Abstract
Recent studies suggest that thiazolidinediones ameliorate diabetic nephropathy (DN) independently of their effect on hyperglycemia. In the current study, we confirm and extend these findings by showing that rosiglitazone treatment prevented the development of DN and reversed multiple markers of oxidative injury in DBA/2J mice made diabetic by low-dose streptozotocin. These diabetic mice developed a 14.2-fold increase in albuminuria and a 53% expansion of renal glomerular extracellular matrix after 12 wk of diabetes. These changes were largely abrogated by administration of rosiglitazone beginning 2 wk after the completion of streptozotocin injections. Rosiglitazone had no effect on glycemic control. Rosiglitazone had similar effects on insulin-treated diabetic mice after 24 wk of diabetes. Podocyte loss and glomerular fibronectin accumulation, other markers of early DN, were prevented by rosiglitazone in both 12- and 24-wk diabetic models. Surprisingly, glomerular GLUT1 levels did not increase and nephrin levels did not decrease in the diabetic animals; neither changed with rosiglitazone. Plasma and kidney markers of protein oxidation and lipid peroxidation were significantly elevated in the 24-wk diabetic animals despite insulin treatment and were reduced to near-normal levels by rosiglitazone. Finally, urinary metabolites were markedly altered by diabetes. Of 1,988 metabolite features identified by electrospray ionization time of flight mass spectrometry, levels of 56 were altered more than twofold in the urine of diabetic mice. Of these, 21 were returned to normal by rosiglitazone. Thus rosiglitazone has direct effects on the renal glomerulus to reduce reactive oxygen species accumulation to prevent type 1 diabetic mice from development of DN.
Keywords: diabetes, thiazolidinedione, kidney, complications, metabolomics, mass spectrometry
early clinical diabetic nephropathy (DN) is characterized by progressive increases in albuminuria which are associated with the development of characteristic histopathological features including thickening of the glomerular basement membrane and mesangial expansion due to accumulation of extracellular matrix proteins (25). One of the areas of general agreement about the pathogenesis of DN is that hyperglycemia and altered hemodynamic properties of diabetic glomeruli lead to multiple changes. These include enhanced expression of the facilitative glucose transporter, GLUT1, leading to enhanced glucose uptake (5, 17), enhanced expression and activation of PKC isoforms, and activation of transforming growth factor (TGF)-β, which in turn induce increased synthesis and decreased degradation of extracellular matrix proteins such as fibronectin and collagen IV by mesangial cells in the glomerulus (52). Other signals including activation of pathways involving ERK, advanced glycation end product receptors, VEGF, and other growth factors (for reviews, see Refs. 4, 14, 18, 54) have been implicated in the mesangial and podocyte responses to diabetes and elevated levels of extracellular glucose. A potential unifying factor in integrating many if not all of these abnormalities is the increase in both cytoplasmic and mitochondrial oxidative stress generated by enhanced glucose metabolic flux and activation of the aforementioned pathways. Multiple studies have now strongly implicated the increased oxidative stress induced by diabetes in the development of diabetic complications, including DN (for a review, see Ref. 49).
Over the past few years, a number of studies have found that thiazolidinediones (TZDs) have substantial ameliorative effects on DN (for a review, see Ref. 44) and that these effects appear to be due to direct effects on renal glomerular cells. While most of these studies are in patients or models of type 2 diabetes, a number of provocative studies in animal models have indicated that TZDs have similar efficacy in models of type 1 diabetes (11, 14, 34, 55, 56). While the mechanisms of action of TZDs are manifold and the specific pathways by which TZDs interrupt DN changes have not been elucidated, these agents clearly have effects on many of the pathways identified to play significant roles in the pathogenesis of DN. For example, TZDs have been shown to ameliorate PKC activation (14), enhanced TGF-β signaling (19, 34, 51), ERK activation (7), oxidative stress (22), and increased VEGF (36) in both in vitro and in vivo DN models. Which TZD actions are most important for their ameliorative effects in DN is not yet clear.
In the current study, we have tested the effects of the TZD rosiglitazone on a recently described, robust murine model of type 1 DN and have found that it dramatically normalized albuminuria, mesangial expansion, fibronectin expression, and podocyte number after 12 wk of diabetes. Plasma and kidney oxidant marker levels of 13-hydroxyoctadecadienoic acid (HODE) and dityrosine were significantly elevated in the 24-wk diabetic animals despite insulin treatment, and their rise was largely prevented by rosiglitazone. Finally, a survey of urinary metabolites in the mice found that 21 altered metabolites were returned to normal by rosiglitazone treatment, despite little or no effect on systemic metabolic parameters, suggesting that a subset of these metabolites could serve as biomarkers to help identify the most important factors involved in TZD renoprotection.
MATERIALS AND METHODS
Mouse model.
Male 10-wk-old DBA/2J mice (Jackson Laboratory, Bar Harbor, ME) were used. Mice were allowed to acclimate to their environment for at least 3 days before injection. Mice were fasted for 4 h and then given intraperitoneal injections of 40 mg/kg streptozotocin (STZ) or the vehicle (control mice) daily for 5 consecutive days as previously reported (3, 48). Beginning 2 wk after completion of STZ injections, rosiglitazone (3 mg/kg po) was given orally in a very small amount of Nutri-Cal (EVSCO Pharmaceuticals, Buena, NJ) daily to one-half of the STZ and control animals. The remainder were given Nutri-Cal only. For each study, we began with different numbers in each of the four groups (see Table 1) because of anticipated losses in the diabetic groups (41). Animals injected with STZ that did not develop fasting blood glucose >300 mg/dl (a total of 5 mice) were excluded from the study. In addition, as expected (41), several animals died over the duration of the study, especially in the diabetic groups, and a greater number appeared to have infections. These animals were excluded from further analysis. There was no increased death rate seen with rosiglitazone. Liver transaminases were measured and were not different in mice treated with rosiglitazone and those receiving vehicle alone, diabetic or not (data not shown). Beginning and final numbers in each group are indicated in Table 1.
Table 1.
