Abstract
The primate posterior thalamus has been proposed to contribute to pain sensation, but its precise role is unclear. This is in part because spinothalamic tract (STT) neurons that project to the posterior thalamus have received little attention. In this study, antidromic mapping was used to identify individual STT neurons with axons that projected specifically to the posterior thalamus in Macaca fascicularis. Each axon was located by antidromic activation at low stimulus amplitudes (<30 μA) and was then surrounded distally by a grid of stimulating points in which 500-μA stimuli were unable to activate the axon antidromically, thereby indicating the termination zone. Several nuclei within the posterior thalamus were targets of STT neurons: the posterior nucleus, suprageniculate nucleus, magnocellular part of the medial geniculate nucleus, and limitans nucleus. STT neurons projecting to the ventral posterior inferior nucleus were also studied. Twenty-five posterior thalamus-projecting STT neurons recorded in lumbar spinal cord were characterized by their responses to mechanical, thermal, and chemical stimuli. Sixteen of 25 neurons were recorded in the marginal zone and the balance was located within the deep dorsal horn. Thirteen neurons were classified as wide dynamic range and 12 as high threshold. One-third of STT neurons projecting to posterior thalamus responded to noxious heat (50°C). Two-thirds of those tested responded to cooling. Seventy-one percent responded to an intradermal injection of capsaicin. These data indicate that the primate STT transmits noxious and innocuous mechanical, thermal, and chemical information to multiple posterior thalamic nuclei.
INTRODUCTION
The posterior thalamus is proposed to be an important region for the transmission of pain and thermal information, but debate about its anatomy, function, and input from the spinal cord remains. The posterior thalamus consists of several nuclei located caudal to the primary somatosensory nuclei [the ventral posterior lateral (VPL) and ventral posterior medial (VPM) are referred to as VP here]. It includes the posterior nucleus (Po), suprageniculate nucleus (Sg), limitans nucleus (L), and the magnocellular part of the medial geniculate nucleus (MGmc), all of which are sites of termination for spinothalamic tract (STT) axons (Apkarian and Hodge 1989; Boivie 1979; Craig 2004; Jones 2007; Mehler 1969 Mehler et al. 1960; Ralston and Ralston 1992; Willis and Coggeshall 2004). The ventral posterior inferior nucleus (VPI), which we include in the term “posterior thalamus” for this report, also receives input from the STT, and it is located adjacent to the dorsal and rostral border of Po.
Single neurons within the primate posterior thalamus are activated by noxious mechanical and thermal stimuli (Apkarian and Shi 1994; Casey 1966; Craig et al. 1994; Perl and Whitlock 1961). In several studies, the percentage of nociceptive neurons within the posterior thalamus has been found to be greater than in VP (Apkarian and Shi 1994; Guilbaud et al. 1977; Poggio and Mountcastle 1960). In humans, low-amplitude stimulation within thalamic regions caudal and inferior to VP leads to the sensation of pain and temperature, and neurons in the same area exhibited responses to noxious mechanical and thermal stimuli (Lenz et al. 1993a,b; Ohara and Lenz 2003). A nucleus within monkey and human posterior thalamus specific for pain and temperature information, the posterior part of the ventral medial nucleus (VMpo), has been proposed to be the major target of marginal zone STT terminals (Blomqvist et al. 2000; Craig 2004; Craig et al. 1994). The proposal of this nucleus has generated controversy and renewed efforts to understand the role of the posterior thalamus in somatosensation (Craig 2004; Craig and Blomqvist 2002; Graziano and Jones 2004; Jones 2002, 2007; Willis et al. 2002).
The relative contributions to the perception of pain and temperature by VP and the posterior thalamus are not clear. Recently, two reports (Kim et al. 2007; Montes et al. 2005) examined patients who have lesions of VP that spared the medial posterior thalamus where VMpo is thought to be located. The lesions were sufficient to produce poststroke central pain and cold dysesthesia, which was interpreted to indicate that some areas of posterior thalamus are not required for these disorders. Consistent with those conclusions, cool and mechanically responsive neurons have been found in primate VP (Bushnell et al. 1993; Kenshalo et al. 1980; Lee et al. 1999; Lenz and Dougherty 1998). Also VPL has been identified as a projection target of cool responsive, nociceptive neurons from the rat marginal zone (Zhang et al. 2006). These studies indicate that VP is a candidate region for cold and mechanical processing. However, stimulation of the posterior thalamus produced the perception of cold in humans, and neurons recorded in the posterior thalamus responded to cool stimuli (Davis et al. 1999), suggesting that cold may be processed in areas caudal to VP as well. Furthermore, responses to cooling were recorded from neurons in primate marginal zone with axons antidromically activated from VMpo (Dostrovsky and Craig 1996). The distal target of those STT axons was not determined however, leaving open the possibility that they may have passed through posterior thalamus en route to VP (Willis et al. 2002).
Recent retrograde tract tracing studies have shown that marginal zone STT neurons can be preferentially labeled from the posterior thalamus, whereas neurons located in the deep dorsal horn were preferentially labeled from injections in VP (Craig 2006; Craig and Zhang 2006). Because many marginal zone neurons respond selectively to and encode the intensity of noxious mechanical and thermal stimuli (Christensen and Perl 1970; Craig et al. 2001; Ferrington et al. 1987; Hylden et al. 1986; Zhang et al. 2006), the posterior thalamus may play a qualitatively different role in pain sensation from VP. Other studies in which tract tracing (Gauriau and Bernhard 2004a; Graziano and Jones 2004; Willis et al. 2001) or electrophysiological methods (Applebaum et al. 1979) were used have shown that marginal zone neurons project both to the posterior thalamus and to VP. Tracing studies lack functional assessment and electrophysiological studies have not specifically examined the population of spinal cord cells projecting to posterior thalamus in primates. Here we present findings on functionally characterized primate STT neurons with axons that terminated specifically within the posterior thalamus as revealed by the antidromic mapping technique.
METHODS
Animal preparation
Macaca fascicularis between 2.5 and 10 kg were used in accordance with guidelines from the University of Minnesota. Monkeys were initially sedated with ketamine (10 mg/kg im), and then a catheter was placed in the median vein of the forelimb. Sodium pentobarbital (20 mg/ml) was given intravenously to produce a depth of anesthesia sufficient for intubation. Alpha chloralose (65 mg/kg iv) was administered, and then monkeys were placed on a feedback-controlled heating pad and fixed in a stereotaxic frame. A mixture of sodium pentobarbital (3–10 mg·kg−1·h−1), gallamine triethiodide (Flaxedil; 5–14 mg·kg−1·h−1), and saline was delivered intravenously continuously with a pump. A laminectomy was performed over the lumbar enlargement and a bilateral craniotomy (interaural 0–20 mm rostral and from the midline to 15 mm lateral, bilaterally) exposed the cortex over both thalami. The dura was opened over the exposed areas of the brain and spinal cord. The spinal cord was covered with warm mineral oil, and the brain was covered with a mixture of mineral oil and petroleum jelly. Pneumothoraces were placed to reduce movement of the spinal cord. The monkey was artificially ventilated, and CO2 was monitored continuously along with arterial blood pressure, heart rate, and body temperature.
