Abstract
Most of the transepithelial transport of sodium in proximal tubules occurs through the coordinated action of the apical sodium/proton exchanger and the basolateral Na-K-ATPase. Hormones that regulate proximal tubule sodium excretion regulate the activities of these proteins. We have previously demonstrated that the level of intracellular sodium concentration modulates the regulation of Na-K-ATPase activity by angiotensin II and dopamine. An increase of a few millimolars in intracellular sodium concentration leads to increased Na-K-ATPase activity without a statistically significant increase in the number of plasma membrane Na-K-ATPase molecules, as determined by cell surface protein biotinylation. Using total internal reflection fluorescence, we detected an increased number of Na-K-ATPase molecules in cytosolic compartments adjacent to the plasma membrane, suggesting that the increased intracellular sodium concentration induces a movement of Na-K-ATPase molecules toward the plasma membrane. While intracellular compartments containing Na-K-ATPase molecules are very close to the plasma membrane, compartments containing type 1 dopamine receptors (D1Rs) are distributed in different parts of the cell cytosol. Fluorescence determinations indicate that an increased intracellular sodium concentration induces the increased colocalization of dopamine receptors with Na-K-ATPase molecules in the region of the plasma membrane. We propose that under in vivo conditions, in response to a sodium load in the lumen of proximal tubules, an increased level of intracellular sodium in epithelial cells is an early event that triggers the cellular response that leads to dopamine inhibition of proximal tubule sodium reabsorption.
Keywords: renal sodium excretion, renal sodium reabsorption, renal sodium regulation, dopamine, angiotensin II, monensin, TIRF, epifluorescence, GFP
in renal proximal tubule epithelia, locally produced dopamine modulates sodium excretion via regulation of the apical Na/H exchanger and basolateral Na-K-ATPase molecules (1, 21, 26, 33). The effect of dopamine is associated with a cellular redistribution of these transporters that results in a reduced capacity of proximal tubule cells to transport sodium (25, 36, 37, 51). We have previously demonstrated that dopamine regulates Na-K-ATPase activity by endocytosis of Na-K-ATPase molecules (12, 14, 20, 37). Thus the action of this hormone results in the shuttling of Na-K-ATPase molecules between the plasma membrane and intracellular compartments. Under basal conditions, most of the type 1 dopamine receptors (D1Rs) are located in intracellular compartments and dopamine induces their translocation to the plasma membrane (5, 46). Activation of plasma membrane D1Rs stimulates a specific signaling cascade that leads to activation of protein kinase C isoform ζ and phosphorylation of Na-K-ATPase-α1 Ser-18 (11). This results in a conformational change of the Na-K-ATPase-α1 NH2 terminal (16) and the binding of class IA phosphoinositide 3-kinase (PI3K) to a polyproline domain located in the Na-K-ATPase-α1 NH2-terminal end, upstream from the phosphorylated Ser-18 (50). The activated PI3K generates phosphoinositides that likely participate in the recruitment and activation of adaptor protein 2, dynamin 2, and clathrin (20).
Although phosphorylation of Na-K-ATPase-α1 is an essential step for dopamine-induced Na-K-ATPase endocytosis, it does not per se inhibit the Na-K-ATPase enzymatic activity. Phosphorylation of Na-K-ATPase-α1 constitutes the triggering signal for removal of plasma membrane phosphorylated Na-K-ATPase molecules via clathrin-coated vesicles to intracellular compartments (8, 9). Thus dopamine does not affect the intrinsic activity of the Na-K-ATPase (turnover rate) but the number of Na-K-ATPase molecules that are available at the plasma membrane involved in sodium and potassium transport (37). We have recently described that intracellular compartments containing Na-K-ATPase molecules are located just underneath the plasma membrane (17). We have also shown that the level of intracellular sodium concentration modulates the plasma membrane recruitment of D1Rs and dopamine-induced endocytosis of Na-K-ATPase molecules in proximal tubule epithelial cells (14). In this study, we present evidence of intracellular trafficking of Na-K-ATPase molecules and D1Rs in response to changes in intracellular sodium concentration.
MATERIALS AND METHODS
Materials.
Ouabain, dopamine, and ExtrAvidin peroxidase were obtained from Sigma (St. Louis, MO). Alexa Fluor 488-tagged anti-mouse and Cy3-tagged anti-rabbit antibodies were purchased from Molecular Probes (Eugene, OR). Sulfo-NHS-biotin was obtained from Pierce (Rockford, IL). Na-K-ATPase-α1 antibody (27) was a generous gift from Dr. Michael J. Caplan (Yale University, New Haven, CT). Other reagents were of the highest quality available.
Cell culture and transfection.
The basic functional unit of Na-K-ATPase is composed of two subunits, α and β. Several different isoforms of these subunits are expressed in different mammalian tissues; however, the kidney only expresses the α1- and β1-polypeptides (30). Because of this, in our experiments we only transfected opossum kidney (OK) cells with the α1-isoform. OK cells stably expressing the rat Na-K-ATPase α1-wt or the S18A mutant of this protein were cultured in DMEM containing 10% calf serum and antibiotics. Plasmid preparation, site-directed mutagenesis, and stable expression of Na-K-ATPase-α1 were performed as previously described (38, 39). Cells expressing Na-K-ATPase α1-wt or α1-S18A were selected and maintained in a 3 μM ouabain medium. While Na-K-ATPase molecules with the endogenous OK cell α1 are totally inhibited, Na-K-ATPase molecules containing the rodent α1 are resistant to this amount of ouabain (39). Thus Na-K-ATPase molecules containing the OK cell endogenous α1 should not interfere with determinations performed in cells expressing rodent α1 (38, 39). Before treatment with monensin, cells were incubated for 30 min in the same culture medium without serum and buffered with 50 mM HEPES. The optimal concentrations of reagents and the extension of the treatments used in these experiments were as previously determined (12, 13, 19). Antibodies were blocked with 1% milk in PBS before use.
Determination of Rb+ uptake.
Measurements of Na-K-ATPase-mediated Rb+ uptake were performed with attached cells as previously described (38, 39). In short, transfected cells were transferred to serum-free DMEM containing 50 mM HEPES, pH 7.4, and either 3 μM or 5 mM ouabain. Cells were incubated with these concentrations of ouabain for 20 min at 37°C in an air atmosphere and 10 min at 23°C before their treatment with 5 μM monensin at 23°C for 30 min. Then, 1 mM dopamine and a trace amount of [86Rb+]RbCl were added. After 20-min incubation at 23°C, cells were washed three times with ice-cold saline, dissolved with SDS, and accumulated radioactivity was determined in a gamma counter. Na-K-ATPase-mediated Rb+ uptake was estimated from the difference in tracer uptake between samples incubated in 3 μM and 5 mM ouabain.
