Abstract
In urinary bladder smooth muscle (UBSM), stimulation of β-adrenergic receptors (β-ARs) leads to activation of the large-conductance Ca2+-activated K+ (BK) channel currents (Petkov GV and Nelson MT. Am J Physiol Cell Physiol 288: C1255–C1263, 2005). In this study we tested the hypothesis that the BK channel mediates UBSM relaxation in response to β-AR stimulation using the highly specific BK channel inhibitor iberiotoxin (IBTX) and a BK channel knockout (BK-KO) mouse model in which the gene for the pore-forming subunit was deleted. UBSM strips isolated from wild-type (WT) and BK-KO mice were stimulated with 20 mM K+ or 1 μM carbachol to induce phasic and tonic contractions. BK-KO and WT UBSM strips pretreated with IBTX had increased overall contractility, and UBSM BK-KO cells were depolarized with ∼12 mV. Isoproterenol, a nonspecific β-AR agonist, and forskolin, an adenylate cyclase activator, decreased phasic and tonic contractions of WT UBSM strips in a concentration-dependent manner. In the presence of IBTX, the concentration-response curves to isoproterenol and forskolin were shifted to the right in WT UBSM strips. Isoproterenol- and forskolin-mediated relaxations were enhanced in BK-KO UBSM strips, and a leftward shift in the concentration-response curves was observed. The leftward shift was eliminated upon PKA inhibition with H-89, suggesting upregulation of the β-AR-cAMP pathway in BK-KO mice. These results indicate that the BK channel is a key modulator in β-AR-mediated relaxation of UBSM and further suggest that alterations in BK channel expression or function could contribute to some pathophysiological conditions such as overactive bladder and urinary incontinence.
Keywords: BK channel knockout mouse, isoproterenol, forskolin, iberiotoxin
during voiding, the urinary bladder smooth muscle (UBSM) contracts forcefully to expel urine. UBSM exhibits spontaneous action potentials that are associated with the phasic nature of the contractions in this tissue (2, 6, 17). Ca2+ entry through dihydropyridine-sensitive L-type voltage-gated Ca2+ (CaV) channels is responsible for the upstroke of the action potential and leads to an increase in global intracellular Ca2+, which activates the UBSM phasic contractions. Blocking L-type CaV channels eliminates the spontaneous action potentials and contractions in UBSM (2, 6). The repolarization phase of the UBSM action potential is mediated by the activity of the large-conductance Ca2+-activated K+ (BK) channels (6) and perhaps the voltage-gated K+ (KV) channels (23). Pharmacological inhibition of UBSM BK channels with a highly specific inhibitor, iberiotoxin (IBTX), increases the action potential duration and frequency, causes membrane potential depolarization (6, 19), and increases the amplitude of phasic contractions (8, 17). The UBSM resting membrane potential is controlled by BK channels, ATP-sensitive K+ channels, and probably KV channels (6, 17, 18, 23). UBSM from knockout (KO) mice lacking either the BK channel pore-forming α- or regulatory β-subunits exhibit increased phasic and nerve-evoked contractions, associated with increased bladder pressure, urine leakage, and significant changes in the urination pattern (14, 17, 22, 24). In essence, the BK channels play a fundamental role in urinary bladder function by opposing increases in UBSM excitability and contractility.
During the bladder filling phase, UBSM accommodates for increasing volumes of urine, providing adequate, low-pressure bladder storage capacity. A population of three different subtypes (β1–β3) of β-adrenergic receptors (β-ARs) mediates the UBSM relaxation in response to norepinephrine released from the sympathetic nerves (1). This sustained β-adrenergic UBSM relaxation facilitates the urine storage by increasing the capacity of the bladder during its filling phase. In UBSM, β-ARs mediate their effect using cAMP as a second messenger and the cAMP-dependent protein kinase (PKA) (for reviews, see Refs. 1 and 25). In guinea pig UBSM, isoproterenol, a nonselective β-AR agonist, inhibits spontaneous action potentials with the associated Ca2+ transients and hyperpolarizes the membrane through PKA activation to mediate relaxation (16). In addition, BK channels can be activated by isoproterenol or by forskolin, an adenylyl cyclase activator that increases intracellular cAMP levels and PKA activity (12), suggesting they play a role in β-AR-mediated relaxation. Using the patch-clamp technique, we have further shown that in guinea pig, UBSM stimulation of β-ARs with isoproterenol activates the BK channels via modulation of localized intracellular Ca2+ signals, known as “Ca2+ sparks,” and by increasing the Ca2+ influx via L-type CaV channels (20).
To test the hypothesis of whether the BK channel mediates the functional relaxation of mouse UBSM in response to β-AR stimulation, we applied the BK channel inhibitor IBTX to wild-type (WT) mouse UBSM that normally expresses a functional BK channel and also used a BK channel knockout (BK-KO) mouse model in which the gene for the pore-forming α-subunit was ablated (Slo1−/− or KCNMA1−/−) (14). Inhibition of the BK channel with IBTX in WT mouse UBSM significantly reduced the β-AR and PKA-mediated UBSM relaxations in response to isoproterenol or forskolin, respectively. However, we found an opposite effect in UBSM tissue from the BK-KO mouse, in which we observed responses indicating an enhanced sensitivity to isoproterenol and forskolin. This suggests that compensatory mechanisms in the β-AR/cAMP/PKA signal transduction pathway may partially overcome the permanent loss of BK channels in β-AR-mediated relaxation, even though the BK channel is a critical element of UBSM excitability and contractility. Collectively, the present data indicate the key importance of the BK channel in UBSM function and reveal differential outcomes after channel pharmacological inhibition or genetic ablation.
