Abstract
The renal Na-K-2Cl cotransporter (NKCC2, BSC1) is selectively expressed in the apical membrane of cells of the thick ascending limb of the loop of Henle (TAL) and macula densa. NKCC2-dependent salt transport constitutes the major apical entry pathway for transepithelial salt reabsorption in the TAL. Although NKCC2 is encoded by a single gene (Slc12a1), differential splicing of the NKCC2 pre-mRNA results in the formation of several alternate transcripts. Thus three full-length splice isoforms of NKCC2 differ in their variable exon 4, resulting in transcripts for NKCC2B, NKCC2A, and NKCC2F. In addition to full-length isoforms, variants with truncated COOH-terminal ends have been described. The various splice isoforms of NKCC2 differ in their localization along the TAL and in their transport characteristics. Data in the literature are reviewed to assess the principles of NKCC2 differential splicing, the localization of NKCC2 splice isoforms along the TAL in various species, and the functional characteristics of the splice isoforms. In addition, we discuss the functional significance of NKCC2 isoforms for TAL salt retrieval and for the specific salt sensor function of macula densa cells based on studies using isoform-specific NKCC2-knockout mice. We suggest that different NKCC2 splice variants cooperate in salt retrieval along the TAL and that the coexpression of two splice variants (NKCC2B and NKCC2A) in the macula densa cells facilitates efficient salt sensing over wide ranges of fluctuating salt concentrations.
Keywords: differential splicing, macula densa, tubuloglomerular feedback, renin
the reabsorption of NaCl in the thick ascending limb of the loop of Henle (TAL) accounts for 20–25% of total renal salt retrieval, and it is the process that generates the energy for the urinary concentrating mechanism. NaCl uptake across the apical membrane of TAL cells is mediated to at least 80% by a cotransport process in which the influx of Na+ drives the uptake of Cl− and K+. At the molecular level, apical Na+/K+/2Cl− reabsorption is mediated by Na-K-2Cl cotransporter NKCC2 (also called BSC1 for bumetanide-sensitive cotransporter) (20, 26, 81). To achieve net salt absorption, apical electroneutral cotransport of Na+/K+/2Cl− (or Na+/NH4+/2Cl−; Refs. 20, 34) via NKCC2 is complemented by recycling of K+ via apical K+ channels (ROMK), efflux of Cl− via basolateral Cl− channels (21, 44), and basolateral extrusion of Na+ by Na+-K+-ATPase (Fig. 1). Since the reabsorptive capacity of downstream portions of the nephron is limited, inhibition of NKCC2 results in marked natriuresis and diuresis, making specific NKCC2 inhibitors like furosemide or bumetanide the most potent class of all diuretics.
Fig. 1.
NaCl reabsorption in the thick ascending limb (TAL). Salt reabsorption in the TAL involves apical electroneutral cotransport of Na+/K+/2Cl− via NKCC2, recycling of K+ via apical K+ channels (ROMK), efflux of Cl− via basolateral Cl− channels, and basolateral salt extrusion by Na+-K+-ATPase. Water permeability of the TAL is low; consequently, salt reabsorption results in successively more dilute ion concentrations in the tubular lumen.
Apart from its function in TAL salt retrieval, NKCC2-dependent transport activity constitutes the initial step in the tubulovascular signaling pathway that connects the macula densa cells in the juxtaglomerular region with the afferent arteriole (38, 61). By transforming an increase in luminal NaCl concentration into a constriction of the afferent arteriole and a subsequent decrease in single-nephron glomerular filtration rate (SNGFR), this connection acts as a negative feedback loop known as tubuloglomerular feedback (TGF) (78). Macula densa cells also control the secretion of renin from granular cells of the afferent arteriole, one of several control mechanisms that serve to regulate the activity of the renin-angiotensin system (70). The notion that NKCC2 plays a critical role in TGF- and macula densa-dependent renin secretion is founded on the profound inhibitory effect of loop diuretics on TGF and on the finding that renin secretion becomes independent of tubular luminal NaCl concentration in the presence of loop diuretics (40, 62, 79, 82).
Slc12a1, the gene encoding for NKCC2 (15, 29), gives rise to different NKCC2 transcripts derived from differential splicing (29, 51, 80, 83). Specifically, three full-length NKCC2 isoforms (NKCC2B, NKCC2A, and NKCC2F) and three additional isoforms with truncated COOH-terminal ends have been described (45, 54). The various splice isoforms of NKCC2 differ in their localization along the TAL and in their transport characteristics. In this review we discuss the principles of differential NKCC2 splicing, the localization of the splice isoforms along the TAL in various species, and the function of the splice isoforms in regard to NaCl transport and macula densa signaling.
Differential Splicing of NKCC2
Differential splicing of pre-mRNA is a widely observed and cell-specific process that allows for the generation of proteins far in excess of the limited number of genes. It has been estimated that >50% of all human primary transcripts undergo differential splicing, and one may assume that this is also the case for other mammalians (31). In addition to NKCC2, modifications by differential splicing are found in the transport proteins for phosphate (41, 74), sulfate (3), bicarbonate (1, 5, 8), organic cations (22, 85), inositol (37, 55, 56, 86), urea (11, 33, 65, 75, 84), and other inorganic ions (28, 46, 58). The function of many of these transporter isoforms derived from differential splicing has yet to be determined (for a comprehensive review see Ref. 52).