Biotinylation of plasma membrane proteins.
These experiments were performed as previously described (12) using OK cells expressing either α1-wt or α1-S18A and grown to 80% confluence. The cells were treated with monensin and dopamine as indicated above for the Rb+ uptake assay. Then, the cell medium was changed to ice-cold 10 mM Tris·HCl (pH 7.5), 2 mM CaCl2, and 150 mM NaCl, 1.5 mg/ml sulfo-NHS-biotin. The cells were incubated in this medium for 1 h at 4°C.
Following biotin labeling, the cells were scraped in immunoprecipitation buffer (20 mM Tris, 2 mM EDTA, 2 mM EGTA, 30 mM sodium pyrophosphate, pH 7.3) containing a protease inhibitor cocktail, frozen in liquid nitrogen, thawed rapidly, probe-sonicated twice on an ice-water bath, and frozen-thawed again. The cell suspension was centrifuged at 14,000 g at 4°C for 5 min. The supernatants were transferred to clean tubes, and 1% Triton X-100 and 0.2% SDS were added. Protein concentration of the different samples was determined. To tubes containing the same amount of protein, anti-Na-K-ATPase-α1 antibody was added and incubated for 1 h at 4°C with end-over-end shaking. The immunoprecipitation was performed under conditions that precipitate all cellular Na-K-ATPase molecules (12). Then, protein A/G-agarose, prewashed three times with PBS and once with immunoprecipitation buffer containing 1% Triton X-100, was added and incubated for 2 h at 4°C with end-over-end shaking. The pellet was washed four times with immunoprecipitation buffer containing 1% Triton X-100 and 0.1% SDS, once with 50 mM Tris·HCl (pH 7.4), and finally resuspended in Laemmli sample buffer (29). Electrophoresis and Western blot analysis with ExtrAvidin peroxidase were performed as previously described (12, 20). The blotted membranes were scanned, and the bands were quantified using an AlphaImager EC camera for gel documentation and analysis (Alpha Innotech, San Leandro, CA).
Fluorescence determinations.
Cells attached to glass coverslips and grown to 90% confluence were incubated with monensin and dopamine as described in determination of Rb+ uptake. The cells were then washed twice with PBS containing 1.2% sucrose (PBSS), fixed with freshly prepared 4% paraformaldehyde in PBSS for 10 min, washed twice with PBSS, incubated with l-lysine/Na-m-periodate for 20 min, washed with PBSS, permeabilized with 0.2% BSA, 0.2% Triton X-100 in PBSS for 10 min, and washed again twice with PBSS. Cells were blocked with 5% BSA, 1% normal goat serum, 0.2% Tween 20 for 1 h, incubated with mouse anti-Na-K-ATPase-α1 and rabbit anti-D1R antibodies and washed with PBSS. In the dark, cells were incubated with anti-mouse-Cy3 and anti-rabbit-Alexa Fluor 488 for 1 h. Then, the coverslips were washed, air dried, and mounted on glass slides using Gel-Mount containing antifading agents (Biomeda, Foster City, CA).
To obtain high-resolution three-dimensional fluorescence images of the cells, the imaging workstation consisted of an Olympus IX-81 inverted fluorescence microscope (Olympus, Tokyo, Japan), equipped with a ×60/×100 oil-immersion objective lens, cooled Hamamatsu ORCA-ER CCD camera (Hamamatsu Photonics, Hamamatsu-city, Japan), a halogen 100-W light source, a motorized filter and shutter, and a scan wizard for collection of x/y and z image sequences and wavelength positions over time, all controlled by the SimplePCI software (Compix, Cranberry Township, PA). Images of cells labeled with both Cy3 and Alexa Fluor 488 were acquired sequentially through the two respective channels. To obtain deconvolved images, at least nine z-stacks of the field with a step of 0.3–0.5 μm were acquired, and deconvolution was performed using AutoDeblur software (AutoQuant Imaging, Troy, NY). For colocalization analysis, the two single-channel images of the same field were merged using SimplePCI software.
Total internal reflection fluorescence microscopy.
For total internal reflection fluorescence (TIRF) determinations, OK cells expressing Na-K-ATPase molecules containing the fluorescent protein GFP (for green fluorescent protein) fused to the NH2 terminal of α1 were used (10). The presence of GFP does not affect the level of protein expression, the amount of Na-K-ATPase expressed at the plasma membrane, the level of Rb+ transport mediated by the Na-K-ATPase, or the hormone regulation (10). The TIRF setup is based on a modified inverted fluorescence microscope (Olympus, Melville, NY) with a video camera (Pentamax, Princeton Instruments, Trenton, NJ) attached to its video port. Light from a 4-W Argon laser (Lexel 88) was directed through a software-controlled shutter (Univlitz, Vincent Associates, Rochester, NY) that opens during camera exposure. The 488-nm argon line (∼1,000 mW) was focused into one end of a 2-mm-diameter quartz fiber-optic setup fixed to the optical system attached to the microscope stage and passed through a set of collimation lenses, which focuses the light on the prism surface. An annulus in this path ensures that only central parallel laser rays reached the prism at the critical angle. The use of fiber optics has two main advantages. First, a fiber-optic setup allows the rapid alignment of the experimental chamber and the corresponding prism in the microscope stage with the selected angle of light incidence. Second, it produces uniform illumination, since it was vibrated at 5 kHz so that the typical laser-interfering patterns (“speckles”) were cancelled out by their averaging during the exposure time of an image frame. Fluorescence images were detected by the camera after the emitted light was passed through the dichroic mirror and emission filters. The calibration of the evanescent wave depth was performed in a cell culture dish without cells by imaging a fluorescent bead through a calibrated piezoelectric positioning drive (35).
TIRF determinations at different concentrations of sodium were performed by permeabilizing the cell membrane with 10 μM gramicidin D and superfusing the sample with different standard sodium concentrations (13). Alternatively, the cells were treated with monensin and TIRF determinations were performed at different times, before and after the addition of monensin.
Statistical analysis.