METHODS
Tissue preparation.
BK-KO and control littermate WT mice were used in the experiments. The targeted disruption of the BK channel α-subunit gene has been described previously (14). Adult mice of both sexes (3–9 mo old; 25–35 g) were euthanized with an overdose of pentobarbital sodium (150 mg/kg ip), followed by exsanguination. This procedure was carried out in accordance with guidelines of the Animal Welfare Act, the Association for Assessment and Accreditation of Laboratory Animals and the Institutional Animal Care and Use Committee of the University of South Carolina (Animal Use Protocol no. 1426). The entire urinary bladder was removed and placed in ice-cold nominally Ca2+-free dissection solution (see Solutions and drugs for composition). The bladder was then pinned to the bottom of a Sylgard-coated petri dish containing dissection solution. After the surrounding adipose and connective tissue were removed, the bladder was cut open with a longitudinal incision beginning from the urethral orifice. The urothelium and entire mucosal layer of the bladder were removed, and the bladder was pinned serosal side up for dissection.
Contractility studies.
Up to eight small UBSM strips (1–2 mm wide and 5–6 mm long) were cut free from each bladder and transferred to a small petri dish containing dissection solution. Individual strips were placed in thermostatically controlled (37°C) tissue baths (5-ml volume). One end of the strip was attached to a stationary metal hook, and the other end was connected to a force-displacement transducer for isometric tension recording. The force generation by the muscle strips was recorded using a MyoMed myograph and MyoViewer data acquisition system (both from MedAssociates, St. Albans, VT). The UBSM strips were suspended under initial 5 mN tension. These procedures were carried out in a nominally Ca2+-free dissection solution. Five minutes later, the bath solution was replaced with a Ca2+-containing physiological saline solution (see Solutions and drugs for composition) to initiate contractions. An ∼60-min equilibration period ensued during which the bath solution was changed every 15 min. Unlike rat and guinea pig UBSM, mouse UBSM strips often do not exhibit spontaneous phasic contractions. Therefore, the mouse UBSM strips in this study were stimulated by either mild depolarization with 20 mM K+ or muscarinic receptor activation with 1 μM carbachol to initiate phasic contractions.
Membrane potential recordings.
Cell membrane potential was recorded from freshly isolated single UBSM cells using the current-clamp mode of the amphotericin-perforated whole cell configuration of the patch-clamp technique. The signals were filtered using an eight-pole Bessel filter 900CT/9L8L (Frequency Devices, Inc) and recorded using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP version 10.1 software (Molecular Devices, Union City, CA). Patch-clamp pipettes were pulled from borosilicate glass (Sutter Instruments, Novato, CA) using a Narishige PP-830 vertical puller, coated with sticky dental wax to reduce capacitance, and polished with a Micro Forge MF-830 fire polisher (Narishige) to give a final tip resistance of ∼4–6 MΩ. Only cells for which a sufficient degree of amphotericin-induced perforation was achieved (series resistance <26 MΩ) were used in this study. All membrane potential recordings were carried out at room temperature (22–23°C). To isolate single UBSM cells, we placed several UBSM strips in a vial containing 2 ml of the nominally Ca2+-free dissection solution supplemented with 1 mg/ml bovine serum albumin (BSA), 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dithioerythritol and incubated for 25 min at 37°C. After that, the tissue strips were transferred to 2 ml of nominally Ca2+-free dissection solution containing 1 mg/ml BSA, 1 mg/ml collagenase (type II; Sigma), and 100 μM CaCl2 for 5–6 min at 37°C. After the incubation, the digested tissue was washed several times in nominally Ca2+-free dissection solution and then gently triturated to yield single smooth muscle cells. Several drops of the solution containing the dissociated cells were then placed in a recording chamber. Cells were allowed to adhere to the glass bottom of the chamber for about 20 min. Most cells were elongated and had a bright, shiny appearance when examined with a phase-contrast microscope. Cells were used for membrane potential recordings within 4–6 h after isolation.
Solutions and drugs.
The nominally Ca2+-free dissection solution had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, with pH adjusted to 7.3 with NaOH. The Ca2+-containing physiological salt solution was prepared daily and contained (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose and was aerated with 95% O2-5% CO2 to obtain pH 7.4. The extracellular (bath) solution used for membrane potential recordings contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, with pH adjusted to 7.4 with NaOH. The patch pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, with pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin-B. All drugs used in this study (atropine, carbachol, forskolin, H-89, isoproterenol, IBTX, and tetrodotoxin) were purchased from Sigma.
Data analysis and statistics.
UBSM contractions were analyzed using the following software MyoMed (MedAssociates), MiniAnalysis (Synaptosoft), and Origin (OriginLab). We analyzed the changes in all four major parameters of the UBSM phasic and tonic contractions (tone): phasic contraction amplitude, phasic contraction frequency, muscle force integral, and muscle tone. Data were further analyzed with Prism (GraphPad, version 4) and presented using CorelDraw Graphic Suite X3 software (Corel). Cumulative concentration-response curves for isoproterenol and forskolin were obtained by adding increasing concentrations of the drugs directly to the tissue baths every 10 min. A 3-min period from the 7th to the 10th minute after exposure to each drug concentration was taken as the analysis period. To compare the phasic contraction parameters, we normalized data to the 20 mM KCl- or 1 μM carbachol-induced contractions and expressed data as a percentage. Muscle force integral (average muscle force) was determined by integrating the area under the curve of the phasic contractions. Relative increase in tone was determined by measuring changes of the phasic contraction baseline curve. Membrane potential data were analyzed using Clampfit version 10.1. Summary data are presented as means ± SE for n number of separate UBSM strips or single UBSM cells isolated from different mice. The data were assessed for statistical significance using Student's t-test, paired or unpaired, as applicable. A P value <0.05 was considered significant.