Antidromic technique
To locate VPL, a low-impedance recording electrode was placed into the thalamus (initial coordinates: interaural: +8.0 mm, midline: +8.0 mm, depth from cortical surface: −18.0 mm) in search of neurons activated by tactile stimulation of the contralateral body. Once VPL was somatotopically mapped and its caudal boundary identified, the electrode was repositioned 2 mm caudal, 2–3 mm medial, and 1–2 mm ventral from the most caudal coordinates at which activity could be recorded from mechanical stimulation of the hindlimb. This location was presumed to be within the posterior thalamus. While the electrode remained in place, the headstage was removed and replaced by the cathode from a stimulus isolator; the anode was placed in contact with the animal, which had been grounded. A second electrode was placed in caudal VPL within the area representing the hindlimb. Once both stimulating electrodes were in place, a repeating square-wave pulse (search stimulus: 5 Hz, 200 μs, 500 μA) was simultaneously delivered through both electrodes allowing for the stimulation of a wide area of the posterior thalamus and caudal VPL. During the search stimulation, a stainless steel recording electrode (epoxylite insulated, ∼10 MΩ) was inserted through a perforation in the pia-arachnoid into the dorsal horn of the spinal cord. The recording electrode was repositioned until an action potential was found meeting the criteria for antidromic activation: constant latency from the stimulation, ability to follow a >300-Hz train, and collision between an orthodromic and an antidromic action potential (Lipski 1981). The stimulating electrode located within the hindlimb representation of VPL and the electrode within the posterior thalamus were then alternately switched off to determine which of the two electrodes provided the stimulus for the action potential recorded in the spinal cord. Often action potentials could be generated by both stimulating electrodes. In this situation, the more rostral VPL electrode typically generated action potentials with longer latency than the electrode located in the posterior thalamus, indicating that the axon passed through the posterior thalamus en route to VPL. Action potentials generated by the search stimulus in the posterior thalamus that could not initially be antidromically activated by the VPL electrode were investigated further by moving the VPL electrode medially and then laterally from the initial position in 500-μm increments. At each new position, the stimulating electrode was lowered ventrally 400 μm at a time while the recording was continuously monitored for the identified antidromic action potential. If no antidromic action potential could be generated within the plane, the electrode was moved 1 mm caudal, and a new mediolateral plane was stimulated as described. This procedure was repeated until an antidromic action potential was reliably established or, if none could be established even in caudal planes with the VPL electrode, then antidromic mapping was continued with the electrode in the posterior thalamus. Once an antidromically activated action potential was reliably established, the stimulus amplitude was then lowered to such a level that the action potentials were generated at a 50% success rate. This amplitude of current was recorded, and the electrode then repositioned to determine the current thresholds around the axon. The most rostral position at which antidromic action potentials could be generated by ≤30 μA was named the low-threshold point (LTP). This amplitude has been demonstrated only to activate axons that are within 400 μm of the tip of the stimulating electrode (Burstein et al. 1991; Dado et al. 1994; Ranck 1975; Zhang et al. 2000b). Axons that were antidromically activated at LTPs within the posterior thalamus and that were surrounded in three dimensions by current pulses >500 μA that were unable to generate antidromic action potentials were identified as posterior thalamus projecting STT neurons and functionally assessed.
Functional assessment
For each posterior thalamus projecting STT neuron, the receptive field location, size, and shape were determined using innocuous mechanical stimuli. Mechanical sensitivity was determined during brushing of the receptive field with a soft-bristled brush, pressure applied using an arterial clip, and pinch with a smaller, frankly painful arterial clip. Cells that did not respond to these mechanical stimuli were tested further with a more intense stimulus: squeezing the skin with forceps. Cells were classified as either high threshold (HT; those that did not respond to innocuous stimuli), or wide dynamic range (WDR; those that responded to both innocuous stimuli and, at higher frequencies, to more intense mechanical stimuli). Thermal stimuli were applied to the receptive field with a contact thermal probe (9 × 9 mm surface area; Yale Instruments). Stimuli of 40, 45, and 50°C were applied at a rate of 3°C/s from a holding temperature of 30°C. Cold stimuli of 20, 15, 10, and 5°C were applied from a base temperature of 30°C. All thermal stimuli were held at the test temperature for 5 s. For some cells, only the 50°C stimulus was applied. Cells for which no response to 50°C was observed were tested with stronger heat stimuli ≤55°C. Subsequent trials were begun after >90 s and after activity returned to baseline levels. A cell was considered responsive if the mean firing rate changed ≥150% from baseline during the 5-s thermal stimulus. Baseline was calculated as the mean firing rate over 30 s during the holding temperature prior to the thermal stimulus. Finally, cells were tested for sensitivity to an intradermal injection of capsaicin (10 μg in 10 μl of 7% Tween-80 in normal saline) using a 28-gauge tuberculin syringe. Action potentials were collected for 1 min prior to the injection and then until 10 min had elapsed after the injection of capsaicin. Analog voltage recordings were amplified, filtered (10–30,000 Hz), digitized at 33 kHz, and then wave-form discriminated and saved on a PC using DAPSYS software (www.dapsys.net).
Histology
At the end of the experiment, an electrolytic lesion was made at the LTP in the thalamus (15 μA, 45 s) and at the recording point in the spinal cord (25 μA, 20 s). Monkeys were perfused with 1 l of room temperature normal saline and then with 3 l of cold 10% formalin with 1% ferrocyanide to produce a Prussian blue reaction at the locations of the lesions. The brain and spinal cord were removed and placed in 10% formalin/1% ferrocyanide overnight and then blocked the next day. Sections were cut on a freezing microtome in 75- or 50-μm sections for the brain and spinal cord, respectively. The tissue was stained in neutral red, and the sites of the lesions were identified. All thalamic nuclei were identified in histological sections by location, intensity of staining, size and orientation of cells, and the pattern of fibers passing through the area. The nomenclature for the macaque thalamus in this study was derived from Olszewski (1952) as modified by Hirai and Jones (1989) and Jones (2007). The nuclei included in the posterior thalamus are the suprageniculate, limitans, Po, and the MGmc. We have additionally included cells in this study with axons that projected to VPI because STT neurons projecting to VPI have not previously been examined.
Data analyses
Stimulation grids were superimposed over traced representations of thalamic sections using the lesion marking the LTP as an anchor point and the distance between track marks and the track mark orientation as guides. Color contour plots of the minimum current amplitude required to produce antidromic action potentials at a 50% success rate at each grid point were generated with SigmaPlot v10.0 (Systat Software). Conduction velocity was estimated by dividing the distance between the stimulating electrode and the recording point by the latency of recording an action potential in the dorsal horn after stimulation in the thalamus (Zhang et al. 1999). Receptive fields were drawn onto standard monkey hindlimb templates using vector graphics software (CorelDRAW Graphics Suite 12), and the number of pixels contained within the illustration of the receptive field was calculated to compare receptive field size.
RESULTS
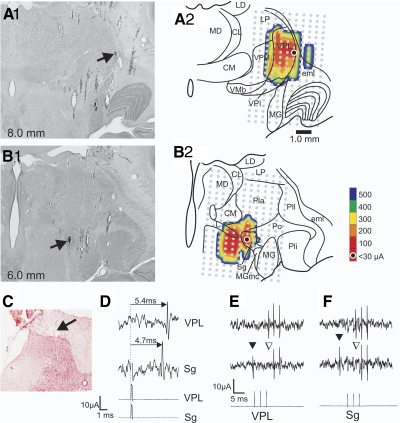
Figure 1 illustrates antidromic mapping of an STT axon that passed through posterior thalamus en route to VPL. Figure 1, A1 and B1, shows the locations of lesions marking the LTPs in VPL and in Sg, respectively. The LTPs identify the locations where antidromic activation required ≤30 μA and indicate the closest approach of the stimulating electrode to the axon. The lesion sites suggest that the axon was routed through Sg and then continued to project rostrally and laterally to VPL. Figure 1, A2 and B2, shows tracings of the sections in A1 and B1 superimposed with a contour plot indicating the minimum amplitude of current required for antidromic activation at each location within the grid of stimulation points. The LTPs in VPL and Sg were both surrounded in the mediolateral plane by stimulating points at which the current required for antidromic activation increased with the distance from the LTP until the axon could not be activated with 500 μA. The recording site was located in the marginal zone of the dorsal horn (Fig. 1C). The antidromic action potential had a longer latency from VPL than from Sg indicating the axon passed through posterior thalamus en route to VPL (Fig. 1D). Antidromic stimulation with ≤30 μA from either of the LTPs generated action potentials at the recording site that maintained a constant latency, followed a train of stimuli at >300 Hz, and could demonstrate collision between an antidromic and orthodromic spike (Fig. 1, E and F). Thirty-three neurons recorded over the course of this study were antidromically activated from VPL. Thirteen were recorded from the marginal zone, and five of these were classified HT. Twenty were recorded from deeper laminae, and five of these were classified HT. The mean conduction velocity of marginal zone and deeper cells was 28.0 ± 11.6 and 34.2 ± 7.4 m/s, respectively. Previous studies have shown a small projection of the STT to ventral lateral (VL) nucleus in cats (Jones and Burton 1974) and primates (Boivie 1979; Craig 2008). In this study, we did not surround the rostral end of axons that were antidromically activated in VPL. Therefore it is possible that some axons antidromically activated within VPL continued rostrally into VL.
