Abstract
Both B- and T-cell immunogenicity of a chlamydial 75-kDa protein was analyzed by using 131 partially overlapped decapeptide homologs of the 75-kDa protein from Chlamydia trachomatis serovar L2. Six rabbit antiserum specimens raised with serovars B, C, and L2 were used to assay the antibody reactivities of the decapeptides. Seventy-five of the 131 decapeptides were recognized by at least one antiserum specimen, and two peptides were found to be immunodominant and surface accessible on native organisms. The same set of decapeptides were cleaved from the pins and tested for their T-cell-stimulating activity in an in vitro proliferation assay. A single decapeptide was able to stimulate proliferation of chlamydial antigen-primed lymph node T cells from BALB/c mice.
Full text
PDF
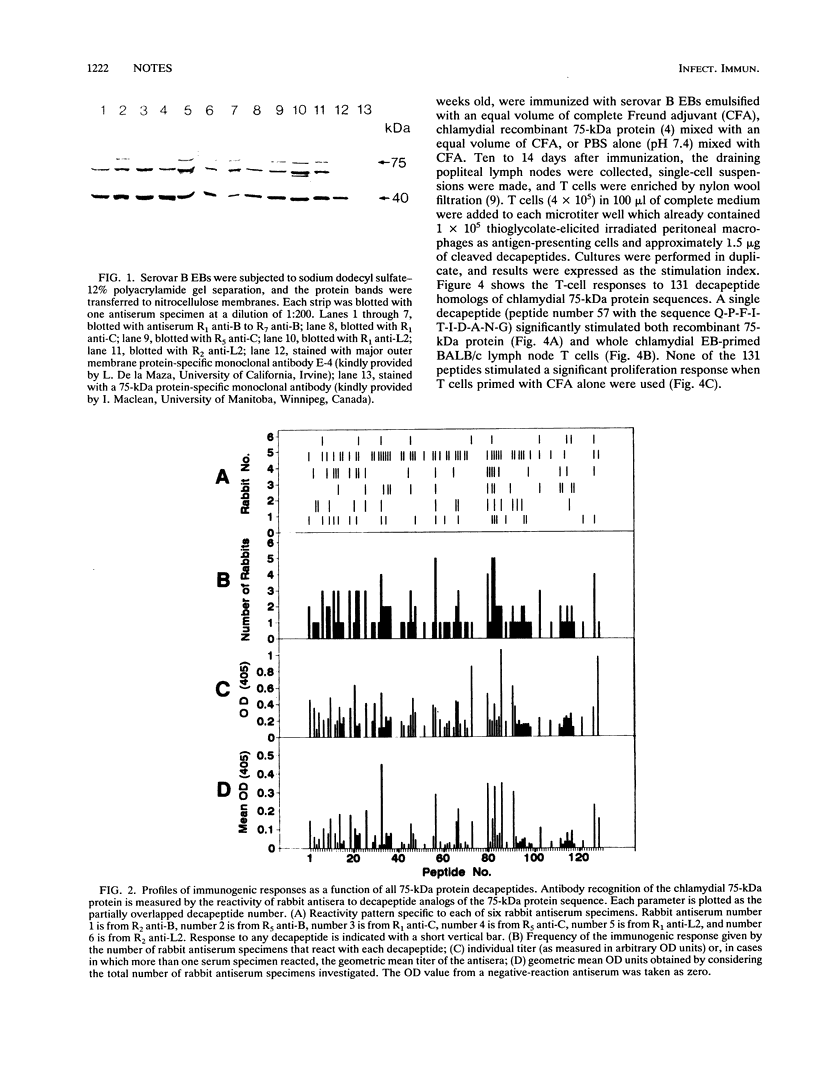
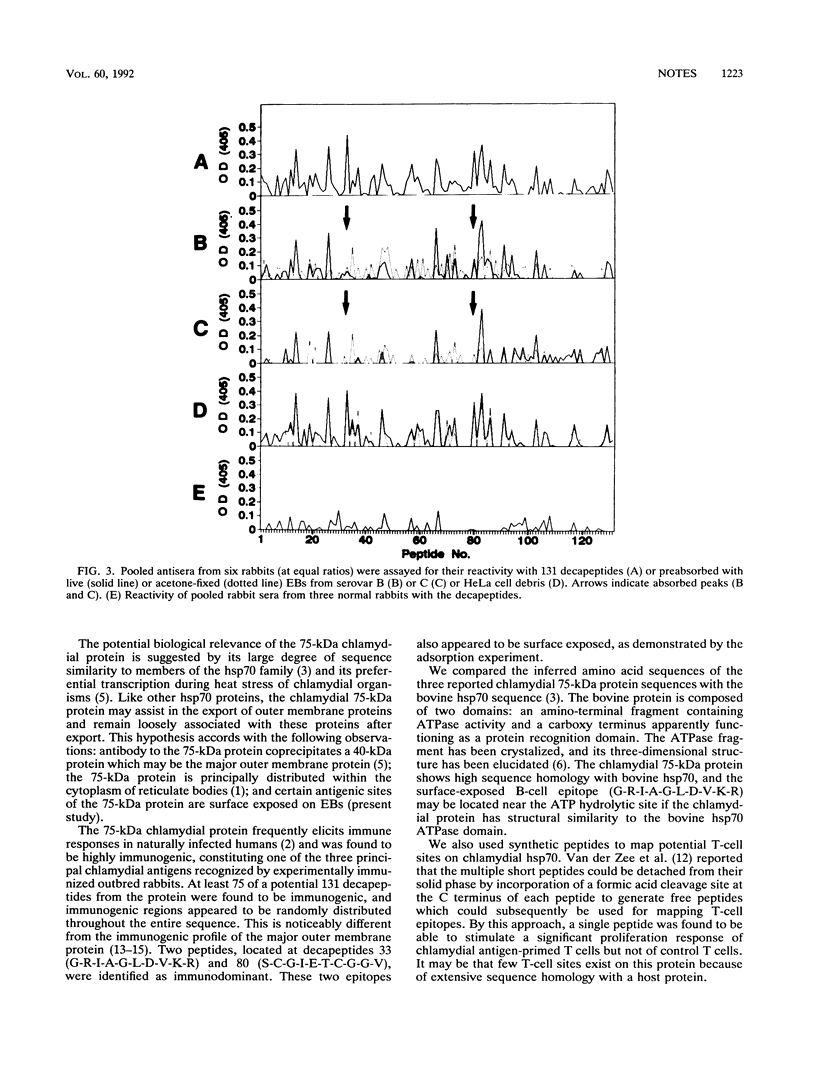
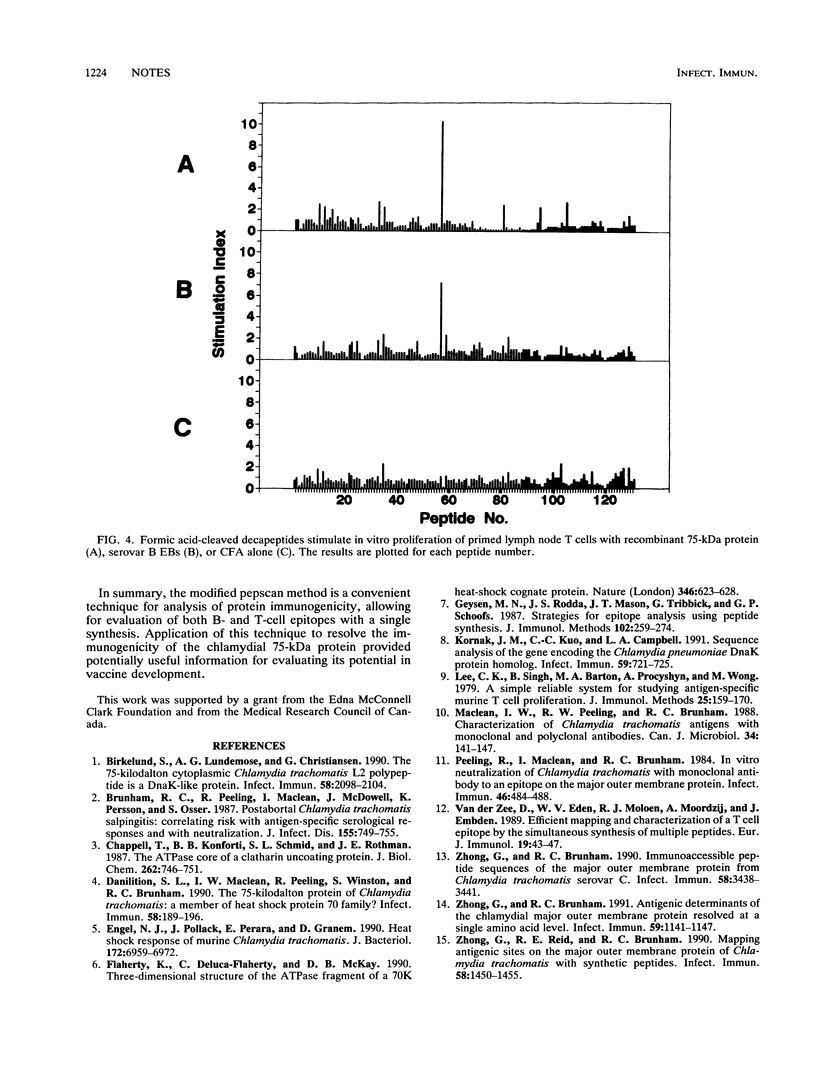
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkelund S., Lundemose A. G., Christiansen G. The 75-kilodalton cytoplasmic Chlamydia trachomatis L2 polypeptide is a DnaK-like protein. Infect Immun. 1990 Jul;58(7):2098–2104. doi: 10.1128/iai.58.7.2098-2104.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Pollack J., Perara E., Ganem D. Heat shock response of murine Chlamydia trachomatis. J Bacteriol. 1990 Dec;172(12):6959–6972. doi: 10.1128/jb.172.12.6959-6972.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Kornak J. M., Kuo C. C., Campbell L. A. Sequence analysis of the gene encoding the Chlamydia pneumoniae DnaK protein homolog. Infect Immun. 1991 Feb;59(2):721–725. doi: 10.1128/iai.59.2.721-725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Singh B., Barton M. A., Procyshyn A., Wong M. A simple reliable system for studying antigen-specific murine T cell proliferation. J Immunol Methods. 1979;25(2):159–170. doi: 10.1016/0022-1759(79)90051-6. [DOI] [PubMed] [Google Scholar]
- Maclean I. W., Peeling R. W., Brunham R. C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988 Feb;34(2):141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee R., Van Eden W., Meloen R. H., Noordzij A., Van Embden J. D. Efficient mapping and characterization of a T cell epitope by the simultaneous synthesis of multiple peptides. Eur J Immunol. 1989 Jan;19(1):43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- Zhong G. M., Brunham R. C. Antigenic determinants of the chlamydial major outer membrane protein resolved at a single amino acid level. Infect Immun. 1991 Mar;59(3):1141–1147. doi: 10.1128/iai.59.3.1141-1147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Brunham R. C. Immunoaccessible peptide sequences of the major outer membrane protein from Chlamydia trachomatis serovar C. Infect Immun. 1990 Oct;58(10):3438–3441. doi: 10.1128/iai.58.10.3438-3441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., Reid R. E., Brunham R. C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990 May;58(5):1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



