Abstract
Differences in the developmental origin and relative proportions of biochemically distinct classes of cortical neurons have been found between rodents and primates. In addition, species differences in the properties of certain cell types, such as neurogliaform cells, have also been reported. Consequently, in this study we compared the anatomical and physiological properties of parvalbumin (PV)-positive basket interneurons in the prefrontal cortex of macaque monkeys and rats. The somal size, total dendritic length, and horizontal and vertical spans of the axonal arbor were similar in monkeys and rats. Physiologically, PV basket cells could be identified as fast-spiking interneurons in both species, based on their short spike and high-frequency firing without adaptation. However, important interspecies differences in the intrinsic physiological properties were found. In monkeys, basket cells had a higher input resistance and a lower firing threshold, and they generated more spikes at near-threshold current intensities than those in rats. Thus monkey basket cells appeared to be more excitable. In addition, rat basket cells consistently fired the first spike with a substantial delay and generated spike trains interrupted by quiescent periods more often than monkey basket cells. The frequency of miniature excitatory postsynaptic potentials in basket cells was considerably higher in rats than that in monkeys. These differences between rats and monkeys in the electrophysiological properties of PV-positive basket cells may contribute to the differential patterns of neuronal activation observed in rats and monkeys performing working-memory tasks.
INTRODUCTION
The existence of a microcircuit template that is conserved across different species and different neocortical regions has been widely discussed (Douglas and Martin 2004; Nelson et al. 2006; Silberberg et al. 2002). The idea of a canonical cortical circuit, however, has been challenged by recent evidence of interspecies differences in the inhibitory component of neocortical circuitry. For example, interneurons constitute a considerably larger proportion of cortical neurons in primates (25–34%) than that in rodents (15–25%) (Beaulieu 1993; DeFelipe et al. 2002; Gabbott and Bacon 1996; Gabbot et al. 1997; Gonchar and Burkhalter 1997; Meinecke and Peters 1987). In addition, the proportions of chemically defined subpopulations of neocortical interneurons might differ between rodents and primates. Thus cells immunoreactive for the Ca-binding protein parvalbumin (PV) seem to predominate in rodent neocortex, but constitute a much smaller proportion of interneurons in primates (Conde et al. 1994; Desgent et al. 2005; Gabbott and Bacon 1996; Gabbott et al. 1997; Glezer et al. 1998; Gonchar and Burkhalter 1997; Kubota et al. 1994), although whether these differences represent species differences in specific subtypes of interneurons or differences only in relative levels of Ca-binding protein expression must yet be determined. Furthermore, the frequency of colocalization of neurochemical markers in the same cell is lower in monkeys than that in rodents (Conde et al. 1994; Kawaguchi and Kubota 1997; Xu et al. 2006; Zaitsev et al. 2005), although a relatively small overlap of neurochemical markers was reported in rat visual cortex (Gonchar and Burkhalter 1997). Moreover, interneurons in primates and rodents might differ in their anatomical site of origin. In rodents, most neocortical γ-aminobutyric acid (GABA) cells originate in the subcortical ganglionic eminence of the telencephalon (Butt et al. 2005; Xu et al. 2004), whereas in humans, as well as in nonhuman primates, GABAergic neuronal progenitors were found not only in the ganglionic eminence, but also in the cortical subventricular zone (Letinic et al. 2002; Petanjek et al. 2008). Electrophysiological differences between interneurons of monkey and rat prefrontal cortex (PFC) were first suggested (Krimer et al. 2005) and then explicitly shown for neurogliaform (NGF) cells (Povysheva et al. 2007). NGF cells in monkey PFC appear to be more excitable than those in rat PFC because they have higher input resistance (Rin), lower firing threshold, and higher firing frequency. Monkey NGF cells fired without a pronounced delay at threshold and thus do not seem to have the “late spiking” phenotype described for rat NGF cells (Kawaguchi 1995).