The existence of different isoforms of NKCC2 was first reported by Payne and Forbush (51) when they cloned NKCC2 from a rabbit kidney cDNA library. These isoforms, NKCC2B, NKCC2A, and NKCC2F, are derived from the differential splicing of the variable exon 4 of the Slc12a1 gene. Corresponding NKCC2 transcripts were later discovered in other species, specifically humans (80), rats (83), and mice (29). Exon 4 is a short 96-bp exon that encodes the second transmembrane domain and parts of the adjacent intracellular loop of the transporter (51). During differential splicing, exon 3 gets fused to exon 4B, exon 4A, or exon 4F. The three variants of exon 4 are then linked to exon 5. In addition to NKCC2B, NKCC2A, and NKCC2F, transcripts with a tandem exon 4B/4F (own unpublished data) and 4A/4F have been described, as shown in Fig. 2A (14, 29, 83). NKCC2A/F tandem proteins appear to exert a dominant-negative effect in the regulation of NKCC2 activity. Thus 86Rb+ and 22Na+ fluxes in oocytes have been shown to be reduced by 50% when both the A and A/F isoforms were coexpressed, compared with single NKCC2A expression (6).
Fig. 2.
A: the Slc12a1 gene and differential splicing of exon 4. During splicing of the pre-mRNA, variant B, A, or F of exon 4 is linked to exon 3. The 3 variants of exon 4 are then fused to exon 5, resulting in transcripts for NKCC2B, NKCC2A, and NKCC2F, respectively (A, B, C). In addition, tandem exons have been described consisting of a combination of exon 4B/F and 4A/F (D, E). B: gene targeting of NKCC2B and NKCC2A. A mutation consisting of the coding sequence for the FLAG peptide and in-frame stop codons followed by a floxed neomycin (neo) resistance expression cassette was introduced in exon 4B (top) or exon 4A (bottom) by homologous recombination in embryonic stem cells. The neomycin cassette was subsequently removed by crossing NKCC2B/neo and NKCC2A/neo mice with the Cre-expressing strain EIIa-Cre. After neo removal, correct splicing was preserved in NKCC2B- and NKCC2A-deficient mice. In NKCC2A-deficient mice, however, an additional aberrant transcript was present that incorporated genomic sequences upstream of exon 4A, the genomic sequence between exon 4A and 4F, and the full sequence of exon 4F.
In addition to the three full-length NKCC2 isoforms (1,095 amino acids), additional isoforms with truncated 3′-ends (770 amino acids) have been identified in the mouse. These alternative transcripts use a polyadenylation site in the intron between exons 16 and 17, resulting in a protein with a shorter COOH-terminal tail with an additional 55 alternate amino acids, which are not present in the full-length isoforms (45, 54). Splicing that results in the formation of a truncated transcript is independent of variable exon 4 splicing, resulting in at least six different isoforms. Heterologous expression studies in Xenopus laevis oocytes first suggested that the truncated isoforms of the murine NKCC2 were not functionally active (54). However, these isoforms had a dominant-negative effect on the full-length isoforms during coexpression in oocytes, indicating a possible regulatory function (54). This dominant-negative effect was prevented after activation of protein kinase A (54). Precedence for the dominant-negative effect of splice variants exists in the literature, and it has been shown, for example, for a splice variant of the long-QT syndrome K+ channel (9). A later study revealed that a reduction in osmolarity (to ∼100 mosM) is necessary to activate the short isoforms of NKCC2 and initiate a K+-independent NaCl cotransport that could be inhibited by protein kinase A activation (52). Despite alteration of ion transport from a Na+/K+/2Cl− to a Na+/Cl− mode (71), transport activity of the truncated NKCC2 isoforms remained loop diuretic sensitive (52). Thus, under hyposmotic conditions and low vasopressin levels and, by inference, low protein kinase A activity, NKCC2 operates in Na+/Cl− mode, resulting in an estimated reduction of overall salt reabsorptive capacity of the TAL by ∼50% compared with antidiuresis (24, 25, 71). It remains to be determined whether similar short NKCC2 isoforms exist in other species and what the exact in vivo function of these isoforms might be.
Localization of NKCC2 Isoforms Along the TAL
Despite certain interspecies differences, the renal localization of the NKCC2 full-length isoforms follows a general pattern. NKCC2 protein is found in the apical membrane and in subapical vesicles along the TAL and in the macula densa segment (32, 47). All localization studies rely on the detection of the respective NKCC2 isoform mRNA transcripts since isoform-specific antibodies are not available. The localization of NKCC2 isoforms was first addressed in the rabbit by isoform-specific Northern blotting (51). NKCC2F transcripts were exclusively found in the renal medulla, NKCC2A transcripts were observed in both the medulla and cortex, and NKCC2B transcripts were detected in the cortex (51). Similarly, in microdissected segments of the rat TAL, NKCC2F was detected by isoform-specific RT-PCR in the medullary TAL, NKCC2A in both the medullary and cortical TAL, and NKCC2B in the cortical TAL including the macula densa-containing segment (83). In mice, as determined by isoform-specific in situ hybridization, NKCC2F is localized to the inner (and in lower density in the outer) stripe of the outer medulla, NKCC2A is observed in the outer stripe of the outer medulla and the cortical TAL, and NKCC2B is observed in the cortical TAL, where its expression overlaps with NKCC2A (29, 49). A detailed investigation of the mouse macula densa segment of the TAL revealed expression of both the B and A isoforms (49). The percent contribution of single isoforms to the total number of NKCC2 transporters in the mouse TAL, as judged from the levels of their respective transcripts, is in the range of 70:20:10 for NKCC2F, NKCC2A, and NKCC2B, respectively (own unpublished data determined by isoform-specific RNase protection assays).1 For humans, no data are available regarding the exact localization of the NKCC2 isoforms along the TAL; however, it appears likely that the expression pattern of the B, A, and F isoforms of NKCC2 in the human kidney does not differ markedly from that in other mammalian species (80).
