Abstract
NKCC1 is a widely expressed isoform of the Na-2Cl-K cotransporter that mediates several direct and indirect vascular effects and regulates expression and release of renin. In this study, we used NKCC1-deficient (NKCC1−/−) and wild-type (WT) mice to assess day/night differences of blood pressure (BP), locomotor activity, and renin release and to study the effects of high (8%) or low (0.03%) dietary NaCl intake on BP, activity, and the renin/aldosterone system. On a standard diet, 24-h mean arterial blood pressure (MAP) and heart rate determined by radiotelemetry, and their day/night differences, were not different in NKCC1−/− and WT mice. Spontaneous and wheel-running activities in the active night phase were lower in NKCC1−/− than WT mice. In NKCC1−/− mice on a high-NaCl diet, MAP increased by 10 mmHg in the night without changes in heart rate. In contrast, there was no salt-dependent blood pressure change in WT mice. MAP reductions by hydralazine (1 mg/kg) or isoproterenol (10 μg/mouse) were significantly greater in NKCC1−/− than WT mice. Plasma renin (PRC; ng ANG I·ml−1·h−1) and aldosterone (aldo; pg/ml) concentrations were higher in NKCC1−/− than WT mice (PRC: 3,745 ± 377 vs. 1,245 ± 364; aldo: 763 ± 136 vs. 327 ± 98). Hyperreninism and hyperaldosteronism were found in NKCC1−/− mice during both day and night. High Na suppressed PRC and aldosterone in both NKCC1−/− and WT mice, whereas a low-Na diet increased PRC and aldosterone in WT but not NKCC1−/− mice. We conclude that 24-h MAP and MAP circadian rhythms do not differ between NKCC1−/− and WT mice on a standard diet, probably reflecting a balance between anti- and prohypertensive factors, but that blood pressure of NKCC1−/− mice is more sensitive to increases and decreases of Na intake.
Keywords: radiotelemetry, running wheels, renin, aldosterone, vasodilators, sodium-2 chloride-potassium cotransporter-deficient mice
nkcc1 is a Na-2Cl-K cotransporter that is widely expressed in both epithelial and nonepithelial cells (14, 22, 23). NKCC1 has been shown to play a role in the regulation of cell volume and of transepithelial ion fluxes in secretory tissues and to influence vascular contractility through effects on intracellular ion concentrations (9). In the kidney, NKCC1 is expressed in the basolateral membrane of inner medullary collecting ducts (12, 16) and in extraglomerular mesangial and renin-producing juxtaglomerular (JG) cells of the afferent arteriole (4, 16).
Because of the expression of NKCC1 in vascular smooth muscle cells, attention has been paid to its role in the regulation of vascular contractility and blood pressure. Vascular responses to blocking NKCC1 with loop diuretics include direct vasodilatation, indirect vasodilatation through mediation of vasorelaxing prostaglandins, and reductions in the contractile responses to vasoconstrictors such as norepinephrine and ANG II (11, 15). A reduction of arterial blood pressure has been reported in NKCC1−/− mice using the tail cuff method (19, 26). Furthermore, bumetanide caused a small reduction of blood pressure in wild-type (WT) but not NKCC1−/− mice, suggesting a tonic role of NKCC1 in blood pressure regulation (11). On the other hand, Castrop et al. (4) reported that NKCC1 deficiency is associated with a marked increase of renin secretion that is due to a direct stimulation of renin synthesis and secretion in JG cells. The state of the extracellular volume of NKCC1−/− mice is unclear, but the upregulation of various renal NaCl transporters would lead one to expect an impaired renal excretory ability and concomitant extracellular volume expansion (26). Thus the integrated effect of NKCC1 deficiency on blood pressure regulation has remained uncertain.
The current experiments were performed in WT and NKCC1−/− mice to assess blood pressure regulation over a 24-h period using radiotelemetry. In view of the profound effect of the renin-angiotensin-aldosterone system on blood pressure, we also performed day and night time measurements to assess whether circadian changes in the renin system may vary between WT and NKCC1−/− mice. Finally, we determined the effects of high (8%) or low (0.03%) dietary NaCl intake on blood pressure and the renin-aldosterone system. Our results show that circadian variations of blood pressure do not differ between WT and NKCC1−/− mice on a normal NaCl diet and that hyperreninism and hyperaldosteronism are maintained during both day and night. NKCC1 deficiency is associated, however, with a reduced ability to maintain constancy of blood pressure during changes in dietary salt intake, indicating impaired overall homeostatic capacity.
METHODS
Animals.
WT and NKCC1−/− mice (NKCC1−/−) of both genders of the strain originally generated by Flagella et al. (10) were supplied from a colony maintained at Emory University (Atlanta, GA). Genetically, these mice have a 129/SvJ-Black Swiss mixed background; WT and NKCC1−/− mice were generated by breeding of heterozygous animals. All animals were between 13 and 17 wk of age at the beginning of the study. Animal care and experimentation was approved by the NIDDK Animal Care and Use Committee and carried out in accordance with National Institutes of Health principles and guidelines for the Care and Use of Laboratory Animals.
Salt diets and study protocols.