FIG. 1.
Example of a single antidromically activated spinothalamic tract (STT) neuron showing the course of its axon through suprageniculate nucleus (Sg) to ventral posterior lateral (VPL). A1: photomicrograph of the lesion marking the low-threshold point (LTP) in VPL (→). A2: illustration of A1 with a superimposed grid ( ) indicating each point at which antidromic stimulation was tested. Contour plot indicates the current amplitude required for antidromic activation. B1: the lesion marking the LTP in Sg (→) of the same axon. B2: illustration as in A2. C: recording point in the marginal zone. D: antidromic activation from VPL had a longer latency than from Sg. E: antidromic activation from VPL. Top: 3 antidromic action potentials recorded in the dorsal horn. Middle: collision of an orthodromic action potential (▾) with an antidromic action potential (▿). Bottom: train of 333-Hz stimuli at the VPL LTP (12 μA). F: antidromic activation from Sg as in E. Stimulus artifacts reduced for clarity. CL, center lateral; CM, center median; eml, external medullary lamina; L, limitans; LD, lateral dorsal; LP lateral posterior; MG, medial geniculate; MGmc, magnocellular part of medial geniculate; MD, medial dorsal; Pla, anterior pulvinar; Pli, inferior pulvinar; Pll, lateral pulvinar; Po, posterior nucleus; VMb, basal ventral medial; VPI, ventral posterior inferior; VPM, ventral posterior medial.
) indicating each point at which antidromic stimulation was tested. Contour plot indicates the current amplitude required for antidromic activation. B1: the lesion marking the LTP in Sg (→) of the same axon. B2: illustration as in A2. C: recording point in the marginal zone. D: antidromic activation from VPL had a longer latency than from Sg. E: antidromic activation from VPL. Top: 3 antidromic action potentials recorded in the dorsal horn. Middle: collision of an orthodromic action potential (▾) with an antidromic action potential (▿). Bottom: train of 333-Hz stimuli at the VPL LTP (12 μA). F: antidromic activation from Sg as in E. Stimulus artifacts reduced for clarity. CL, center lateral; CM, center median; eml, external medullary lamina; L, limitans; LD, lateral dorsal; LP lateral posterior; MG, medial geniculate; MGmc, magnocellular part of medial geniculate; MD, medial dorsal; Pla, anterior pulvinar; Pli, inferior pulvinar; Pll, lateral pulvinar; Po, posterior nucleus; VMb, basal ventral medial; VPI, ventral posterior inferior; VPM, ventral posterior medial.
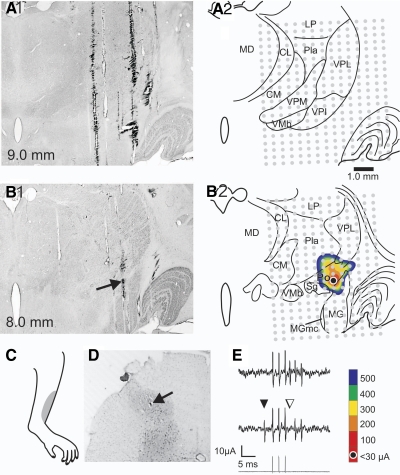
In contrast to the STT neurons that could be antidromically activated from VPL, 25 cells could only be activated within the posterior thalamus. Figure 2 illustrates an example of this type of STT neuron. The lesion marking the LTP (Fig. B1, →) was located in Po, just medial to the dorsal part of the medial geniculate nucleus. The current required to activate the axon at the LTP was 15 μA and increased as the distance from the LTP increased (Fig. 2B2). One millimeter rostral to the plane of the LTP the axon could not be antidromically activated with current amplitudes ≥500 μA (Fig. 2A, 1 and 2). This neuron had a cutaneous receptive field over the shin of the left hindlimb (Fig. 2C) and was recorded in nucleus proprius (Fig. 2D). The neuron was classified as a WDR type. Figure 2E demonstrates that an antidromic action potential occurred in the soma at a constant latency, followed a >300-Hz train and that an orthodromic action potential collided with an antidromic action potential. The latency for the antidromic action potential to reach the recording site was 7.6 ms (conduction velocity = 30.7 m/s).
FIG. 2.
Example of an STT neuron projecting directly to the Po of the thalamus. A1: photomicrograph of a section 1.0 mm rostral to the LTP where stimulation with ≥500 μA was unable to generate antidromic action potentials. A2: illustration of the same section with each antidromic stimulation test site marked ( ). B1: photomicrograph of a lesion marking the LTP in the Po (→). B2: illustration of B1 with each stimulation test site indicated and a contour plot showing the amplitude of current required to activate the neuron antidromically. C: receptive field. D: the neuron was recorded in nucleus proprius (→). E: antidromic activation from Po: Top: series of 3 antidromic action potentials time locked to the 3 stimuli. Middle: collision between an orthodromic (▾) and blocked antidromic action potential (▿). Bottom: 333-Hz, 8-μA stimulus train.
). B1: photomicrograph of a lesion marking the LTP in the Po (→). B2: illustration of B1 with each stimulation test site indicated and a contour plot showing the amplitude of current required to activate the neuron antidromically. C: receptive field. D: the neuron was recorded in nucleus proprius (→). E: antidromic activation from Po: Top: series of 3 antidromic action potentials time locked to the 3 stimuli. Middle: collision between an orthodromic (▾) and blocked antidromic action potential (▿). Bottom: 333-Hz, 8-μA stimulus train.
An example of a second posterior thalamus-projecting STT neuron, and its functional characterization, is shown in Fig. 3. Antidromic thresholds in a combined horizontal and coronal view are indicated in Fig. 3A. Three parallel mediolateral rows were examined for stimulation sites that could produce antidromic action potentials. Two rostral rows passing through VPL are shown in the horizontal view. Within these rows, 500-μA stimuli were unable to produce antidromic activation. However, within the most caudal row (interaural: +6.7 mm) the axon could be activated. The lowest antidromic threshold within each mediolateral track is indicated (Fig. 3A). An illustration of this caudal plane is shown in the coronal view with antidromic thresholds indicated at each stimulation point (Fig. 3A). A photomicrograph of the coronal section from this caudal plane is shown in Fig. 3B with an arrow indicating the lesion at the LTP (21 μA) within the Po. The cell did not respond to brushing the receptive field but did respond to more intense stimuli and was classified as an HT type neuron (Fig. 3C). The receptive field is shown (Fig. 3D). The cell was recorded from nucleus proprius (Fig. 3E).
FIG. 3.
Example of a functionally characterized STT neuron projecting directly to the Po. A, top: horizontal view of stimulating electrode penetration sites through the thalamus. The lowest amplitude of current that produced antidromic action potentials is indicated for each mediolateral position. The current amplitude of the LTP is circled (21 μA).  , electrode penetrations that were unable to evoke antidromic action potentials with ≥500 μA. Bottom: coronal view corresponding to the most caudal plane above. B: photomicrograph of the coronal plane shown in A with a lesion marking the LTP in Po. Scale bar = 1.0 mm. C: characterization of the response to mechanical stimuli. B, brush; Pr, pressure; Pi, pinch; S, squeeze. This cell was classified as a high-threshold (HT) neuron. D: receptive field. E: recording point in nucleus proprius.
, electrode penetrations that were unable to evoke antidromic action potentials with ≥500 μA. Bottom: coronal view corresponding to the most caudal plane above. B: photomicrograph of the coronal plane shown in A with a lesion marking the LTP in Po. Scale bar = 1.0 mm. C: characterization of the response to mechanical stimuli. B, brush; Pr, pressure; Pi, pinch; S, squeeze. This cell was classified as a high-threshold (HT) neuron. D: receptive field. E: recording point in nucleus proprius.