Less information is available with respect to the localization of the truncated NKCC2 isoforms. Mount et al. (45) used an immunohistochemical approach using an antibody to specifically detect the unique COOH-terminal ends of the truncated isoforms of NKCC2. The truncated NKCC2 proteins were found to colocalize with their full-length counterparts along the TAL, but in contrast to the full-length protein not all TAL cells stained for the truncated isoforms, with the percentage of positive TAL cells declining from the medullary toward the cortical portions of the TAL. Also, the short NKCC2 isoforms appeared to be in some cases localized to the apical membrane of the tubular cells but were frequently observed intracellularly. This raises the possibility that the dominant-negative effect of truncated isoforms on NKCC2 transport activity may be related to the intracellular formation of dimers/multimers between truncated and full-length isoforms, rendering the latter nonfunctional (45). This possible regulatory mechanism of NKCC2 activity appears to be more relevant in medullary compared with cortical portions of the TAL, which would be in line with the pivotal role of the renal medulla in urine concentrating ability.
Functional Characteristics of NKCC2 Isoforms: Expression Studies
Most of our knowledge regarding the transport characteristics of the NKCC2 isoforms has been derived from in vitro heterologous expression studies using the Xenopus laevis oocyte system. Flux studies after injection of cRNAs of the various full-length isoforms of NKCC2 have shown that the B, A, and F isoforms differ markedly in their ion affinities. For rabbit NKCC2, Km values of NKCC2B, NKCC2A, and NKCC2F were reported to be 21, 16, and 67 mM for Na+, 0.9, 0.8, and 2.9 mM for K+, and 9, 45, and 111 mM for Cl−, respectively (17). A similar apparent affinity pattern was reported in a second study using a similar approach, although for unknown reasons some results differed considerably compared with the preceding study, with Km of NKCC2A for Na+ being <10 mM and for Cl− being ∼15 mM (14). For the murine NKCC2, Plata et al. (53) determined Km values of NKCC2B, NKCC2A, and NKCC2F of 3, 5, and 21 mM for Na+, 0.8, 1.0, and 1.5 mM for K+, and 12, 22, and 29 mM for Cl−, respectively. Thus, despite some variability between different studies and species, the order of apparent affinities for Cl− as the transport-limiting ion in the TAL tubular fluid is NKCC2B > NKCC2A > NKCC2F.
Site-directed mutagenesis has shown that the differences in ion affinities do in fact reside in the alternatively spliced exon 4 that encodes the second transmembrane domain and parts of the adjacent intracellular loop of the transporter (51). Replacing as little as six amino acids of which three were located in the second transmembrane domain and three were situated in the adjacent intracellular loop changed the apparent affinity of the B isoform for Na+ and Cl− into the ion affinities of the F isoform (16). Similarly, changing six amino acid residues altered the transport characteristics of NKCC2B into those of NKCC2A (16). Similar results were obtained from studies using chimeras of the shark NKCC2A and NKCC2F (12). Furthermore, the introduction of point mutations in exon 4 resulted in marked changes of cation affinities (13), emphasizing the key role of the second transmembrane domain and parts of the following intracellular loop in modulating the transport characteristics of various NKCC2 isoforms.
Evidence that the Cl− affinity of the loop diuretic-sensitive salt transport in the TAL differs in cells from medullary compared with cortical TAL segments preceded the discovery of NKCC2 isoforms (19, 36). The data regarding the transport characteristics of the NKCC2 isoforms, in combination with their localization along the TAL, are congruent with earlier functional studies using the isolated perfused tubule technique. In the isolated perfused TAL, salt transport occurred more rapidly in medullary TAL relative to cortical TAL segments, consistent with the presence of high levels of the low-affinity F isoform in the medullary TAL. Cortical TAL segments, conversely, had a greater diluting power, which is congruent with the presence of high-affinity NKCC2B (7, 59).
Functional Characteristics of NKCC2 Isoforms: Deletion Studies
Complete NKCC2 deletion.
Loss of NKCC2 function is associated with a severe salt-losing nephropathy in humans called type 1 Bartter syndrome (68). Type 1 Bartter syndrome is characterized by the inability of patients to generate concentrated urine. Symptoms include the development of antenatal polyhydramnios (48, 66), salt and volume loss after birth accompanied by hypokalemic alkalosis (2), hypercalciuria (43), excessive prostanoid formation (10, 64), and hyperreninemia (18, 63). Patients, especially infants, suffer from dehydration and low blood pressure (4) and are affected to various degrees by muscle weakness, fever, tetany, seizures, and growth and mental retardation (27, 30, 42, 69).
A recently generated mouse model with inactivation of NKCC2 mirrors its human counterpart in most critical aspects (73). The majority of homozygous animals died within the first 2 wk of life. Suppression of the excessive prostanoid formation with the cyclooxygenase inhibitor indomethacin increased the survival rate, but survivors were growth retarded and developed progressive hydronephrosis (73). Conversely, heterozygous NKCC2+/− mice showed no gross phenotype despite a 50% reduction in NKCC2 mRNA levels (72). Analysis of NKCC2 protein levels revealed that posttranscriptional mechanisms fully compensated for the reduced mRNA expression and restored NKCC2 protein to wild-type levels (72). Since NKCC2 mRNA levels in this model apparently do not reflect protein levels, one may speculate that the contribution of NKCC2 transcriptional regulation (i.e., modulation of NKCC2 promoter activity and mRNA stability) to the overall setting of NKCC2 activity is limited.
Isoform-specific deletion.
