Abstract
Loss of physiological regulation of the renal thiazide-sensitive Na+-Cl− cotransporter (NCC) by mutant WNK1 or WNK4 results in pseudohypoaldosteronism type II (PHAII) characterized by arterial hypertension and hyperkalemia. WNK4 normally inhibits NCC, but this effect is lost by eliminating WNK4 catalytic activity or through PHAII-type mutations. In contrast, another member of the WNK family, WNK3, activates NCC. The positive effect of WNK3 on NCC also requires its catalytic activity. Because the opposite effects of WNK3 and WNK4 on NCC were observed in the same expression system, sequences within the WNKs should endow these kinases with their activating or inhibiting properties. To gain insight into the structure-function relationships between the WNKs and NCC, we used a chimera approach between WNK3 and WNK4 to elucidate the domain of the WNKs responsible for the effects on NCC. Chimeras were constructed by swapping the amino or carboxyl terminus domains, which flank the central kinase domain, between WNK3 and WNK4. Our results show that the effect of chimeras toward NCC follows the amino-terminal domain. Thus the amino terminus of the WNKs contains the sequences that are required for their activating or inhibiting properties on NCC.
Keywords: distal convoluted tubule, diuretics, hypertension, kinase
the wnks [with no lysine (K)] are a subfamily of serine/threonine kinases that lack the conserved lysine (substituted by cysteine), which in all other serine/threonine kinases is located in the β-strand 3 of kinase subdomain II of the catalytic domain (35). A total of four genes encoding WNK isoforms are present in the human genome: WNK1, WNK2, WNK3, and WNK4, located in chromosomes 12, 9, X, and 17, respectively. The degree of identity among WNKs is >80% in the kinase domain and <17% in the flanking amino- or carboxy-terminal domains. Mutations in WNK1 and WNK4 are the cause of pseudohypoaldosteronism type II (PHAII) (33), an inherited disease that features arterial hypertension, hyperkalemia, and metabolic acidosis. PHAII patients are highly sensitive to thiazide diuretics (18). Clinically, PHAII is the mirror image of Gitelman's disease, an inherited illness characterized by arterial hypotension, hipokalemia, and metabolic alkalosis due to inactivating mutations of the SLC12A3 gene that encodes the thiazide-sensitive Na+-Cl− cotransporter NCC (28). Thus PHAII seems to be the consequence of overactivity of NCC due to a loss of physiological regulation by the WNKs (15, 34, 41). This hypothesis is supported by recent studies in Xenopus laevis oocytes (11, 34, 37), culture cells (2), and transgenic mice harboring WNK4 with PHAII-type mutations (15, 41). It is not surprising that the WNKs have emerged as powerful regulators not only of NCC but also of all the members of the cation-coupled chloride cotransporter family (SLC12) (9, 14).
The SLC12 family of membrane transporters is composed of seven members that are divided into two branches. The Na+-driven branch that includes SLC12A3 encodes NCC and two other genes: SLC12A1 (encoding the kidney-specific Na+-K+-2Cl− cotransporter NKCC2) and SLC12A2 (encoding the ubiquitously expressed Na+-K+-2Cl− cotransporter NKCC1). The K+-driven branch is composed of four genes, SLC12A4 to SLC12A7, which encode K+-Cl− cotransporters KCC1 to KCC4, respectively. Studies from several laboratories have shown that phosphorylation activates Na+-driven and inactivates K+-driven cotransporters, whereas dephosphorylation has the opposite effect (for review, see Refs. 1, 5, 6, 9, 12, 16). With the use of X. laevis oocytes as a heterologous expression system, it has been observed that WNK4 is a negative regulator of NCC (11, 34, 37) and all four KCCs (7, 10). The effect on both NCC (2, 11, 34) and KCCs (10) is eliminated by the D318A substitution, which renders WNK4 catalytically inactive, proving that the catalytic activity of WNK4 is required. In addition, PHAII-type missense mutations of WNK4 in a conserved acidic region of the carboxy-terminal domain prevent the negative effect of WNK4 on NCC (2, 34). WNK3 is also a powerful regulator of all members of the SLC12 family. However, WNK3 activates NCC, NKCC1, and NKCC2 (13, 27) while inhibiting all four KCCs (3). Interestingly, eliminating WNK3 catalytic activity by the D294A mutation not only prevents it from acting on the cotransporters but also inverts the kinase effect. In other words, WNK3-D294A becomes a powerful inhibitor of NCC, NKCC1, and NKCC2 (13, 27) and an activator of all KCCs (3), regardless of the cell volume changes that are usually required to activate or inhibit these transporters.
All these studies together demonstrate that, with the same expression system of X. laevis oocytes, the effect of WNK3 and WNK4 on NCC is opposite, whereas their effect on KCCs is similar. Thus it is reasonable to propose that sequences within WNK3 and WNK4 endow these kinases with the ability to activate or inhibit NCC, respectively. In the present study we undertook a chimeric approach between WNK3 and WNK4 to begin to define structure-function relationships between WNKs and NCC. Our results show that information required for being a positive or a negative regulator of NCC in WNK3 and WNK4 is located within the short amino terminal domain.