Physiological data
|
12-wk Study |
24-wk Study
|
|||
|---|---|---|---|---|
| 0 Wk | 12 Wk | 0 Wk | 24 Wk | |
| BW, g | ||||
| Control | 23.0±0.70 (13) | 27.6±0.88 (13) | 24.7±0.57 (16) | 29.8±1.01 (16) |
| Diabetic | 24.4±0.48 (17) | 21.9±0.52 (17)* | 25.4±0.39 (27) | 23.9±0.48 (13)* |
| Control+Rosi | 22.6±0.74 (12) | 26.8±0.70 (12) | 25.3±0.54 (15) | 29.2±0.87 (14) |
| Diabetic+Rosi | 24.2±0.52 (16) | 21.8±0.48 (16)* | 26.3±0.38 (22) | 23.5±0.35 (11)* |
| FBG, mg/dl | ||||
| Control | 142.7±1.90 (13) | 140.3±5.62 (13) | 24.7±0.57 (16) | 112.6±5.62 (16) |
| Diabetic | 139.5±2.80 (17) | 561.6±23.50 (17)* | 25.4±0.39 (27) | 489.6±20.06 (13)*† |
| Control+Rosi | 136.3±2.08 (12) | 126.6±2.71 (12) | 25.3±0.54 (15) | 123.4±4.59 (14) |
| Diabetic+Rosi | 141.4±2.70 (16) | 507.7±27.43 (16)* | 26.3±0.38 (22) | 331.2±41.18 (11)* |
| GHb, % | ||||
| Control | 5.62±0.08 (13) | 5.83±0.24 (15) | ||
| Diabetic | 14.9±0.43 (16)* | 14.6±0.428 (13)* | ||
| Control+Rosi | 5.71±0.15 (12) | 6.06±0.19 (14) | ||
| Diabetic+Rosi | 14.8±0.31 (16)* | 13.8±0.54 (11)* | ||
| Left KW, g | ||||
| Control | 0.32±0.015 (13) | 0.37±0.02 (16) | ||
| Diabetic | 0.36±0.014 (17) | 0.36±0.00 (13) | ||
| Control+Rosi | 0.29±0.012 (12) | 0.36±0.01 (14) | ||
| Diabetic+Rosi | 0.32±0.33 (16) | 0.33±0.00 (11) | ||
| L KW/BW, g KW/g BW | ||||
| Control | 0.011±0.0004 (13) | 0.012±0.001 (16) | ||
| Diabetic | 0.017±0.001 (17)† | 0.015±0.001 (13)* | ||
| Control+Rosi | 0.011±0.0003 (12) | 0.012±0.001 (14) | ||
| Diabetic+Rosi | 0.015±0.0003 (16)* | 0.014±0.001 (11) | ||
| HDL, mg/dl | ||||
| Control | 44.62±1.60 (13) | 46.0±1.38 (16) | ||
| Diabetic | 55.3±1.37 (16)* | 49.0±2.70 (13) | ||
| Control+Rosi | 45.6±1.37 (12) | 44.8±2.65 (14) | ||
| Diabetic+Rosi | 55.1±1.79 (16)* | 47.2±1.96 (11) | ||
| Total CHOL, mg/dl | ||||
| Control | 67.3±2.59 (13) | 64.1±2.56 (16) | ||
| Diabetic | 92.6±2.887 (16)* | 71.8±4.19 (13) | ||
| Control+Rosi | 65.5±1.70 (12) | 60.1±3.77 (14) | ||
| Diabetic+Rosi | 84.0±2.83 (16)* | 66.8±2.62 (11) | ||
Values are means ± SE with total number of animals in parentheses. Rosi, rosiglitazone; BW, body wt; FBG, fasting blood glucose; GHb, glycated hemoglobin; KW, kidney wt; L KW/BW, left kidney wt/body wt; CHOL, cholesterol.
P < 0.05: vs. control(s),
P < 0.05: vs. rosiglitazone-treated diabetic animals.
In the first study, animals were euthanized 12 wk after completion of STZ injections. In the second study, mice were subcutaneously implanted with pellets impregnated with bovine insulin (LinBit tablets, LinShin Canada, Toronto, Ontario, Canada) at 10, 15, and 20 wk post-STZ to prevent ketosis, protein catabolism, and significant weight loss. The administered dose of insulin was ∼0.1 U/day (8). Blood glucose levels were measured from tail-vein blood (Accu-Chek Advantage, Roche Diagnostics, Indianapolis, IN). Values for blood lipids and glycosylated hemoglobin were determined using terminal blood samples by the Michigan Diabetes Research and Training Center Chemistry Laboratory. Blood liver enzyme values were determined by the University of Michigan University for Laboratory Animal Medicine Animal Diagnostic Lab. Urine samples were collected in murine metabolic cages (Hatteras Instruments, Cary, NC) or in plastic spot urine containers. Spot urinary albumin and creatinine levels were collected with the Albuwell M and Companion Creatinine systems (Exocell, Philadelphia, PA) (3).
The procedures used in this study were in accordance with the guidelines of the University of Michigan Committee on the Use and Care of Animals. Veterinary care was provided by the University of Michigan Unit for Laboratory Animal Medicine. The University of Michigan is accredited by the American Association of Laboratory Animal Care. The animal care and use program conformed to the standards in Guide for the Care and Use of Laboratory Animals [Department of Health, Education, and Welfare Publication No. (NIH) 86-23].
Kidney preparation.
Under general anesthesia, a blood sample was drawn and then both kidneys were flushed under constant 100-mmHg pressure with PBS containing 50 U/ml sodium heparin through a cannula placed in the abdominal aorta. Once flushed of blood, the left kidney from each mouse was ligated, and the right kidney was perfused with a ferric oxide slurry in PBS via the abdominal aorta. The left kidney was removed, weighed, and fixed overnight in a solution 2% paraformaldehyde in PBS. The right kidney with the iron-containing glomeruli was then isolated with a technique modified from that of Meezan et al. (26). Isolated glomeruli were lysed and used for immunoblotting. Some kidney cortical regions were dissected before fixation and placed into lysis buffer for immunoblotting or flash frozen for oxidant marker analysis. Sections (3 and 9 μm) were cut from the fixed kidneys and used for periodic acid-Schiff (PAS) staining (3 μm only) or were used for immunohistochemistry for podocyte counting (3 and 9 μm).
Podocyte counting.
Methods were performed as previously published (48) following the methods of Sanden et al. (43). Thin and thick sections were cut from the 2% paraformaldehyde-fixed, paraffin-embedded kidney samples. These sections were deparaffinized and rehydrated through ethanols (70, 95, 100%). All sections were incubated in Retrieve One buffer (Signet Laboratories, Dedham, MA) at 90°C for 2 h to enhance antigen retrieval. Using an ABC Staining Kit (Vector Laboratory, Dedham, MA), podocyte nuclei were detected with a rabbit polyclonal anti-WT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 20 μg/ml. Images of all glomeruli from both thick (9 μm) and thin (3 μm) kidney cortical cross sections were collected using the MetaMorph Image System (version 6.1, Molecular Devices, Downingtown, PA) by a blinded observer (J. Saha). Glomerular volume/podocyte (GV/P) was also calculated as previously reported (48). GV/P is a variable that incorporates the relationship between both podocyte number and glomerular basement membrane surface area, is the reciprocal of podocyte density, and is a useful measure of the degree of podocyte reserve (43).
Mesangial extracellular matrix determination.
For quantification of mesangial extracellular matrix, 3-μm sections from paraformaldehyde-fixed, paraffin-embedded kidney slices were stained using PAS reagent. The mesangial area was expressed quantitatively by calculating the percentage of the total glomerular area that was PAS positive. Fifteen glomerular tufts per animal were chosen randomly for analysis. Quantification of glomerular and PAS-positive areas was performed with MetaMorph Imaging Software (version 6.1, Molecular Devices) calibrated for the microscope and a digital camera used to capture the images.
Immunoblotting.
Glomerular or cortical samples were sonicated and/or mechanically disrupted in lysis buffer [0.1% SDS, 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and complete protease inhibitor cocktail (Roche Applied Science) in 10 mM Tris·HCl, pH 7.4] and used for SDS-PAGE as previously reported (47). Lysates were run on 7.5 or 10% SDS-PAGE and immunoblotted with antibodies for fibronectin and β-tubulin (mab1926 and 05–661, respectively, Millipore, Temecula, CA), GLUT1 (a gift from Dr. Carter-Su, Univ. of Michigan), and nephrin or Neph1 (12) (gifts from Dr. L. Holzman, Univ. of Michigan). Blots were exposed to film after incubation in Roche LumiLight Western Blotting Substrate. All exposures were within the linear range of the film and normalized to β-tubulin content whenever feasible.
HODE and dityrosine levels.
Kidneys were harvested immediately, and the kidney cortex was quickly dissected. To prevent ex vivo oxidation, cortex and plasma samples were submerged in ice-cold antioxidant buffer A [100 μM diethylene tetramino pentaacetic acid (DTPA; metal chelator), 50 μM butylated hydroxyl toluene (BHT; lipid soluble antioxidant), 1% (vol/vol) ethanol,10 mM 3-amino-1,2,4-triazole (peroxidase inhibitor), 50 mM sodium phosphate buffer, pH 7.4], rapidly frozen by immersion in liquid nitrogen, and stored at −80°C until analysis. Before acid hydrolysis, kidney cortex samples were homogenized at 4°C in buffer A, frozen, and then thawed. Protein was precipitated from kidney cortex homogenates or plasma with ice-cold trichloroacetic acid (10% vol/vol), collected by centrifugation, washed with 10% trichloroacetic acid, and delipidated twice with water/methanol/water-washed diethyl ether (1:3:7 vol/vol).