To generate mice with targeted disruption of single NKCC2 isoforms, the alternate exons 4B or 4A were altered by the introduction of in-frame stop codons resulting in the premature termination of translation. Thus these strains of isoform-specific knockout mice lack both the full-length and the corresponding truncated NKCC2 isoforms (49, 50). After removal of the neomycin selection marker by Cre recombinase, the modification of exon 4B was found not to perturb subsequent splicing, resulting in a correct B isoform transcript including the mutation (Fig. 2B). Mutation of exon 4A, on the other hand, yielded a second transcript resulting from incorrect splicing in addition to producing a correctly spliced transcript (Fig. 2B).2 The phenotype of mice with specific inactivation of NKCC2B or NKCC2A was relatively mild (49, 50). No gross abnormalities and no reduced survival rates were observed, and ambient urine osmolarity and concentrating ability in NKCC2B- or NKCC2A-deficient mice were only slightly reduced compared with wild-type control mice. These data indicate that the contribution of NKCC2B and NKCC2A to overall salt retrieval is minor, or that it can be fully compensated by increased reabsorption in more distal portions of the nephron. As mentioned above, results from isoform-specific RNase protection assays indicate that NKCC2B, NKCC2A, and NKCC2F transcripts in the mouse account for 10%, 20%, and 70% of total NKCC2 transcripts, respectively (own unpublished data). Thus the presence of the dominant medullary F isoform in combination with either the A or the B isoform appears to be sufficient to prevent major electrolyte disturbances.
The mild phenotype of mice with specific loss of NKCC2B (and similarly with loss of NKCC2A) resembles rare cases of human patients with unusually mild manifestations of type 1 Bartter syndrome (80). A sequence analysis of NKCC2 in one of these patients revealed a missense mutation in exon 4B (80), rendering NKCC2B nonfunctional as determined in vitro (16). One may speculate that mutations in single isoforms of NKCC2 with a concomitant mild salt-losing phenotype may be erroneously diagnosed as type 3 Bartter syndrome (mutations in the basolateral Cl− channel clcnkb; Ref. 67).
NKCC2A- and NKCC2B-deficient mice were used to assess the contribution of NKCC2B and NKCC2A to net Cl− reabsorption along the loop of Henle. To determine loop Cl− reabsorption in vivo, loops of Henle were perfused from the late proximal tubule and fluid samples were collected by micropuncture from the early distal convoluted tubule (57). At low perfusion flow rates (and by inference low Cl− concentrations in downstream portions of the TAL), Cl− reabsorption was significantly reduced in NKCC2B-deficient mice compared with wild-type mice, whereas no differences were observed for NKCC2A-deficient mice (50). Conversely, the lack of NKCC2A resulted in reduced Cl− absorption at high perfusion flow. These in vivo data are in line with the in vitro experiments indicating that NKCC2B is a high-affinity and NKCC2A a lower-affinity/higher-capacity isoform. Apparently, under conditions of low Cl− load (low perfusion flow rate), TAL reabsorption becomes dependent on the activity of the high-Cl−-affinity NKCC2B isoform. Conversely, the loss of NKCC2A becomes obvious under conditions of high loop perfusion flow (high Cl− concentration), indicating that Cl− concentrations under these conditions engage the transport activity of the lower-affinity NKCC2A. These data also suggest that NKCC2A transport capacity exceeds that of NKCC2B, which might be related to both the higher transport capacity of NKCC2A per se and its higher expression levels.
Considering the marked differences in transport characteristics of the various NKCC2 isoforms, it is conceivable that the ratio of isoform expression may not be stable, but that a possible modulation of differential splicing of the cotransporter may offer a measure for adaptations to changes in reabsorptive needs. Congruent with this concept, Brunet et al. (6) demonstrated that chronic water loading of rats resulted in a decrease in F and A/F isoform expression levels, whereas the abundance of the A transcript remained unaltered. During furosemide administration, the expression levels of the A isoform increased whereas those of the F and A/F isoforms decreased (6). The mechanism of this regulation was not addressed experimentally, but the authors speculated that intracellular Cl− concentrations may influence the splicing machinery; in this context, intracellular Cl− has been shown to inversely influence NKCC transport activity (23, 60).
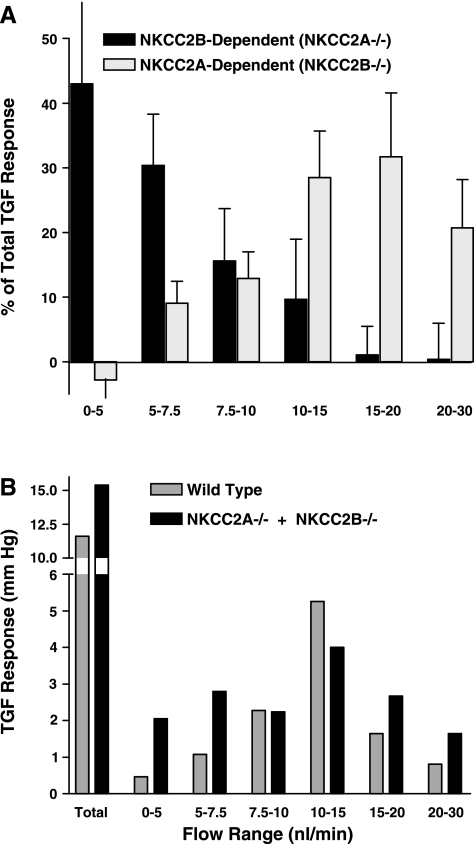
The vascular response caused by the TGF mechanism is one of the end points of the juxtaglomerular signaling pathway. TGF responses as determined by stop-flow pressure (PSF) measurements in the proximal tubule as a measure of glomerular capillary pressure and, by inference, preglomerular resistance revealed distinct abnormalities in NKCC2B- and NKCC2A-deficient mice relative to wild-type mice (49, 50). In NKCC2B-deficient mice, TGF response curves were right-shifted compared with wild-type mice, whereas a left shift of the TGF curve and a reduction of the maximum response magnitude was observed in NKCC2A-deficient animals (49, 50). These results imply that there is a marked redistribution in the fractional TGF responses that are elicited by increasing flow rates. As shown in Fig. 3, almost 75% of the total TGF response attributable to NKCC2B occurs in the flow range <7.5 nl/min while NKCC2A contributes very little to TGF at these low flows. In contrast, NKCC2A carries most of the TGF response at flow rates >7.5 nl/min. These data suggest the successive cooperation of NKCC2B (high Cl− affinity) and NKCC2A (low Cl− affinity) in their salt-sensing function when NaCl concentrations at the macula densa are experimentally altered within the TGF-relevant Cl− concentration range (62).