MATERIAL AND METHODS
The cDNAs of rat NCC, mouse WNK4, human WNK3, and human KCC2 have been described previously and are the clones that were used in the studies that demonstrated the effects of WNK3 or WNK4 on NCC or KCC2 (8, 27, 29, 34). Chimeric clones were constructed between WNK3 and WNK4 by swapping the amino- or the carboxy-terminal domain. To this end, unique silent KpnI and NsiI sites were introduced by site-directed mutagenesis (QuickChange; Stratagene) at the beginning and at the end, respectively, of the kinase domain of both WNK3 and WNK4. Amino- and carboxy-terminal domains were then swapped by cutting and pasting cDNA fragments to construct the chimeric proteins shown in Fig. 1. Restriction analysis and sequencing were used to corroborate the appropriate creation of silent sites and to rule out unwanted mutations. In addition, all experiments were performed using the constructs of wild-type WNK3 and WNK4 in which the KpnI and NsiI sites were introduced.
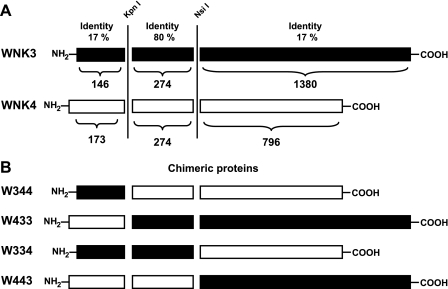
Fig. 1.
Identity degree and chimeric proteins between WNK3 and WNK4. A: identity degree between WNK3 (black) and WNK4 (white) divided by the amino-terminal, kinase, and carboxy-terminal domains. Vertical lines indicate the regions in which silent restriction sites for KpnI and NsiI were introduced. Numbers above WNKs represent the percentage of identity for each segment. Numbers below WNKs represent the number of amino acid residues of each domain. B: chimeric constructs obtained between WNK3 (black) and WNK4 (white) by swapping the amino- or carboxy-terminal domains.
Assessment of the Na+-Cl− and K+-Cl− cotransporter function.
NCC and KCC2 activity was assessed by functional expression in X. laevis oocytes following previously described protocols (3, 8, 20, 24, 29). Briefly, oocytes were surgically harvested from adult female X. laevis frogs (Nasco) under tricaine anesthesia (0.17%) and incubated in frog Ringer ND96 (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl, and 5 HEPES/Tris, pH 7.4) in the presence of collagenase B (2 mg/ml) for 1 h. Oocytes were washed four times in ND96, defolliculated, and incubated overnight at 18°C in ND96 supplemented with 2.5 mM sodium pyruvate and 5 mg/100 ml gentamicin. The next day, mature oocytes were injected with 50 nl of water alone or containing cRNA at 0.2 μg/μl NCC or KCC2 cRNA alone or together with a wild-type, mutant, or chimeric WNK cRNA. After injection, oocytes were maintained for 3 days in frog Ringer.
For NCC influx experiments 2 h before uptake, oocytes were incubated in a Cl−-free isotonic medium (in mM: 96 Na+ isethionate, 2 K+-gluconate, 1.8 Ca2+-gluconate, 1.0 Mg2+-gluconate, and 5 HEPES, pH 7.4). Tracer 22Na+ uptake (New England Nuclear) was then assessed in groups of 10–15 oocytes exposed to isotonicity using our usual isotonic uptake solution [in mM: 40 NaCl, 56 N-methyl-d-glucamine (NMDG)-Cl, 1.8 CaCl2, 1.0 MgCl2, and 5.0 HEPES, pH 7.4; 210 mosmol/kgH2O] containing 1 mM ouabain, 0.1 mM amiloride, and 0.1 mM bumetanide plus 2 μCi/ml 22Na+. To determine the 22Na+ uptake due to NCC, we exposed parallel groups to the same uptake solution but in the presence of 100 μM metolazone.
For KCC2 influx experiments, we assessed tracer 86Rb+ uptake (New England Nuclear) in experimental groups of 10–15 oocytes. 86Rb+ influx was measured in two different osmolar conditions. For hypotonic conditions, oocytes were incubated for 30 min in hypotonic K+- and Cl−-free medium (in mM: 50 NMDG-gluconate, 4.6 Ca2+-gluconate, 1.0 Mg2+-gluconate, and 5 HEPES/Tris, pH 7.4; 110 mosmol/kgH2O) with 1 mM ouabain, followed by 60 min in hypotonic Na+-free medium containing 10 mM KCl, 40 mM NMDG-Cl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH 7.4, and supplemented with 1 mM ouabain and 2.0 μCi/ml 86Rb+. Parallel groups of oocytes were exposed to Cl−-free uptake solution in which Cl− was substituted with gluconate. For uptake in isotonic conditions, the same solutions were used but supplemented with 3.5 g/100 ml sucrose to reach picomolar conditions for oocytes (∼210 mosmol/kgH2O).
In all experiments, tracer activity was determined for each oocyte dissolved in 10% SDS by beta-scintillation counting. All results presented are based on a minimum of three different experiments with at least 10 oocytes per group in each experiment. Statistical significance is defined as two-tailed, with P < 0.05, and the results are presented as means ± SE. The significance of the differences between groups was tested by one-way ANOVA with multiple comparisons using Bonferroni correction.