Lipid extracts were saved for HODE analysis. HODEs were quantified by reverse-phase C18 HPLC analysis of triphenylphosphine-reduced lipid extracts after base hydrolysis, as described previously (39). Briefly, analyses for HODE were performed on a Jasco HPLC system equipped with a reverse-phase column (Ultrasphere; 250 × 4.6 mm, particle size 5 μm, Beckman). Oxidized lipids were detected by monitoring absorbance at 234 nm. Authentic 13-HODE was prepared from linoleic acid with soybean lipoxygenase (6, 46) and reduced with triphenylphosphine before analysis. The identity of the compound was confirmed by identical retention time (6.1 min) with authentic 13-HODE. Quantitation was performed by comparison with standard curves generated using authentic 13-HODE (6, 46). The protein content of tissue pellets was determined by a modified Lowry protein assay using bovine serum albumin as a standard, and the HODEs were normalized to protein content (24).
Protein pellets were hydrolyzed in 4 N methane sulfonic acid at 110°C for 24 h under argon, and amino acids were isolated from the acid hydrolysate using a solid-phase C18 column (Supelclean SPE, Supelco, Bellefonte, PA) as described previously (38). Isolated amino acids were derivatized with dabsyl chloride as described previously (23). Analysis of the dabsylated derivatives of tyrosine and o,o′-dityrosine were performed by reverse-phase HPLC (Jasco HPLC Column, Beckman ODS Ultrasphere C18 column, 250 × 4.6 mm; particle size 5 μm) as described previously (23). The identity of the compounds was confirmed by identical retention time with authentic o,o′-dityrosine and tyrosine (12.5 and 16.8 min, respectively) by monitoring absorbance at 460 nm. Quantitation was performed by comparison with standard curves generated using authentic o,o′-dityrosine and tyrosine.
Urine metabolite screening.
The urine samples were stored at −80°C with 1 mM NaN3 before analysis. An Agilent Technologies (New Castle, DE) 6410 Triple Quadrapole mass spectrometer (MS) system, equipped with an Agilent 1200 series HPLC system and an electrospray (ESI) source, was used to determine the concentration of creatinine in the urine samples. Positive LC/ESI/tandem MS (MS2) was performed using the following parameters: spray voltage 4,000 V, drying gas flow 11 l/min, drying gas temperature 325°C, and nebulizer pressure 40 psi. Flow injection analysis (FIA) was used to optimize the fragmentor voltage and collision energy. An MS2 scan was performed to optimize the fragmentor voltage. The product ion scans were used to optimize collision energy in the FIA and injection on the column. The urine samples were injected on a Phenomenex LUNA hydrophilic interaction chromatography (HILIC) column (3 μm, 2.1 × 150 mm) using premixed H2O/acetonitrile (15/85) in 20 mM ammonium acetate, pH = 6.8, with an isocratic gradient. To determine the level of creatinine, a known amount of [2H3]creatinine was spiked into each sample. A full-scan mass spectrum revealed molecular ions of m/z 114 and 117 for authentic creatinine and [2H3]creatinine, respectively. The transitions of the m/z 114 to 44 and m/z 117 to 47 were monitored in multiple-reaction monitoring mode for authentic and [2H3]creatinine, respectively. The creatinine concentration in the urine sample was determined by comparing the peak areas for authentic and [2H3]creatinine for the above transitions.
To allow for normalization of the data, each urine sample was diluted to have an identical creatinine concentration (500 fmol/μl). Five microliters of the diluted urine samples were then subjected to reverse-phase HPLC on an Agilent 1200 series HPLC system using an Agilent Zobax SB-Aq 1.8-μm, 2.1 × 100-mm column. The HPLC conditions are as follows: flow rate: 0.4 μl/min; solvent A: 0.2% acetic acid in water; solvent B: 0.2% acetic acid in methanol; and gradient: solvent B 2–98% over 16 min. The samples were then analyzed by ESI in both positive- and negative-ion modes using an Agilent 6410 Time of Flight (TOF) MS. Mass spectral data were acquired with an acquisition rate of 1.35 spectra/s, averaging 10,000 transients. The source parameters were adjusted as follows: drying gas temperature 250°C, drying gas flow rate 12 l/min, nebulizer pressure 45 psi, and fragmentor voltage 150 V. Differential expression analysis by positive- and negative-ion mode ESI of urine demonstrated unique signatures specific for each group. 2D visualization tools in the MassHunter Profiler differential analysis software (Agilent Technologies, Santa Clara, CA) enabled the features to be rapidly sorted into multiple categories. Only metabolites that were present in at least five of seven samples were considered for analysis. Automated data-analysis algorithms have recently been used for the data mining of electrospray liquid chromatography/mass spectrometry (LC/MS) data (50). Herein, the Agilent Molecular Feature Extractor algorithm was utilized to identify discrete molecular entities defined by the combination of retention time (in seconds), mass (m/z to 4 decimal places), and peak intensity. Mouse urine samples were extracted using a signal-to-noise threshold of two, and the resulting molecular feature list was sorted by peak areas. Each molecular feature was a discrete molecular entity defined by a combination of retention time, mass, and response in an LC/MS analysis. Groups were then compared using the MassHunter program to generate lists of chemically qualified molecular features: persistent chemical background was removed by subtracting solvent-generated features; coeluting interferences were resolved; isotopic cluster was recognized and grouped; charge-state assignments and molecular adducts were recognized; and 2D data were visualized. The common metabolites found in urine samples were searched in an exact mass database of >14,000 metabolites using the MassHunter METLIN Metabolite Database software (http://metlin.scripps.edu) and the 2,180 endogenous human metabolites in the Metabolome tool box (53) (http://hmdb.ca/index.html). The endogenous metabolites with mass error of <200 ppm were chosen as potential identifications.
Cultured mesangial cell reactive oxygen species detection.
The rat mesangial cell line (MC LacZ) was cultured as previously reported (16, 17) in RPMI 1640 with 20% NuSerum IV (BD Biosciences, Bedford, MA) on coverslips, and cells were exposed to 8 or 20 mM glucose ± rosiglitazone (10 μM) or vehicle (DMSO) together for indicated periods. The cells were loaded with carboxymethyl-H2-dichlorofluorescein diacetate (CM-H2-DCFDA; Molecular Probes, Eugene, OR) for 30 min before incubation in 8 mM or 20 mM glucose concentrations. DCF fluorescence was detected by an Olympus FluoView 500 Laser-Scanning Confocal Microscope set for an excitation of 480 nm and an emission of 530 nm. Fluorescence density was analyzed by Image J (National Institutes of Health, Bethesda, MD).
Real-time quantitative PCR.
RNA was isolated from cultured rat mesangial cells. Total RNA (1 μg) was reversed transcribed by Superscript. Real-time PCR was performed with TGF-β primers (TaqMan Gene Expression Assay, catalog no. Rn00572010, Applied Biosystems, Foster City, CA) on a TaqMan ABI 7900 Sequence Detection System (Applied Biosystems) and normalized to 18S rRNA.
Data analysis.
Data are presented as means ± SE. For metabolite analysis, the MassHunter Profiler software and Genespring MS statistical analysis software packages (Agilent Technologies) were used to compare LC/MS data between groups. When two groups were compared, a Student's t-test was used. For multiple comparisons, a one-way ANOVA followed by Tukey-Kramer post hoc analysis was performed. P values ≤0.05 were considered statistically significant. For metabolite analysis, the MassHunter Profiler software (Agilent Technologies) was used to compare LC/MS data between groups.