PV-positive cells in both monkey and rat PFC have been identified morphologically as basket or chandelier cells (Conde et al. 1994; Gabbot et al. 1997). These interneurons innervate the soma and proximal dendrites or the axon initial segment of pyramidal cells, respectively (Somogyi et al. 1998). Physiologically, in both species PV-positive cells are characterized by brief action potentials (APs) and lack of spike adaptation, characteristic features of the “fast-spiking (FS) phenotype” and, vice versa, cells identified as fast-spiking usually express PV (Kawaguchi and Kubota 1997; Toledo-Rodriguez et al. 2004; Zaitsev et al. 2005).
In single-unit recordings in vivo in the PFC, FS units play an important role in working-memory (WM) function in monkey since they are spatially tuned during visual WM tasks (Rao 1999). In addition, they contribute to sharpening the tuning of pyramidal cells during WM in modeling studies (Tanaka 1999; Wang et al. 2004) and GABAA-receptor blockade in the PFC produces an expansion of the receptive fields of FS units (Rao et al. 2000). Importantly, the firing frequency of FS units, putative FS interneurons, in monkey PFC during the delay period of oculomotor delayed response task is 40–60 Hz (Wilson et al. 1994), which is considerably higher than that in prefrontal FS units of rats performing a figure-eight maze delayed spatial alternation task (∼12 Hz; Jung et al. 1998). The differences in the spiking behavior of putative FS interneurons between monkey and rat PFC might be related to the differences in their intrinsic membrane properties.
Our recent physiological studies of interneurons in monkey and rat PFC (Krimer et al. 2005; Povysheva et al. 2006) suggested that PV-positive basket cells in primates might differ from those in rats. However, a definitive interspecies comparison was not possible in those studies because of significant differences in the developmental stages of rats (prepubertal) and monkeys (young adults) that were studied. Here, we directly compared morphological and physiological properties of PV-positive basket cells in adult monkey and adult rat PFC. We report that monkey basket cells are more excitable and also fire a first spike with less delay than that in rat basket cells. In monkeys, these cells have fewer quiescent periods at near-suprathreshold current intensities and, accordingly, generate more spikes at a near-threshold current level. Finally, the frequency of miniature excitatory postsynaptic potentials (mEPSPs) is considerably higher in rat than that in monkey basket cells and positively correlates with the rheobase current, indicating a possible important association between membrane and synaptic properties in basket cells. These differences in subthreshold and suprathreshold membrane properties of PV-positive basket cells in rats and monkeys could serve as a basis for the different patterns of activation of FS units reported previously in animals performing WM tasks (Constantinidis and Goldman-Rakic 2002; Jung et al. 1998; Wilson et al. 1984).
METHODS
Brain slices were obtained from adult (56–135 days, 350–550 g; n = 20) male Wistar rats and adult (3.5–6.0 kg; 4–5 yr old; n = 15) male long-tailed macaque monkeys (Macaca fascicularis). Slices from the same monkeys were also used in the other studies (Krimer et al. 2005; Povysheva et al. 2006; Zaitsev et al. 2005). All animals were treated in accordance with the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Rats were deeply anesthetized with halothane and decapitated. The brain was quickly removed and immersed in ice-cold preoxygenated artificial cerebrospinal fluid (ACSF). Tissue block containing the prelimbic cortex in rats (Paxinos and Watson 1998) was excised for slicing. The protocol used to obtain tissue blocks from monkey PFC was previously described (Gonzalez-Burgos et al. 2000). Coronal slices (350 μm thick) were cut with a vibratome (Model VT1000S, Leica, Nussloch, Germany). Slices were incubated at 37°C for 0.5–1 h and further stored at room temperature until transfer to a recording chamber perfused with ACSF at 31–32°C. The recording temperatures were identical for both species. Through all steps of the experiments, ACSF of the following composition was used (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 24 NaHCO3, and 10–20 dextrose. ACSF was perfused with 95% O2-5% CO2 gas mixture.