Each experiment was repeated at least three times. Comparison between two experimental groups was determined by the nonpaired Student's t-test. P < 0.05 was indicated with an “*” in the figures. For the microscopic images, at least eight randomly chosen sets of cells were used in each experimental condition. Figures show representative images for each experiment.
RESULTS
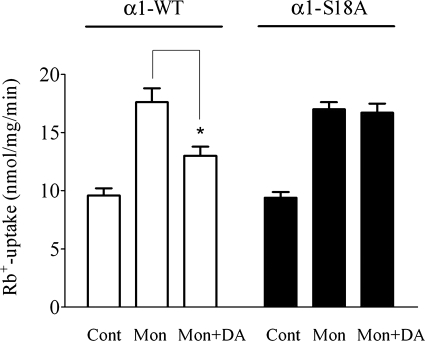
Experiments were performed with OK cells, which are widely used as a cell culture model of proximal tubule epithelial cells (24, 31, 34). Cells were grown in DMEM containing calf serum and antibiotics. Under these conditions, OK cells attach to the bottom of cell culture flasks and form a monolayer. In animal models, the dopamine natriuretic effect was only observed under conditions of sodium load (1, 4, 26). Consistently, we and other researchers have observed that a slight increase in the level of intracellular sodium concentration is essential for dopamine-induced endocytosis of Na-K-ATPase molecules and the concomitant reduced capacity of the cell to transport sodium (13, 41). The use of the ionophore monensin allows us to change the intracellular sodium concentration in a stepwise manner and maintain the electrogenic gradient across the plasma membrane (13). The elevated intracellular sodium concentration elicited by 5 μM monensin produced a stimulation of Na-K-ATPase-mediated Rb+ uptake (Fig. 1). Using fluorescence microscopy, we have previously determined that OK cells have a basal level of intracellular sodium concentration of ∼9 mM and that 5 μM monensin doubles the basal intracellular sodium concentration (13). Subsequent addition of dopamine results in a 25% reduction of Na-K-ATPase-mediated Rb+ uptake (Fig. 1).
Fig. 1.
Effect of monensin (Mon) and dopamine (DA) on Na-K-ATPase-mediated Rb+ uptake. Opposum kidney (OK) cells expressing the wild-type (WT, left) or S18A mutant form of Na-K-ATPase-α1 (right) were treated with 5 μM monensin for 30 min and 1 μM dopamine for 5 min before Rb+ uptake assay. *P < 0.05.
Dopamine induces Na-K-ATPase-α1-subunit Ser-18 phosphorylation, which is essential for dopamine-induced Na-K-ATPase endocytosis (8). When Na-K-ATPase activity was measured in OK cells expressing the α1-S18A mutant, dopamine has no effect on Na-K-ATPase activity (Fig. 1). However, the effect of monensin on Na-K-ATPase activity was not affected. The level of Na-K-ATPase-mediated Rb+ uptake was the same in cells expressing α1-wt and α-S18A. As the S18A mutation in α1 does not affect the catalytic turnover of the Na-K-ATPase (8, 9), the previous result indicates that both cell lines express the same amount of active Na-K-ATPase molecules at the plasma membrane.
Increased intracellular sodium concentration does not affect the abundance of plasma membrane Na-K-ATPase.
To determine whether monensin changes the size of the Na-K-ATPase plasma membrane pool, we used the technique of cell membrane protein biotinylation (12, 14, 15, 18, 23, 28, 47, 48). Sulfo-NHS-biotin reacts with primary amino groups and does not permeate the plasma membrane; thus, proteins containing primary amines exposed to the extracellular medium are the only ones that are biotinylated. As the reaction is performed at 4°C, intracellular protein trafficking does not occur and there is no exchange between the intracellular compartments and plasma membrane pool of Na-K-ATPase molecules. For determination of the absolute number of plasma membrane Na-K-ATPase molecules using cell surface protein biotinylation, the efficiency of reaction between the protein amino groups and the biotinylation reagent is very critical since the intent is to label optimally 100% of the Na-K-ATPase molecules present at the plasma membrane (23). This is not the case when biotinylation is used to determine a change in the size of the plasma membrane pool of Na-K-ATPase molecules. In this case, the critical consideration is that the labeling efficiency is the same in treated samples and controls. Although we may not have labeled all of the Na-K-ATPase molecules at the plasma membrane, care was taken to produce the biotinylation reaction in the same conditions in treated samples and controls.
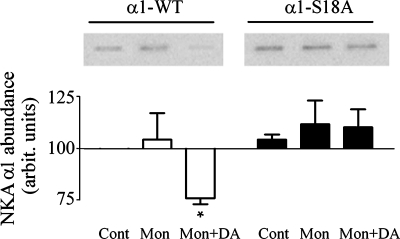
Consistent with results presented in Fig. 1, there was no significant difference in the amount of plasma membrane Na-K-ATPase of cells expressing α1-wt and α1-S18A (Fig. 2). The same level of biotinylation was observed in cells treated or not with monensin, which suggests that increased intracellular sodium does not affect the size of the plasma membrane Na-K-ATPase pool. Subsequent addition of dopamine reduced the plasma membrane Na-K-ATPase abundance in cells expressing α1-wt, but had no effect on cells expressing the α1-S18A mutant. Results illustrated in Figs. 1 and 2 are consistent with previous observations by other investigators, in different cell types, showing that an acute increase in intracellular sodium concentration does not affect the number of plasma membrane Na-K-ATPase molecules (3, 40, 43, 47–49).
Fig. 2.
Effect of monensin and dopamine on plasma membrane Na-K-ATPase-α1 abundance. OK cells expressing the wild-type or S18A mutant form of Na-K-ATPase-α1 were treated with 5 μM monensin for 30 min and 1 μM dopamine for 5 min. The abundance of Na-K-ATPase molecules at the plasma membrane was determined by biotinylation, as described in materials and methods. After biotinylation, the cells were dissolved and, from samples containing the same amount of protein, Na-K-ATPase molecules were precipitated using a specific antibody. The amount of biotin present in the immunoprecipitated Na-K-ATPase molecules was determined by Western blot analysis. Top: representative Western blots. Bottom: quantitation of Western blots. *P < 0.05 with respect to cells treated with monensin alone.
Determination of TIRF.