RT-PCR of WNKs in X. laevis oocytes.
X. laevis genome data bases were analyzed with BLAST using protein sequences of the carboxy-terminal domain of human WNK1, WNK2, WNK3, and WNK4 as bait. EST sequences with significant identity to each WNK were found. The percentages of identity of sequences from accession nos. EG575770, CA983538, BF048601, and BJ067481 were 75, 68, 76, and 84 with WNK1, WNK2, WNK3, and WNK4 carboxy-terminal domain, respectively. We then designed primer pairs from each sequence to amplify a fragment of each WNK from the oocytes' mRNA by RT-PCR. Total RNA from X. laevis oocytes was isolated using the Tripure system (Roche) following the manufacturer's recommendations and treated with DNase I for 1 h to eliminate possible contamination with genomic DNA. Reverse transcription was carried out using 2.5 μg of total RNA extracted from oocytes at 37°C for 60 min in a total volume of 20 μl using 200 units of the Moloney murine leukemia virus reverse transcriptase (Invitrogen). Simultaneous PCR for each WNK was carried out in the absence of reverse transcription to rule out the possibility of DNA genomic contamination. The cDNA fragments obtained from PCR were resolved in 5% acrylamide gels. Fragments from WNK3 and WNK4 were also resolved in agarose gel, excised, and sequenced by automatic DNA sequencing.
RESULTS
As shown in Fig. 1, WNKs can be divided into three structurally defined domains. The first is a short amino-terminal domain that, in WNK3 and WNK4, is made up of 146 and 173 amino acid residues, respectively. This is followed by the serine/threonine kinase domain of 274 amino acid residues in all WNKs and a long carboxy-terminal domain of 1,380 and 796 amino acid residues in WNK3 and WNK4, respectively. To analyze the role of the amino- and carboxy-terminal domains on NCC regulation, we constructed four chimeras between WNK3 and WNK4 by swapping their amino- or carboxy-terminal domains. To this end, silent restriction sites for KpnI and NsiI were introduced by site-directed mutagenesis at the beginning and end of the kinase domain. Figure 1B shows the chimeras denoted by three numbers corresponding to the amino-, kinase, and carboxy-terminal domains, respectively. The numbers 3 and 4 represent the origin of each domain. Thus chimeras 344 and 433 contain the amino-terminal domain of WNK3 and WNK4, respectively, fused into the kinase and carboxy-terminal domains of the other WNK. Chimeras 334 and 443 were made by swapping the carboxy-terminal domain of WNK3 or WNK4, respectively, into the amino-terminal and kinase domains of the other WNK. All chimeric constructs were corroborated by a restriction map and sequencing. The effect of each chimera on NCC and KCC2 activity was then assessed in control experiments that used wild-type WNK3 or WNK4 harboring the silent KpnI and NsiI sites (see materials and methods).
Effect of WNK3 or WNK4 on NCC follows the amino-terminal domain.
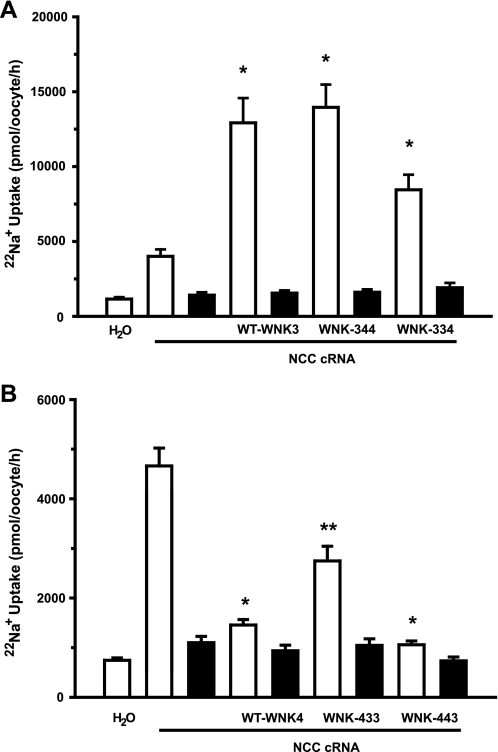
Figure 2 depicts the compiled results of three different experiments in which X. laevis oocytes were injected with NCC cRNA alone or together with wild-type or chimeric cRNAs. As we had previously shown (27), coinjection with NCC cRNA and wild-type WNK3 cRNA resulted in increased NCC activity. Figure 2A shows that activation induced by WNK3 increased 22Na+ uptake by NCC from 4,006 ± 464 pmol·oocyte−1·h−1 in oocytes injected with NCC cRNA alone to 12,925 ± 1,651 pmol·oocyte−1·h−1 in oocytes coinjected with NCC and wild-type WNK3 cRNA (P < 0.01). Interestingly, coinjection with the chimera 344 cRNA resulted in a similar extent of NCC activation that reached 13,956 ± 1,520 pmol·oocyte−1·h−1 (P < 0.01 vs. NCC alone). The chimera 334 cRNA also induced a significant increase in NCC activity to 8,449 ± 1,010·oocyte−1·h−1 (P < 0.05 vs. NCC alone). Therefore, chimeras containing the amino-terminal domain of WNK3 were able to increase NCC activity.
Fig. 2.