Electrophysiological recordings
Whole cell voltage recordings were made from layer 2/3 neurons visualized by infrared differential interference contrast videomicroscopy using a Zeiss Axioskop 2 FS microscope, equipped with a ×40 water-immersion objective and a Dage-MTI NC-70 video camera (Dage-MTI Television, Michigan City, IN). Interneurons were identified based on their round or oval cell body and lack of apical dendrite. Pyramidal cells were recognized by their apical dendrites and triangular somata. Patch electrodes were filled with an internal solution containing (in mM): 114 K-gluconate, 6 KCl, 10 HEPES, 4 ATP-Mg, and 0.3 GTP; pH was adjusted to 7.25 with KOH. Biocytin (0.5%; Molecular Probes, Eugene, OR) was added to the solution for later morphological identification of the recorded neurons. Electrodes had 5- to 12-MΩ open-tip resistance. Voltages were amplified with an IE-210 electrometer (Warner Instruments, Hamden, CT) or a MultiClamp 700A amplifier (Axon Instruments, Union City, CA) operating in bridge-balance mode. Signals were filtered at 5 or 4 kHz in the IE-210 and the MultiClamp, respectively, and acquired at a sampling rate of 20 kHz using a 16-bit-resolution Power 1401 interface and Signal software (CED, Cambridge, UK). Access resistance and capacitance were compensated on-line. Access resistance typically was 15–30 MΩ and remained relatively stable during experiments (≤30% increase) for the cells included in the analysis. Membrane potential was not corrected for the liquid junction potential. During some of the experiments, gabazine (4.5–6 μM; Sigma, St. Louis, MO) was added to the bath to block GABAA receptors. d-2-Amino-5-phospho-pentanoic acid (APV, 50 μM; Sigma) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μM; Sigma) were included in the bath to block N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, respectively. α-Dendrotoxin (α-DTX, 150–200 nM; Alomone Labs, Jerusalem, Israel) was used to block Kv1 channels and 4-(N-ethyl-N-phenylamino-1,2-dimethyl-6-methylamino)pyrimidinium chloride (ZD7288, 20 μM; Alomone Labs) was used to block Ih channels.
Miniature EPSPs were recorded at a membrane potential of about −75 mV in both rat and monkey PFC neurons. Tetrodotoxin (TTX, 0.5–1 μM; Alomone Labs) and bicuculline methiodide (BMI, 10–20 μM; Sigma) were added to the bath to block APs and GABAA-mediated inhibitory postsynaptic potentials, respectively.
Electrophysiological data analysis
To characterize the membrane properties of neurons, hyper- and depolarizing current steps were applied for 500 ms in 5- to 10-pA increments at 0.5 Hz. Input resistance (Rin) was measured from the slope of a linear regression fit to the voltage–current relation in a hyperpolarizing range. The membrane time constant was determined by single-exponential fitting to the on-phase of the average voltage responses to the initial (5–15 pA) hyperpolarizing current steps, which presumably are less affected by voltage-dependent membrane conductances. All AP measures were taken from the first AP of the first evoked sweep that reached the spike threshold (level of voltage deflection exceeding 10 mV/1 ms). Peak amplitude of the AP was measured from the AP threshold. The spike rise time was measured as the time taken to rise from 10 to 90% of the peak amplitude. The spike decay time was measured as the time taken to decay from 10 to 90% of the amplitude between peak and spike threshold. Duration of the AP was measured at its half-amplitude. Depolarizing sag was estimated as the difference between the most negative membrane potential during a 500-ms hyperpolarizing current step and the membrane potential at the end of the step as the percentage relative to the voltage deflection from the resting membrane potential (RMP) at the end of the step. Hump was estimated at the depolarizing current step that preceded spiking as the difference between the most positive membrane potential and the membrane potential at the end of the step as the percentage relative to the voltage deflection from the RMP at the end of the step. Adaptation ratio (AR) coefficient was used to describe spike frequency adaptation in spike trains. First, the ratio between the first and the last interspike interval (ISI) was calculated for each stimulation current intensity. Then, the AR coefficient was estimated from the linear regression of AR versus current at 60 pA above threshold. The coefficient of variation (CV) of ISIs was measured within the last 350 ms of the response to depolarizing current pulses at the level of 20 pA above spike threshold.
Basket cells were identified as FS based on the results of the previously performed cluster analysis, ANOVA, and Fisher least significant difference post hoc test (Krimer et al. 2005). The parameters with the most discriminative values, action potential duration (APD; average value 0.37 ± 0.09), and AR (average value 0.82 ± 0.21) were used as criteria for FS interneurons: APD − 1.5SDs as the high limit and AR coefficient +SD as the low limit. Accordingly, only basket cells with the spike half-duration <0.51 ms and AR coefficient >0.61 were included in the analysis. The aforementioned criteria correspond to those previously used in the neocortex of young rats (Kawaguchi 1995) and adult rats (Thomson et al. 1996).