Fig. 3.
A: relative contribution of NKCC2A and NKCC2B isoforms to the tubuloglomerular feedback (TGF) response. Relative response magnitudes for the flow ranges indicated along the x-axis are expressed as % of the total stop-flow pressure (PSF) response (NKCC2A−/−: 100% = 7.1 ± 1 mmHg; NKCC2B−/−: 100% = 8.4 mmHg). Data are calculated from Refs. 49 and 50. B: TGF responses in wild-type mice compared with the sum of TGF responses in NKCC2B−/− and NKCC2A−/− mice. Response magnitudes for the flow ranges indicated along the x-axis are expressed as changes in PSF (mmHg). The enhanced responses in the low flow ranges (0–5 and 5–7.5 nl/min) are most likely due to the increased expression of the B isoform in NKCC2A−/− mice (50).
Further investigations may be necessary to detail whether the expression levels of either NKCC2B or NKCC2A in the macula densa may be altered under conditions of long-term changes in salt delivery and therefore may be involved in the “resetting” of macula densa function that occurs under situations of prolonged exposure of the macula densa cells to high (or low) salt concentrations (76, 77). Thus coexpression of the B and A isoforms of NKCC2 in the macula densa and their cooperation in salt reabsorption appear to allow efficient salt sensing to occur over a wide range of fluctuating tubular Cl− concentrations. The presence of two NKCC2 isoforms in the macula densa that perform the cell-specific task of salt sensing in different concentration ranges may serve as an example for how the functional capacity of proteins can be expanded and fine-tuned by means of differential splicing.
Macula Densa Control of Renin Secretion
The functional significance of macula densa NKCC2 isoforms for the regulation of renin secretion, the other end point of macula densa signaling, was assessed by examining the effect of an acute intravenous infusion of isotonic saline. This experimental maneuver has been shown to increase distal tubular NaCl concentration and thereby to result in a suppression of renin secretion (35, 39). In NKCC2B-deficient mice, saline administration caused a reduction in plasma renin concentration by 64%, slightly greater than the 45% reduction seen in wild-type mice (Fig. 4). Thus the presence of the remaining low-affinity isoform, NKCC2A, in the macula densa is sufficient to mediate suppression of renin secretion in response to increased salt concentration (49). In contrast, the renin-inhibitory response of saline was nearly absent in NKCC2A−/− mice, consistent with the notion that NKCC2A-dependent Cl− transport activity initiates macula densa-dependent regulation of renin secretion in the high NaCl concentration range.
Fig. 4.
NKCC2 isoforms and macula densa control of renin secretion. Suppression of plasma renin concentration (PRC) after acute salt loading by saline infusion (data are shown as % change compared with baseline). A reduction in PRC after salt loading is significantly more pronounced in NKCC2B-deficient mice compared with control mice, whereas the suppression of renin secretion is markedly reduced in NKCC2A-deficient mice. Combined from Refs. 49 and 50.
Summary
Differential splicing of pre-mRNAs has presumably evolved to permit an expansion of protein diversity and to overcome functional limitations imposed by the relatively small number of genes in the genome. This also applies to NKCC2 as the major salt transport protein in the TAL of the kidney. Differential splicing of NKCC2 pre-mRNA results in the formation of three full-length and three truncated isoforms. The corresponding transport proteins differ in their localization along the TAL, and heterologous expression studies showed that these NKCC2 isoforms also differ markedly in their in vitro transport characteristics. Furthermore, mouse strains with specific functional deletion of single splice variants revealed the in vivo significance of NKCC2 splicing. Thus various NKCC2 isoforms appear to cooperate in TAL salt retrieval and thereby facilitate efficient salt reabsorption despite spatially and temporally highly variable ion concentrations. The same applies to the macula densa cell-specific function of salt sensing, which initiates subsequent signaling to influence preglomerular resistance and renin secretion. Thus the coexpression of NKCC2B and NKCC2A (with different ion affinities) enables macula densa cells to serve as efficient salt sensors over a wide range of fluctuating tubular Cl− concentrations.
GRANTS
The work in the laboratories of the authors was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 699/A4 to H. Castrop) and by the intramural program of National Institute of Diabetes and Digestive and Kidney Diseases (J. Schnermann).
Footnotes
For the quantification of murine NKCC2 isoforms, isoform-specific probes were generated for RNase protection assay. In brief, isoform-specific cDNA fragments consisting of exon 4 and parts of exon 5 were cloned into vector pSP73 (Promega, Madison, WI). Antisense transcripts were generated by in vitro transcription and were labeled with [32P]GTP. Antisense transcripts were then hybridized to total kidney RNA, digested with a mixture of RNase A and T1, and separated on an 8% polyacrylamide gel. Protected fragments were visualized and 32P incorporation was measured by a phosphorimager. Expression of β-actin was determined for normalization. Relative expression levels of NKCC2F, NKCC2A, and NKCC2B were 158.3 ± 22, 42.8 ± 8, and 21.9 ± 5 cpm/μg RNA, respectively (n = 5).