We recently described that intracellular compartments containing Na-K-ATPase molecules are just underneath the plasma membrane (17). To detect Na-K-ATPase traffic in these compartments, we used the TIRF technique. In this method, a collimated laser beam is directed to the base wall of the cell dish. The laser beam angle of incidence is such that after entering into the cell dish wall, the beam is “trapped” inside the wall by total internal reflection and does not enter into the cell medium (35). Although the laser beam cannot directly excite cell fluorescent molecules, the reflected laser beam generates an electromagnetic field that extends into the cell medium. The electromagnetic field is stronger just against the dish wall in which the laser beam is trapped and decays exponentially with the distance from this wall. Thus this “evanescent wave” has the capacity to excite fluorescent molecules that are very close to the bottom of the cell dish (32). When confluent, OK cells form an epithelium-like monolayer in which the Na-K-ATPase-containing basolateral cell membrane domain is against the cell culture dish. We used TIRF in cells expressing the Na-K-ATPase GFP-α1 fusion protein. We demonstrated that the fusion of GFP to the α1 NH2 terminal does not affect the level of expression of Na-K-ATPase, its transport activity, or its endocytosis induced by dopamine (10, 20).
A brightfield image, a fluorescence image, and a TIRF image of the same cell are illustrated in Fig. 3, A, B, and C, respectively. Both epifluorescence and TIRF images were acquired at a 490-nm excitation wavelength. The evanescent wave depth was determined to be 247 ± 8.8 nm (mean ± SD; n = 8) by imaging a fluorescent bead through a calibrated piezoelectric positioning drive, as described by Oheim et al. (35). This determination was performed using a free-cell system, and it may reflect the maximum evanescent wave thickness under optimal conditions. Other researchers estimated that the evanescent wave may extend 100–150 nm from the surface containing the trapped laser beam (2, 42, 44). Considering that the plasma membrane has an average thickness of 50 nm, the evanescent wave extends beyond the plasma membrane and has the capacity to excite GFP-tagged Na-K-ATPase molecules that are just underneath the plasma membrane. We have determined that the attached OK cells have a depth of ∼9 μm (17).
Fig. 3.
Images of cells expressing green fluorescent protein (GFP)-tagged α1 observed under epifluorescence and total internal reflection fluorescence (TIRF) illumination. A: brightfield image of a typical OK cell taken using Hoffman modulation contrast optics. B and C: fluorescence images of the same cell acquired using epi- and evanescent wave illumination, respectively. Bar = 5 μm.
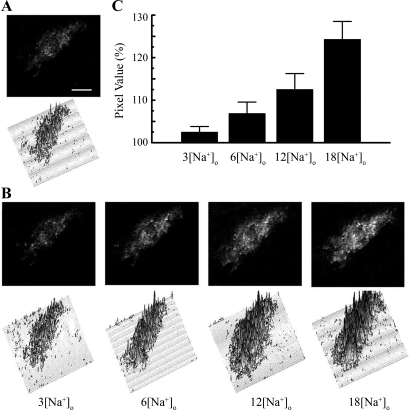
TIRF emission levels of GFP-tagged Na-K-ATPase were determined at different concentrations of intracellular sodium after the cells were permeabilized with 10 μM gramicidin D (13). Figure 4A illustrates the TIRF image and pixel-by-pixel fluorescence determinations of a typical OK cell obtained by evanescent wave illumination, under basal conditions. TIRF increases stepwise at increasing sodium concentrations (Fig. 4, B and C). Bars in Fig. 4C represent the percentage of fluorescence change (mean ± SD) in relation to control, from 18 different cells. In Fig. 4B, top, the number and intensity of white spots increase with the increasing sodium concentration (increased density of fluorescent pixels). In Fig. 4B, bottom, the total area occupied by the fluorescent pixels at 3 mM intracellular sodium concentration is smaller than at 18 mM intracellular sodium concentration, which indicates that there are more fluorescent pixels at increasing intracellular sodium concentration, where intracellular sodium concentration is XXX. Also, the height of the fluorescence pixels at 3 mM is lower than at 18 mM intracellular sodium concentration, which indicates that the intensity corresponding to individual pixels increases as the intracellular sodium concentrations increases. As each pixel displays the fluorescence of many GFP-tagged Na-K-ATPase molecules, the increases in both the density of pixels displaying fluorescence and the intensity of fluorescence (height of the individual peaks) suggest that as the intracellular sodium increased, there was a movement of GFP-tagged Na-K-ATPase from a low-intensity to a high-intensity evanescent field as well as an incorporation of GFP-tagged Na-K-ATPase that was outside of the evanescent field into the evanescent field generated by the laser.
Fig. 4.
Increased TIRF level of GFP-tagged Na-K-ATPase-α1 in response to increased intracellular sodium concentration. A: control fluorescence image and corresponding pixel-by-pixel fluorescence determination of an OK cell obtained by evanescent wave illumination. Bar = 5 μm. B: TIRF images obtained at different sodium concentrations after the cell membrane was permeabilized with 10 μM gramicidin D. C: percentage of signal changes (means ± SD) observed in relation to control fluorescence levels from 18 different cells. Under basal conditions, these cells have an average of 9 mM intracellular sodium (10). Numbers on the abscissa indicate the increase of intracellular sodium with respect to the control basal intracellular sodium concentration level.