After baseline studies were completed, four mice of each genotype were placed on either a high (8.0%) or low (0.03%) NaCl in random order (Purina). All diet changes were followed by 1 wk with the transmitter turned off. Thus telemetric data were collected, or plasma for renin and aldosterone measurements was taken after 7 days of adjustment. The β-adrenergic receptor agonist isoproterenol (10 mg/kg) or the direct vasodilator hydralazine (1 mg/kg) was given by intraperitoneal injection with baseline blood pressure measurements starting 120 min before the injection.
Radiotelemetry.
Mice were anesthetized with ketamine and xylazine (90 and 10 mg/kg, respectively), and the left carotid artery was isolated. Transmitters (model TA11PA-C10) were magnetically activated >24 h before implantation. The tip of the telemeter catheter was inserted in the carotid artery and advanced in the aortic arch, with the telemeter body positioned in a subcutaneous pocket on the right flank (2, 3). After surgery (1 day), each animal was returned to its home cage and provided with ad libitum food and water for the duration of the study. The telemeter signal was processed using a model RPC-1 receiver, a 20-channel data exchange matrix, APR-1 ambient pressure monitor, and a Data Quest ART Silver 2.3 acquisition system (Data Sciences International, St. Paul, MN). At 2-min intervals, the system was set to sample the mean, systolic, and diastolic blood pressure, pulse pressure, heart rate (HR), and locomotor activity over a 10-s interval and record their average values. The recording room was maintained at 21–22°C with a 12:12-h light-dark cycle. The implanted telemeter was activated on the morning of the 7th-10th day, and data were collected for the following 7 days. Circadian rhythmicity had returned in all mice. Data shown represent the measurements from the last 3 days of the recording period. To determine whether salt-induced changes in extracellular fluid volume affect blood pressure and HR in NKCC1−/− mice, we continued the telemetric studies in four WT (2 male, 2 female) and four NKCC1−/− (2 male and 2 female) mice by administering a low- or a high-NaCl diet for 1 wk and performing telemetry on the following 3 days.
Wheel running activity.
Wheel running activity was examined in cages equipped with stainless steel running wheels (Mini-Mitter). Animals had free access to food and water and were kept on a 12:12-h light-dark lighting cycle. Cages contained wood-chunk bedding to prevent mechanical blockade of wheel revolutions. Running wheel revolutions were quantified by a magnetic reed switch next to the wheel, and data were stored on a personal computer using Vital View software (Mini-Mitter). Following a training interval, data from an 8-day period were analyzed using Matlab software (The Mathworks) and the Clocklab Toolbox (Actimetrics).
Blood collection and plasma assays.
Blood was taken from conscious mice by tail vein puncture and collected in a 75-μl hematocrit tube that contained 1 μl of 125 mM EDTA in its tip. Tubes were centrifuged to separate red blood cells and plasma, and plasma was frozen until used for renin determination. Plasma renin concentration (PRC) was measured as the generation of ANG I after addition of excess rat substrate with final plasma dilutions varying between 1:500 and 1:1,000 (ANG I radioimmunoassay Gammacoat; Diasorin, Stillwater, MN). ANG I generation was determined for a 1-h incubation period at 37°C. In each assay, background ANG I levels were determined in a plasma aliquot kept frozen without the addition of substrate until assay. Blood collections were made at 11:00 A.M.–1:00 P.M. for daytime PRC measurements and at 11:00 P.M.–1:00 A.M. for nighttime measurements. To assess how representative a single PRC determination is for a given animal, we collected two blood samples at 48-h intervals from 25 mice for repeat PRC measurements. Plasma aldosterone was measured by RIA (Coat-A Count Aldosterone Kit TKAL1; DPC, Los Angeles, CA).
Glomerular filtration rate measurements.
Glomerular filtration rate (GFR) of conscious mice was measured by fluorescein isothiocyanate inulin clearance after a single retroorbital injection and consecutive blood sampling from the tail vein. The method was modified in our laboratory to reduce the required blood withdrawal as described in detail recently (9).
Statistical analysis.
Data are expressed as means ± SE. Statistical comparisons were done by paired t-test for comparisons of PRC before and after an intervention in the same animals, by nonparametric Mann-Whitney test for comparisons between two groups of animals, for example of different genotypes, and by ANOVA (with repeated measures if adequate) for comparisons of more than two data sets. Analysis of telemetry blood pressure data was performed using the Analysis Program of Dataquest A.R.T. 2.3 (DSI). P values <0.05 were considered to indicate a significant difference.
RESULTS
Blood pressure and HR.