RESULTS
β-AR-mediated inhibition of UBSM phasic and tonic contractions in WT mice.
We sought to investigate how BK channels modulate the basic contractile properties of UBSM strips upon pharmacological β-AR stimulation. It is well known that UBSM contractions are modulated by neurotransmitters released from autonomic nerves located in the bladder wall. To minimize any possible effects caused by neurotransmitter release, we performed all experiments in the presence of 1 μM tetrodotoxin, a neuronal Na+ channel blocker.
In WT UBSM strips, a nonselective stimulation of β-ARs with isoproterenol (0.1 nM–1 μM) caused a concentration-dependent decrease in phasic contractions and muscle tone (Fig. 1, top trace). Isoproterenol, over a range of 1–100 μM, almost completely inhibited the contractility. Figure 2 shows the cumulative concentration-response curves for the effect of isoproterenol on the phasic contractions amplitude, frequency, muscle force integral, and muscle tone in WT UBSM strips. As shown, isoproterenol (0.1 nM–100 μM) inhibited the phasic contraction amplitude, frequency, and muscle force integral. It also significantly reduced the muscle tone in WT UBSM strips. In UBSM, a phasic contraction reflects an elevation of Ca2+ entry via L-type CaV channels, caused by a single action potential or a burst of action potentials (5). The amplitude of a phasic contraction depends on the increase in Ca2+ entry caused by membrane depolarization during an action potential, whereas the duration of a phasic contraction depends on a single action potential duration or on a whole burst of action potentials (5–7). The frequency of phasic contractions reflects mechanisms that temporarily cause action potentials to cease, such as an increase in K+ channel conductance (2, 5, 17, 18, 20).
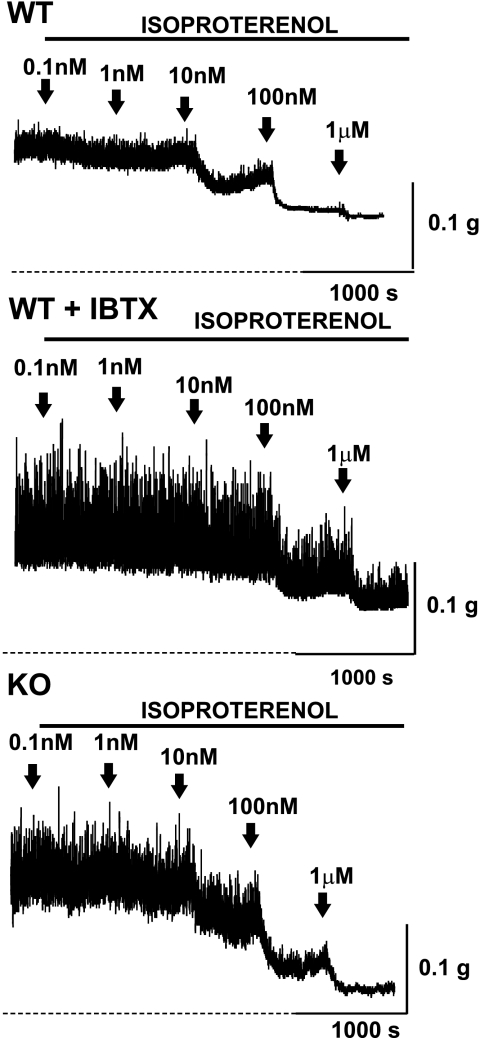
Fig. 1.
Original recordings illustrating the inhibitory effect of isoproterenol on the contractility of urinary bladder smooth muscle (UBSM) strips isolated from wild-type (WT) and large-conductance Ca2+-activated K+ (BK) channel α-subunit knockout (KO) mice. Isoproterenol (0.1 nM–1 μM) inhibited in a concentration-dependent manner both phasic and tonic contractions of WT UBSM strips (top trace), WT UBSM strips pretreated with the BK channel inhibitor iberiotoxin (IBTX; 200 nM; middle trace), and KO UBSM strips (bottom trace). The overall contractility was increased in the presence of IBTX and in the absence of functional BK channel (BK-KO mice). Note that isoproterenol was less effective when the BK channel was inhibited with IBTX but had similar potency in the BK-KO compared with WT mice. Phasic and tonic contractions were induced by addition of 20 mM K+ in the bath solution. Dotted lines indicate the initial baseline level, which corresponds to the muscle tone.
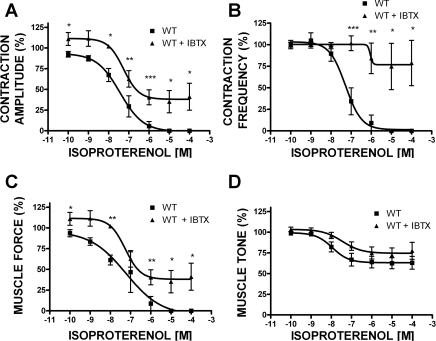
Fig. 2.