Effect of WNK3, WNK4, and chimeras on the renal thiazide-sensitive Na+-2Cl− cotransporter (NCC) activity. A: Xenopus laevis oocytes were injected with water or NCC cRNA alone or together with WNK3, chimera 344, or chimera 334 cRNAs, as indicated. B: oocytes were injected with water or NCC cRNA alone or together with WNK4, chimera 433, or chimera 443 cRNAs, as indicated. 22Na+ uptake was assessed in isotonic medium 3 days later in the absence (open bars) or presence (filled bars) of 100 μM metolazone. *Significantly different from the uptake observed in oocytes injected with NCC cRNA alone. **Significantly different from the uptake observed in oocytes injected with NCC cRNA alone or NCC + wild-type WNK4 or 443 chimera.
The opposite results were observed with chimeras containing the WNK4 amino-terminal domain (Fig. 2B). As we had previously shown (34), coinjection of NCC cRNA with WNK4 cRNA resulted in a significant reduction of NCC-induced 22Na+ uptake (Fig. 2B) from 4,562 ± 348 pmol·oocyte−1·h−1 in NCC oocytes to 1,456 ± 109 pmol·oocyte−1·h−1 in oocytes coinjected with NCC and wild-type WNK4 cRNA (P < 0.01). Chimeras 433 and 443 also induced a significant reduction of NCC activity to 2,747 ± 296 and 1,058 ± 75 pmol·oocyte−1·h−1, respectively. Thus data shown in Fig. 2 suggest that WNK3 activating effects and WNK4 inhibiting effects on NCC correspond to the WNKs' amino-terminal domain. Interestingly, the inhibitory effect of chimera 433 was significantly lower than that observed in wild-type WNK4 or chimera 443, suggesting that activity and/or stability of this chimera in these experiments was lower than that of wild-type WNK4 or chimera 443 or that the catalytic domain of WNK3 still exerted some positive effect that somehow competes with the negative effect of the amino terminus. The first possibility is most likely, since in the series of experiments done in Fig. 5 (see below) the inhibitory effect of chimera 433 and wild-type WNK4 was similar.
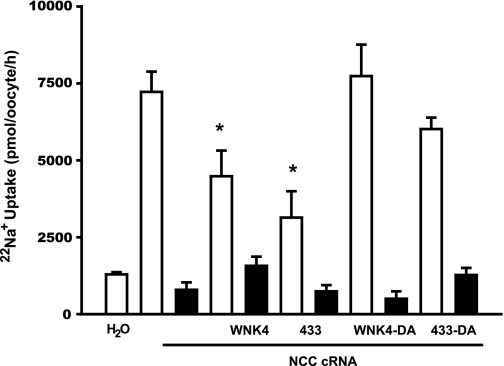
Fig. 5.
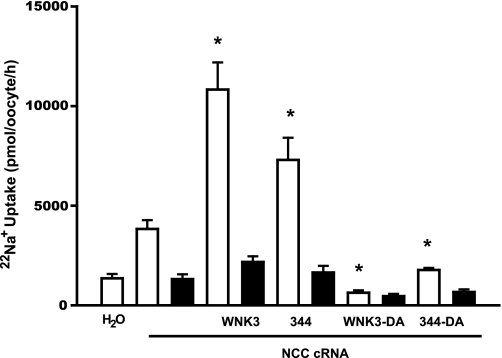
Effect of aspartic acid substitution in wild-type WNK4 and chimera 433 on NCC activity. Oocytes were injected with NCC cRNA alone or together with wild-type WNK4, WNK4-D318A, chimera 433, or chimera 433-D318A cRNA. Three days later, 22Na+ uptake was assessed in the absence (open bars) or presence (filled bars) of 100 μM metolazone. *Significantly different from the uptake observed in oocytes injected with NCC cRNA alone.
All chimeras are powerful inhibitors of K+-Cl− cotransporter KCC2.