We have attempted to generate NKCC2F-deficient mice with the same general approach. However, in two attempts so far we have not been able to obtain recombination in embryonic stem cells or to achieve germ line transmission.
REFERENCES
- 1.Abuladze N, Song M, Pushkin A, Newman D, Lee I, Nicholas S, Kurtz I. Structural organization of the human NBC1 gene: kNBC1 is transcribed from an alternative promoter in intron 3. Gene 251: 109–122, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bartter FC, Pronove P, Gill JR Jr, Maccardle RC. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33: 811–828, 1962. [DOI] [PubMed] [Google Scholar]
- 3.Beck L, Markovich D. The mouse Na+-sulfate cotransporter gene Nas1. Cloning, tissue distribution, gene structure, chromosomal assignment, and transcriptional regulation by vitamin D. J Biol Chem 275: 11880–11890, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bettinelli A, Bianchetti MG, Girardin E, Caringella A, Cecconi M, Appiani AC, Pavanello L, Gastaldi R, Isimbaldi C, Lama G. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr 120: 38–43, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–C1211, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Brunet GM, Gagnon E, Simard CF, Daigle ND, Caron L, Noel M, Lefoll MH, Bergeron MJ, Isenring P. Novel insights regarding the operational characteristics and teleological purpose of the renal Na+-K+-Cl2 cotransporter (NKCC2s) splice variants. J Gen Physiol 126: 325–337, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burg MB Thick ascending limb of Henle's loop. Kidney Int 22: 454–464, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3− cotransporter. J Biol Chem 272: 19111–19114, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Demolombe S, Baro I, Pereon Y, Bliek J, Mohammad-Panah R, Pollard H, Morid S, Mannens M, Wilde A, Barhanin J, Charpentier F, Escande D. A dominant negative isoform of the long QT syndrome 1 gene product. J Biol Chem 273: 6837–6843, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Dunn MJ Prostaglandins and Bartter's syndrome. Kidney Int 19: 86–102, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Fenton RA, Cottingham CA, Stewart GS, Howorth A, Hewitt JA, Smith CP. Structure and characterization of the mouse UT-A gene (Slc14a2). Am J Physiol Renal Physiol 282: F630–F638, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gagnon E, Bergeron MJ, Brunet GM, Daigle ND, Simard CF, Isenring P. Molecular mechanisms of Cl− transport by the renal Na+-K+-Cl− cotransporter. Identification of an intracellular locus that may form part of a high affinity Cl−-binding site. J Biol Chem 279: 5648–5654, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon E, Bergeron MJ, Daigle ND, Lefoll MH, Isenring P. Molecular mechanisms of cation transport by the renal Na+-K+-Cl− cotransporter: structural insight into the operating characteristics of the ion transport sites. J Biol Chem 280: 32555–32563, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon E, Forbush B, Caron L, Isenring P. Functional comparison of renal Na-K-Cl cotransporters between distant species. Am J Physiol Cell Physiol 284: C365–C370, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA, Hebert SC. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994. [PubMed] [Google Scholar]
- 16.Gimenez I, Forbush B. The residues determining differences in ion affinities among the alternative splice variants F, A, and B of the mammalian renal Na-K-Cl cotransporter (NKCC2). J Biol Chem 282: 6540–6547, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gimenez I, Isenring P, Forbush B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277: 8767–8770, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Goodman AD, Vagnucci AH, Hartroft PM. Pathogenesis of Bartter's syndrome. N Engl J Med 281: 1435–1439, 1969. [DOI] [PubMed] [Google Scholar]
- 19.Greger R Coupled transport of Na+ and Cl− in the thick ascending limb of Henle's loop of rabbit nephron. Scand Audiol Suppl 14: 1–15, 1981. [PubMed] [Google Scholar]
- 20.Greger R Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Greger R, Bleich M, Schlatter E. Ion channels in the thick ascending limb of Henle's loop. Renal Physiol Biochem 13: 37–50, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature 372: 549–552, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Forbush B 3rd. The Na-K-Cl cotransporters. J Bioenerg Biomembr 30: 161–172, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hebert SC, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle. II. Determinants of the ADH-mediated increases in transepithelial voltage and in net Cl− absorption. J Membr Biol 80: 221–233, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Hebert SC, Friedman PA, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle. I. ADH increases transcellular conductance pathways. J Membr Biol 80: 201–219, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Hebert SC, Reeves WB, Molony DA, Andreoli TE. The medullary thick limb: function and modulation of the single-effect multiplier. Kidney Int 31: 580–589, 1987. [DOI] [PubMed] [Google Scholar]
- 27.Hetzel W, Molitor H. [Paralysis, pain syndrome, consciousness disorders: on the neurologic manifestations of Bartter syndrome]. Nervenarzt 62: 500–505, 1991. [PubMed] [Google Scholar]