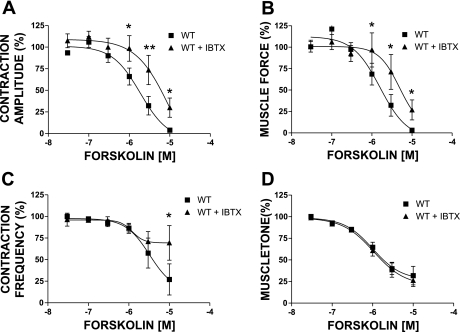
Concentration-response curves for the effects of isoproterenol (0.1 nM–100 μM) on the phasic contraction amplitude (A), phasic contraction frequency (B), muscle force integral (C), and muscle tone (D) in UBSM strips isolated from WT mice under control conditions and after the BK channel was blocked with IBTX (200 nM). In the presence of IBTX, the concentration-response curves were shifted to the right. Data are normalized to the 20 mM K+-induced contractions. Values are means ± SE (n = 4). *P < 0.05; **P < 0.01; ***P < 0.005 (paired test).
BK channel role in the β-AR-mediated relaxation of UBSM strips from WT mice.
Blocking the BK channel with 200 nM IBTX increased the phasic contraction amplitude and the overall UBSM contractility in the WT mouse (Fig. 1, middle trace; see also Ref. 17). In the presence of 200 nM IBTX, the concentration-response curves for isoproterenol (0.1 nM–100 μM) were shifted significantly to the right (Fig. 2). After the BK channel pharmacological inhibition, isoproterenol, even at a concentration of 100 μM, could not completely inhibit the phasic contraction amplitude, frequency, muscle force integral, and muscle tone. This clearly indicates a key role of the BK channel in UBSM β-AR-mediated relaxation.
Increase in β-AR-mediated relaxation of UBSM strips from BK-KO mice.
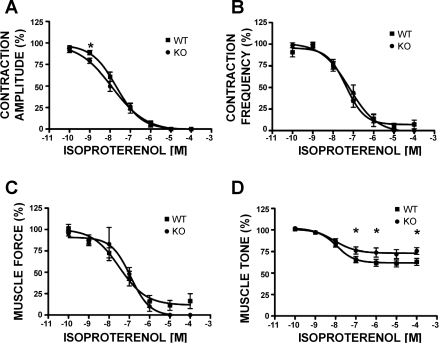
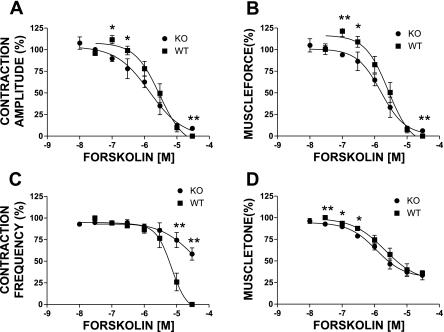
To test this hypothesis further, we used UBSM strips isolated from BK-KO mice. Deletion of the pore-forming BK channel α-subunit (Slo gene) completely eliminates the activity of BK channels in UBSM from BK-KO mice (14, 22, 24). Theoretically, this should have functional consequences that are equivalent to pharmacologically blocking the BK channels with IBTX. Indeed, UBSM strips from BK-KO mice had increased overall contractility, similar to UBSM strips from WT mice in the presence of 200 nM (Fig. 1, bottom trace; see also Ref. 14). Interestingly, however, in UBSM strips isolated from the BK-KO mouse, isoproterenol (0.1 nM–10 μM) completely inhibited the phasic contractions amplitude, frequency, and muscle force integral and significantly reduced the muscle tone, similar to the effect observed in UBSM from WT mice (Figs. 1, bottom trace, and 3). Interestingly, the concentration-response curves for isoproterenol (0.1 nM–100 μM) in UBSM strips from BK-KO mice did not show a rightward shift as was observed when the BK channels were blocked with IBTX in UBSM strips from WT mice (Fig. 3). In the case of phasic contraction amplitude, even a leftward shift in the isoproterenol concentration-response curves was noted (Fig. 3). These results imply that the genetic deletion of the BK channels differentially modulates the outcomes of β-AR-mediated relaxation and that new compensatory mechanisms are developed.
Fig. 3.
Concentration-response curves for the effects of isoproterenol (0.1 nM–100 μM) on the phasic contraction amplitude (A), phasic contraction frequency (B), muscle force integral (C), and muscle tone (D) in UBSM strips isolated from WT and BK-KO mice. Surprisingly, there was no rightward shift in the concentration-response curves for the contraction amplitude (A), frequency (B), and muscle force (C) such as that observed in the WT animals in the presence of IBTX (see Fig. 2), and even a leftward shift was noted on the effects on the phasic contraction amplitude (A). Data are normalized to the 20 mM K+-induced contractions. Values are means ± SE (n = 14–21). *P < 0.05 (unpaired test).
PKA-induced relaxation of UBSM phasic and tonic contractions in WT mice.
In UBSM, β-ARs mediate their intracellular effects primarily by using cAMP-PKA signal transduction pathways (for reviews, see Refs. 1 and 25). To further test the PKA role in transmitting the β-AR effects to the BK channels, we employed forskolin, an adenylyl cyclase activator that increases intracellular cAMP levels and PKA activity.
UBSM strips from WT mice exposed to cumulatively increasing concentrations of forskolin (30 nM–10 μM) responded with relaxation similar to the effect of isoproterenol (Fig. 4, top trace). As shown in Fig. 4, a complete inhibition of contractility was observed at a concentration of 10 μM forskolin. Figure 5 shows the cumulative concentration-response curves for the effect of forskolin on phasic contraction amplitude, frequency, muscle force integral, and muscle tone in WT UBSM strips. As shown, forskolin (30 nM–10 μM) inhibited all four parameters of contractility in WT UBSM strips (Fig. 5).