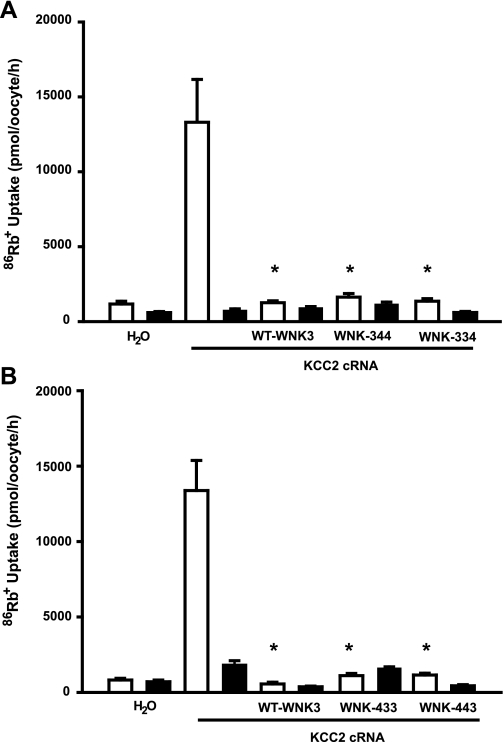
It has been shown previously that both wild-type WNK3 and WNK4 are potent inhibitors of K+-Cl− cotransporters KCC1 to KCC4. Catalytically inactive forms of WNK3 and WNK4 not only lose their inhibitory effect but also become powerful activators of K+-Cl− cotransporters (3, 7, 10). We thus reasoned that if the WNK3 and WNK4 chimeras conserved their catalytic activity, then it would follow that they were able to inhibit K+-Cl− cotransporter expression, since this is the effect of both wild-type WNK3 and WNK4. Thus we assessed the effect of wild-type WNK3 and all four chimeras on the activity of KCC2 in hypotonic conditions, in which this cotransporter is maximally active (Fig. 3, A and B). Oocytes were injected with KCC2 cRNA alone or together with each of the chimeras' cRNA. Three days later, the activity of KCC2 was assessed in hypotonic conditions by measuring the Na+-independent and Cl−-dependent 86Rb+ influx. As we had previously shown (27, 29), injection of oocytes with KCC2 cRNA resulted in a remarkable increase in 86Rb+ uptake during cell swelling (Fig. 3, A and B). When KCC2 was coinjected with wild-type WNK3 or any one of the four chimeras, regardless of their effect on NCC, 86Rb+ uptake in these groups was similar to that of water-injected oocytes (Fig. 3, A and B). This finding implies that all cRNAs induced a complete inhibition of KCC2 activity. Because catalytic activity of WNK3 and WNK4 is required for the inhibition of K+-Cl− cotransporters (10, 27), these observations strongly suggest that all four chimeras are functional.
Fig. 3.
Effect of WNK3 and chimeras on K+-Cl− cotransporter KCC2. A: oocytes were injected with water or KCC2 cRNA alone or together with WNK3, chimera 344, or chimera 334 cRNAs, as indicated. B: oocytes were injected with water or KCC2 cRNA alone or together with WNK3, chimera 433, or chimera 443 cRNAs, as indicated. 86Rb+ uptake was assessed in hypotonic medium 3 days later in the presence (open bars) or absence (filled bars) of extracellular Cl−. *Significantly different from the uptake observed in oocytes injected with KCC2 cRNA alone.
Effect of chimeras 344 and 433 on NCC and KCC2 requires their catalytic activity.
We have previously shown that the elimination of catalytic activity in wild type WNK3 (WNK3-D294A) not only prevents its activating effect on NCC but also turns kinase-dead WNK3 into an NCC inhibitor (27). Thus, to further study the chimera 344 properties, we introduced the mutation D294A into this chimera to render it catalytically inactive (344-DA). We then assessed the effect of wild-type WNK3, WNK3-D294A, the chimera 344, and the kinase-dead chimera 344-D294A on NCC activity (Fig. 4). We observed that 22Na+ uptake in oocytes injected with NCC cRNA alone was 3,830 ± 444 pmol·oocyte−1·h−1. However, in those coinjected with NCC and either WNK3 or chimera 344 cRNA, the tracer uptake increased to 10,826 ± 1,364 and 7,296 ± 1,114 pmol·oocyte−1·h−1, respectively (P < 0.01 vs. NCC alone). In contrast, uptake in oocytes coinjected with NCC and kinase-dead WNK3 or kinase-dead chimera 344 cRNA was significantly reduced. Na+ uptake in NCC plus WNK3-D294A was 631 ± 120, and in oocytes injected with NCC plus 344-DA, it was 1,772 ± 103 pmol·oocyte−1·h−1 (P < 0.01 vs. NCC alone). Thus, similar to WNK3-D294A (27), chimera 344-DA lost the activating effect on NCC and became an inhibitor of this cotransporter. This observation, together with results shown in Fig. 3, implies that increased activity of NCC by the chimera 344 requires its catalytic activity.
Fig. 4.
Effect of aspartic acid substitution in wild-type WNK3 and chimera 344 on NCC activity. Oocytes were injected with NCC cRNA alone or together with wild-type WNK3, WNK3-D294A, chimera 344, or chimera 344-D294A cRNA. Three days later, 22Na+ uptake was assessed in the absence (open bars) or presence (filled bars) of 100 μM metolazone. *Significantly different from the uptake observed in oocytes injected with NCC cRNA alone.
We and others (2, 11, 34) have shown that the WNK4 inhibitory effect on NCC requires its catalytic activity. Therefore, to further analyze the functional properties of chimera 433, we assessed the effect of wild-type WNK4, the kinase-dead WNK4-D318A, chimera 433, and the kinase-dead chimera 433-D318A (433-DA) on NCC activity. As shown in Fig. 5, the NCC-induced basal 22Na+ uptake of 7,225 ± 658 pmol·oocyte−1·h−1 was reduced to 4,483 ± 832 and 3,138 ± 855 pmol·oocyte−1·h−1 by WNK4 and chimera 433, respectively. In contrast, no significant reduction of NCC activity was induced by WNK4-D318A or chimera 433-DA. Therefore, as in the case of the catalytically inactive WNK4-D318A, eliminating the catalytic activity of chimera 433 prevented its inhibition of NCC. This observation, together with results shown in Fig. 2, also implies that to inhibit NCC, the 433 chimera requires catalytic activity.