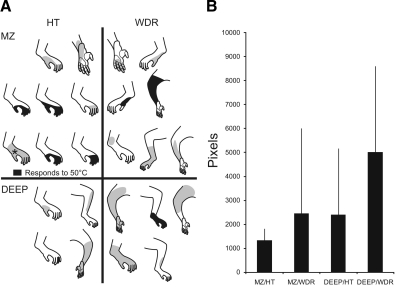
All STT neurons that projected to the posterior thalamus had a mechanically sensitive receptive field. These varied in size from a single toe to most of the hindlimb. Figure 4A shows the receptive field for posterior thalamus-projecting STT neurons grouped by mechanical sensitivity and the region of the dorsal horn where the recording site was lesioned. Receptive fields colored in black indicate that the cell was responsive to a 50°C heat stimulus. The mean size and SD of receptive fields in each of the different groups is shown in Fig. 4B.
FIG. 4.
Receptive fields of STT neurons that projected directly to the posterior thalamus. A: receptive fields organized by mechanical classification and location of the recording site. MZ, marginal zone. black, neurons that responded to noxious heat (50°C). Receptive fields are not shown to scale. *, receptive field of neuron that responded to 52°C but not 50°C. B: size of receptive fields for each group (mean ± SD).
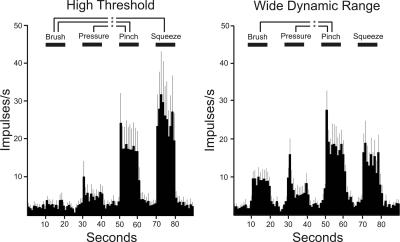
Posterior thalamus-projecting STT neurons could be classified as HT (n = 12) or WDR (n = 13; Fig. 5). Both HT- and WDR-type cells significantly increased their firing rates when the stimulus intensity was increased from pressure to pinch, suggesting that they can distinguish between innocuous and noxious mechanical stimuli. The mean spontaneous activity assessed before mechanical testing was not different for HT and WDR type neurons: 2.2 Hz ± 0.1 and 2.2 Hz ± 0.2 Hz, respectively.
FIG. 5.
Mean ± SE responses to mechanical stimuli for high-threshold and wide-dynamic-range (WDR) type posterior thalamus-projecting STT neurons. Both HT and WDR type cells significantly increased their firing rates from pressure to pinch, suggesting that they can encode innocuous and noxious mechanical stimuli. One-way ANOVA with Tukey posttest (P < 0.05). WDR-Squeeze was not included in the statistical analysis because the number of tests was small (n = 3).
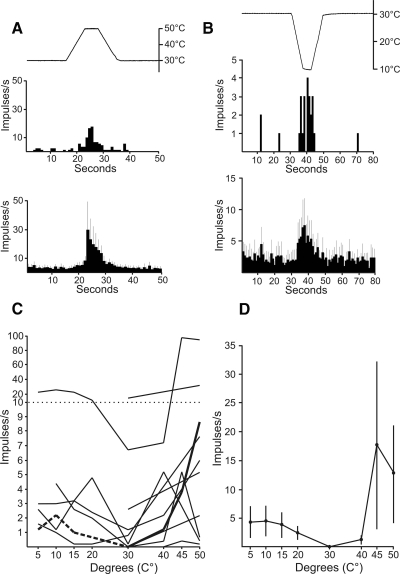
Thirty-three percent (8/24) of posterior thalamus-projecting STT neurons were excited by a 5-s 50°C heat stimulus. An example of a response to 50°C and the mean of all eight responses are shown in Fig. 6A. Neurons that did not respond to 50°C were often tested further with heat ≤55°C but only 1/11 responded (Fig. 4A). That particular neuron also responded to cool temperatures. Nine posterior thalamus-projecting STT neurons were tested with cooling stimuli and six responded. Five of these six also responded to heat ≥50°C. An example of a response to cold (10°C) and the mean response to cold is shown in Fig. 6B. The response of each thermally excitable neuron and the overall mean response to a range of stimulus temperatures are shown in Fig. 6, C and D. These results indicate that some posterior thalamus-projecting STT neurons are capable of responding to thermal stimuli. However, discharge rates were typically low and graded changes in the thermal stimuli did not consistently elicit graded changes in the magnitude of responses.
FIG. 6.
Responses of posterior thalamus-projecting STT neurons to thermal stimuli. A, top: an example of a response to noxious heat (50°C). Bottom: mean ± SE of all heat responsive neurons to 50°C. B, top: example of a response to cold (10°C). Bottom: mean ± SE of all cold responsive neurons to 10°C. C: responses to a range of thermal stimuli for each thermally responsive neuron. Each point represents the mean firing rate during the 5-s thermal stimulus. Note the scale change on the ordinate. Bold and dashed lines indicate data used for examples in A and B, respectively. D: mean ± SE response of all thermally responsive neurons with the baseline activity at the holding temperature subtracted.
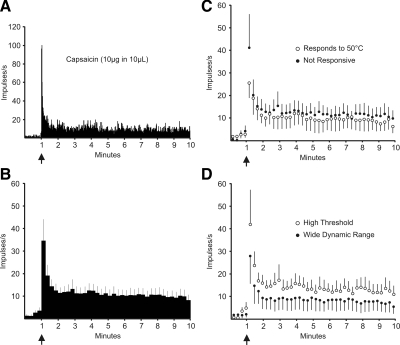
Seventy-one percent (12/17) of posterior thalamus projecting STT neurons responded to an intradermal injection of capsaicin with a discharge ≥1.5 times baseline lasting >1 min. An example of a typical response is shown in Fig. 7A. The average response of the 12 STT neurons projecting to posterior thalamus that were responsive to capsaicin is shown in Fig. 7B. All neurons that responded to 50°C that were subsequently tested with capsaicin responded (4/4). Surprisingly, 67% (8/12) of neurons that responded to capsaicin did not respond to heat. The Mann-Whitney U test was used to determine whether groups of capsaicin-sensitive, posterior-thalamus-projecting STT neurons responded differently to capsaicin. Neurons that were not responsive to heat had a greater response to capsaicin than neurons that were heat sensitive (Fig. 7C; P < 0.001). Also, HT type neurons responded more vigorously to capsaicin than did WDR type neurons (Fig. 7D; P < 0.001).
FIG. 7.
Responses to capsaicin of posterior thalamus-projecting STT neurons. A: example of a typical response to capsaicin. ↑, the time of injection. B: mean ± SE response of each capsaicin responsive neuron calculated in 15-s bins. C: mean ± SE response to capsaicin of neurons responsive and not responsive to a ≥50°C heat stimulus. D: mean ± SE response to capsaicin of HT and WDR type neurons.
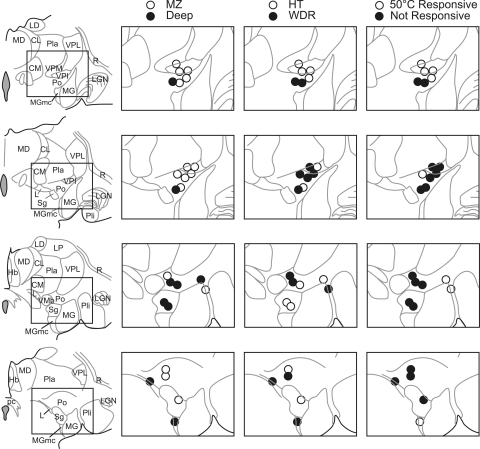
The location of the termination zone of each posterior thalamus-projecting STT axon is shown in one of four anterior-posterior levels spanning ∼3 mm (Fig. 8). Axons projected to several nuclei of the posterior thalamus: Po (13), VPI (6), Sg (3), MG (1), MGmc (1), and L (1). The location of each LTP in the posterior thalamus is indicated along with the location of the cell of origin in the dorsal horn. Each axon projecting to VPI originated from a neuron located in the marginal zone. Other nuclei in posterior thalamus received input from the marginal zone as well as the deep dorsal horn. The projection target of each STT neuron and the class of mechanical sensitivity (WDR or HT) of the cell of origin is indicated (Fig. 8). Termination zones of neurons that were responsive to a 50°C heat stimulus are shown. A large proportion of heat responsive neurons projected to or around VPI. These results indicate that the STT provides innocuous and noxious mechanical and thermal information to several nuclei of the posterior thalamus.
FIG. 8.
Location of all lesions in the posterior thalamus marking LTPs of functionally characterized STT neurons. Left: camera lucida tracings of four sections through the anterior-posterior extent of the posterior thalamus. Nuclei within the posterior thalamus receive input from HT and WDR type neurons located both in the marginal and the deep dorsal horn. VPI appears to receive a high percentage of its input from marginal zone neurons. Each section represents ∼750 μm. Hb, habenula; LGN, lateral geniculate nucleus; pc, posterior commissure; R, reticular nucleus.