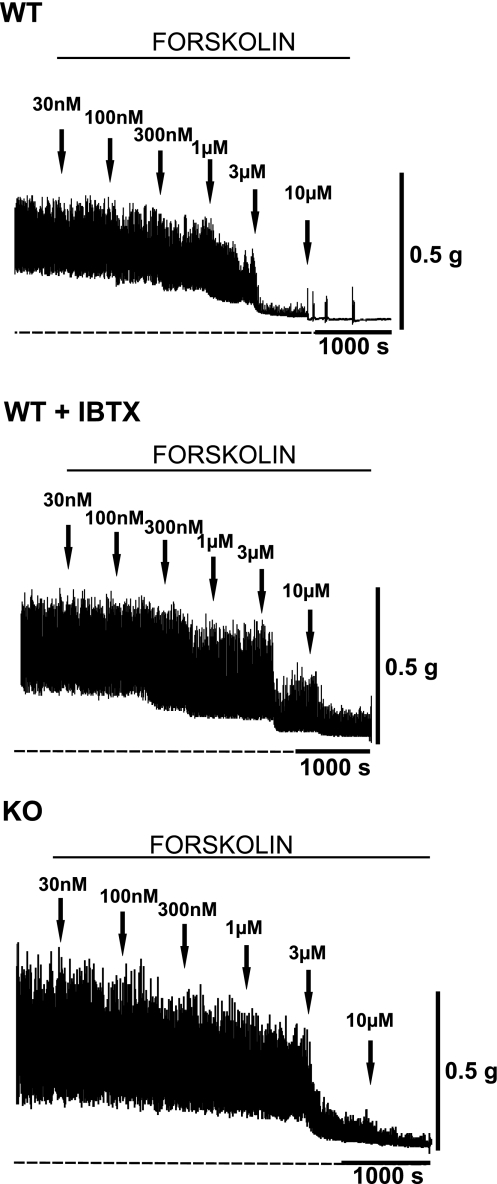
Fig. 4.
Original recordings illustrating the inhibitory effect of forskolin on the contractility of UBSM strips isolated from WT and BK-KO mice. Forskolin (30 nM–10 μM) inhibited in a concentration-dependent manner both phasic and tonic contractions of WT UBSM strips (top trace), WT UBSM strips pretreated with the BK channel inhibitor IBTX (200 nM; middle trace), and KO UBSM strips (bottom trace). The overall contractility was increased in the presence of IBTX and in the absence of functional BK channel (KO mice). Note that forskolin was less effective when the BK channel was inhibited with IBTX but had similar potency in the KO compared with WT mice. Phasic and tonic contractions were induced by addition of 1 μM carbachol in the bath solution. Dotted lines indicate the initial baseline level, which corresponds to the muscle tone.
Fig. 5.
Concentration-response curves for the effects of forskolin (30 nM–10 μM) on the phasic contraction amplitude (A), muscle force integral (B), phasic contraction frequency (C), and muscle tone (D) in UBSM strips isolated from WT mice under control conditions and after the BK channel was blocked with IBTX (200 nM). In the presence of IBTX, the concentration-response curves were shifted to the right. Data are normalized to the 1 μM carbachol-induced contractions. Values are means ± SE (n = 5). *P < 0.05; **P < 0.01 (paired test).
BK channel role in the PKA-induced relaxation of UBSM strips from WT mice.
In WT UBSM strips, forskolin (even at 10 μM) could not completely inhibit the contractility after the BK channel was pharmacologically inhibited with IBTX (Figs. 4, middle trace, and 5). After BK channel inhibition with 200 nM IBTX, the concentration-response curves for forskolin (30 nM–10 μM) on the phasic contraction amplitude, frequency, and muscle force integral were shifted significantly to the right (Fig. 5). These results suggest the importance of the BK channel in UBSM PKA-induced relaxation in WT mice.
Increased PKA-induced relaxation of UBSM strips from BK-KO mice.
To further evaluate the role of the BK channels in the cAMP-PKA signaling pathway, we used UBSM strips isolated from BK-KO mice. In these strips, forskolin (30 nM–10 μM) inhibited phasic contraction amplitude, frequency, muscle force integral, and muscle tone, similar to the effect in UBSM from WT mice (Fig. 4, bottom trace). Figure 6 compares the concentration-response curves for forskolin (30 nM–10 μM) effects on phasic contraction amplitude, frequency, muscle force integral, and muscle tone in UBSM strips from WT and BK-KO mice. In UBSM strips from BK-KO mice, forskolin effects on the contractions, with the exception of contraction frequency, were significantly more pronounced than in UBSM from WT mice, and leftward shifts in the concentration-response curves were observed. This observation suggests that the lack of BK channels could cause compensatory upregulation of the cAMP-PKA signal transduction pathway in UBSM.
Fig. 6.
Concentration-response curves for the effects of forskolin (10 nM–30 μM) on the phasic contraction amplitude (A), muscle force integral (B), phasic contraction frequency (C), and muscle tone (D) in UBSM strips isolated from WT and BK-KO mice. Surprisingly, with the exception of the forskolin effect on contraction frequency (C), there was no rightward shift in the concentration-response curves such as that observed in the WT animals in the presence of IBTX (see Fig. 5). Instead, a leftward shift was noted on the effects on the phasic contraction amplitude (A), muscle force (B), and muscle tone (D). Data are normalized to the 1 μM carbachol-induced contractions. Values are means ± SE (n = 8–10). *P < 0.05; **P < 0.01 (unpaired test).