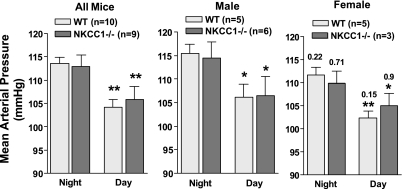
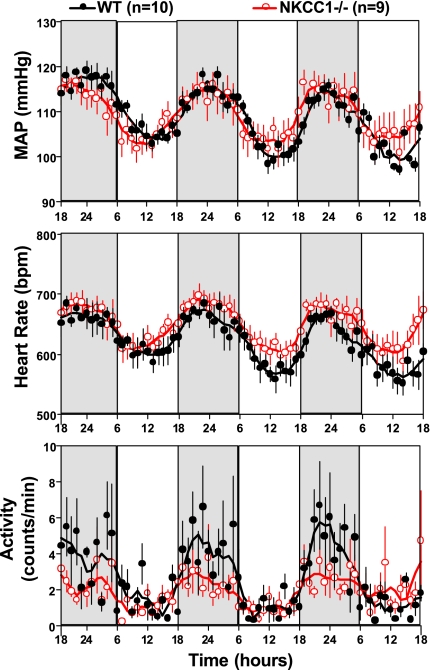
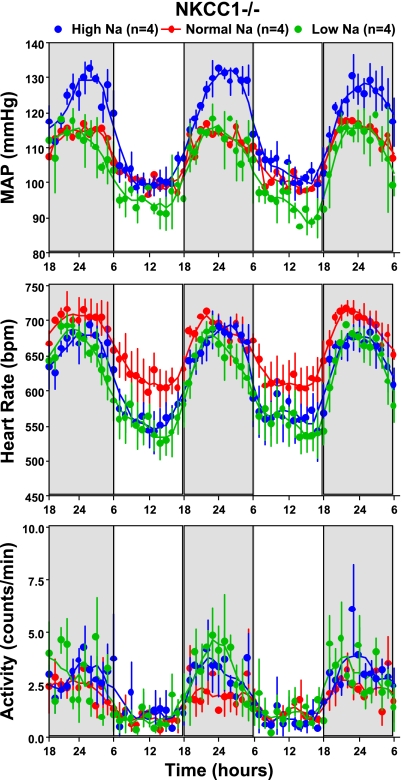
With the use of radiotelemetry, cardiovascular function was assessed in 10 WT (5 male, 5 female) and 9 NKCC1−/− (6 male, 3 female) mice over a 1-wk observation period. Mean arterial pressure (MAP, 24 h) was not significantly different between WT and NKCC1−/− mice when analyzed for the entire group (109 ± 1.6 vs. 109.5 ± 2.4 mmHg; P = 0.84) or when analyzed for male and female mice separately (male: 112 ± 3 vs. 110.6 ± 3.4 mmHg, P = 0.79; female: 108 ± 1.7 vs. 107 ± 2 mmHg; P = 1.0). Similarly, HR was not significantly different between WT and NKCC1−/− animals (entire group: 629 ± 17 vs. 650 ± 14 beats/min, P = 0.6; male: 634 ± 29 vs. 660 ± 17 beats/min, P = 0.33; female: 625 ± 23 vs. 628 ± 23 beats/min; P = 0.78). Figure 1 shows MAP for the 12-h dark and 12-h light periods as averages of the 12-h means of the last three recording days for the entire group of animals and for the animals separated by gender. There was a significant difference in MAP and HR between daytime and nighttime that was of similar magnitude in WT and NKCC1−/− mice but no difference between genotypes either during the night or during the day [WT vs. knockout (KO) night: MAP 114 ± 1 vs. 113 ± 2.5 mmHg, P = 0.72; HR 654 ± 18 vs. 670 ± 15 beats/min, P = 0.5; WT vs. KO day: MAP 104 ± 2 vs. 106 ± 3 mmHg, P = 0.84; HR 596 ± 17 vs. 625 ± 16 beats/min; P = 0.44]. MAP tended to be lower in female than male mice during both day and night, but, as indicated in Fig. 1, differences did not reach significance levels. Thus the circadian MAP and HR amplitudes for the entire group were not statistically different between WT and NKCC1−/− mice (MAP: 9.4 ± 1 vs. 7 ± 1.5 mmHg; P = 0.28; HR: 57 ± 4.4 vs. 45 ± 8.9 beats/min; P = 0.55). Mean hourly values of MAP, HR, and locomotor activity over the last three observation days are shown in Fig. 2. As can be seen, both WT and NKCC1−/− mice demonstrated robust circadian rhythms of arterial pressure, HR, and spontaneous locomotor activity.
Fig. 1.
Mean arterial blood pressure for the 12-h night and day periods in wild-type (WT) and Na-2Cl-K cotransporter-deficient (NKCC1−/−) mice. Results are shown without and with grouping by gender. *P < 0.05 and **P < 0.01 vs. night in same genotype (paired t-test). Nos. for the female data are P values for comparison with corresponding male data (Mann Whitney test).
Fig. 2.
Hourly averages of mean arterial blood pressure (MAP), heart rate, and locomotor activity measured over three successive days (nighttime indicated by darker shading) in 10 WT and 9 NKCC1−/− mice. Connecting lines represent smoothing using the weighted average of the 9 nearest points.
Locomotor activity.