The average conduction velocity (mean ± SD) of all 25 posterior thalamus-projecting STT neurons was 22.3 ± 11.6 m/s. Marginal zone and deep neurons did not propagate at significantly different rates (marginal zone = 19.2 ± 9.7 m/s; deep = 27.4 ± 13.3 m/s) nor were conduction velocities dependent on the thalamic projection target (Po = 25.2 ± 12.8 m/s; VPI = 20.6 ± 12.6 m/s; Sg/l/MGmc = 17.4 ± 5.3 m/s). The mean conduction velocity of axons that projected to the posterior thalamus was significantly slower compared with axons that projected to VPL (31.7 ± 9.7 m/s; P < 0.05).
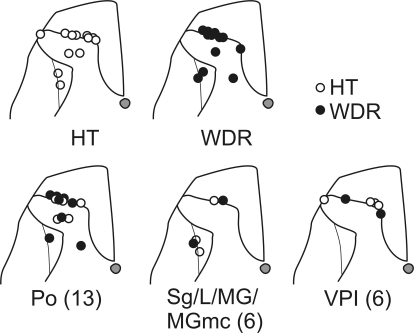
Lesions marking the recording points in the dorsal horn are shown in Fig. 9. Both WDR and HT type STT neurons projected to the posterior thalamus. Sixteen neurons in the marginal zone and eight neurons from the deeper laminae projected to several nuclei within the posterior thalamus.
FIG. 9.
Locations of lesions marking recording sites of 24 neurons in the lumbar dorsal horn. Top: recording sites of HT and WDR type neurons. Bottom: the recording sites of HT and WDR type neurons and to which nucleus in the posterior thalamus they project. One recording site from a WDR type cell was not recovered.
DISCUSSION
In this study, 25 STT neurons were identified that projected directly to the posterior thalamus in monkeys. These cells were characterized by responses to mechanical, thermal, and chemical stimuli. Sixteen of 25 neurons were recorded in the marginal zone, and the rest were located in the deep dorsal horn. Half of the total was classified as WDR and half as HT. One-third of STT neurons projecting to posterior thalamus responded to noxious heat (50°C). Two-thirds of those tested responded to cooling. Seventy-one percent of neurons responded to the noxious chemical capsaicin. Axons ending in the posterior thalamus were found to project mainly to the Po and VPI. The Sg, MGmc, and the L received fewer projections. These data indicate that the primate STT transmits noxious and innocuous mechanical, thermal, and chemical information to multiple nuclei within the posterior thalamus.
To identify STT axons that project to the posterior thalamus, it is important to surround distal axon projections using antidromic mapping techniques because STT axons often pass through this region en route to rostral targets. Here we localized the axons of STT neurons in the posterior thalamus by using antidromic activation at low stimulus amplitudes (<30 μA). The distal end of each axon was then surrounded by a large three-dimensional grid of stimulating points where 500-μA stimulus pulses were unable to activate the neuron antidromically, indicating that the axon did not pass through the stimulated area. These methods allowed us to map the location of the termination zone for each axon that was antidromically activated from the posterior thalamus.
Although the focus of this study was on STT neurons that projected solely to the posterior thalamus, in several experiments, we observed that the same STT axon could sometimes be antidromically activated from VPL as well as from multiple locations within posterior thalamus. At some of those locations in the posterior thalamus, the latency was longer than the latency from VPL (unpublished observations). We could not achieve LTPs at these additional locations nor did we surround the locations with ineffective stimulating tracks to determine the endpoint of the axon, and therefore we did not include these neurons as part of the posterior thalamus population. However, the longer latency from posterior thalamus can be explained by the activation of a collateral branch off of a parent axon. Collateral branches might target posterior thalamic nuclei even though the parent axon continues rostrally to VPL. Consistent with this idea, double-labeling experiments have shown that as many as 10–20% of STT axons branch and terminate in both medial and lateral thalamic nuclei (Applebaum et al. 1979; Craig et al. 1989; Giesler et al. 1981; Kevetter and Willis 1983). A subpopulation of STT neurons that project to both the posterior thalamus as well as to VPL could contribute to nociceptive processing at both sites.
Previously we described STT neurons projecting directly to posterior thalamic nuclei in rats using similar antidromic mapping techniques (Zhang and Giesler 2005). A majority of the rat STT neurons projecting to posterior thalamus were located in the marginal zone, a finding that is consistent with the present results in monkeys. Receptive fields in rats ranged from a single digit to most of the limb, a result that is also similar to present observations in monkeys. Seventy-seven percent of STT neurons projecting to the posterior thalamus were classified as HT in rat, and half were HT in monkey. In rats, the posterior triangular nucleus received the most projections from STT neurons followed by the medial geniculate, Po, posterior intralaminar nucleus, and suprageniculate. Monkey STT projections to posterior thalamus were mostly to Po, followed by VPI, Sg, L, MG, and MGmc. The rat posterior triangular nucleus and the monkey Po share the position of having the highest proportion of STT input in the posterior thalamus, suggesting that they may possess overlapping functions. Interestingly, in the rat ∼70% of neurons responded to noxious heat (≤55°C); however, in monkeys, only 38% responded to noxious heat (≤55°C). Also only 1 neuron of 13 tested in rats responded to cold stimuli, but 6 of 9 tested in monkey responded to cold. The differences between rodent and primate might reflect different roles of the posterior thalamus in thermal coding in these species.
The input to the posterior thalamus from the STT has been studied using degeneration (Boivie 1979; Mehler 1969; Mehler et al. 1960), retrograde (Craig 2006; Craig and Zhang 2006; Willis et al. 2001), and anterograde tracing methods (Apkarian and Hodge 1989; Beggs et al. 2003; Craig 2004; Gauriau and Bernard 2004a; Ralston and Ralston 1992; Stepneiwska et al. 2003). Each method has confirmed that neurons of the dorsal horn send projections to the posterior thalamus. Craig and colleagues have recently advanced a hypothesis that marginal zone neurons preferentially target VMpo and that VPL receives input from neurons located within the deeper laminae (Craig 2006; Craig and Zhang 2006). The present study confirms that the posterior thalamus receives a relatively high proportion of marginal zone projections. However, we also found that marginal zone neurons projected frequently to VPL consistent with earlier anatomic (Gauriau and Bernhard 2004a; Graziano and Jones 2004; Willis et al. 2001) and antidromic activation studies (Ferrington et al. 1987; Simone et al. 2004; Zhang et al. 2000a,b).
Poggio and Mountcastle (1960) first observed that more than half of the neurons in the posterior thalamus of cats could be activated by noxious mechanical stimulation. Nociceptive responses elicited by mechanical, thermal, and electrical stimuli from neurons in the posterior thalamus have since been observed in rats (Bordi and LeDoux 1994; Gauriau and Bernard 2004b; Guilbaud et al. 1980), cats (Benedek et al. 1997; Brinkhus and Carstens 1979; Calma 1965; Carstens and Yokota 1980; Curry 1972; Curry and Gordon 1972; Guilbaud et al. 1977; Matsumoto et al. 1988; Nyquist and Greenhoot 1974), and monkeys (Casey 1966; Craig et al. 1994; Perl and Whitlock 1961; Whitlock and Perl 1961). In humans, neurons recorded caudal to the principle somatosensory nuclei (presumably within the posterior thalamus) were found to be responsive to noxious mechanical and thermal stimuli, and electrical stimulation within the microampere range elicited thermal and pain sensations (Davis et al. 1999; Lenz et al. 1993a,b; Ohara and Lenz 2003). Our results show that several nuclei within the posterior thalamus receive direct projections from nociceptive and thermoreceptive spinal cord neurons and suggest that the STT makes an important contribution to the response properties of neurons in the posterior thalamus.