Miniature events were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). Peak events were first detected automatically using an amplitude threshold of twofold the average root mean square noise, which was about 0.3 mV for mEPSPs. After automatic analysis, events were rechecked by visual inspection of the traces and were accepted for analysis if they had a monophasic rising phase and decayed to baseline in an exponential manner. Approximately 100–300 events in each cell were included in the analysis. Amplitudes of miniature responses were determined from baseline to peak. The time constants of single-exponential fits were used to describe the decay time. The rise time was estimated as the time necessary to rise between 10 and 90% of the peak response.
Morphological data analysis
To identify cell morphology after the electrophysiological experiments, neurons were filled with biocytin (0.5%) added to the internal solution. After recordings, slices were immersed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) and then were kept in storing solution (33% glycerol, 33% ethylene glycol, in 0.1 M PBS) at −80°C. PV immunoreactivity (and calretinin and/or calbindin in some cells) in a sample of FS interneurons from monkey (n = 21) and rat (n = 14) PFC was detected by an indirect immunofluorescence technique as previously described (Zaitsev et al. 2005). Briefly, slices were incubated for 24–48 h at 4°C in blocking serum (10% normal goat serum; 2% bovine serum albumin; 0.4% Triton X-100 in phosphate buffer [PB]) containing streptavidin-Alexa Fluor 633 conjugate (dilution 1:500; Invitrogen). After this, cells were confocally reconstructed for morphological identification. Next, slices were serially resectioned at 40–50 μm and then incubated in a mixture of antibodies against two of the three calcium-binding proteins: PV (1:2,000; Sigma or Swant, Bellinzona, Switzerland), calretinin (1:2,000; Swant or Chemicon, Temecula, CA), or calbindin (1:2,000; Swant) and, subsequently, in a mixture of the two secondary antibodies (Alexa Fluor 594 conjugated goat anti-mouse IgG, 1:500 and Alexa Fluor 488 conjugated goat anti-rabbit IgG, 1:500, Molecular Probes) diluted in the blocking serum. This procedure yielded differential fluorescent covisualization of Alexa Fluor 633-biocytin-filled interneurons and Alexa Fluor 488- and 594-labeled calcium-binding proteins. Rinsed sections were kept in a storing solution until they were analyzed using an Olympus FluoviewTM 500 confocal laser scanning microscope (Olympus America, Melville, NY). After that, the sections were treated with 1% H2O2 for 2–3 h at room temperature, rinsed, and incubated with the avidin-biotin-peroxidase complex (1:100; Vector Laboratories, Burlingame, CA) in PB for 4 h. Sections were rinsed, stained with Ni-3,3′-diaminobenzidine (DAB), mounted on gelatin-coated glass slides, dehydrated, and coverslipped. Slices from some experiments were processed for biocytin only.
Cells were morphologically identified as basket cells based on the confocal reconstructions or/and development of biocytin. Some cells were three-dimensionally reconstructed and their dendritic trees were quantitatively described using the Neurolucida neuron tracing system with NeuroExplorer software (MBF Bioscience, Williston, VT). The horizontal and vertical axonal spans were measured in the cells developed for biocytin as the mean distance between the three most distal axonal endings on each side of the soma of individual interneurons.
Statistical analysis
Two-tailed t-tests were used for group comparisons in most cases. Pearson correlation coefficient was used to measure the relation between mEPSP frequency and the rheobase current. Statistical significance of between-group differences for CV of ISIs and number of spikes measured at different depolarizing current steps was evaluated using repeated-measures ANOVA and Fisher's post hoc test.
Unless otherwise noted values are presented as means ± SD. Statistical tests were performed using Excel (Microsoft, Redmond, WA) or Statistica 6.1 (Statsoft, Tulsa, OK).
Twenty of 39 basket cells and 23 of 27 pyramidal cells from monkey dorsolateral PFC were reported in our previous publications (Krimer et al. 2005; Povysheva et al. 2006; Zaitsev et al. 2005).