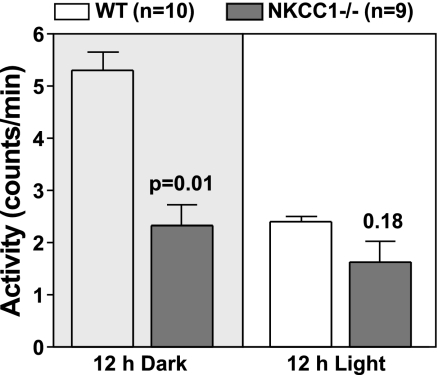
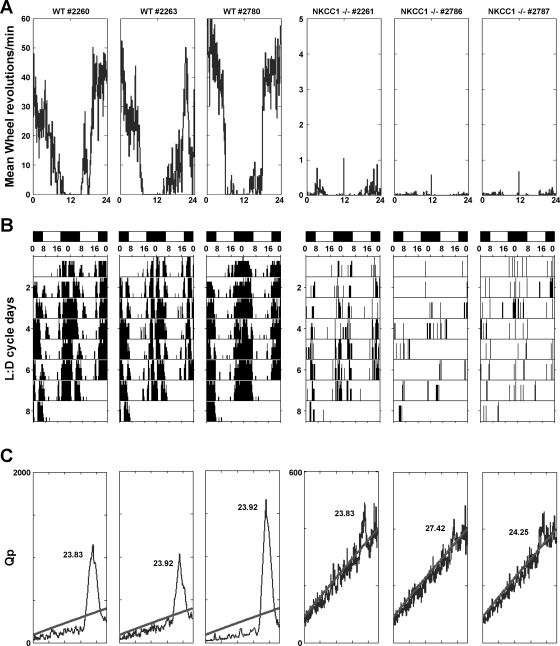
The 24-h mean spontaneous locomotor activity measured by telemetry was found to be significantly higher in WT than NKCC1−/− mice when analyzed for the entire group (3.7 ± 0.2 vs. 1.6 ± 0.4 movement counts/min; P = 0.0006; Fig. 3). When split into gender groups, a significantly higher activity was only seen in male WT mice (4.7 ± 1.4 vs. 1.4 ± 0.7, P = 0.016) but not in female WT mice due to normal activity in one of the three female animals (2.67 ± 0.3 vs. 2.7 ± 1.5, P = 0.78). As shown in Fig. 3, this difference in activity was restricted to the dark period, whereas daytime activity was at comparably low levels in WT and NKCC1−/− mice. It is to be noted that the shaker-type head movements of NKCC1−/− mice are not registered as lateral excursions by the telemetric receiver. To further examine the locomotor behavior of NKCC1−/− mice, we studied the wheel running activities in three female WT and three female mutant animals. As shown in Fig. 4A, wheel running activity was dramatically depressed in NKCC1−/− mice. Furthermore, double-plotted actograms indicate that wheel running activity in WT mice occurred in a robust circadian fashion (Fig. 4B) with a period length of ∼24 h as dictated by the light cycle (Fig. 4C). In contrast, NKCC1−/− mice showed either absence of significant periodicity or a greatly reduced period strength as indicated by the low Qp values (Fig. 4C).
Fig. 3.
Mean locomotor activity during the night (left) and during the day (right) in WT and NKCC1−/− mice. Significance is given for genotype comparison (Mann Whitney test).
Fig. 4.
Wheel running activity in three individual WT (3 boxes on left) and three NKCC1−/− mice (3 boxes on right). A: mean wheel revolution counts over a period of 24 h (0–6 h = dark, 6–18 h = light, 18–24 h = dark); note change in axis in the NKCC1−/− plots on right. B: activity shown as actograms where wheel rotations are double plotted over 48 h; lighting conditions are indicated by black (dark, D)/white (light, L) bars; note clustering of activity during the nighttime. C: chi-square periodogram showing circadian rhythm strength (expressed as Qp value) on the ordinate vs. 24-h period on the abscissa. Values above the oblique line indicate times with significantly higher activity than background (P < 0.05). Peak height indicates rhythm strength, and time given is mean period length. Note change in axis in the NKCC1−/− plots on right.
Plasma renin and aldosterone concentrations.
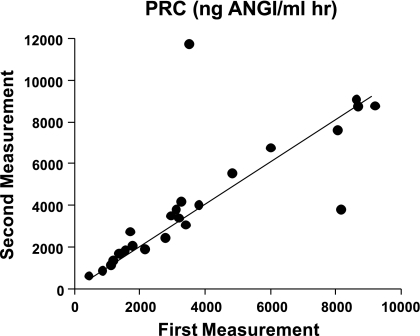
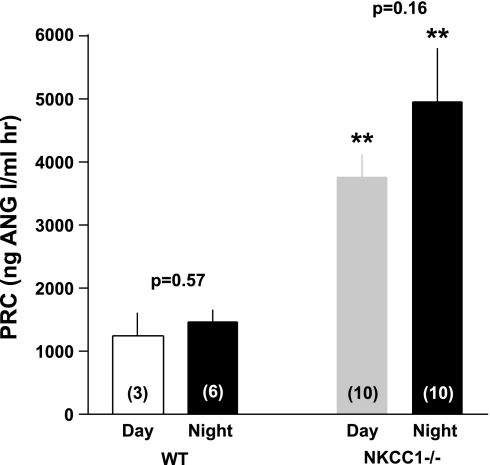
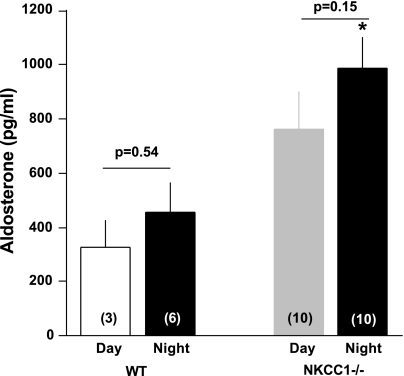
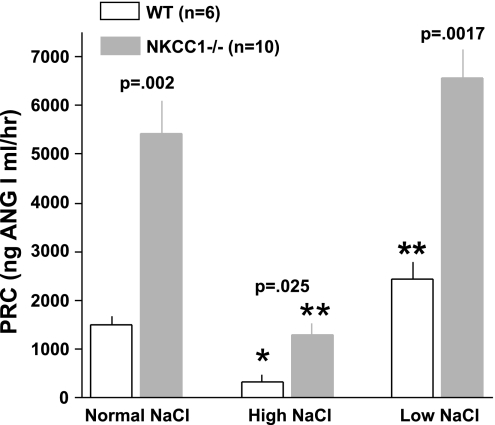
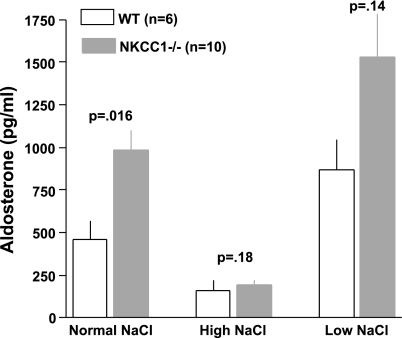