PKA inhibition eliminates the increase in β-AR-mediated relaxation of UBSM strips from BK-KO mice.
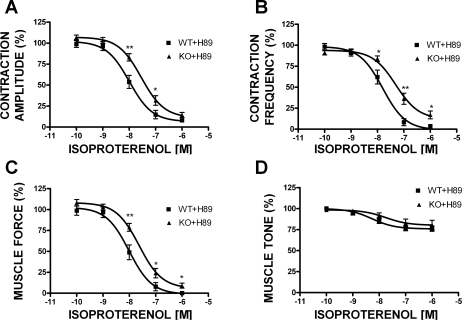
To test the hypothesis that permanent deletion of BK channels in BK-KO animals causes compensatory upregulation of PKA in UBSM, we used the PKA-specific inhibitor H-89. This inhibitor was selected because of its membrane permeability. In this experimental series, UBSM strips isolated from WT and BK-KO mice were pretreated with 3 μM H-89 for 30 min, and then increasing concentrations of isoproterenol were applied to build concentration-response curves. In the presence of 3 μM H-89, the concentration-response curves for isoproterenol in UBSM strips from BK-KO mice showed a rightward shift, as was originally expected and similar to the effect of blocking the BK channels with IBTX in UBSM strips from WT mice (Fig. 7). This experimental series confirms that permanent deletion of BK channels causes compensatory upregulation of PKA, which is eliminated upon PKA inhibition.
Fig. 7.
Concentration-response curves for the effects of isoproterenol (0.1 nM–1 μM) on the phasic contraction amplitude (A), phasic contraction frequency (B), muscle force integral (C), and muscle tone (D) in UBSM strips isolated from WT and BK-KO mice in the presence of 3 μM H-89, a PKA inhibitor. When the PKA was inhibited with H-89 (3 μM), a rightward shift in the concentration-response curves for the contraction amplitude (A), frequency (B), and muscle force (C) was observed in the BK-KO mice. Data are normalized to the 20 mM K+-induced contractions. Values are means ± SE (n = 11–16). *P < 0.05; **P < 0.01 (unpaired test).
IBTX did not affect the contractility of UBSM strips isolated from BK-KO strips.
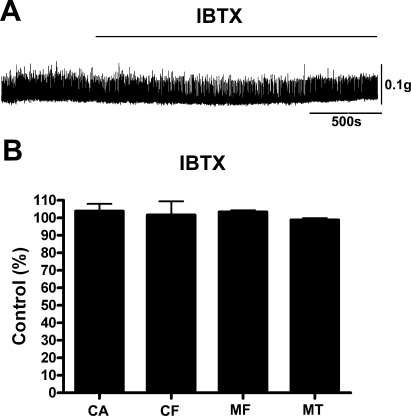
Deletion of the BK channel should have functional consequences that are identical to blocking BK channels with IBTX. Therefore, IBTX should have no effect on the BK-KO mouse. Indeed, application of 200 nM IBTX did not change any of the contraction parameters in UBSM strips from BK-KO mice (n = 5, P > 0.05; Fig. 8).
Fig. 8.
IBTX has no effect on the contractility of UBSM strips isolated from the BK-KO mice. A: original recordings illustrating the lack of effect of IBTX (200 nM) on the contractility of a UBSM strip isolated from the BK-KO mouse. Phasic and tonic contractions were induced by addition of 20 mM K+ in the bath solution. B: summary data illustrating the effect of IBTX (200 nM) on contraction amplitude (CA), contraction frequency (CF), muscle force (MF), and muscle tone (MT) in UBSM strips isolated from BK-KO mice. Data are normalized to the 20 mM K+-induced contractions (control). Values are means ± SE (n = 5). P > 0.05 (paired test).
UBSM cells from the BK-KO mouse have depolarized resting membrane potential.
In UBSM, the BK channel contributes to the maintenance of the resting membrane potential, and pharmacological inhibition of the BK channel with IBTX causes depolarization of the resting membrane potential (6, 19). The deletion of the BK channel in UBSM cells from the BK-KO mouse should also lead to membrane potential depolarization. To test this hypothesis, we performed current-clamp experiments using the perforated whole cell patch-clamp technique when the cells’ native environment was preserved. Under current-clamp conditions, the resting membrane potential in UBSM cells isolated from WT mice was −34.9 ± 4.6 mV (n = 6), and that in UBSM cells from BK-KO animals was −22.5 ± 2.2 mV (n = 5, P < 0.05). There was no difference in the cell capacitance. UBSM cells isolated from WT mice had a cell capacitance of 35.4 ± 9.3 pA/pF (n = 6), and UBSM cells from BK-KO animals had a cell capacitance of 34.6 ± 12.1 pA/pF (n = 5, P > 0.05). These experiments indicate that permanent deletion of the BK channel in UBSM cells from the BK-KO mouse is associated with membrane potential depolarization leading to an increased Ca2+ influx via L-type CaV channels and, thus, increased contractility (Fig. 9).
Fig. 9.