RESULTS
DBA/2J mice became rapidly and consistently diabetic after STZ treatment. In the 12-wk study, administration of rosiglitazone from 2–12 wk after completion of STZ injections had no effect on body weight, kidney weight, fasting blood sugar, or glycosylated hemoglobin in either the control or diabetic groups (Table 1). In the 24-wk study, in which animals received low-dose insulin, administration of rosiglitazone from 2 to 24 wk resulted in a modest reduction in fasting blood sugar by the end of the study but had no statistically significant effect on glycosylated hemoglobin levels or on other baseline characteristics (Table 1). The relative lowering of blood glucose in the 24-wk study corresponded to a gradual worsening of the general health of the mice, which did not occur until the final 4 wk of life. Liver enzymes (ALT and AST) were monitored. These enzymes levels were somewhat higher in diabetic animals, but rosiglitazone had no effect on these levels in either control or diabetic animals (not shown).
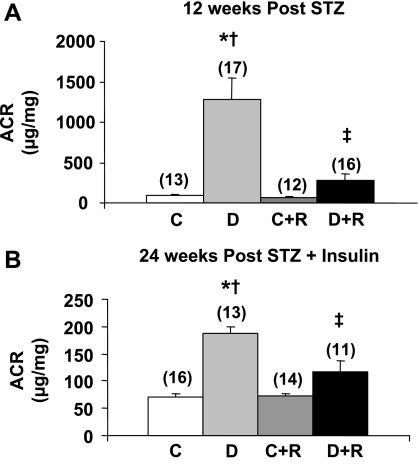
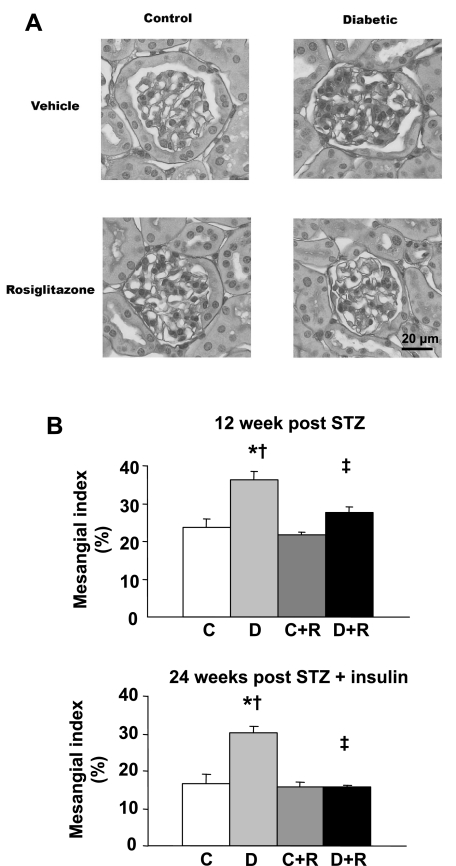
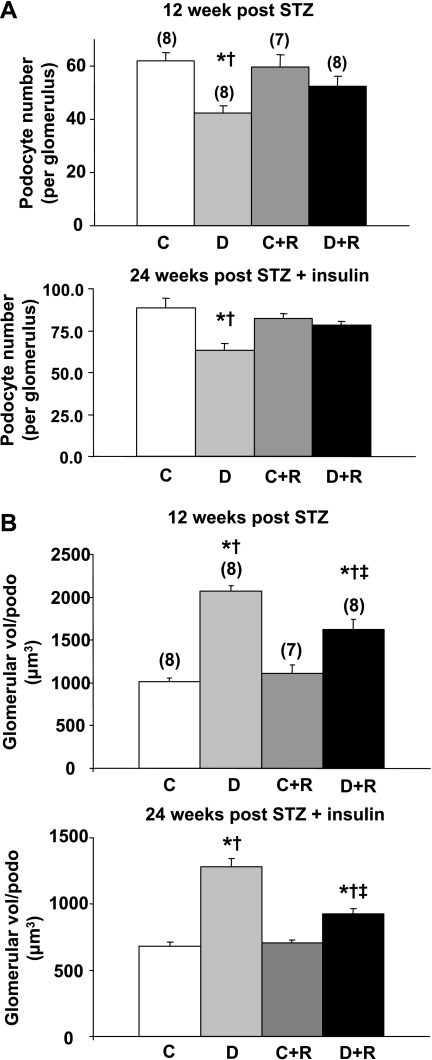
In the 12-wk study, diabetic DBA/2J mice developed profound albuminuria, which was ∼14-fold greater than in control mice (Fig. 1). Rosiglitazone treatment reduced albuminuria almost fivefold in the diabetic animals, but had no effect on albuminuria in the control animals (Fig. 1). In the 24-wk study, insulin treatment prevented much of the albuminuria seen in the 12-wk study as albuminuria in the diabetic mice after 12 wk of diabetes was only about one-eighth the levels in the 12-wk mice who received no insulin (data not shown). This reduction in albuminuria was maintained at 24 wk (Fig. 1). Despite this insulin protection, rosiglitazone further reduced albuminuria virtually back to control levels (Fig. 1). Similarly, rosiglitazone reduced the expansion in mesangial matrix seen in both 12- and 24-wk diabetic mice (Fig. 2A). Quantified as the mesangial index (percent PAS-positive material in the mesangial tuft), 12 wk of untreated diabetes induced a 54% increase in the mesangial matrix that was limited to only a 17% increase by rosiglitazone (Fig. 2B). In the 24-wk group, the mesangial index was increased by 80.2% in the diabetic animals; this increase was completely prevented in the rosiglitazone-treated diabetic mice (Fig. 2B). Podocyte number was decreased in the 12- and 24-wk diabetic animals (Fig. 3A). This reduction in podocyte number was largely prevented by rosiglitazone (Fig. 3A). GV/P, a measure of the amount of glomerular volume “covered” by each podocyte, and the reciprocal of podocyte density (43), was increased about twofold in diabetic animals in both 12- and 24-wk studies. This increase was largely prevented by rosiglitazone (Fig. 3B).
Fig. 1.
Effect of rosiglitazone on urinary albumin creatinine ratios (ACR) at 12 and 24 wk after streptozotocin (STZ) injection. C, control; D, diabetic; C+R, rosiglitazone-treated control; D+R, rosiglitazone-treated diabetic. Values are means ± SE with total number of animals in parentheses. P < 0.05: *vs. controls; †vs. rosiglitazone-treated controls; ‡vs. diabetics.
Fig. 2.
Effect of rosiglitazone on mesangial extracellular matrix area. A: representative examples of glomerular periodic acid-Schiff (PAS) staining at 12 wk after STZ treatment. B: mesangial area was expressed quantitatively by calculating the percentage of the total glomerular area that was PAS positive at 12 and 24 wk after STZ injection. Values are means ± SE; n = 9 (12 wk) or 7 (24 wk) for each group. P < 0.05: *vs. controls; †vs. rosiglitazone-treated controls; ‡vs. diabetics.
Fig. 3.
Effect of rosiglitazone on podocyte number in diabetic mice 12 and 24 wk after STZ injection. A: average number of podocytes per glomerulus. B: degree of podocyte reserve as measured in glomerular volume per podocyte. Values are means ± SE; total number of animals are n = 9 (12 wk; A) or 7 (24 wk; A and B) or are in parentheses (12 wk; B). P < 0.05: *vs. controls; †vs. rosiglitazone-treated controls; ‡vs. diabetics.