Figure 5 summarizes our experiments in which we determined PRC two times in the same 25 mice with blood taken 48 h apart. The overall regression line was the same as the line of identity (slope 1.01), indicating that over a limited time span under the same conditions single PRC measurements can be taken as representative for a given mouse in >90% of cases. Previous studies have shown that the expression and secretion of renin is stimulated in NKCC1−/− mice (4). To determine whether renin secretion undergoes circadian variations and whether absence of NKCC1 modifies day-night differences, we measured PRC in the same mice around noon and around midnight in WT and NKCC1−/− mice (Fig. 6). Baseline PRC (ng ANG I·ml−1·h−1) in WT averaged 1,245 ± 364 at day (n = 3, all male) and 1,461 ± 186 at night (n = 6; 3 male, 3 female; P = 0.57 comparing day and night values; all determinations are the average of two repeat measurements). In NKCC1−/− mice, mean PRC in the same mice was 3,745 ± 377 at day and 4,932 ± 871 at night (n = 10; night, but not day determinations are the average of two repeat measurements; P = 0.16 for day/night paired comparison). PRC was about threefold higher in NKCC1−/− mice than in WT mice during both day and night (P = 0.007 for day, and P = 0.0002 for night comparison). Plasma aldosterone concentrations (Fig. 7) paralleled those of renin: they were generally lower in WT than in NKCC1−/− mice during both day (327 ± 98 vs. 763 ± 136 pg/ml; n = 3 vs. 10; P = 0.16) and night (456 ± 108 vs. 985 ± 117 pg/ml; n = 6 vs. 10; P = 0.036), whereas day/night differences in a given genotype were not statistically different. GFR as an index of renal function was found to be unaltered by the absence of NKCC1. GFR averaged 344.2 ± 15.8 μl/min in WT (n = 10) and 349.9 ± 19.4 μl/min in NKCC1−/− mice (n = 6).
Fig. 5.
Repeat measurements of plasma renin concentration (PRC) in blood collected from the same animals at 48-h intervals. Line is the linear regression line relating values from first and second determinations. Experiments were done in 25 mice (9 WT and 16 NKCC1−/−) during both day (3 WT and 3 NKCC1−/−) and night (6 WT and 13 NKCC1−/−).
Fig. 6.
Mean PRC in WT and NKCC1−/− mice at day and night (animal nos. given in bars); the same mice were used for day and night measurements with the exception of 3 additional female mice that were only studied at night. P values are given for day/night comparisons; **P < 0.01 comparing genotypes at given day period.
Fig. 7.
Mean plasma aldosterone concentration in WT and NKCC1−/− mice at day and night. Animal nos. are given in bars; the same mice were used during both day and night with the exception of 3 female WT mice that were only assessed at night. P values are given for day/night comparisons (Mann Whitney nonparametric test). *P < 0.05 comparing WT and NKCC1−/− mice.
Na intake and blood pressure.