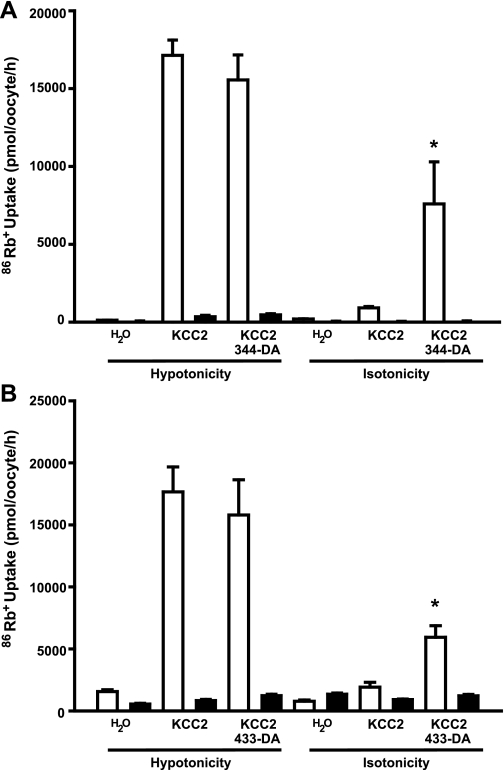
Elimination of catalytic activity in both WNK3 and WNK4 also results in interesting effects on the K+-Cl− cotransporters. The mutant WNK3-D294A and WNK4-D318A not only lost their inhibitory effect on the K+-Cl− cotransporters expressed in oocytes under hypotonic conditions but also became powerful activators of these cotransporters under isotonic conditions (3, 10). Thus we also tested the effect of kinase-dead chimeras 344-D294A and 433-D318A on KCC2 activity under both hypotonic and isotonic conditions. As shown in Fig. 6, oocytes injected with KCC2 cRNA alone exhibited a remarkable increase in 86Rb+ uptake. The level of 86Rb+ uptake was not affected by coinjection with the catalytically inactive chimeras 344-D294A and 433-D318A. We showed in Fig. 2 that all chimeras were able to inhibit KCC2 activity under hypotonic conditions. Thus kinase-dead chimeras lost their inhibitory properties on KCC2.
Fig. 6.
Effect of chimeras 344-D294A and 433-D318A on KCC2 activity under hypotonic and isotonic conditions. A: oocytes were injected with KCC2 cRNA alone or together with 344-D294A cRNA. B: oocytes were injected with KCC2 cRNA alone or together with 433-D318A. Three days later, tracer 86Rb+ uptake was assessed exposing oocytes to hypotonic or isotonic uptake medium in the presence (open bars) or absence (filled bars) of extracellular Cl−. *Significantly different from the uptake observed in oocytes injected with KCC2 cRNA alone.
When expressed in oocytes and exposed to isotonic conditions during the uptake, KCC2 was minimally active (Fig. 6). Coinjection with catalytically inactive chimeras 344-DA and 433-DA cRNA resulted in a significant activation of KCC2. The observation that D294A or D318A mutations in 344 and 433 chimeras, respectively, as in wild-type WNK3 or WNK4, prevented the inhibitory effect of these chimeras on KCC2 activity in hypotonic conditions (Fig. 3 vs. Fig. 6) is also strong evidence that chimeras 344 and 433 possess catalytic activity. In addition, similar to what we observed for WNK3 (3) and WNK4 (10), the activating effect on KCC2 under isotonic conditions is generated in chimeras 344 or 433 by eliminating their catalytic activity.
DISCUSSION
WNK kinases have emerged as powerful regulators of several transport pathways including transporters, channels, and paracellular proteins (for recent review, see Refs. 14 and 25). NCC is one of the most important targets, because impairing its regulation by WNK1 or WNK4 mutations results in the arterial hypertension observed in PHAII patients (15, 18, 41). However, little is known regarding the critical domains within WNKs that endow these kinases with the ability to activate or inhibit NCC. In the present study, we took advantage of the fact that in the same expression system, WNK3 and WNK4 have opposite effects on NCC. WNK3 is an activator (27), whereas WNK4 is an inhibitor of this cotransporter (34). In contrast, both kinases are inhibitors of K+-Cl− cotransporters (3, 10). Thus unique sequences within WNK3 and WNK4 should be critical in defining their effects on NCC. WNK3 and WNK4 exhibit a very high degree of identity within the serine/threonine kinase domain but a very low degree of identity in the amino- and carboxy-terminal domains. Therefore, we constructed chimeric proteins by interchanging these two domains and tested their effects on NCC and KCC2. Our experiments showed that chimeras containing the amino-terminal domain of WNK3 (344 and 334) increased the activity of NCC, whereas chimeras containing the amino-terminal domain of WNK4 (433 and 443) inhibited NCC (Fig. 2). Similar to wild-type WNK3 and WNK4, these effects were lost with DA mutations that render the WNKs kinase-dead (Figs. 4 and 5). Furthermore, all chimeras were able to inhibit KCC2 activity under hypotonic conditions (Fig. 3) in which we had previously shown that catalytic activity is required for such effects of WNK3 or WNK4 (3, 10). In addition, kinase-dead chimeras 344-DA or 433-DA also became KCC activators (Fig. 6). Taking all these observations together, we conclude that behavior of chimeras toward NCC follows the amino-terminal domain and that chimeras are catalytically active.