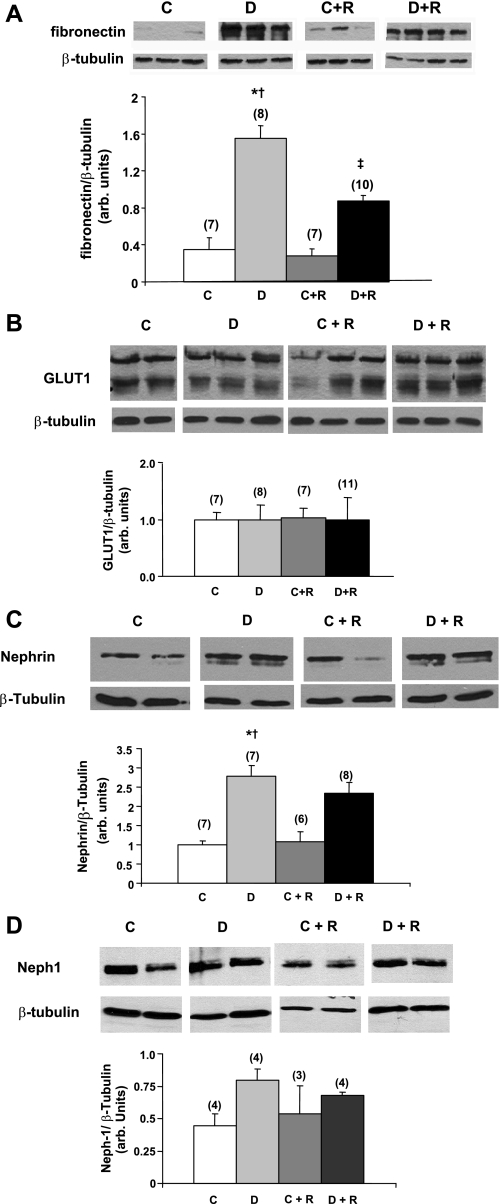
Immunoblots of glomerular samples from 12-wk diabetic animals showed a significant increase in fibronectin accumulation, which was reduced by rosiglitazone (Fig. 4A). Interestingly, glomerular levels of the glucose transporter GLUT1 were not elevated in the diabetic mice (12 wk) and were unchanged by rosiglitazone in this model (Fig. 4B). Similarly, surprisingly, at 24 wk glomerular nephrin levels went up with DN in this model and appeared to increase even more with rosiglitazone treatment (Fig. 4C). Neph1 showed a similar pattern at 12 wk (Fig. 4D), but the differences were not significant.
Fig. 4.
Effect of rosiglitazone on glomerular levels of fibronectin (A), GLUT1 (B), nephrin (C), and Neph1 (D). Immunoblots of glomerular lysates from rosiglitazone treated and untreated control and diabetic mice (12 wk for fibronectin, GLUT1 and Neph1 and 24 wk for nephrin) are shown. β-Tubulin was used as a loading control. Relative intensities are demonstrated by densitometry. Values are means ± SE with the number of animals for each group in parenthesis. P < 0.05: *vs. controls; †vs. rosiglitazone-treated controls; ‡vs. diabetics.
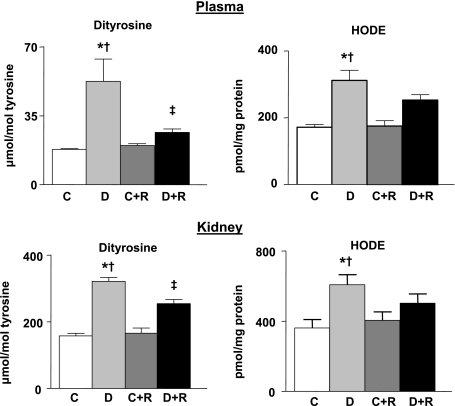
Oxidative stress is a major mechanism of hyperglycemia-induced DN in humans, particularly through the oxidation of proteins and lipids. To determine whether elevated oxidative stress correlated with DN and functional measures of kidney damage in this diabetic model, we quantified levels of protein-bound o,o′-dityrosine and HODE, highly sensitive markers of protein oxidation and lipid peroxidation, respectively (39), in plasma and renal cortex from five animals in each group. Dityrosine levels were significantly increased in the plasma of diabetic animals (Fig. 5). This effect was markedly reduced by treatment with rosiglitazone. Plasma HODE levels were also significantly increased by diabetes and appeared to be attenuated by rosiglitazone treatment, but this latter effect did not reach statistical significance. A similar pattern was noted in the renal cortex of diabetic animals in which dityrosine and HODE levels were significantly increased, and the dityrosine increases were markedly attenuated by rosiglitazone. Rosiglitazone treatment did not affect dityrosine or HODE levels in plasma or renal cortex of nondiabetic DBA/2J mice.
Fig. 5.
Effect of rosiglitazone treatment on protein oxidation and lipid peroxidation in plasma and renal cortex. Protein-bound dityrosine, a highly sensitive and specific marker for protein oxidation and hydroxyoctadecadienoic acid (HODE), a marker for lipid peroxidation, were measured at the end of the 24-wk study. Values are means ± SE; n = 5 for all groups. P < 0.05: *vs. controls; †vs. rosiglitazone-treated controls; ‡vs. diabetics.
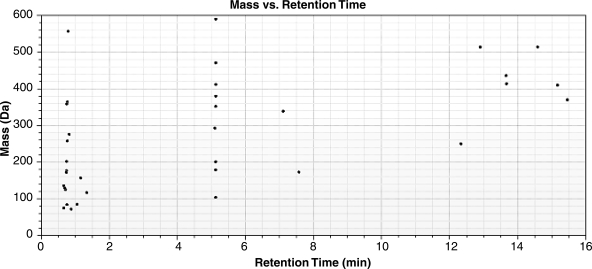
To determine whether the urinary metabolite profile was altered in diabetes and whether rosiglitazone therapy reversed these alterations, we analyzed the urine by using HPLC/ESI/TOF. Urine samples were diluted and normalized to creatinine content so that the final concentration was 500 fmol/μl. Profiling of mouse urine with ESI-positive/negative modes demonstrated unique signatures specific for each group. A total of 1,988 molecular features were identified in all the samples. Of these, 1,023 features were present in all diabetic and/or diabetic+rosiglitazone samples, and 668 features were present in all control and/or control+rosiglitazone samples. A total of 56 features showed up- or downregulation by >2-fold in either ESI-positive or -negative modes in diabetic animals. Levels of 35 features (Fig. 6) in ESI-positive mode and 26 features in negative mode (not shown) were altered in the urine of diabetic mice, and 5 features were present in both modes. Of these, 21 features (12 features in ESI-positive mode and 10 features in ESI-negative mode with 1 shared feature) were returned to normal by rosiglitazone therapy.
Fig. 6.
Electrospray ionization time of flight mass spectrometry (ESI-TOF-MS) analysis of urine. Metabolites that were present in at least 5 of the 7 samples were considered for analysis. A total of 56 features showed up- or downregulation by >2-fold in either ESI-positive or -negative modes in diabetic animals compared with controls. Levels of 35 features in ESI-positive mode which showed such alterations are depicted.
To obtain preliminary identification of the molecular features, we utilized publicly available exact mass databases including METLIN Metabolite Database (http://metlin.scripps.edu/) and the endogenous metabolites in the Metabolome tool box (53) (http://hmdb.ca/index.html). These results are summarized in Table 2. Of the 56 molecular features, 32 were identified with a mass error of < 200 ppm. Of these, we were able to identify nine compounds that returned back to baseline with rosiglitazone therapy, raising the possibility that they could be potential biomarkers for resolution of the DN phenotype.
Table 2.