- 28.Hiki K, D'Andrea RJ, Furze J, Crawford J, Woollatt E, Sutherland GR, Vadas MA, Gamble JR. Cloning, characterization, and chromosomal location of a novel human K+-Cl− cotransporter. J Biol Chem 274: 10661–10667, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi P, Vanden Heuvel GB, Payne JA, Forbush B 3rd. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am J Physiol Renal Fluid Electrolyte Physiol 269: F405–F418, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Iwata F, Hanawa Y, Takashima H. Chronic hypomagnesemia and hypokalemia due to renal wasting in siblings. Acta Paediatr Jpn 35: 252–257, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Kan Z, Rouchka EC, Gish WR, States DJ. Gene structure prediction and alternative splicing analysis using genomically aligned ESTs. Genome Res 11: 889–900, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan MR, Plotkin MD, Lee WS, Xu ZC, Lytton J, Hebert SC. Apical localization of the Na-K-Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int 49: 40–47, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol 10: 230–237, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kim GH, Ecelbarger C, Knepper MA, Packer RK. Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935–942, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol 290: F1016–F1023, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Koenig B, Ricapito S, Kinne R. Chloride transport in the thick ascending limb of Henle's loop: potassium dependence and stoichiometry of the NaCl cotransport system in plasma membrane vesicles. Pflügers Arch 399: 173–179, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, Burg MB, Handler JS. Cloning of the cDNA for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem 267: 6297–6301, 1992. [PubMed] [Google Scholar]
- 38.Lapointe JY, Bell PD, Cardinal J. Direct evidence for apical Na+:2Cl−:K+ cotransport in macula densa cells. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1466–F1469, 1990. [DOI] [PubMed] [Google Scholar]
- 39.Lorenz JN, Kotchen TA, Ott CE. Effect of Na and Cl infusion on loop function and plasma renin activity in rats. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1328–F1335, 1990. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz JN, Weihprecht H, Schnermann J, Skott O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol Renal Fluid Electrolyte Physiol 260: F486–F493, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci USA 90: 5979–5983, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marco-Franco JE, Morey A, Ventura C, Gasco JM, Alarcon A. Long-term evolution and growth patterns in a family with Bartter's syndrome. Clin Nephrol 42: 33–37, 1994. [PubMed] [Google Scholar]
- 43.McCredie DA, Rotenberg E, Williams AL. Hypercalciuria in potassium-losing nephropathy: a variant of Bartter's syndrome. Aust Paediatr J 10: 286–295, 1974. [DOI] [PubMed] [Google Scholar]
- 44.Molony DA, Reeves WB, Andreoli TE. Na+:K+:2Cl− cotransport and the thick ascending limb. Kidney Int 36: 418–426, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Mount DB, Baekgaard A, Hall AE, Plata C, Xu J, Beier DR, Gamba G, Hebert SC. Isoforms of the Na-K-2Cl cotransporter in murine TAL. I. Molecular characterization and intrarenal localization. Am J Physiol Renal Physiol 276: F347–F358, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Mount DB, Mercado A, Song L, Xu J, George AL Jr, Delpire E, Gamba G. Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J Biol Chem 274: 16355–16362, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol 275: F885–F893, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Ohlsson A, Sieck U, Cumming W, Akhtar M, Serenius F. A variant of Bartter's syndrome. Bartter's syndrome associated with hydramnios, prematurity, hypercalciuria and nephrocalcinosis. Acta Paediatr Scand 73: 868–874, 1984. [DOI] [PubMed] [Google Scholar]
- 49.Oppermann M, Mizel D, Huang G, Li C, Deng C, Theilig F, Bachmann S, Briggs J, Schnermann J, Castrop H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J Am Soc Nephrol 17: 2143–2152, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Oppermann M, Mizel D, Kim SM, Chen L, Faulhaber-Walter R, Huang Y, Li C, Deng C, Briggs J, Schnermann J, Castrop H. Renal function in mice with targeted disruption of the A isoform of the Na-K-2Cl co-transporter. J Am Soc Nephrol 18: 440–448, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Payne JA, Forbush B 3rd. Alternatively spliced isoforms of the putative renal Na-K-Cl cotransporter are differentially distributed within the rabbit kidney. Proc Natl Acad Sci USA 91: 4544–4548, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plata C, Meade P, Hall A, Welch RC, Vazquez N, Hebert SC, Gamba G. Alternatively spliced isoform of apical Na+-K+-Cl− cotransporter gene encodes a furosemide-sensitive Na+-Cl− cotransporter. Am J Physiol Renal Physiol 280: F574–F582, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Plata C, Meade P, Vazquez N, Hebert SC, Gamba G. Functional properties of the apical Na+-K+-2Cl− cotransporter isoforms. J Biol Chem 277: 11004–11012, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G. Isoforms of the Na-K-2Cl cotransporter in murine TAL. II. Functional characterization and activation by cAMP. Am J Physiol Renal Physiol 276: F359–F366, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Porcellati F, Hlaing T, Togawa M, Stevens MJ, Larkin DD, Hosaka Y, Glover TW, Henry DN, Greene DA, Killen PD. Human Na+-myo-inositol cotransporter gene: alternate splicing generates diverse transcripts. Am J Physiol Cell Physiol 274: C1215–C1225, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Porcellati F, Hosaka Y, Hlaing T, Togawa M, Larkin DD, Karihaloo A, Stevens MJ, Killen PD, Greene DA. Alternate splicing in human Na+-MI cotransporter gene yields differentially regulated transport isoforms. Am J Physiol Cell Physiol 276: C1325–C1337, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Ramsey JA, Brown RHJ, Croghan PC. Electrometric titration of chloride in small volumes. J Exp Biol 32: 822, 1955. [Google Scholar]