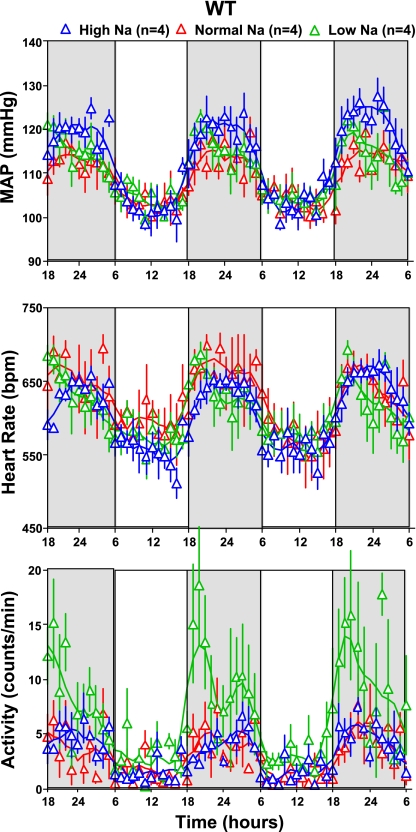
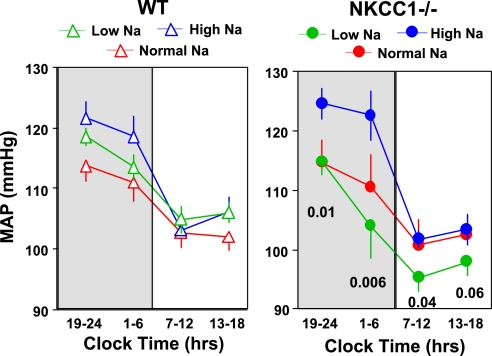
In WT mice (Fig. 8), MAP and HR were not significantly different between animals fed either a low- or high-Na diet although there was a tendency for slightly higher MAP values at night with high Na (night: 119 ± 2 mmHg at high Na vs. 115 ± 2 mmHg at low Na, P = 0.25; day: 105 ± 2 mmHg at high Na, and 106 ± 2 mmHg at low Na). In contrast (Fig. 9), there was a marked elevation of MAP in NKCC1−/− mice fed a high-Na diet that was most pronounced during night and that was not accompanied by a difference in HR (MAP night: high Na 124 ± 3.2 mmHg, low Na 110 ± 3.2 mmHg, P = 0.005; HR night: high Na 661 ± 18 beats/min, low Na 627 ± 31 beats/min, P = 0.12). Data analysis in 6-h bins showed that MAP of NKCC1−/− mice was significantly higher with salt excess than with salt restriction in three of the four time periods (Fig. 10). On the other hand, MAP decreased in salt-restricted NKCC1−/− mice compared with normal or high-Na intake in the inactive phase (low Na: 96 ± 3.3 mmHg, high Na 103 ± 1.9 mmHg, P = 0.007). An additional observation was that a low-Na diet increased locomotor activity compared with high salt at night in WT mice (from 4.3 ± 0.6 to 9.1 ± 1.2 counts/min; P = 0.023), but not in NKCC1−/− animals (Figs. 8 and 9).
Fig. 8.
Hourly averages of MAP, heart rate, and locomotor activity measured over three successive days (night time indicated by darker shading) in 4 WT mice kept on normal (red), low-Na (green), and high-Na (blue) diets. Connecting lines represent smoothing using the weighted average of the 9 nearest points.
Fig. 9.
Hourly averages of MAP, heart rate, and locomotor activity measured over three successive days (nighttime indicated by darker shading) in 4 NKCC1−/− mice kept on normal (red), low-Na (green), and high-Na (blue) diets. Connecting lines represent smoothing using the weighted average of the 9 nearest points.
Fig. 10.
MAP in 6 h bins for WT (left) and NKCC1−/− (right) mice on normal (red), low-Na (green), and high-Na (blue) diets. Nighttime periods are shaded. P values are given for comparison between high- and low-Na diets.
Additional studies were performed to assess whether the regulation of the renin-angiotensin system by dietary NaCl intake is altered in NKCC1−/− mice. As shown in Fig. 11, both WT and NKCC1−/− mice responded to a low-NaCl intake with a slight increase of PRC and to a high-NaCl intake with a significant decrease. NKCC1−/− mice remained relatively hyperreninemic at all levels of salt intake. Changes of plasma aldosterone concentration during changes in NaCl intake (Fig. 12) parallel those of PRC in that a low-NaCl diet caused slight increases and a high-NaCl diet significant decreases in both WT and NKCC1−/− mice.
Fig. 11.
PRC in WT (open bars) and NKCC1−/− mice (gray bars) on normal, high-, and low-NaCl diets. All blood collections were done at night. P values are given for comparison between genotypes (Mann Whitney test). *P < 0.05 and **P < 0.001 compared with normal NaCl diet (one-way ANOVA).
Fig. 12.
Plasma aldosterone concentration in WT (open bars) and NKCC1−/− (gray bars) mice on normal, high-, and low-NaCl diets. All blood collections were done at night. P values are given for comparison between genotypes (Mann Whitney test).
Effects of isoproterenol and hydralazine.
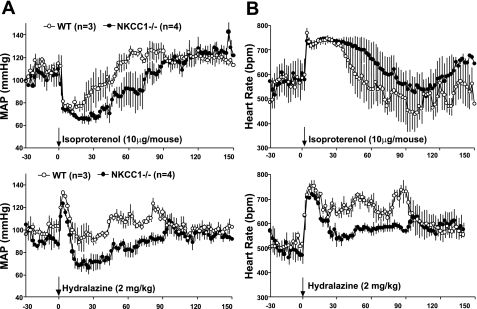
To examine the effect of NKCC1 absence on vasodilator responses, we determined the acute blood pressure response to administration of isoproterenol and hydralazine. As summarized in Fig. 13, both agents reduced blood pressure and increased HR. However, the absolute decreases or durations of the blood pressure reduction were consistently greater in NKCC1−/− than WT mice. In the period 30–60 min after injection of isoproterenol or hydralazine, the average MAP reduction was 3.2 and 0 mmHg in WT and 21.4 and 13.4 mmHg in NKCC1−/− mice, respectively. Areas under the MAP curve were greater in NKCC1−/− than WT mice for both isoproterenol (1,567 vs. 1,105) and hydralazine (975 vs. 678).
Fig. 13.
Effect of an ip injection of isoproterenol and hydralazine in indicated doses on MAP (A) and heart rate (B) in WT and NKCC1−/− mice.