Identification of urinary metabolites
| Mass Observed | Mass Theoretical | Name | Formula | |
|---|---|---|---|---|
| 1 | 72.0214 | 72.0211 | Methylglyoxal | C3H4O2 |
| 72.0211 | Pyruvaldehyde | C3H4O2 | ||
| 72.0211 | Malondialdehyde | C3H4O2 | ||
| 2 | 74.0007* | 74.0004 | Glyoxylic acid | C2H2O3 |
| 3 | 75.0687 | 75.0684 | Trimethylamine oxideoxide | C3H9NO |
| 4 | 84.0163 | 84.0324 | Imidazolone | C3H4N2O |
| 5 | 97.9672 | 97.9769 | Phosphoric acid | H3O4P |
| 6 | 102.0311 | 102.0317 | Succinic semialdehyde | C4H6O3 |
| 102.0317 | Acetoacetic acid | C4H6O3 | ||
| 102.0317 | 4-Hydroxycrotonic acid | C4H6O3 | ||
| 102.0317 | 2-Ketobutyric acid | C4H6O3 | ||
| 102.0317 | 2-Methyl-3-oxopropanoic acid | C4H6O3 | ||
| 7 | 104.028 | 104.011 | Malonic acid | C3H4O4 |
| 104.011 | Hydroxypyruvic acid | C3H4O4 | ||
| 8 | 115.038 | 115.0395 | 3-Methyl-2-oxobutanoate | C5H7O3 |
| 9 | 117.078 | 117.079 | Valine | C5H11NO2 |
| 117.079 | Betaine | C5H11NO2 | ||
| 10 | 125.014 | 125.0147 | Taurine | C2H7NO3S |
| 11 | 129.042 | 129.043 | 1-Pyrroline-4-hydroxy 2-carboxylic acid | C5H7NO3 |
| 129.043 | Pyroglutamic acid | C5H7NO3 | ||
| 129.043 | Pyrroline hydroxycarboxylic acid | C5H7NO3 | ||
| 129.043 | N-Acryloylglycine | C5H7NO3 | ||
| 129.043 | Pyrrolidonecarboxylic acid | C5H7NO3 | ||
| 12 | 135.068* | 135.0684 | N-Methylformamide | C8H9NO |
| 13 | 136.063 | 136.0637 | n-methylnicotinamide | C7H8N2O |
| 14 | 159.089 | 159.0895 | 3-Dehydrocarnitine | C7H13NO3 |
| 159.0895 | Isovalerylglycine | C7H13NO3 | ||
| 159.0895 | Valerylglycine | C7H13NO3 | ||
| 159.0895 | 2-Methylbutyrylglycine | C7H13NO3 | ||
| 15 | 172.077 | 172.0736 | 2-Octenedioic acid | C8H12O4 |
| 172.0736 | Cis-4-Octenedioic acid | C8H12O4 | ||
| 16 | 173.105 | 173.1052 | Isovalerylsarcosine | C8H15NO3 |
| 173.1052 | Hexanoylglycine | C8H15NO3 | ||
| 173.1052 | Isovalerylalanine | C8H15NO3 | ||
| 17 | 173.999 | 174.0164 | Dehydroxyascorbic acid | C6H6O6 |
| 174.0164 | cis-Aconitic acid | C6H6O6 | ||
| 174.0164 | trans-Aconitic acid | C6H6O6 | ||
| 18 | 177.059* | 177.046 | N-Formyl-l-methionine | C6H11NO3S |
| 177.079 | 5-Hydroxytryptophol | C10H11NO2 | ||
| 19 | 179.058 | 179.0582 | Hippuric acid | C9H9NO3 |
| 20 | 213.009* | 213.010 | Indoxyl sulfate | C8H7NO4S |
| 21 | 250.167* | 250.1205 | Ubiquinone | C14H18O4 |
| 22 | 255.108 | 255.0981 | Nicotinamide riboside | C11H15N2O5 |
| 255.0968 | 7,8-Dihydroneopterin | C9H13N5O4 | ||
| 23 | 258.081* | 258.0852 | Imidazoleacetic acid riboside | C10H14N2O6 |
| 258.0852 | 3-Methyluridine | C10H14N2O6 | ||
| 258.0852 | Ribothymidine | C10H14N2O6 | ||
| 24 | 276.079 | 276.078 | Biotin sulfone | C10H16N2O5S |
| 25 | 302.100* | 302.1582 | 6-Ketoestriol | C18H22O4 |
| 26 | 339.095 | 339.0469 | 5-Amino-1-(5-phospho-d-ribosyl) imidazole-4-carboxylate | C9H14N3O9P |
| 27 | 342.1154 | 342.1162 | Sucrose | C12H22O11 |
| α-Lactose | C12H22O11 | |||
| Isomaltose | C12H22O11 | |||
| Maltose | C12H22O11 | |||
| Trehalose | C12H22O11 | |||
| 3-β-Galactopyranosyl glucose | C12H22O11 | |||
| 28 | 364.097 | 364.042 | Xanthylic acid | C10H13N4O9P |
| 29 | 370.095 | 370.1475 | Amylose | C14H26O11 |
| 30 | 380.098* | 380.0475 | Thiamine monophosphate | C12H18ClN4O4PS |
| 31 | 412.035* | 412.0185 | 2′-Deoxyinosine-5′-diphosphate | C10H14N4O10P2 |
| 32 | 436.187 | 436.259 | 1-Oleoyl-lysophosphatidic acid | C21H41O7P |
Urinary metabolites which showed a >twofold change in positive or negative ESI mode from animals with diabetic nephropathy compared with control animals (<200 ppm mass error) are listed in order of ascending observed mass. Identification is based on METLIN and Human Metabolome Database search results.
Molecular features returning to normal after rosiglitazone treatment (in bold font).
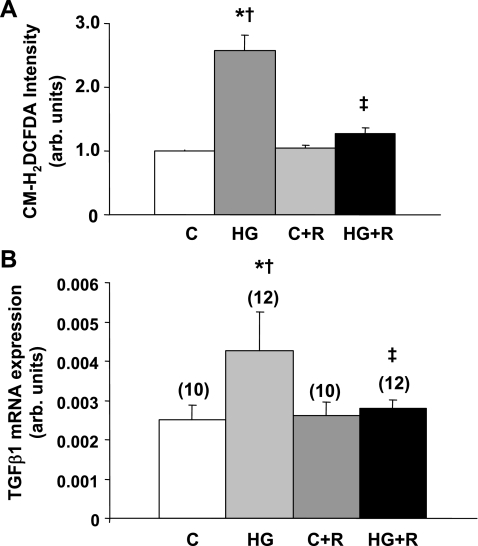
Finally, in cultured glomerular mesangial cells exposed to high (20 mM) glucose, rosiglitazone (10 μg/ml) prevented the dramatic increase in DCFDA fluorescence caused by incubation in high glucose, consistent with the antioxidant effects of rosiglitazone (Fig. 7A). Similarly, TGF-β mRNA levels were enhanced in the cultured cells by high glucose, and this increase was prevented by rosiglitazone (Fig. 7B).
Fig. 7.
Effect of rosiglitazone on reactive oxygen species production and transforming growth factor (TGF)-β mRNA levels in rat mesangial cells cultured in media containing 8 or 20 mM glucose. A: dichlorofluorescein diacetate (DCFDA) levels as a marker of reactive oxygen species. B: TGF-β mRNA levels. Values are means ± SE; n = 10 in each group (A) or in parentheses (B). C, 8 mM glucose; HG, 20 mM glucose; C+R, 8 mM glucose+rosiglitazone; D+R, 20 mM glucose+rosiglitazone. P < 0.05: *vs. 8 mM glucose-incubated cells; †vs. rosiglitazone-treated, 8 mM glucose-incubated cells; ‡vs. rosiglitazone-treated. 20 mM glucose-incubated cells.