Two previous studies have suggested that essential sequences for NCC regulation in WNK4 and WNK3 are located within the carboxy-terminal domain. Yang et al. (40) analyzed the effect of coinjecting X. laevis oocytes with cRNA from NCC and different fragments of WNK4. Constructs from the amino-terminal and kinase domains (WNK4 1–444) were not able to inhibit NCC, whereas those containing the carboxy-terminal domain, with or without the kinase domain (WNK4 168–1222 or 445–1222), reduced NCC activity with efficacy similar to full-length WNK4. More recently, Yang et al. (39) confirmed our previous observations (27) that wild-type WNK3 activates NCC and that catalytically inactive WNK3-D294A becomes an inhibitor. Nevertheless, they observed that injecting oocytes with a construct containing only the WNK3 carboxy-terminal domain (WNK3 421–1743), without the associated kinase domain, resulted in increased NCC activity. Thus, although catalytic activity in WNK3 is required for activating NCC (34, 39), the authors observed that the carboxy-terminal domain by itself contains the information required for the WNK3 effects on NCC and proposed that WNK3 and WNK4 interact each other for NCC regulation (19). The active site or motif for such regulation was not elucidated. However, these studies did not rule out the possibility of a dominant negative effect induced by fragments of WNKs, particularly since transcripts corresponding to all four WNKs are expressed in X. laevis oocytes. As shown in Supplemental Fig. 1 (supplemental data for this article is available online at the American Journal of Physiology-Renal Physiology website), a fragment of the expected size for each WNK was amplified from oocytes total RNA, using primers that were designed based on est sequences from X. laevis that showed a high degree of identity with the carboxy-terminal domain of each mammalian WNK. No fragments were amplified in the absence of reverse transcription. The DNA sequence of excised bands corresponding to WNK3 and WNK4 exhibit a 75 and 82% identity with the corresponding fragment of mammalian WNK3 or WNK4, respectively.
It has been shown that WNKs can interact between each other physically and/or through phosphorylation processes (17, 30, 36, 39). One important example is seen in the WNK1 regulation of WNK4-NCC activity. WNK1 can interact with, and inhibit the effect of, WNK4 on NCC (38, 40). Under physiological conditions, this effect is not observed because the PRKWNK1 gene produces an alternative spliced variant (S-WNK1), which is expressed exclusively in the distal convoluted tubule (DCT) and the connecting tubule (CNT) of the nephron (22, 23). S-WNK1 lacks the entire amino-terminal and kinase domains and prevents the WNK1-WNK4 interaction by hijacking the long form of WNK1 through protein-protein interaction (30). In PHAII patients, because of the intronic deletion of WNK1, the expression of the long WNK1 isoform is increased, diminishing the ratio of S-WNK1 to L-WNK1 in DCT/CNT. This allows WNK1 to interact with WNK4, preventing its inhibitory effect on NCC (30). These studies show that fragments of WNKs can affect cotransporters through dominant negative action on other WNKs, and this mechanism seems to occur in vivo, since expression of the S-WNK1 protein occurs naturally in DCT and CNT (4, 23). In contrast, there is no evidence for the existence of spliced truncated variants of WNK3 or WNK4 in DCT. Since WNKs are expressed in oocytes, it is possible that the carboxy terminus of WNK3 or WNK4 interacting with endogenous WNK3 or WNK4 prevents their respective effect on NCC. Thus, although the effect of wild-type WNK3 or WNK4 on NCC can be emulated by the carboxy-terminal domain of WNKs in oocytes, there is no implication for a shared mechanism.
The above proposal might explain why the inhibitory effect of WNK4 on NCC has been shown to be kinase dependent by three groups (2, 11, 34) as well as the present study, but not by another group (40). An important difference between these studies could be the WNK4 construct that was used. Wilson et al. (34), Golbang et al. (11), Cai et al. (2), and the present study were performed using the complete WNK4 (1–1222 residues), with or without the D318A mutation that eliminates catalytic activity. In contrast, Yang et al. (40) used a truncated form of WNK4 lacking the first 168 residues of the amino-terminal domain (168–1222 residues). Because WNK3 and WNK4 fragments can interact at the protein level (39) and X. laevis oocytes express all four WNKs, it is possible that the truncated WNK4 (168–1222) has a dominant negative effect on endogenous WNK3 or WNK4 resulting in NCC inhibition that is not expected to be kinase dependent.
