicate that different spinal circuits are involved in tonic and bursting external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292: F1044–F1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Havton LA. Re-established micturition reflexes show differential activation patterns after lumbosacral ventral root avulsion injury and repair in rats. Exp Neurol 212: 291–297, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z The effects of isoflurane and propofol on intraoperative neurophysiological monitoring during spinal surgery. J Clin Monit Comput 18: 303–308, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CL, de Groat WC. The role of capsaicin-sensitive afferent fibers in the lower urinary tract dysfunction induced by chronic spinal cord injury in rats. Exp Neurol 187: 445–454, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 45: 1709–1718, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Field KJ, Lang CM. Hazards of urethane (ethyl carbamate): a review of the literature. Lab Anim 22: 255–262, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Hoang TX, Pikov V, Havton LA. Functional reinnervation of the rat lower urinary tract after cauda equina injury and repair. J Neurosci 26: 8672–8679, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King BS, Rampil IJ. Anesthetic depression of spinal motor neurons may contribute to lack of movement in response to noxious stimuli. Anesthesiology 81: 1484–1492, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: other systems and conclusions. Experientia 42: 531–537, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am J Physiol Regul Integr Comp Physiol 251: R250–R257, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15:157–167, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Marson L Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol 389: 584–602, 1997. [PubMed] [Google Scholar]
- 16.Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn 19: 87–99, 2000. [DOI] [PubMed] [Google Scholar]
- 17.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol 248: 532–549, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Morikawa K, Ichihashi M, Kakiuchi M, Yamauchi T, Kato I, Ito Y, Gomi Y. Effects of various drugs on bladder function in conscious rats. Jpn J Pharmacol 50: 369–376, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol 226: 238–245, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Peng CW, Chen JJ, Cheng CL, Grill WM. Role of pudendal afferents in voiding efficiency in the rat. Am J Physiol Regul Integr Comp Physiol 294: R660–R672, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Schrøder HD Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol 192: 567–587, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama O, Yoshiyama M, Namiki M, de Groat WC. Influence of anesthesia on bladder hyperactivity induced by middle cerebral artery occlusion in the rat. Am J Physiol Regul Integr Comp Physiol 273: R1900–R1907, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Yoshiyama M, Roppolo JR, de Groat WC. Alteration by urethane of glutamatergic control of micturition. Eur J Pharmacol 264: 417–425, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Zhou HH, Jin TT, Qin B, Turndorf H. Suppression of spinal cord motoneuron excitability correlates with surgical immobility during isoflurane anesthesia. Anesthesiology 88: 955–961, 1998. [DOI] [PubMed] [Google Scholar]