A nucleus specific for nociceptive and thermal input located within the primate posterior thalamus has been proposed (VMpo) (Blomqvist et al. 2000; Craig 2004; Craig et al. 1994). VMpo was originally based on the location in the posterior thalamus of labeled axon terminations that ascended from the marginal zone (Craig et al. 1994). Nearly all of the neurons that were recorded from the thalamic region where these terminations were found were reported to exhibit responses to noxious or cool stimuli (Craig et al. 1994). VMpo was further defined by a discrete axonal plexus that was immunoreactive for the calcium binding protein calbindin (Blomqvist et al. 2000; Craig 2004; Craig et al. 1994). There are several reasons to be cautious about accepting the VMpo hypothesis (Graziano and Jones 2004; Jones 2007; Ralston 2003; Willis et al. 2002). Studies differ on the extent and concentration of calbindin immunoreactivity in the posterior thalamus and whether fibers arising from the marginal zone are themselves immunoreactive for calbindin (Blomqvist et al. 2000; Craig 2004; Craig et al. 1994; Graziano and Jones 2004; Rausell et al. 1992; Stepneiwska et al. 2003). Because VMpo is positioned within previously named nuclei (i.e., Po, suprageniculate, and VPM nucleus) to which spinothalamic and trigeminothalamic fibers have been shown to project, it is difficult to identify the borders of VMpo without the use of an aid such as the calbindin stain. Also the claim that VMpo is a specific pain relay nucleus is difficult to assess because the projections from VMpo to cortical or subcortical regions have not been specifically demonstrated. Additionally, a study examining STT neurons thought to project to and therefore contribute to the function of VMpo (Dostrovsky and Craig 1996) did not establish the distal projection targets of the antidromically activated axons. Therefore axons of passage may have been inadvertently stimulated (Willis et al. 2002). The hypothesis that VMpo is necessary for the generation of poststroke central pain was recently examined and challenged (Kim et al. 2007; Montes et al. 2005). Finally, the present data do not support the hypothesis of a direct marginal zone projection to a discrete nucleus located posteromedial to VP and ventral to the anterior pulvinar and center median nucleus. Our stimulating electrode penetrations passed through the area described as VMpo, and we have antidromically activated only a few axons that projected there. Instead marginal zone neurons in this study projected to several nuclei of the posterior thalamus, and these nuclei also received input from neurons located deeper within the dorsal horn. Additionally, many of the neurons projecting to the posterior thalamus were not nociceptive-specific (i.e., HT) or thermoreceptive, observations that are also inconsistent with the VMpo hypothesis. In this study, the VPI received the highest proportion of HT marginal zone input.
We observed cool responsive STT neurons that projected to the posterior thalamus. This is consistent with a study in which stimulation in human posterior thalamus evoked cold sensations, and neurons recorded from the same area were responsive to cooling the skin (Davis et al. 1999). Noxious heat processing has been hypothesized to take place within the posterior thalamus because lesions within VP failed to disrupt the sensation of noxious heat (Montes et al. 2005). In this study, 1/3 of neurons projecting to posterior thalamus responded to noxious heat (50°C). These results support the hypothesis that some thermal processing occurs in the posterior thalamus. However, most of these neurons had low discharge rates to thermal stimuli and displayed a limited ability to discriminate between temperatures. In contrast, 77% (22/29) of STT neurons antidromically activated from VPL responded to noxious heat. In previous studies, STT neurons projecting to VPL often responded to graded temperatures with graded responses indicating that the neurons likely encode the intensity of thermal stimulation (Ferrington et al. 1987; Kenshalo et al. 1979; Willis et al. 1974). Our data suggest that the STT provides different thermosensory information to the posterior thalamus and VPL.
A large proportion (71%) of STT neurons projecting to the posterior thalamus responded to an intradermal injection of capsaicin. All of the heat-responsive neurons tested responded to capsaicin, but surprisingly, 67% of neurons responsive to capsaicin did not respond to a ≥50°C heat stimulus. Furthermore, heat-insensitive STT neurons had a greater discharge to capsaicin than did the heat-responsive STT neurons. Ringkamp et al. (2001) described myelinated primary afferent fibers in monkeys that were powerfully activated by capsaicin but were insensitive to heat. TRPV1 is thought to be the primary molecular mechanism underlying responses to both capsaicin and heat (Caterina et al. 1997; Patapoutian 2003). On the basis of recordings from excised patches of DRG, Nagy and Rang (1999) hypothesized that different molecular configurations of the TRPV1 channel could be selectively responsive to either heat or capsaicin. In addition, Lu et al. (2005) have found that one splice variant of the TRPV1 gene is activated by heat but not capsaicin, suggesting that different molecular configurations of TRPV1 channels confer selective sensitivity. Although the molecular mechanisms that produce capsaicin-sensitive cells unresponsive to heat are unknown, our results suggest that STT neurons that project to the posterior thalamus are capable of conveying chemical nociceptive information independently from information about noxious heat.
Responses in the posterior thalamus have been evoked by both somatosensory and auditory stimuli, sometimes in the same cell (Bordi and LeDoux 1994; Curry 1972; Poggio and Mountcastle 1960). This is likely because of overlapping axon terminations in the posterior thalamus from cells in the spinal cord and the inferior colliculus (Jones and Burton 1974; LeDoux et al. 1987; Rockel et al. 1972). These data suggest that a role of the posterior thalamus might be to process convergent sensory information from multiple systems. Indeed several studies have shown that the posterior thalamus is involved in fear conditioning, which is established by pairing an auditory tone with a noxious foot shock (LeDoux 2000; Shi and Davis 1999). In rats, the posterior thalamus has been shown to project to the amygdala, a structure necessary for the acquisition of fear conditioning (Bordi and LeDoux 1994; Ledoux et al. 1990). The efferent pathways from the posterior thalamus also project to SII and to the insula (Burton and Jones 1976; Calma 1965; Friedman and Murray 1986; Stevens et al. 1993). Recently electrophysiological and anatomical methods were combined to show that nociceptive neurons recorded in the posterior thalamus of rats projected to SII, whereas nonnociceptive neurons projected to the insula (Gauriau and Bernard 2004b). The insula and SII are frequently activated in positron emission tomography and functional magnetic resonance imaging studies of responses of human cortical regions to noxious stimuli (Apkarain et al. 2005; Casey et al. 1994; Coghill et al. 1994; Treede et al. 2000). These studies indicate that STT neurons projecting to the posterior thalamus provide information about noxious stimuli to supraspinal systems that are identified to be involved in the processing of pain.
The projection of the STT to the posterior thalamus arises from neurons in both the marginal zone and the deep dorsal horn and terminates within several nuclei including Po and VPI. In addition, both HT and WDR type neurons project to the posterior thalamus in primates. Our results indicate that the STT projection to the posterior thalamus is more complex than previously suggested. It will be interesting in future studies to explore the functional contributions of each of these posterior thalamic nuclei to cortical nociceptive processing.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-047399 and NS-059199 and by the Graduate School of the University of Minnesota.