The extent of identity between WNK3 and WNK4 amino-terminal domains is very low (17%). In essence, the two domains are extremely different. It is not known how WNK3 or WNK4 affects the activity of NCC. This could be due to direct interaction and phosphorylation between WNK and NCC, or alternatively, WNKs could interact with other proteins that in turn define the type of effect on NCC. Supporting the first possibility, it has been shown that WNK4 and NCC form a protein complex that is not affected by the kinase activity (31) or by PHAII type mutations (15), suggesting that at least WNK4 and NCC interact directly. Supporting the second possibility, several studies have shown that WNK1 and WNK4 interact with STE-20 kinases such as SPAK and OSR1 and that this interaction is critical for the effect of WNKs on NKCC1 (7, 21, 31). In this regard, we have shown that SPAK is expressed in X. laevis oocytes (10) and that interaction between WNK3 and SPAK is required for WNK3-induced activation of NKCC2 (26). Finally, we also have provided evidences that protein phosphatases are involved in the effect of WNK3 and its catalytically inactive form on the K+-Cl− cotransporters (3). Thus it is possible that information that allows WNK3 or WNK4 to activate or inhibit NCC contained within the WNKs' amino-terminal domain endows the kinase with the capacity to associate with NCC or other intermediary proteins (SPAK, protein phosphatases, or others). However, no particular protein patterns or motifs are found when both WNK3 and WNK4 amino-terminal domains are analyzed using multiple database algorithm sites. Interestingly, WNK3 and WNK4 amino-terminal domains drastically differ in the number of proline residues, 7 of 146 for WNK3 (4.7%) and 32 of 173 for WNK4 (18.4%), suggesting a different structural conformation for each domain. In this regard, a recent report clearly shows that an amino-terminal proline-rich domain of WNK1 is necessary and sufficient for inhibition of ROMK1 by this kinase (32). The identity degree of the amino-terminal domain of WNK1 with WNK3 or WNK4 is, however, also below 15%. Thus further investigation is required to define the motif or active site within the WNK3 or WNK4 amino-terminal domain responsible for their activating or inhibiting properties.
In summary, our data using WNK3/WNK4 chimeras strongly suggest that the activating effect of WNK3 and the inhibitory effect of WNK4 on NCC follow the amino-terminal domain.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-64635 and Consejo Nacional de Ciencia y Tecnologia Grant 59992 (to G. Gamba) and by a grant from the Foundation Leducq for the Transatlantic Network on Hypertension-Renal Salt Handling in the Control of Blood Pressure.
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adragna NC, Fulvio MD, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol 201: 109–137, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int 69: 2162–2170, 2006. [DOI] [PubMed] [Google Scholar]
- 3.De Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci USA 103: 1976–1981, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flatman PW Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta 1566: 140–151, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Flatman PW Cotransporters, WNKs and hypertension: important leads from the study of monogenetic disorders of blood pressure regulation. Clin Sci (Lond) 112: 203–216, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure and characterization of two members of the mammalian electroneutral sodium-(potassium)- chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 9.Gamba G Molecular physiology and pathophysiology of the electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Garzon-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vazquez N, Ponce-Coria J, Moreno E, Delpire E, Gamba G. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol 292: F1197–F1207, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Golbang AP, Cope G, Hamad A, Murthy M, Liu CH, Cuthbert AW, O'Shaughnessy KM. Regulation of the expression of the Na/Cl cotransporter (NCCT) by WNK4 and WNK1: evidence that accelerated dynamin-dependent endocytosis is not involved. Am J Physiol Renal Physiol 291: F1369–F1376, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl− cotransport: the SLC12 family. Pflügers Arch 447: 580–593, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kahle KT, Rinehart J, De Los HP, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl− in and out of cells: Implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci USA 102: 16783–16788, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahle KT, Rinehart J, Ring A, Gimenez I, Gamba G, Hebert SC, Lifton RP. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda ) 21: 326–335, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem 10: 341–354, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87: 3248–3254, 2002. [DOI] [PubMed] [Google Scholar]
- 19.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease. An integrated view. Hypertension 51: 588–596, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno E, San Cristobal P, Rivera M, Vazquez N, Bobadilla NA, Gamba G. Affinity defining domains in the Na-Cl cotransporter: different location for Cl− and thiazide binding. J Biol Chem 281: 17266–17275, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42685–42693, 2005. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol 14: 2447–2456, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco-Alvarez D, San Cristobal P, Meade P, Moreno E, Vazquez N, Munoz E, Diaz A, Juarez ME, Gimenez I, Gamba G. The Na-Cl cotransporter is activated and phosphorylated at the amino terminal domain upon intracellular chloride depletion. J Biol Chem 281: 28755–28763, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Peng JB, Warnock DG. WNK4-mediated regulation of renal ion transport proteins. Am J Physiol Renal Physiol 293: F961–F973, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Ponce-Coria J, San Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, De Los HP, Juarez P, Munoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA 105: 8458–8463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinehart J, Kahle KT, De Los HP, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA 102: 16777–16782, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon DB, Nelson-Williams C, Johnson-Bia M, Ellison D, Karet FE, Morey-Molina A, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitelman HJ, Lifton RP. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Song L, Mercado A, Vazquez N, Xie Q, Desai R, George AL, Gamba G, Mount DB. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res Mol Brain Res 103: 91–105, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Subramanya AR, Yang CL, Zhu X, Ellison DH. Dominant-negative regulation of WNK1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 290: F619–F624, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J 391: 17–24, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang HR, Liu Z, Huang CL. Domains of WNK1 kinase in the regulation of ROMK1. Am J Physiol Renal Physiol 295: F438–F445, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Xu BE, Min X, Stippec S, Lee BH, Goldsmith EJ, Cobb MH. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J Biol Chem 277: 48456–48462, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang CL, Ellison DH. WNK1 interacts physically with WNK4. J Am Soc Nephrol 14: 77A, 2003. [Google Scholar]
- 39.Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest 115: 1379–1387, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.