DISCUSSION
The pathogenesis of DN is complex and involves alterations in multiple signaling pathways in several cell types in the kidney. Moreover, the pathological features of DN are equally complex and include, at a minimum, glomerular mesangial matrix and basement membrane expansion, leading to glomerulosclerosis, podocyte damage and loss, arteriolar hyalinosis, and ultimately tubulointerstitial fibrosis (3). Clinical features of the disease include a gradual increase in albuminuria and a progressive decline in glomerular filtration rate. While no rodent models recapitulate all these features (3), the streptozotocin DBA/2J mouse is an excellent model of early type 1 DN with many features that better mimic the disease in humans than most conventional mouse or rat models (13, 41). We therefore investigated this robust murine model to determine the impact of the TZD rosiglitazone on features of DN. We found that rosiglitazone treatment beginning 2 wk after initiation of diabetes prevented or markedly attenuated virtually all of the features of early DN, regardless of whether the mice received insulin, and did so independently of effects on glycemic control in this type 1 diabetes model.
A number of studies in animal models of type 1 and type 2 DN (reviewed in Ref. 44) and in type 2 diabetic humans (2, 28, 30, 40, 44) have shown that TZDs can prevent or retard DN via mechanisms that appear to be in part independent of their effects on glycemic control or blood pressure. It appears that multiple mechanisms of action are involved in kidney protection by TZDs in diabetes. For example, TZDs have been found to reduce levels of oxidative stress (22, 35), TGF-β (35), endothelin-1 (29, 42, 45), PAI-1 (20, 21, 31), and advanced glycation end products (34). They also appear to reduce inflammation in tubulointerstitial and glomerular compartments (1).
Our study has extended and clarified some of the protective effects of TZDs in the evolution of DN. We have found that rosiglitazone prevents podocyte loss, a critical early feature of DN that predicts progression in humans (37), and have found that it has similar protective effects in both the presence and absence of insulin in a robust animal model of type 1 diabetes. While previous reports have suggested podocyte protection with TZDs in type 2 models of DN (22) and in humans with type 2 diabetes (28), this is the first report, to our knowledge, to show such protection in type 1 diabetes, indicating that these protective effects are independent of insulin-induced glucose metabolism, and the first to show preservation of glomerular podocytes by TZDs.
Although other reports have suggested a link between TZD protection against DN and reduction of oxidative stress in type 2 diabetic models (22, 35), in this report we demonstrate that rosiglitazone reverses several markers of oxidant injury that have been found to be critical in diabetic humans with vascular disease (39) and does so in a type 1 diabetic model already receiving insulin. Specifically, we detected substantial increases in both protein-bound dityrosine and HODE, highly sensitive markers of protein oxidation and lipid peroxidation, respectively (39), in plasma and renal cortex from diabetic DBA/2J mice, and that rosiglitazone treatment very effectively reduced dityrosine levels in both plasma and the kidney, indicating very efficient inhibition of protein oxidation in the diabetic kidney by TZDs.
Metabolite profiling by ESI/TOF is a powerful technique for identifying novel candidate metabolites (50) that may be signatures of diabetic kidney disease. We matched 32 of the 56 potential mass features that are altered in diabetic state compared with control animals, utilizing exact mass measurements from the METLIN and the Human Metabolome databases (Table 2). Some molecular features have multiple isomers as potential matches. Further studies are required to confirm the identity of these compounds, utilizing authentic standards and tandem MS/MS analysis. Many of the identified metabolites in diabetic urine are consistent with perturbed metabolism and include amino acid metabolites (valine, taurine, glycine metabolites, hippurate); reactive carbonyls (malonate, methylglyoxal, glyoxylic acid); lipids (lysophosphatidic acid, dehydrocarnitine); products of redox regulation (malondialdehyde, dehydroascorbate, aconitate); products of intermediary metabolism (biotin, n-formyl methionine, thiamine monophosphate); and nucleotides (deoxyionisine monophosphate).
Interestingly, 21 urinary metabolites altered in the setting of DN were returned to normal by rosiglitazone. Of these 21 metabolites, we were able to match 13 molecular features to the databases. Ubiquinone (m/z 250.12), a key redox active metabolite, exhibited such a change. It is found in the membranes of endoplasmic reticulum, peroxisomes, lysosomes, vesicles, and the inner membrane of the mitochondria, where it plays an important role in the electron transport chain and mitochondrial biogenesis. Levels of indoxyl sulfate (m/z 213.01) also returned back to baseline following rosiglitazone therapy. This compound is a metabolite of tryptophan metabolism. Indoxyl sulfate has been postulated to be a circulating uremic toxin in hemodialysis patients (32) and has been described to stimulate glomerular sclerosis and interstitial fibrosis in experimental models of kidney disease (27, 33). In plasma, indoxyl sulfate is a protein-bound solute that can induce endothelial dysfunction by inhibiting endothelial proliferation and migration and induce oxidative stress in vitro (10). These metabolites are thus excellent candidates for markers of pathways that play a critical role for the development of DN and for those responsible for the protective effects of TZDs.
GLUT1, the important glucose transporter whose increased expression has been implicated in the pathogenesis of DN (5, 15), and nephrin, the critical component of the podocyte slit diaphragm and glomerular filtration barrier that has often been reported to decline with progressive DN (9), and Neph1 were not changed in the expected directions in the diabetic DBA/2J mice, questioning the role of increased GLUT1 expression and decreased nephrin expression in the pathogenesis of DN at least in this model of relatively early DN. Moreover, rosiglitazone prevented DN without affecting the expression of GLUT1 or Neph1 and further increased nephrin expression. Since we have demonstrated novel effects of rosiglitazone on urinary metabolites in DN, these protective effects were similarly independent of effects on glomerular expression of these three proteins. While these findings by themselves do not undermine the importance of GLUT1 and nephrin in the development and progression of DN, they do show that increased GLUT1 and decreased nephrin expression are not required for development of significant early DN.
Given the pleiotropic nature of TZDs, it will be difficult to elucidate the most critical mechanisms responsible for their protective effects. Precise elucidation of the 56 metabolite features altered in DN in our model, especially those 21 that are returned to normal levels by TZDs, should allow us to identify candidate pathways that are critical for the evolution of nephropathy and the protective effect of TZDs. After validation in humans, some of these features may ultimately serve as biomarkers of DN and response to therapy. Studies that address these issues could reveal new pathways of injury that may be efficiently prevented or treated by TZDs, or by more specific and potent analogs that remain to be developed.