Acknowledgments
We thank H. Truong for excellent technical assistance and E. G. Jones for providing aid in the identification of thalamic structures.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Apkarian et al. 2005.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005. [DOI] [PubMed] [Google Scholar]
- Apkarian and Hodge 1989.Apkarian AV, Hodge CJ. Primate spinothalamic pathways. III. Thalamic termination of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol 288: 493–511, 1989. [DOI] [PubMed] [Google Scholar]
- Apkarian and Shi 1994.Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 14: 6779–6795, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum et al. 1979.Applebaum AE, Leonard RB, Kenshalo DR Jr, Martin RF, Willis WD. Nuclei in which functionally identified spinothalamic tract neurons terminate. J Comp Neurol 188: 575–586, 1979. [DOI] [PubMed] [Google Scholar]
- Beggs et al. 2003.Beggs J, Jordan S, Ericson A-C, Blomqvist A, Craig AD. Synaptology of trigemino- and spinothalamic lamina I terminations in the posterior ventral medial nucleus of the Macaque. J Comp Neurol 459: 334–354, 2003. [DOI] [PubMed] [Google Scholar]
- Benedek et al. 1997.Benedek G, Pereny J, Kovacs G, Fischer-Szatmari L, Katok YY. Visual, somatosensory, auditory and nociceptive modality properties in the feline suprageniculate nucleus. Neuroscience 78: 179–189, 1997. [DOI] [PubMed] [Google Scholar]
- Blomqvist et al. 2000.Blomqvist A, Zhang ET, Craig AD. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain 123: 601–619, 2000. [DOI] [PubMed] [Google Scholar]
- Boivie 1979.Boivie J An anatomical reinvestigation of the termination of the spinothalamic tract in the monkey. J Comp Neurol 186: 343–370, 1979. [DOI] [PubMed] [Google Scholar]
- Bordi and LeDoux 1994.Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdale stimulation. Exp Brain Res 98: 275–286, 1994. [DOI] [PubMed] [Google Scholar]
- Brinkhus et al. 1979.Brinkhus HB, Carstens E, Zimmermann M. Encoding of graded noxious skin heating by neurons in posterior thalamus and adjacent areas in the cat. Neurosci Lett 15: 37–42, 1979. [DOI] [PubMed] [Google Scholar]
- Burstein et al. 1991.Burstein R, Dado RJ, Giesler GJ Jr. Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol 66: 261–284, 1991. [DOI] [PubMed] [Google Scholar]
- Burton and Jones 1976.Burton H, Jones EG. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol 168: 249–301, 1976. [DOI] [PubMed] [Google Scholar]
- Bushnell et al. 1993.Bushnell MC, Duncan GH, Tremblay N. Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol 69: 739–752, 1993. [DOI] [PubMed] [Google Scholar]
- Calma 1965.Calma N The activity of the posterior group of thalamic nuclei in the cat. J Physiol 180: 350–370, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens and Yokota 1980.Carstens E, Yokota T. Viscerosomatic convergence and responses to intestinal distension of neurons at the junction of midbrain and posterior thalamus in the cat. Exp Neurol 70: 392–402, 1980. [DOI] [PubMed] [Google Scholar]
- Casey 1966.Casey KL Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29: 727–750, 1966. [DOI] [PubMed] [Google Scholar]
- Casey et al. 1994.Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA. Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71: 802–807, 1994. [DOI] [PubMed] [Google Scholar]
- Caterina et al. 1997.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- Christensen and Perl 1970.Christensen BN, Perl ER. Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J Neurophysiol 33: 293–307, 1970. [DOI] [PubMed] [Google Scholar]
- Coghill et al. 1994.Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci 14: 4095–4108, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig 2004.Craig AD Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol 477: 119–148, 2004. [DOI] [PubMed] [Google Scholar]
- Craig 2006.Craig AD Retrograde analyses of spinothalamic projections in the Macaque monkey: input to ventral posterior nuclei. J Comp Neurol 499: 965–978, 2006. [DOI] [PubMed] [Google Scholar]
- Craig 2008.Craig AD Retrograde analyses of spinothalamic tract projections in the Macaque monkey: input to the ventral lateral nucleus. J Comp Neurol 508: 315–328, 2008. [DOI] [PubMed] [Google Scholar]
- Craig and Blomqvist 2002.Craig AD, Blomqvist A. Is there a specific lamina I spinothalamocortical pathway for pain and temperature sensations in primates? J Pain 3: 95–101, 2002. [DOI] [PubMed] [Google Scholar]
- Craig et al. 1994.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773, 1994. [DOI] [PubMed] [Google Scholar]
- Craig et al. 2001.Craig AD, Krout K, Andrew D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol 86: 1459–1480, 2001. [DOI] [PubMed] [Google Scholar]
- Craig et al. 1989.Craig AD, Linington AJ, Kniffki KD. Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol 289: 568–585, 1989. [DOI] [PubMed] [Google Scholar]
- Craig and Zhang 2006.Craig AD, Zhang E-T. Retrograde analyses of spinothalamic projections in the Macaque monkey: input to posterolateral thalamus. J Comp Neurol 499: 953–964, 2006. [DOI] [PubMed] [Google Scholar]
- Curry 1972.Curry MJ The exteroceptive properties of neurons in the somatic part of the posterior group (PO). Brain Res 44: 439–462, 1972. [DOI] [PubMed] [Google Scholar]
- Curry and Gordon 1972.Curry MJ, Gordon G. The spinal input to the posterior group in the cat: an electrophysiological investigation. Brain Res 44: 417–437, 1972. [PubMed] [Google Scholar]
- Dado et al. 1994.Dado RJ, Katter JT, Giesler GJ Jr. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of antidromically identified axons in the thalamus and hypothalamus. J Neurophysiol 71: 959–980, 1994. [DOI] [PubMed] [Google Scholar]
- Davis et al. 1999.Davis KD, Lozano RM, Manduch M, Tasker RR, Kiss ZH, Dostrovsky JO. Thalamic relay site for cold perception in humans. J Neurophysiol 81: 1970–1973, 1999. [DOI] [PubMed] [Google Scholar]
- Dostrovsky and Craig 1996.Dostrovsky JO, Craig AD. Cooling-specific spinothalamic tract neurons in the monkey. J Neurophysiol 76: 3656–3665, 1996. [DOI] [PubMed] [Google Scholar]
- Ferrington et al. 1987.Ferrington DG, Sorkin LS, Willis WD Jr. Responses of spinothalamic tract cells in the superficial dorsal horn of the primate lumbar spinal cord. J Physiol 388: 681–703, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman and Murray 1986.Friedman DP, Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the Macaque. J Comp Neurol 252: 348–373, 1986. [DOI] [PubMed] [Google Scholar]
- Guilbaud et al. 1977.Guilbaud G, Caille D, Besson JM, Benelli G. Single units activities in ventral posterior and posterior group thalamic nuclei during nociceptive and non-nociceptive stimulation in the cat. Arch Ital Bio 115: 35–56, 1977. [PubMed] [Google Scholar]
- Guilbaud et al. 1980.Guilbaud G, Peschanski M, Gautron M, Binder D. Neurons resonding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8: 303–318, 1980. [DOI] [PubMed] [Google Scholar]
- Gauriau and Bernard 2004a.Gauriau C, Bernard J-F. A comparative reappraisal of projections from the superficial laminae of the dorsal horn in the rat: the forebrain. J Comp Neurol 468: 24–56, 2004a. [DOI] [PubMed] [Google Scholar]
- Gauriau and Bernard 2004b.Gauriau C, Bernard J-F. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 24: 752–761, 2004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesler et al. 1981.Giesler GJ, Yezeirski RP, Gerhart KD, Willis WD. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol 46: 1285–1308, 1981. [DOI] [PubMed] [Google Scholar]
- Graziano and Jones 2004.Graziano A, Jones EG. Widespread thalamic terminations of fibers arising in the superficial medullary dorsal horn of monkeys and their relation to calbindin immunoreactivity. J Neurosci 24: 248–256, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai and Jones 1989.Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 14: 1–34, 1989. [DOI] [PubMed] [Google Scholar]
- Hylden et al. 1986.Hylden JL, Hayashi H, Dubner R, Bennett GJ. Physiology and morphology of the lamina I spinomesenchepalic projection. J Comp Neurol 247: 505–515, 1986. [DOI] [PubMed] [Google Scholar]
- Jones 2002.Jones EG A pain in the thalamus. J Pain 3: 102–104, 2002. [DOI] [PubMed] [Google Scholar]
- Jones 2007.Jones EG The Thalamus. Cambridge, UK: Cambridge, 2007.