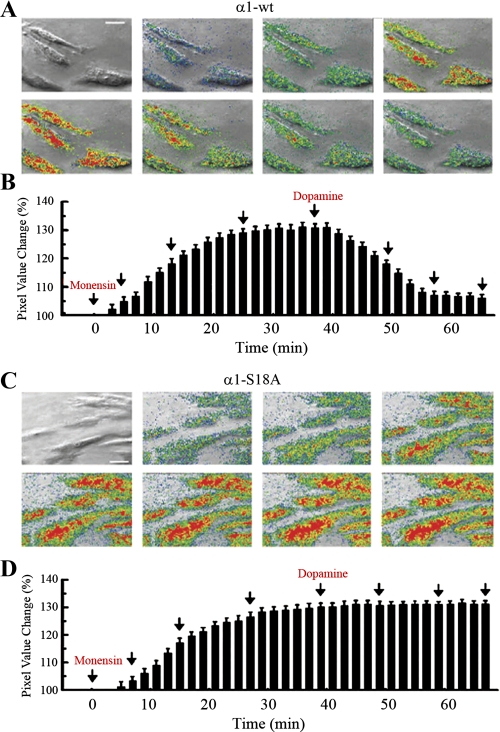
Gramicidin D induces the equilibration of sodium across the plasma membrane (13). Thus, in these experiments, there was no sodium gradient across the plasma membrane. Then, TIRF determinations were repeated using monensin, instead of gramicidin, to increase the cell intracellular sodium concentration. Determinations were performed in OK cells expressing GFP-tagged Na-K-ATPase-α1 (Fig. 5, A and B) or GFP-tagged Na-K-ATPase α1-S18A mutant (Fig. 5, C and D). The basal level of TIRF, before the addition of monensin, is illustrated in Fig. 3C. For quantitation purposes, in Fig. 5, the basal level of TIRF was arbitrarily set at the 100% level. Then, monensin was added to the cell medium and TIRF was determined every 2 min. Figure 5A, top row, illustrates four images displaying the progressive increase in emission light levels over baseline obtained following the application of 5 μM monensin. After a stable fluorescence level was reached, the application of 1 μM dopamine evoked a fluorescence decrease (Fig. 5A, bottom row). The histogram in Fig. 5B corresponds to the signal change percentage (means ± SD) obtained in relation to background fluorescence in the whole image sequence. Images were acquired every 2 min, and arrows indicate the times the images illustrated in Fig. 5A were acquired. Using fluorescence microscopy of cells loaded with the sodium-sensitive dye SBFI, we have previously demonstrated that addition of monensin to the cell medium produces a steady increase in intracellular sodium up to a plateau reached at ∼30 min (13). The corresponding intracellular sodium concentrations were 9, 15, 22, and 27 mM at 0, 5, 15, and 30 min after the addition of monensin (13). Thus the TIRF increase illustrated in Fig. 5, B and D, follows the stepwise increase in intracellular sodium concentration.
Fig. 5.
Fluorescence light changes under evanescent wave illumination evoked in OK cells by monensin and dopamine. TIRF determinations were performed in cells expressing either Na-K-ATPase-α1 (A and B) or the S18A mutant of this protein (C and D), both fused to GFP. Determinations illustrated in the histograms were performed every 2 min (B and D), and arrows indicate the times images illustrated in A and C were captured. Times of application of monensin and dopamine are indicated. Fluorescence increases were coded in pseudocolors and overlapped on a brightfield image of the corresponding OK cell acquired using Hoffman modulation contrast. Blue, green, yellow, and red represent signal increases equivalent to 1, 1.5, 2, 2.5, and 3 SD of the pixel variability in the image background, respectively. Bar = 5 μm.
Treatment with monensin produced enhanced TIRF in cells expressing either GFP-α1 (Fig. 5A) or GFP-α1-S18A (Fig. 5C). However, after treatment with dopamine, TIRF is reduced in cells expressing GFP-α1 (Fig. 5A), but not in cells expressing the mutant GFP-α1-S18A (Fig. 5C). As phosphorylation of Na-K-ATPase-α1 Ser-18 is essential for dopamine-dependent endocytosis of Na-K-ATPase molecules (8), dopamine has no effect on the TIRF level of cells expressing the α-S18A mutant.
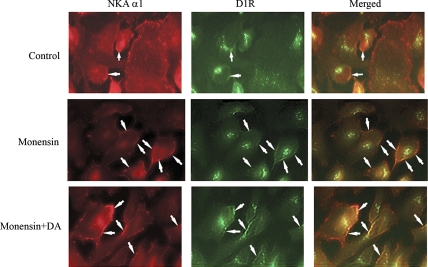
We have previously observed that while most of the Na-K-ATPase molecules are in the area of the plasma membrane (17), under basal conditions, D1Rs are mainly localized in the cell interior (14). Brismar et al. (5) have shown that there is some colocalization of Na-K-ATPase molecules with D1Rs. We wanted to determine whether this colocalization depended on or was affected by the level of intracellular sodium concentration. Thus we performed experiments using nontransfected OK cells and determined the possible colocalization of the endogenous proteins. Figure 6 shows that in cells treated with monensin, the colocalization between the fluorophores attached to Na-K-ATPase and D1Rs is significantly increased (Fig. 6, 2nd row, right). These results are consistent with our previous observations that an increase in intracellular sodium (elicited by monensin) produces the recruitment of D1Rs to the plasma membrane (14), inducing an increased colocalization of D1Rs with Na-K-ATPase molecules. Subsequent treatment of the cells with dopamine further increased the colocalization of Na-K-ATPase and D1R molecules (Fig. 6, 3rd row, right).
Fig. 6.
Colocalization of Na-K-ATPase-α1 and type 1 dopamine receptor (D1R). Images of OK cells expressing Na-K-ATPase wild-type α1 treated with vehicle (top), 5 μM monensin for 30 min (middle), and 5 μM monensin for 30 min, and then with 1 μM dopamine for 5 min (bottom) are shown. Fluorescence images were acquired through the Cy3 channel (Na-K-ATPase-α1) and the Alexa Fluor 488 channel (D1R). Merged images (right) were obtained using SimplePCI software. Cells were treated and images acquired as described in materials and methods. Arrows point to the sites of colocalization.
DISCUSSION
We present evidence that changes in intracellular sodium concentration produce a redistribution of Na-K-ATPase and D1R molecules. The increased Na-K-ATPase-mediated Rb+ uptake and TIRF level observed in cells expressing Na-K-ATPase molecules containing GFP-α1 and treated with monensin to increase the intracellular sodium concentration may suggest that Na-K-ATPase molecules are recruited to the plasma membrane. However, this was not supported by direct determination of the amount of plasma membrane Na-K-ATPase, using cell surface protein biotinylation. As, during the TIRF determinations, the evanescent field produced by the laser has the capacity to stimulate fluorescent molecules that are at the plasma membrane and also in sub-plasma membrane compartments, the combined results from determinations of TIRF and the plasma membrane pool of Na-K-ATPase molecules suggest that the increased level of intracellular sodium concentration induces the movement of compartments containing GFP-tagged Na-K-ATPase toward the plasma membrane, but the Na-K-ATPase molecules are not delivered to the plasma membrane.