DISCUSSION
The main focus of the present study was a reassessment of the role of the NKCC1 transporter in the regulation of cardiovascular function using radiotelemetry in NKCC1−/− mice. Our observations show that MAP and HR did not significantly differ between WT and NKCC1−/− animals using either 24-h mean values or average values for the active nighttime and inactive daytime periods. Furthermore, there were no significant gender-specific differences in blood pressure in either WT or NKCC1−/− mice. The discrepancy between the present data and previous observations showing a reduction in MAP may be technical in nature (10, 19, 26). Whereas telemetry permits acquisition of blood pressure information with minimum disturbance, increases in blood pressure have been shown to result from animal restraint required for the tail cuff method even after the recommended training period, and it is conceivable that this effect differs between mice of different backgrounds and genotypes (13). It is also possible that subtle differences in animal housing and maintenance could be responsible for variations in blood pressure regulation. This scenario is feasible since the phenotypic description of NKCC1−/− mice has identified both anti- and prohypertensive factors so that the specific mix of these variables and therefore the blood pressure endpoint may be determined by specific circumstances.
An increase in renin expression and renin secretion with an associated marked rise of PRC has been described previously and constitutes an important prohypertensive factor in NKCC1−/− mice (4, 26). The current observations confirm these earlier results and show in addition that elevation of PRC persists in the active nighttime phase. Absence of significant blood pressure differences indicates that renin stimulation in NKCC1−/− mice is not baroreceptor-mediated, but probably results from direct stimulation of renin secretion from JG cells (4). A second prohypertensive factor in NKCC1−/− mice is upregulation of renal NaCl transporters (26). Both proximal transporters such as Na/H exchanger 3 and NKCC2, distal transporters such as the NaCl cotransporter (NCC), and ubiquitous transporters such as α-Na-K-ATPase showed increased expression at least on a low-NaCl diet (26). To the extent that NKCC1 mediates net Cl secretion along the collecting duct, a reduction in Cl secretion in NKCC1−/− mice could be an additional reason for fluid retention and blood pressure elevation in these mice (25). Finally, a reduction in plasma atrial natriuretic peptide levels has been observed in NKCC1−/− mice, and this could contribute to a blood pressure elevation (26).
On the other hand, NKCC1−/− mice also have a number of attributes that one would expect to lower blood pressure when present alone. There is considerable evidence to show that NKCC1 expressed in vascular smooth muscle cells contributes to membrane voltage, and that inhibition of NKCC1 causes cell hyperpolarization by lowering intracellular Cl and reduced shunting of the K equilibrium potential (5, 18). Closing of voltage-dependent Ca channels and a reduced cytosolic Ca may be a reason for the vasorelaxation that has been reported to be caused by loop diuretics in both resistance and capacitance vessels (8, 11, 20). In addition, inhibition of NKCC1 caused a reduction of the contractile response to phenylephrine and probably other vasoconstrictor agents (1, 8), and the development of mechanical force in response to phenylephrine was reduced in aortic rings from NKCC1−/− compared with WT mice (19). The present study complements these observations by showing that the blood pressure reduction caused by two different vasodilators is markedly enhanced in NKCC1−/− mice, suggesting that absence of NKCC1-mediated NaCl transport enhances vasorelaxation. The precise mechanism by which lack of NKCC1 enhances the vasorelaxation caused by hydralazine and isoproterenol, two vasodilators with different mechanisms of action, has not been studied in the present work, but one may suspect that it is related to changes in basal membrane polarization and cytosolic Ca concentrations. Regardless of the mode of action, reduced vascular responses to vasoconstrictors and enhanced responses to vasodilators constitute a blood pressure-lowering combination that may vary between animals and conditions, and may therefore explain some of the differences between the present and previous observations. Finally, the clearly reduced locomotor activity in NKCC1−/− mice may be an additional factor in blood pressure control since, in general, decreased locomotor activity is loosely associated with reduced arterial blood pressure (17, 24).
Deafness and imbalance has been found to be associated with inactivation of the secretory Na-2Cl-K cotransporter (6, 10). Indeed, the most apparent phenotype of the NKCC1−/− mice is the shaker-waltzer behavior of circling and head bobbing characteristic for inner ear abnormalities (6, 7). While this may create the impression of hyperactivity, NKCC1−/− mice have a greatly limited range of lateral mobility, as detected by telemetry. Camera tracking has confirmed that NKCC1−/− animals cling to the wall of the cage and that they show little tendency to enter the open central space (unpublished observations). In addition, although WT mice use a running wheel extensively, NKCC1−/− mice were rarely found to perform this type of activity. It is possible that they lack the necessary dexterity because of vestibular abnormalities, but it agrees well with the reduced spontaneous activity. It is noteworthy that the remnant wheel running activity does not show the strong circadian association with darkness seen in WT animals, but appears to be more randomly distributed throughout the day. Nevertheless, since the circadian rhythm of arterial blood pressure and HR is normal, it is unlikely that an altered function of the central suprachiasmatic clock mechanism is causal in the altered locomotor rhythmicity of NKCC1−/− mice.