- 58.Randall J, Thorne T, Delpire E. Partial cloning and characterization of Slc12a2: the gene encoding the secretory Na+-K+-2Cl− cotransporter. Am J Physiol Cell Physiol 273: C1267–C1277, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Rocha AS, Kokko JP. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest 52: 612–623, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell JM Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Schlatter E, Salomonsson M, Persson AE, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+2Cl−K+ cotransport. Pflügers Arch 414: 286–290, 1989. [DOI] [PubMed] [Google Scholar]
- 62.Schnermann J, Briggs J. Concentration-dependent sodium chloride transport as the signal in feedback control of glomerular filtration rate. Kidney Int Suppl 12: S82–S89, 1982. [PubMed] [Google Scholar]
- 63.Seyberth HW, Koniger SJ, Rascher W, Kuhl PG, Schweer H. Role of prostaglandins in hyperprostaglandin E syndrome and in selected renal tubular disorders. Pediatr Nephrol 1: 491–497, 1987. [DOI] [PubMed] [Google Scholar]
- 64.Seyberth HW, Rascher W, Schweer H, Kuhl PG, Mehls O, Scharer K. Congenital hypokalemia with hypercalciuria in preterm infants: a hyperprostaglandinuric tubular syndrome different from Bartter syndrome. J Pediatr 107: 694–701, 1985. [DOI] [PubMed] [Google Scholar]
- 65.Shayakul C, Steel A, Hediger MA. Molecular cloning and characterization of the vasopressin-regulated urea transporter of rat kidney collecting ducts. J Clin Invest 98: 2580–2587, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sieck UV, Ohlsson A. Fetal polyuria and hydramnios associated with Bartter's syndrome. Obstet Gynecol 63: 22S–24S, 1984. [PubMed] [Google Scholar]
- 67.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Simopoulos AP Growth characteristics in patients with Bartter's syndrome. Nephron 23: 130–135, 1979. [DOI] [PubMed] [Google Scholar]
- 70.Skott O, Briggs JP. Direct demonstration of macula densa-mediated renin secretion. Science 237: 1618–1620, 1987. [DOI] [PubMed] [Google Scholar]
- 71.Sun A, Grossman EB, Lombardi M, Hebert SC. Vasopressin alters the mechanism of apical Cl− entry from Na+:Cl− to Na+:K+:2Cl− cotransport in mouse medullary thick ascending limb. J Membr Biol 120: 83–94, 1991. [DOI] [PubMed] [Google Scholar]
- 72.Takahashi N, Brooks HL, Wade JB, Liu W, Kondo Y, Ito S, Knepper MA, Smithies O. Posttranscriptional compensation for heterozygous disruption of the kidney-specific NaK2Cl cotransporter gene. J Am Soc Nephrol 13: 604–610, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O. Uncompensated polyuria in a mouse model of Bartter's syndrome. Proc Natl Acad Sci USA 97: 5434–5439, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tatsumi S, Miyamoto K, Kouda T, Motonaga K, Katai K, Ohkido I, Morita K, Segawa H, Tani Y, Yamamoto H, Taketani Y, Takeda E. Identification of three isoforms for the Na+-dependent phosphate cotransporter (NaPi-2) in rat kidney. J Biol Chem 273: 28568–28575, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Terris JM, Knepper MA, Wade JB. UT-A3: localization and characterization of an additional urea transporter isoform in the IMCD. Am J Physiol Renal Physiol 280: F325–F332, 2001. [DOI] [PubMed] [Google Scholar]
- 76.Thomson SC, Bachmann S, Bostanjoglo M, Ecelbarger CA, Peterson OW, Schwartz D, Bao D, Blantz RC. Temporal adjustment of the juxtaglomerular apparatus during sustained inhibition of proximal reabsorption. J Clin Invest 104: 1149–1158, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomson SC, Blantz RC, Vallon V. Increased tubular flow induces resetting of tubuloglomerular feedback in euvolemic rats. Am J Physiol Renal Fluid Electrolyte Physiol 270: F461–F468, 1996. [DOI] [PubMed] [Google Scholar]
- 78.Thurau K, Schnermann J. [The sodium concentration in the macula densa cells as a regulating factor for glomerular filtration (micropuncture experiments)]. Klin Wochenschr 43: 410–413, 1965. [DOI] [PubMed] [Google Scholar]
- 79.Vander AJ, Carlson J. Mechanism of the effects of furosemide on renin secretion in anesthetized dogs. Circ Res 25: 145–152, 1969. [DOI] [PubMed] [Google Scholar]
- 80.Vargas-Poussou R, Feldmann D, Vollmer M, Konrad M, Kelly L, van den Heuvel LP, Tebourbi L, Brandis M, Karolyi L, Hebert SC, Lemmink HH, Deschenes G, Hildebrandt F, Seyberth HW, Guay-Woodford LM, Knoers NV, Antignac C. Novel molecular variants of the Na-K-2Cl cotransporter gene are responsible for antenatal Bartter syndrome. Am J Hum Genet 62: 1332–1340, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winters CJ, Reeves WB, Andreoli TE. A survey of transport properties of the thick ascending limb. Semin Nephrol 11: 236–247, 1991. [PubMed] [Google Scholar]
- 82.Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest 53: 1695–1708, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang T, Huang YG, Singh I, Schnermann J, Briggs JP. Localization of bumetanide- and thiazide-sensitive Na-K-Cl cotransporters along the rat nephron. Am J Physiol Renal Fluid Electrolyte Physiol 271: F931–F939, 1996. [DOI] [PubMed] [Google Scholar]
- 84.You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature 365: 844–847, 1993. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Dresser MJ, Chun JK, Babbitt PC, Giacomini KM. Cloning and functional characterization of a rat renal organic cation transporter isoform (rOCT1A). J Biol Chem 272: 16548–16554, 1997. [DOI] [PubMed] [Google Scholar]
- 86.Zhou C, Agarwal N, Cammarata PR. Osmoregulatory alterations in myo-inositol uptake by bovine lens epithelial cells. 2. Cloning of a 626 bp cDNA portion of a Na+/myo-inositol cotransporter, an osmotic shock protein. Invest Ophthalmol Vis Sci 35: 1236–1242, 1994. [PubMed] [Google Scholar]