Because of the limited availability of sodium that occurs at the basal level of intracellular sodium concentration (∼10 mM), at any given time, most of the plasma membrane Na-K-ATPase molecules are not working (maximal activity is observed at 120 mM sodium) (22). In cells treated with monensin, the increased intracellular sodium concentration induces that more Na-K-ATPase molecules, already present at the plasma membrane, participate in sodium transport and this is expressed as increased Na-K-ATPase activity. Through this mechanism, the cell can handle small increases in intracellular sodium concentration and does not need an increased plasma membrane pool of Na-K-ATPase to reduce the intracellular sodium concentration. Consistently, using cell surface protein biotinylation, we could not detect any significant change in the size of the plasma membrane pool of Na-K-ATPase molecules (Fig. 2). The biotinylation technique seems to have worked properly since we could detect reductions in the plasma membrane pool of Na-K-ATPase induced by dopamine (Fig. 2), which corresponds to a reduction in the level of TIRF (Fig. 5). However, it can be argued that the increased intracellular sodium concentration promotes the delivery of Na-K-ATPase molecules to the plasma membrane and that these molecules participate in sodium transport, but they are not accessible to labeling by sulfo-NHS-biotin. Several lines of evidence suggest that this is not a likely explanation to the results of cell surface protein biotinylation. Our observation that an increase in intracellular sodium concentration does not produce an acute increase in the size of the plasma membrane pool of Na-K-ATPase molecules has been previously reported by other researchers (3, 40, 43, 47–49). In several cell types, it was described that an increase in intracellular sodium concentration produces an increase in the plasma membrane pool of Na-K-ATPase molecules that is dependent on protein synthesis (40, 43, 49). Thus there is a lag of several hours between the increase in intracellular sodium concentration and the increase in the plasma membrane pool of Na-K-ATPase molecules. Besides these effects that depend on protein expression, it has also been described that an increase in intracellular sodium concentration in cortical collecting tubule cells produces the recruitment of Na-K-ATPase molecules to the plasma membrane from an intracellular latent pool (3, 47, 48). This effect was observed after 1–2 h of intracellular sodium increase. These reports indicate that an increased intracellular sodium concentration induces the delivery of Na-K-ATPase molecules to the plasma membrane and that the just-delivered Na-K-ATPase molecules could be determined using different techniques: binding of an anti-Na-K-ATPase antibody, ouabain binding, and cell surface protein biotinylation. However, this is not an acute response to the increased level of intracellular sodium.
While the experiments reported above were performed with final concentrations of intracellular sodium of 100 mM and higher, our experiments were performed at a final concentration of ∼19 mM (a change in intracellular sodium of ∼10 mM). This is very important to consider because the Na-K-ATPase molecules that are already present at the plasma membrane can easily handle small changes in intracellular sodium concentrations. Furthermore, our experiments were done during the lag period in which the references described above did not observe any increase in the plasma membrane pool of Na-K-ATPase. On the other hand, we have evidence that if there were Na-K-ATPase molecules delivered to the plasma membrane in response to the increased intracellular sodium, we would have determined them. Using the same biotinylation technique described in this manuscript, we determined an increased plasma membrane pool of Na-K-ATPase molecules in cells treated with angiotensin II (14), PMA (12), or the serotonin agonist 8-OH-DPAT (6). Furthermore, while the increased intracellular sodium concentration did not promote an increase in the plasma membrane pool of Na-K-ATPase molecules, it did increase the plasma membrane pool of D1Rs, which we determined using the same biotinylation method described in this manuscript (14). Thus the experimental results from us and other researchers support our conclusion that an increased intracellular sodium concentration does not produce an acute increase in the plasma membrane pool of Na-K-ATPase molecules.
We considered the possibility that the changes in TIRF were not the result of the movement of GFP-tagged Na-K-ATPase molecules inside the evanescent wave field, but due to a change in the intrinsic fluorescence of GFP in response to conformational changes in the Na-K-ATPase molecule. Since the Na-K-ATPase cellular activity is limited by the availability of intracellular sodium (4, 21, 22), treatment with monensin increases the number of Na-K-ATPase molecules that are involved in sodium transport. This is reflected in the increased Na-K-ATPase activity illustrated in Fig. 1. During the process of sodium transport, the Na-K-ATPase that is in the basal E2 conformation switches to the E1 conformation (22). However, the change of conformation E2 → E1→ E2 is very fast, so that most of the time the Na-K-ATPase is in the E2 conformation. Since the molecules under turnover are not synchronized, some of the Na-K-ATPase molecules would be in E1 while others are in the E2 conformation. Even assuming that GFP attached to Na-K-ATPase-α1 in the E1 conformation has more fluorescence than when GFP is attached to the E2 conformation, this would not explain the TIRF increase we observed because the enzyme is only transiently in the E1 conformation. It is important to notice that while the E2 → E1 → E2 conformational change occurs in the millisecond range, we observed a steady increased in TIRF over 20–30 min. Furthermore, we have specifically determined that treating the cells with monensin does not produce any change in the level of fluorescence of a fluorophore attached to the Na-K-ATPase-α1 NH2 terminal (16).
Using fluorescence resonance energy transfer, we have demonstrated that dopamine-induced phosphorylation of α1-Ser-18 results in a conformational change in the α1 NH2 terminal such that this part of the protein is closer to the plasma membrane (16). However, this is not responsible for the changes in TIRF we describe in this report because the TIRF increase was also observed in cells expressing the α1-S18A mutant, which cannot be phosphorylated by the action of dopamine and consequently does not display any conformational change. Furthermore, the TIRF increase is produced by increased concentration of intracellular sodium, which we observed does not produce any conformational change in the Na-K-ATPase-α1 (16). It may be argued that the increased level of TIRF is not produced by an increased level of intracellular sodium concentration but to other undetermined effects of monensin, such as changes in the membrane potential. The experiments with gramicidin suggest that this is not the case. The only characteristic that these experiments have in common with those with monensin is the increased level of intracellular sodium concentration. However, the mechanism to increase intracellular sodium is different, and in the experiments with gramicidin there is no sodium concentration gradient across the plasma membrane.
Dopamine induced a reduction in the TIRF level, which is consistent with the decrease in Na-K-ATPase abundance at the plasma membrane (Fig. 2) and the level of reduction of Na-K-ATPase activity (Fig. 1) elicited by the dopamine treatment. The specificity of this effect for Na-K-ATPase is demonstrated by the fact that none of the three dopamine effects (reduction of TIRF, reduction of plasma membrane Na-K-ATPase, and reduction of Na-K-ATPase activity) were observed when the experiments were performed in OK cells expressing the S18A mutant of α1. The fact that dopamine treatment induced a TIRF reduction and a corresponding decrease in the plasma membrane Na-K-ATPase pool suggests that the Na-K-ATPase molecules withdrawn from the plasma membrane are translocated to intracellular compartments that are beyond the reach of the evanescent field. However, these intracellular compartments are still very close to the plasma membrane (17). As the resolution of fluorescence microscopy is not enough to clearly separate Na-K-ATPase molecules in intracellular compartments from Na-K-ATPase molecules in the plasma membrane, the endocytosis of Na-K-ATPase molecules induced by dopamine is not evident in the results presented in Fig. 6.