A striking finding in the present study is the marked nocturnal rise of blood pressure in NKCC1−/− mice when dietary salt content was increased and, conversely, the somewhat augmented fall in blood pressure during the day with low-salt intake. This is in contrast to WT mice in which comparable salt-dependent effects on MAP were not seen. The present observations are not necessarily inconsistent with prior studies that did not detect an effect of salt intake on blood pressure regulation in NKCC1−/− mice (21, 26), since in those studies blood pressure was measured during the day where the high-salt effect would be absent while the effect of low-salt diet is relatively modest and might have been missed. The causes for the augmented effects of changes in salt intake on blood pressure are not known, but they are likely to reflect an impairment of vascular compensatory mechanisms. Specifically, one may assume that a reduced ability of NKCC1−/− mice to modify vascular tone may affect the vascular adjustments necessary to maintain blood pressure during salt-induced increments and decrements of extracellular fluid volume. In view of the marked disturbance of plasma renin levels, it is conceivable that the renin-angiotensin system may play a role in the effects of salt intake on blood pressure. Although the directional changes of PRC associated with alterations in salt intake were largely identical between WT and NKCC1−/− mice, the magnitude of the changes was exaggerated in the KO mice, and plasma renin did not fall to the same low levels in response to a high-salt diet as in WT mice. Differences in animal behavior may also have to be considered. Specifically, the stimulation of locomotor activity by a low-salt intake may contribute to blood pressure maintenance in WT mice. Further studies are needed to clarify the factors contributing to salt sensitivity of blood pressure in NKCC1−/− mice.
In conclusion, arterial blood pressure over 24 h was not significantly different between NKCC1−/− and WT mice. Normal arterial blood pressures in NKCC1−/− mice are probably the result of a balance between the effect of factors tending to increase and decrease blood pressure. However, NKCC1 deficiency is associated with a greater blood pressure increase during high-salt intake at night and a greater blood pressure decrease during low-salt intake during the day, indicating impaired cardiovascular regulation in response to variations of salt intake.
GRANTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases and by NIDDK Grant PO1 DK-061521, Project 2 (S. M. Wall).
Acknowledgments
We are grateful to Yuning Huang for assistance in certain aspects of these experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akar F, Skinner E, Klein JD, Jena M, Paul RJ, O'Neill WC. Vasoconstrictors and nitrovasodilators reciprocally regulate the Na+-K+-2Cl− cotransporter in rat aorta. Am J Physiol Cell Physiol 276: C1383–C1390, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Carlson SH, Wyss JM. Long-term telemetric recording of arterial pressure and heart rate in mice fed basal and high NaCl diets. Hypertension 35: E1–E5, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Castrop H, Lorenz JN, Hansen PB, Friis U, Mizel D, Oppermann M, Jensen BL, Briggs J, Skott O, Schnermann J. Contribution of the basolateral isoform of the Na-K-2Cl- cotransporter (NKCC1/BSC2) to renin secretion. Am J Physiol Renal Physiol 289: F1185–F1192, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chipperfield AR, Harper AA. Chloride in smooth muscle. Prog Biophys Mol Biol 74: 175–221, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22: 192–195, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Deol MS Inherited diseases of the inner ear in man in the light of studies on the mouse. J Med Genet 5: 137–158, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dormans TP, Pickkers P, Russel FG, Smits P. Vascular effects of loop diuretics. Cardiovasc Res 32: 988–997, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol 19: 722–730, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Garg P, Martin CF, Elms SC, Gordon FJ, Wall SM, Garland CJ, Sutliff RL, O'Neill WC. Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol 292: H2100–H2105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Gross V, Luft FC. Exercising restraint in measuring blood pressure in conscious mice. Hypertension 41: 879–881, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Haas M, Forbush B 3rd. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol 62: 515–534, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Johnston GD, Hiatt WR, Nies AS, Payne NA, Murphy RC, Gerber JG. Factors modifying the early nondiuretic vascular effects of furosemide in man. The possible role of renal prostaglandins. Circ Res 53: 630–635, 1983. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SM, Huang Y, Qin Y, Mizel D, Schnermann J, Briggs JP. Persistence of circadian variation in arterial blood pressure in beta1/beta2-adrenergic receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 294: R1427–R1434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreye VA, Bauer PK, Villhauer I. Evidence for furosemide-sensitive active chloride transport in vascular smooth muscle. Eur J Pharmacol 73: 91–95, 1981. [DOI] [PubMed] [Google Scholar]
- 19.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na+-K+-2Cl− cotransporter. Am J Physiol Heart Circ Physiol 283: H1846–H1855, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol 293: F279–F287, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Pace AJ, Lee E, Athirakul K, Coffman TM, O'Brien DA, Koller BH. Failure of spermatogenesis in mouse lines deficient in the Na(+)-K(+)-2Cl(−) cotransporter. J Clin Invest 105: 441–450, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol 272: C173–C183, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Russell JM Sodium-potassium-chloride cotransport. Physiol Rev 80: 211–276, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549: 313–325, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl- cotransporter NKCC1 to Cl- secretion in rat OMCD. Am J Physiol Renal Physiol 280: F913–F921, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Wall SM, Knepper MA, Hassell KA, Fischer MP, Shodeinde A, Shin W, Pham TD, Meyer JW, Lorenz JN, Beierwaltes WH, Dietz JR, Shull GE, Kim YH. Hypotension in NKCC1 null mice: role of the kidneys. Am J Physiol Renal Physiol 290: F409–F416, 2006. [DOI] [PubMed] [Google Scholar]