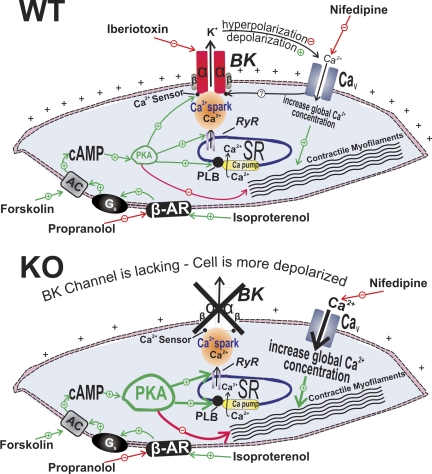
Proposed mechanisms by which BK channels mediate β-adrenergic relaxation in UBSM cells with illustrations of the differential outcomes following channel pharmacological inhibition or genetic ablation in the WT (top) and the BK-KO mouse (bottom), respectively. In UBSM cells from WT mice, functionally active BK channels regulate Ca2+ entry via L-type voltage-gated Ca2+ (CaV) channels, and thus contractility (top). In addition, BK channels are under the local control of the so-called “Ca2+ sparks” caused by Ca2+ release from the ryanodine receptors of the sarcoplasmic reticulum, adjacent to the cell membrane (9, 20). After permanent BK channel gene deletion in the BK-KO mouse, an adaptive compensatory upregulation of the β-adrenergic receptor (β-AR)-cAMP-PKA signaling pathway develops. The enhanced β-AR/PKA activity compensates for the increased Ca2+ entry via L-type CaV channels that occurs due to sustained membrane depolarization in the absence on the BK channels. AC, adenylyl cyclase; Gs, stimulatory G protein; PKA, cAMP-dependent protein kinase; PLB, phospholamban; RyR, ryanodine receptor; SR, sarcoplasmic reticulum.
DISCUSSION
The present study reveals the fundamental role of the BK channels in β-adrenergic and PKA-mediated relaxation in mouse UBSM. Pharmacological inhibition of BK channels (IBTX in WT mice) and genetic ablation of the pore-forming BK channel α-subunit (BK-KO mouse) have opposing effects on β-adrenergic and PKA-mediated relaxation: inhibition vs. enhancement of the relaxation, respectively. Most likely, the unexpected effects in BK-KO mice are due to development of a compensatory adaptive upregulation of the β-AR-cAMP-PKA signal transduction pathway. Such a mechanism may compensate for the enhanced Ca2+ entry via L-type CaV channels, which occurs due to membrane depolarization developed after permanent BK channel gene ablation in the BK-KO mouse.
The central role played by BK channels in modulating UBSM function derives from their functionally antagonistic relationship with the L-type CaV channels that deliver the extracellular Ca2+ influx necessary to activate contraction (Fig. 9). As key regulators of UBSM membrane excitability, the BK channels control the opening and closing of the L-type CaV channels and, therefore, UBSM contractility. In general, inhibition of the UBSM BK channels leads to an increased membrane excitability and contractility, whereas their activation hyperpolarizes the membrane and decreases the contractility (Fig. 9; for reviews, see Refs. 2 and 4). Ca2+ is an important regulator not only of UBSM contractility but also of BK channels (17, 20). In UBSM, BK channels are under the local control of so-called “Ca2+ sparks” caused by Ca2+ release from the ryanodine receptors (RyRs) of the sarcoplasmic reticulum (SR), adjacent to the plasma membrane (Fig. 9; 8, 9, 19, 20). β-AR stimulation with isoproterenol, which leads to PKA activation, has been shown to increase Ca2+ spark activity in guinea pig UBSM (20). The latter effect appears to be mediated by PKA-induced phosphorylation of phospholamban, which, when in a phosphorylated state, activates the SR Ca2+ pump and elevates SR Ca2+ load and, thus, RyRs and Ca2+ spark activity (Fig. 9). Under physiological conditions, when the BK channels are functional, stimulation of β-AR/PKA causes activation of BK channels and, thus, membrane hyperpolarization (12, 16, 20). This leads to closing of the L-type CaV channels, reduction of the global intracellular Ca2+ concentration, and relaxation (Fig. 9). Pharmacological inhibition of the BK channels with IBTX blocks this mechanism and prevents β-AR/PKA-mediated relaxation.
The long-term consequences that might have occurred in mice without functional BK channels (BK-KO mice) may be fundamentally different and involve complex compensatory mechanisms that substitute for the permanent lack of these fundamental channels (Fig. 9). We found that, in this case, the UBSM cell membrane is constantly depolarized with about 12 mV, leading to permanently increased L-type CaV channel activity and massive Ca2+ influx (Fig. 9). Thus one might predict that β-AR/PKA-signaling pathways are enhanced to compensate for the increased L-type CaV channel activity. Indeed, we found that permanent deletion of BK channels in BK-KO mice causes compensatory increase in PKA activity, which is prevented by blocking PKA with its specific inhibitor, H-89 (Fig. 7).
Our data on UBSM are in agreement with a recently published study from Peter Ruth's group on airway smooth muscle using a similar BK channel-deficient animal model (21). Ruth's group also observed that their BK-KO mouse model had an enhanced sensitivity to isoproterenol and that airway smooth muscle cells from BK-KO mice were depolarized with about 10 mV compared with cells from WT mice (21). However, it should be mentioned that in the absence of β-AR/PKA stimulation, IBTX and genetic BK channel deletion had equivalent effects on UBSM contractility (14, 22, 24). These results suggest that in urinary bladder and airway smooth muscle, the cAMP/PKA system in the BK-KO mouse compensates for the lack of a BK channel so that other pathways are engaged.