Under basal conditions, most of the proximal tubule epithelial cell D1Rs are located in intracellular compartments (5, 14, 26), and treatment with dopamine induces the recruitment of the D1Rs to the plasma membrane (5, 26, 46). We have previously demonstrated that the plasma membrane recruitment of D1Rs can also be induced by increasing the intracellular sodium concentration (14). In this report, using fluorescence microscopy, we show that the colocalization of Na-K-ATPase and D1Rs was greatly increased in cells with higher intracellular sodium concentration. Because Na-K-ATPase molecules are in intracellular compartments just beneath the plasma membrane (17) or at the plasma membrane, the increased colocalization between D1Rs and Na-K-ATPase occurs because the D1Rs are being recruited to the plasma membrane. Thus it is possible that in their transit toward the plasma membrane, vesicles containing D1Rs fuse with intracellular compartments containing Na-K-ATPase, and then these vesicles move toward the plasma membrane to discharge D1Rs.
In vivo, dopamine inhibition of proximal tubule sodium reabsorption is only observed under conditions of sodium load (1, 26, 41, 45). Consistent with this, for dopamine to inhibit the Na-K-ATPase, the intracellular sodium concentration of proximal tubule epithelial cultured cells should be increased by a few millimolars above basal levels (13, 41). Thus we propose that a small increase in intracellular sodium is an early signal that triggers the proximal tubule cell to respond to a sodium load. When the proximal tubule cell identifies that an increase in intracellular sodium has occurred, a series of processes are initiated to counteract the increased sodium reabsorption: the recruitment of D1Rs from intracellular storage compartments to the plasma membrane with a parallel decrease of type 1 angiotensin II receptors (7, 14), activation of the dopaminergic intracellular signaling cascade of second messengers, the synthesis by the proximal tubule cells of dopamine, and its facilitated transport to the cell exterior (1), where the hormone binds to the just-recruited receptors. Activation of D1Rs promotes inhibition of proximal tubule sodium reabsorption. Then it is possible that as part of this cellular response, intracellular compartments move toward the plasma membrane to receive the Na-K-ATPase molecules that will be endocytosed in response to activation of D1Rs. After the endocytosed Na-K-ATPase molecules are received by the intracellular compartments, they move back toward the inside of the cell. This would correspond to the reduction of TIRF observed after treatment with dopamine.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-62195 to C. H. Pedemonte.
Acknowledgments
The authors thank Dr. Enrique Torre (Emory University) for advice on fluorescence microscopy, Dr. Mustafa Lokhandwala (University of Houston) for helpful discussions, and Dr. Samina Salim (University of Houston) for critically reading the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aperia AC Intrarenal dopamine: a key signal in the interactive regulation of Na+ metabolism. Annu Rev Physiol 62: 621–647, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Axelrod D, Thompson NL, Burghardt TP. Total internal inflection fluorescent microscopy. J Microsc 129: 19–28, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Barlet-Bas C, Khadouri C, Marsy S, Doucet A. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem 265: 7799–7803, 1990. [PubMed] [Google Scholar]
- 4.Bertorello AM, Katz AI. Short-term regulation of renal Na-K-ATPase activity: physiological relevance and cellular mechanisms. Am J Physiol Renal Fluid Electrolyte Physiol 265: F743–F755, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA 12: 5573–5578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budu CE, Efendiev R, Bertorello AM, Pedemonte CH. Hormonal-dependent recruitment of Na,K-ATPase to the plasmalemma is mediated by PKCβ and modulated by [Na+]i. Brit J Pharmacol 137: 1380–1386, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 97: 2745–2752, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na,K-ATPase is initiated by phosphorylation of Ser-18 in the rat alpha-subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalytic α subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem 273: 8814–8819, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Done SC, Leibiger IB, Efendiev R, Katz AI, Leibiger B, Berggren PO, Pedemonte CH, Bertorello AM. Tyrosine 537 within the Na+,K+-ATPase alpha-subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J Biol Chem 277: 17108–17111, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Efendiev R, Bertorello AM, Pedemonte CH. PKC-beta and PKC-zeta mediate opposing effects on proximal tubule Na+,K+-ATPase activity. FEBS Lett 456: 45–48, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Efendiev R, Bertorello AM, Pressley TA, Rousselot M, Feraille E, Pedemonte CH. Simultaneous phosphorylation of Ser11 and Ser18 in the alpha-subunit promotes the recruitment of Na+,K+-ATPase molecules to the plasma membrane. Biochemistry 39: 9884–9892, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Efendiev R, Bertorello AM, Zandomeni R, Cinelli AR, Pedemonte CH. Agonist-dependent regulation of renal Na+,K+-ATPase activity is modulated by intracellular sodium concentration. J Biol Chem 277: 11489–11496, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Efendiev R, Budu CE, Cinelli AR, Bertorello AM, Pedemonte CH. Intracellular Na+ regulates dopamine and angiotensin II receptors availability at the plasma membrane and their cellular responses in renal epithelia. J Biol Chem 278: 28719–28726, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Efendiev R, Chen Z, Krmar RT, Uhles S, Katz AI, Pedemonte CH, Bertorello AM. The 14-3-3 protein translates the NA+,K+-ATPase α1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem 280: 16272–16277, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Efendiev R, Cinelli AR, Leibiger IB, Bertorello AM, Pedemonte CH. FRET analysis reveals a critical conformational change within the Na,K-ATPase alpha1 subunit N-terminus during GPCR-dependent endocytosis. FEBS Lett 580: 5067–5070, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Efendiev R, Das-Panja K, Cinelli AR, Bertorello AM, Pedemonte CH. Localization of intracellular compartments that exchange Na,K-ATPase molecules with the plasma membrane in a hormone-dependent manner. Br J Pharmacol 151: 1006–1013, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efendiev R, Krmar RT, Ogimoto G, Zwiller J, Tripodi G, Katz AI, Bianchi G, Pedemonte CH, Bertorello AM. Hypertension-linked mutation in the adducin alpha-subunit leads to higher AP2-mu2 phosphorylation and impaired Na+,K+-ATPase trafficking in response to GPCR signals and intracellular sodium. Circ Res 95: 1100–1108, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Efendiev R, Pedemonte CH. Contrary to rat-type, human-type Na,K-ATPase is phosphorylated at the same amino acid by hormones that produce opposite effects on enzyme activity. J Am Soc Nephrol 17: 31–38, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Efendiev R, Yudowski GA, Zwiller J, Leibiger B, Katz AI, Berggren PO, Pedemonte CH, Leibiger IB, Bertorello AM. Relevance of dopamine signals anchoring dynamin-2 to the plasma membrane during Na+,K+-ATPase endocytosis. J Biol Chem 277: 44108–44114, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Féraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Glynn IM, Karlish SJ. The sodium pump. Annu Rev Physiol 37: 13–55, 1975. [DOI] [PubMed] [Google Scholar]