- Jones and Burton 1974.Jones EG, Burton H. Cytoarchitecture and somatic sensory connectivity of thalamic nuclei other than the ventrobasal complex in the cat. J Comp Neurol 154: 395–432, 1974. [DOI] [PubMed] [Google Scholar]
- Kenshalo et al. 1980.Kenshalo DR, Giesler GJ Jr, Leonard RB, Willis WD. Responses of neurons in the primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol 43: 1594–1614, 1980. [DOI] [PubMed] [Google Scholar]
- Kenshalo et al. 1979.Kenshalo DR, Leonard RB, Chung JM, Willis WD. Responses of primate spinothalamic neurons to graded and to repeated noxious heat stimuli. J Neurophysiol 42: 1370–1389, 1979. [DOI] [PubMed] [Google Scholar]
- Kevetter and Willis 1983.Kevetter GA, Willis WD. Collaterals of spinothalamic cells in the rat. J Comp Neurol 215: 453–464, 1983. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2007.Kim JH, Greenspan JD, Coghill RC, Ohara S, Lenz FA. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci 27: 4995–5005, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux 2000.LeDoux JE Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184, 2000. [DOI] [PubMed] [Google Scholar]
- LeDoux et al. 1990.LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 10: 1043–1054, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux et al. 1987.LeDoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264: 123–146, 1987. [DOI] [PubMed] [Google Scholar]
- Lee et al. 1999.Lee JI, Dougherty PM, Antezana D, Lenz FA. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol 410: 541–555, 1999. [DOI] [PubMed] [Google Scholar]
- Lenz and Dougherty 1998.Lenz FA, Dougherty PM. Neurons in the human thalamic somatosensory nucleus (Ventralis caudalis) respond to innocuous cool and mechanical stimuli. J Neurophysiol 79: 2227–2230, 1998. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1993a.Lenz FA, Seike M, Lin YC, Baker FH, Rowland LH, Gracely RH, Richardson RT. Neurons in the area of human thalamic nucleus ventalis caudalis respond to painful heat stimuli. Brain Res 623: 235–240, 1993a. [DOI] [PubMed] [Google Scholar]
- Lenz et al. 1993b.Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70: 200–212, 1993b. [DOI] [PubMed] [Google Scholar]
- Lipski 1981.Lipski J Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981. [DOI] [PubMed] [Google Scholar]
- Lu et al. 2005.Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol 67: 1119–1127, 2005. [DOI] [PubMed] [Google Scholar]
- Matsumoto et al. 1988.Matsumoto N, Sato T, Sawano H, Tochinai A, Suzuki TA. Characteristics of tooth pulp-driven neurons in the posterior group of the cat thalamus. Neurosci Lett 93: 253–258, 1988. [DOI] [PubMed] [Google Scholar]
- Mehler 1969.Mehler WR Some neurological species differences-a posteriori. Ann NY Acad Sci 167: 424–468, 1969. [Google Scholar]
- Mehler et al. 1960.Mehler WR, Feferman ME, Nauta WJH. Ascending axon degeneration following anterolateral cordotomy. An experimental study in the monkey. Brain 83: 718–750, 1960. [DOI] [PubMed] [Google Scholar]
- Montes et al. 2005.Montes C, Magnin M, Maarrawi J, Frot M, Convers P, Mauguiere F, Garcia-Larrea L. Thalamic thermo-algesic transmission: ventral posterior (VP) complex versus VMpo in the light of a thalamic infarct with central pain. Pain 113: 223–232, 2005. [DOI] [PubMed] [Google Scholar]
- Nagy and Rang 1999.Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J Neurosci 19: 10647–10655, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist and Greenhoot 1974.Nyquist JK, Greenhoot JH. Unit analysis of nonspecific thalamic responses to high-intensity cutaneous input in the cat. Exp Neurol 42: 609–622, 1974. [DOI] [PubMed] [Google Scholar]
- Ohara and Lenz 2003.Ohara S, Lenz FA. Medial lateral extent of thermal and pain sensations evoked by microstimulation in somatic sensory nuclei of human thalamus. J Neurophysiol 90: 2367–2377, 2003. [DOI] [PubMed] [Google Scholar]
- Olszewski 1952.Olszewski J The Thalamus of the Macaca mulatta: An Atlas for use with the Stereotaxic Instrument. Basel: Karger, 1952.
- Patapoutian et al. 2003.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4: 529–539, 2003. [DOI] [PubMed] [Google Scholar]
- Perl and Whitlock 1961.Perl ER, Whitlock DG. Somatic stimuli exciting spinothalamic projections to thalamic neurons in cat and monkey. Exp Neurol 3: 256–296, 1961. [DOI] [PubMed] [Google Scholar]
- Poggio and Mountcastle 1960.Poggio GF, Mountcastle VB. Study of the functional contributions of the lemniscal and spinothalamic systems to somatic sensibility. Johns Hopkins Hosp Bull 106: 266–316, 1960. [PubMed] [Google Scholar]
- Price et al. 1976.Price DD, Dubner R, Hu JW. Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal and, nociceptive stimulation of monkey's face. J Neurophysiol 39: 936–953, 1976. [DOI] [PubMed] [Google Scholar]
- Ralston 2003.Ralston HJ Pain, the brain, and the (calbindin) stain. J Comp Neurol 459: 329–333, 2003. [DOI] [PubMed] [Google Scholar]
- Ralston and Ralston 1992.Ralston HJ, Ralston DD. The primate dorsal spinothalamic tract: evidence for a specific termination in the posterior nuclei (Po/SG) of the thalamus. Pain 48: 107–118, 1992. [DOI] [PubMed] [Google Scholar]
- Ranck 1975.Ranck JB Which elements are excited by electrical stimulation of mammalian central nervous system: a review. Brain Res. 98: 417–440, 1975. [DOI] [PubMed] [Google Scholar]
- Rausell et al. 1992.Rausell E, Bae CS, Viñuela A, Huntley GW, Jones EG. Calbindin and parvalbumin cells in monkey VPL thalamic nucleus: distribution, laminar cortical projections, and relations to spinothalamic terminations. J Neurosci 12: 4088–4111, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringkamp et al. 2001.Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci 21: 4460–4468, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel et al. 1972.Rockel AJ, Heath CJ, Jones EG. Afferent connections to the diencephalon in the marsupial phalanger and question of sensory convergence in the “posterior group” of the thalamus. J Comp Neurol 145: 105–129, 1972. [DOI] [PubMed] [Google Scholar]
- Shi and Davis 1999.Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci 19: 420–430, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone et al. 2004.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ, Jr. Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004. [DOI] [PubMed] [Google Scholar]
- Stepniewska et al. 2003.Stepniewska I, Sakai ST, Qi H-X, Kaas JH. Somatosensory input to the ventrolateral thalamic region in the macaque monkey: a potential substrate for Parkinsonian tremor. J Comp Neurol 455: 378–395, 2003. [DOI] [PubMed] [Google Scholar]
- Stevens et al. 1993.Stevens RT, London SM, Apkarian AV. Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res 631: 241–246, 1993. [DOI] [PubMed] [Google Scholar]
- Treede et al. 2000.Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain 87: 113–119, 2000. [DOI] [PubMed] [Google Scholar]
- Whitlock and Perl 1961.Whitlock DG, Perl ER. Thalamic projections of spinothalamic pathways in monkey. Exp Neurol 3: 240–255, 1961. [DOI] [PubMed] [Google Scholar]
- Willis and Coggeshall 2004.Willis WD, Coggeshall RE. Sensory Mechanism of the Spinal Cord. New York: Kluwer Academic/Plenum Publishers, 2004.
- Willis et al. 1974.Willis WD, Trevino DL, Coulter JD, Maunz RA. Responses of primate spinothalamic tract neurons to natural stimulation of hindlimb. J Neurophysiol 37: 358–372, 1974. [DOI] [PubMed] [Google Scholar]
- Willis et al. 2001.Willis WD, Zhang X, Honda CN, Giesler GJ Jr. Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain 92: 267–276, 2001. [DOI] [PubMed] [Google Scholar]
- Willis et al. 2002.Willis WD, Zhang X, Honda CN, Giesler GJ Jr. A critical review of the role of the proposed VMpo nucleus in pain. J Pain 3: 79–94, 2002. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2006.Zhang X, Davidson S, Giesler GJ Jr. Thermally identified subgroups of marginal zone neurons project to distinct regions of the ventral posterior lateral nucleus in rats. J Neurosci 26: 5215–5223, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Giesler 2005.Zhang X, Giesler GJ Jr. Response characteristics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol 93: 2552–2564, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2000b.Zhang X, Honda CN, Giesler GJ Jr. Position of spinothalamic tract axons in upper cervical spinal cord of monkeys. J Neurophysiol 84: 1180–1185, 2000b. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1999.Zhang X, Wenk HN, Gokin AP, Honda CN, Giesler GJ Jr. Physiological studies of spinohypothalamic tract neurons in the lumbar enlargement of monkeys. J Neurophysiol 82: 1054–1058, 1999. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2000a.Zhang X, Wenk HN, Honda CN, Giesler GJ Jr. Locations of spinothalamic tract axons in cervical and thoracic spinal cord white matter in monkeys. J Neurophysiol 83: 2869–2880, 2000a. [DOI] [PubMed] [Google Scholar]