The present findings suggest that loss-of-function mutations in the BK channel gene could contribute to bladder disease in humans. Overactive bladder and urinary incontinence are manifested by increased UBSM phasic contractions that often follow partial bladder outlet obstruction (PBOO), which is most often the result of an enlarged prostate due to benign prostatic disease (for review, see Ref. 1). Because the BK channel is arguably one of the most physiologically relevant modulators of UBSM excitability, mutations of this channel should lead to dramatic functional effects and increased contractility as observed in overactive bladder due to PBOO. Collective evidence, accumulated in the past several years, supports the idea that the BK channel may be involved in the etiology of overactive bladder and urinary incontinence subsequent to PBOO. This evidence can be summarized as follows. 1) The BK channel is one of the most important K+ channels that controls membrane excitability in human UBSM (19), and therefore slight changes in its activity would lead to significant functional consequences in humans. 2) Inhibition of the BK channel with IBTX increases the action potential frequency in guinea pig UBSM (6). An analogous increase in the UBSM action potential frequency also has been observed in animal models with experimental PBOO (13). In addition, BK channel inhibition with IBTX leads to increased overall UBSM contractility (17), resembling overactive bladder behavior following PBOO. 3) Genetic deletion of the BK channel regulatory β1- or pore-forming α-subunits (KO animals; see Fig. 9) leads to UBSM overactivity (14, 17, 22). 4) Overexpression of the BK channel pore-forming α-subunit using naked DNA gene transfer techniques eliminated bladder overactivity due to PBOO in rats (3). The ability of BK channel gene transfer to ameliorate experimental UBSM overactivity is consistent with the opposite phenomenon observed in mice lacking BK channel subunits (see no. 3 above). 5) Expression of RyR, which provides negative feedback regulation of the BK channel to control normal UBSM contractility, is decreased in rat UBSM with experimental PBOO (11). Furthermore, experiments on RyR type 2 (RyR2) heterozygous KO mice (RyR2+/−) suggest that RyR2 deficiency changes UBSM membrane potential and excitation-contraction coupling via BK channel modulation (10). Therefore, changes in BK channel regulatory mechanisms such as RyR, PKA, or β-ARs may be implicated in the pathogenesis of overactive bladder following PBOO. It also has been suggested that long-term PBOO leads to alteration in the β-AR densities or subtypes, changing the bladder response to adrenergic stimuli (15). It is likely that the changes in the β-AR/PKA signaling pathway develop to compensate for the BK channel loss-of-function mutations during PBOO.
In conclusion, our results indicate that BK channels play a key role in mediating the β-adrenergic- and PKA-induced relaxations and that the genetic deletion or pharmacological inhibition of the BK channels differentially modulates the functional outcomes on UBSM contractility. Alterations in BK channel expression or function could be involved in some human pathologies such as overactive bladder that occur following PBOO (19). Our data further suggest that β-adrenergic agonists would be highly effective in alleviating human incontinence that could potentially be due to BK channel loss-of-function mutations.
GRANTS
The work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-070909 (to G. V. Petkov) and DK-053832 and DK-065947 (to M. T. Nelson), as well as a GlaxoSmithKline Young Investigator Grant of The National Kidney Foundation (to G. V. Petkov).
Acknowledgments
We thank Dr. Xiangli Cui (University of South Carolina) and Dr. Jennifer Schnellmann (Medical University of South Carolina) for critical evaluation of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Brading AF Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christ GJ, Day NS, Day M, Santizo C, Zhao W, Sclafani T, Zinman J, Hsieh K, Venkateswarlu K, Valcic M, Melman A. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regul Integr Comp Physiol 281: R1699–R1709, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol 147: S41–S55, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564: 201–212, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Herrera GM, Heppner TJ, Nelson MT. Voltage-dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Hotta S, Morimura K, Ohya S, Muraki K, Takeshima H, Imaizumi Y. Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol 582: 489–506, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang HH, Song B, Lu GS, Wen QJ, Jin XY. Loss of ryanodine receptor calcium-release channel expression associated with overactive urinary bladder smooth muscle contractions in a detrusor instability model. BJU Int 96, 428–433, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Adachi-Akahane S, Nagao T. Involvement of BKCa channels in the relaxation of detrusor muscle via beta-adrenoceptors. Eur J Pharmacol 404: 231–238, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Jiang C, Hao P, Li W, Fan L, Zhou Z, Song B. Changes in T-type calcium channel and its subtypes in overactive detrusor of the rats with partial bladder outflow obstructions. Neurourol Urodyn 26: 870–878, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Moore CK, Levendusky M, Longhurst PA. Relationship of mass of obstructed rat bladders and responsiveness to adrenergic stimulation. J Urol 168: 1621–1625, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Nakahira Y, Hashitani H, Fukuta H, Sasaki S, Kohri K, Suzuki H. Effects of isoproterenol on spontaneous excitations in detrusor smooth muscle cells of the guinea pig. J Urol 166: 335–340, 2001. [PubMed] [Google Scholar]
- 17.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. β1-Subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Petkov GV, Cui X, Hristov K, Rovner E. Electrophysiological and pharmacological evidence for the presence of the large conductance voltage- and Ca2+-activated K+ (BK) channels in human urinary bladder smooth muscle: potential physiological role. In: FASEB Summer Research Conference “Ion Channel Regulation” Abstract Book. Bethesda, MD: FASEB, p. 10, 2007.
- 20.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Sausbier M, Zhou XB, Beier C, Sausbier U, Wolpers D, Maget S, Martin C, Dietrich A, Ressmeyer AR, Renz H, Schlossmann J, Hofmann F, Neuhuber W, Gudermann T, Uhlig S, Korth M, Ruth P. Reduced rather than enhanced cholinergic airway constriction in mice with ablation of the large conductance Ca2+-activated K+ channel. FASEB J 21: 812–822, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi O, Chapple CR. β3-Adrenoceptors in urinary bladder. Neurourol Urodyn 26: 752–756, 2007. [DOI] [PubMed] [Google Scholar]