- 23.Gottardi CJ, Dunbar LA, Caplan MJ. Biotinylation and assessment of membrane polarity: caveats and methodological concerns. Am J Physiol Renal Fluid Electrolyte Physiol 268: F285–F295, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Guimaraes J, Vieira-Coelho M, Serrao M, Soares-da-Silva P. Opossum kidney (OK) cells in culture synthesize and degrade the natriuretic hormone dopamine: a comparison with rat renal tubular cells. Int J Biochem Cell Biol 29: 681–688, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Hussain T, Lokhandwala MF. Renal dopamine receptor function in hypertension. Hypertension 32: 187–197, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Kashgarian M, Biemesderfer D, Caplan M, Forbush B 3rd. Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int 28: 899–913, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Kimura T, Allen PB, Nairn AC, Caplan MJ. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell 18: 4508–4518, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 30.Lingrel J, Moseley A, Dostanic I, Cougnon M, He S, James P, Woo A, O'Connor K, Neumann J. Functional roles of the alpha isoforms of the Na,K-ATPase. Ann NY Acad Sci 986: 354–359, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Malstrom K, Stange G, Murer H. Identification of proximal tubular transport functions in the established kidney cell line, OK. Biochim Biophys Acta 902: 269–277, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Manneville JB Use of TIRF microscopy to visualize actin and microtubules in migrating cells. Methods Enzymol 406: 520–532, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Moe OW Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol 10: 2412–2425, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Nash SR, Godinot N, Caron MG. Cloning and characterization of the opossum kidney cell D1 dopamine receptor: expression of identical D1A and D1B dopamine receptor mRNAs in opossum kidney and brain. Mol Pharmacol 44: 918–925, 1993. [PubMed] [Google Scholar]
- 35.Oheim M, Loerke D, Chow RH, Stuhmer W. Evanescent-wave microscopy: a new tool to gain insight into the control of transmitter release. Philos Trans R Soc Lond B Biol Sci 354: 307–318, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedemonte CH, Bertorello AM. Short-term regulation of the proximal tubule Na,K-ATPase: increased/decreased Na,K-ATPase activity mediated by protein kinase C isoforms. J Bioenerg Biomembr 33: 479–488, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol 25: 322–327, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Pedemonte CH, Pressley TA, Cinelli AR, Lokhandwala MF. Stimulation of protein kinase C rapidly reduces intracellular Na+ concentration via activation of the Na+ pump in OK cells. Mol Pharmacol 52: 88–97, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Pedemonte CH, Pressley TA, Lokhandwala MF, Cinelli AR. Regulation of Na,K-ATPase transport activity by protein kinase C. J Membr Biol 155: 219–227, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Pressley TA Ion concentration-dependent regulation of Na,K-pump abundance. J Membr Biol 105: 187–195, 1988. [DOI] [PubMed] [Google Scholar]
- 41.Seri K, Kone BC, Gullans SR, Aperia A, Brenner BM, Ballermann BJ. Influence of Na intake on dopamine-induced inhibition of renal cortical Na,K-ATPase. Am J Physiol Renal Fluid Electrolyte Physiol 258: F52–F60, 1990. [DOI] [PubMed] [Google Scholar]
- 42.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol 2: 268–275, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Taormino JP, Fambrough DM. Pre-translational regulation of the (Na++K+)-ATPase in response to demand for ion transport in cultured chicken skeletal muscle. J Biol Chem 265: 4116–4123, 1990. [PubMed] [Google Scholar]
- 44.Taraska JW, Perrais D, Ohara-Imaizumi M, Nagamatsu S, Almers W. Secretory granules are recaptured largely intact after stimulated exocytosis in cultured endocrine cells. Proc Natl Acad Sci USA 100: 2070–2075, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tramier M, Piolot T, Gautier I, Mignotte V, Coppey J, Kemnitz K, Durieux C, Coppey-Moisan M. Homo-FRET versus hetero-FRET to probe homodimers in living cells. Methods Enzymol 360: 580–597, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Trivedi M, Narkar VA, Hussain T, Lokhandwala MF. Dopamine recruits D1A receptors to Na-K-ATPase-rich caveolar plasma membranes in rat renal proximal tubules. Am J Physiol Renal Physiol 287: F921–F931, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Vinciguerra M, Arnaudeau S, Mordasini D, Rousselot M, Bens M, Vandewalle A, Martin PY, Hasler U, Feraille E. Extracellular hypotonicity increases Na,K-ATPase cell surface expression via enhanced Na+ influx in cultured renal collecting duct cells. J Am Soc Nephrol 15: 2537–2547, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Vinciguerra M, Deschenes G, Hasler U, Mordasini D, Rousselot M, Doucet A, Vandewalle A, Martin PY, Feraille E. Intracellular Na+ controls cell surface expression of Na,K-ATPase via a cAMP-independent PKA pathway in mammalian kidney collecting duct cells. Mol Biol Cell 14: 2677–2688, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolitzky BA, Fambrough DM. Regulation of the (Na++K+)-ATPase in cultured chick skeletal muscle. Modulation of expression by the demand for ion transport. J Biol Chem 261: 9990–9999, 1986. [PubMed] [Google Scholar]
- 50.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+,K+-ATPase alpha-subunit and regulates its trafficking. Proc Natl Acad Sci USA 97: 6556–6561, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Mircheff AK, Hensley CB, Magyar CE, Warnock DG, Chambrey R, Yip KP, Marsh DJ, Holstein-Rathlou NH, McDonough AA. Rapid redistribution and inhibition of renal sodium transporters during acute pressure natriuresis. Am J Physiol Renal Fluid Electrolyte Physiol 270: F1004–F1014, 1996. [DOI] [PubMed] [Google Scholar]