GRANTS
These studies were supported by grants from the Juvenile Diabetes Research Foundation (1-2005-347 to F. C. Brosius III and 2-2003-149 to S. Pennathur), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants U01-DK-076139 (to F. C. Brosius III) and R37-DK-406960 (to R. T. Kennedy), and from the A. Alfred Taubman Medical Research Institute. This research used the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center supported by NIDDK Grant DK-20572. Mass spectrometry experiments were conducted in part at the Metabolomics Mass Spectrometry Core at the Michigan Metabolomics and Obesity Center.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agarwal R Anti-inflammatory effects of short-term pioglitazone therapy in men with advanced diabetic nephropathy. Am J Physiol Renal Physiol 290: F600–F605, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Saha C, Battiwala M, Vasavada N, Curley T, Chase SD, Sachs N, Semret MH. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int 68: 285–292, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Breyer MD, Bottinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brosius FC 3rd. Trophic factors and cytokines in early diabetic glomerulopathy. Exp Diabesity Res 4: 225–233, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosius FC, Heilig CW. Glucose transporters in diabetic nephropathy. Pediatr Nephrol 20: 447–451, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Byun J, Mueller DM, Fabjan JS, Heinecke JW. Nitrogen dioxide radical generated by the myeloperoxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoprotein. FEBS Lett 455: 243–246, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chang PC, Chen TH, Chang CJ, Hou CC, Chan P, Lee HM. Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via a p38 MAPK-dependent pathway. Kidney Int 65: 1664–1675, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Collins SJ, Alexander SL, Lopez-Guisa JM, Cai X, Maruvada R, Chua SC, Zhang G, Okamura DM, Matsuo S, Eddy AA. Plasminogen activator inhibitor-1 deficiency has renal benefits but some adverse systemic consequences in diabetic mice. Nephron 104: e23–34, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol 22: 393–398, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost 5: 1302–1308, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Fujii M, Takemura R, Yamaguchi M, Hasegawa G, Shigeta H, Nakano K, Kondo M. Troglitazone (CS-045) ameliorates albuminuria in streptozotocin-induced diabetic rats. Metabolism 46: 981–983, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Haneda M, Koya D, Kikkawa R. Cellular mechanisms in the development and progression of diabetic nephropathy: activation of the DAG-PKC-ERK pathway. Am J Kidney Dis 38: S178–S181, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Heilig CW, Brosius FC, Cunningham C. Role for GLUT1 in diabetic glomerulosclerosis. Exp Rev Mol Med 8: 1–18, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Heilig CW, Kreisberg JI, Freytag S, Murakami T, Ebina Y, Guo L, Heilig K, Loberg R, Qu X, Jin Y, Henry D, Brosius FC 3rd. Antisense GLUT-1 protects mesangial cells from glucose induction of GLUT-1 and fibronectin expression. Am J Physiol Renal Physiol 280: F657–F666, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Heilig CW, Liu Y, England RL, Freytag SO, Gilbert JD, Heilig KO, Zhu M, Concepcion LA, Brosius FC 3rd. d-glucose stimulates mesangial cell GLUT1 expression and basal and IGF-I-sensitive glucose uptake in rat mesangial cells: implications for diabetic nephropathy. Diabetes 46: 1030–1039, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res 53: 131–142, 2004. [PubMed] [Google Scholar]
- 19.Katavetin P, Eiam-Ong S, Suwanwalaikorn S. Pioglitazone reduces urinary protein and urinary transforming growth factor-beta excretion in patients with type 2 diabetes and overt nephropathy. J Med Assoc Thai 89: 170–177, 2006. [PubMed] [Google Scholar]
- 20.Kato K, Satoh H, Endo Y, Yamada D, Midorikawa S, Sato W, Mizuno K, Fujita T, Tsukamoto K, Watanabe T. Thiazolidinediones down-regulate plasminogen activator inhibitor type 1 expression in human vascular endothelial cells: A possible role for PPARgamma in endothelial function. Biochem Biophys Res Commun 258: 431–435, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kruszynska YT, Yu JG, Olefsky JM, Sobel BE. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes 49: 633–639, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Makino H, Miyamoto Y, Sawai K, Mori K, Mukoyama M, Nakao K, Yoshimasa Y, Suga S. Altered gene expression related to glomerulogenesis and podocyte structure in early diabetic nephropathy of db/db mice and its restoration by pioglitazone. Diabetes 55: 2747–2756, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Malencik DA, Sprouse JF, Swanson CA, Anderson SR. Dityrosine: preparation, isolation, analysis. Anal Biochem 242: 202–213, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87: 206–210, 1978. [DOI] [PubMed] [Google Scholar]
- 25.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meezan E, Brendel K, Ulreich J, Carlson EC. Properties of a pure metabolically active glomerular preparation from rat kidneys. I. Isolation. J Pharmacol Exp Ther 187: 332–341, 1973. [PubMed] [Google Scholar]
- 27.Miyazaki T, Ise M, Hirata M, Endo K, Ito Y, Seo H, Niwa T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int Suppl 63: S211–S214, 1997. [PubMed] [Google Scholar]
- 28.Nakamura T, Ushiyama C, Osada S, Hara M, Shimada N, Koide H. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism 50: 1193–1196, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Ushiyama C, Shimada N, Hayashi K, Ebihara I, Koide H. Comparative effects of pioglitazone, glibenclamide, and voglibose on urinary endothelin-1 and albumin excretion in diabetes patients. J Diabetes Complications 14: 250–254, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Ushiyama C, Suzuki S, Shimada N, Sekizuka K, Ebihara L, Koide H. Effect of troglitazone on urinary albumin excretion and serum type IV collagen concentrations in type 2 diabetic patients with microalbuminuria or macroalbuminuria. Diabet Med 18: 308–313, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas SB, Kawano Y, Wakino S, Collins AR, Hsueh WA. Expression and function of peroxisome proliferator-activated receptor-gamma in mesangial cells. Hypertension 37: 722–727, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Niwa T, Emoto Y, Maeda K, Uehara Y, Yamada N, Shibata M. Oral sorbent suppresses accumulation of albumin-bound indoxyl sulphate in serum of haemodialysis patients. Nephrol Dial Transplant 6: 105–109, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med 124: 96–104, 1994. [PubMed] [Google Scholar]
- 34.Ohga S, Shikata K, Yozai K, Okada S, Ogawa D, Usui H, Wada J, Shikata Y, Makino H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am J Physiol Renal Physiol 292: F1141–F1150, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Ohtomo S, Izuhara Y, Takizawa S, Yamada N, Kakuta T, van Ypersele de Strihou C, Miyata T. Thiazolidinediones provide better renoprotection than insulin in an obese, hypertensive type II diabetic rat model. Kidney Int 72: 1512–1519, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Onozaki A, Midorikawa S, Sanada H, Hayashi Y, Baba T, Katoh T, Watanabe T. Rapid change of glucose concentration promotes mesangial cell proliferation via VEGF: inhibitory effects of thiazolidinedione. Biochem Biophys Res Commun 317: 24–29, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennathur S, Bergt C, Shao B, Byun J, Kassim SY, Singh P, Green PS, McDonald TO, Brunzell J, Chait A, Oram JF, O'Brien K, Geary RL, Heinecke JW. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J Biol Chem 279: 42977–42983, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem 280: 22706–22714, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Pistrosch F, Herbrig K, Kindel B, Passauer J, Fischer S, Gross P. Rosiglitazone improves glomerular hyperfiltration, renal endothelial dysfunction, and microalbuminuria of incipient diabetic nephropathy in patients. Diabetes 54: 2206–2211, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension 43: 661–666, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms' tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol 14: 2484–2493, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int 70: 1223–1233, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S, Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun 254: 757–763, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein. J Biol Chem 269: 20394–20400, 1994. [PubMed] [Google Scholar]
- 47.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC 3rd. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol Renal Physiol 276: F658–F665, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC 3rd. Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment (Abstract). BMC Nephrol 7: 6, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy. Adv Chronic Kidney Dis 12: 146–154, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Want EJ, Nordstrom A, Morita H, Siuzdak G. From exogenous to endogenous: the inevitable imprint of mass spectrometry in metabolomics. J Proteome Res 6: 459–468, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Weigert C, Brodbeck K, Bierhaus A, Haring HU, Schleicher ED. c-Fos-driven transcriptional activation of transforming growth factor beta-1: inhibition of high glucose-induced promoter activity by thiazolidinediones. Biochem Biophys Res Commun 304: 301–307, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Weigert C, Brodbeck K, Brosius FC, 3rd Huber M, Lehmann R, Friess U, Facchin S, Aulwurm S, Haring HU, Schleicher ED, Heilig CW. Evidence for a novel TGF-beta1-independent mechanism of fibronectin production in mesangial cells overexpressing glucose transporters. Diabetes 52: 527–535, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, Macinnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L. HMDB: the Human Metabolome Database. Nucleic Acids Res 35: D521–526, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita H, Nagai Y, Takamura T, Nohara E, Kobayashi K. Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism 51: 403–408, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Yotsumoto T, Naitoh T, Kanaki T, Matsuda M, Tsuruzoe N. A novel peroxisome proliferator-activated receptor (PPAR)gamma agonist, NIP-222, reduces urinary albumin excretion in streptozotocin-diabetic mice independent of PPARgamma activation. Metabolism 52: 1633–1637, 2003. [DOI] [PubMed] [Google Scholar]