RESULTS
Morphological properties of monkey and rat basket cells
Interneurons from monkey and rat PFC were classified as “basket” based on previously described morphological features (Jones and Hendry 1984; Kawaguchi 1995; Lund and Lewis 1993). Because PV-positive basket cells are known to be FS in the neocortex of different species, only neurons with basket cell morphology and FS electrophysiological properties (based on short spike duration and firing without significant adaptation; see methods) were included in this study. Basket cell somata had a round or slightly vertical oval shape and were located in layers 2/3. The cells possessed smooth and multipolar dendrites (Fig. 1, A and C). The axon of the cells originated from the cell body or one of the primary dendrites. Axons spread either in all directions or predominantly horizontally, rarely extending into layer I or layer V. Therefore the basket cell axonal tree occupied a significant volume, but still was mainly located within layers 2/3, the same cortical layers as the soma.
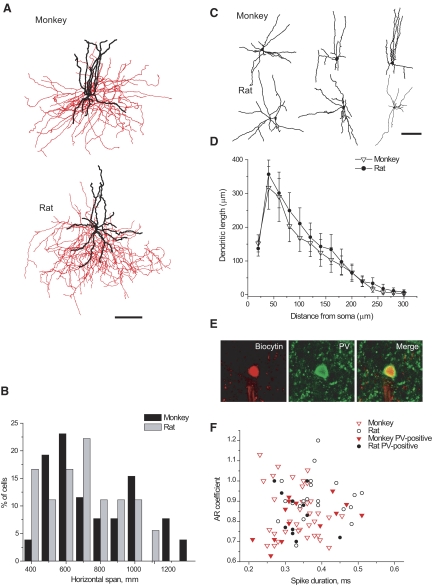
FIG. 1.
Morphological and neurochemical features of monkey and rat basket cells. A: representative examples of monkey and rat basket cells (axons are indicated in red; dendrites are indicated in black; scale bar 100 μm). B: distributions of horizontal axonal span values are comparable in monkeys (n = 26) and rats (n = 19). C: morphological varieties of dendritic trees of basket cells (scale bar 100 μm). D: Sholl analysis of dendritic arbors from monkey (n = 10) and rat (n = 12) basket cells. Average dendritic length is plotted against the distance from the soma. Error bars represent SE. E: parvalbumin (PV)-immunoreactivity in rat basket cell. F: graphs of adaptation ratio (AR) coefficient against spike duration revealed that 2 populations of fast-spiking (FS) basket cells from monkeys and rats (those that were shown to be PV-positive and those that did not go through immunohistochemical analysis) totally overlap in the distributions.
Since basket cells are known to have a great range of their axonal horizontal dimension, and are often viewed as a morphologically heterogeneous group of cells (see e.g. Lund and Lewis 1993), we evaluated the spatial distribution of their axonal trees in monkeys and rats. We found that the average axonal vertical and horizontal spans were comparable in both species (Table 1). Distributions of horizontal span did not significantly differ between species (Kolmogorov–Smirnov test, P > 0.1; Fig. 1B), indicating that basket cells from monkeys and rats were equally represented by smaller and larger cells.
TABLE 1.
Morphometric analysis of basket cells from monkeys and rats
| Parameter Measured | Monkeys (n = 11) | Rats (n = 14) |
|---|---|---|
| Soma area, μm2 | 92 ± 25 | 94 ± 19 |
| Number of primary dendrites | 6.8 ± 2.1 | 6.1 ± 1.8 |
| Total dendritic length, μm | 2,000 ± 970 | 2,268 ± 936 |
| Number of dendritic nodes | 12 ± 5 | 14 ± 5 |
| Axonal horizontal span, μm | 810 ± 394 (n = 26) | 750 ± 531 (n = 19) |
| Axonal vertical span, μm | 576 ± 173 (n = 26) | 553 ± 92 (n = 19) |
| Axonal length, μm | 21,912 ± 4,033 (n = 4) | 34,655 ± 5,190 (n = 3) |
| Number of axonal nodes | 161 ± 36 (n = 4) | 359 ± 83 (n = 3) |
Values are means ± SD.
Additional quantitative analysis was performed on the cells with fully reconstructed on the transmitted light microscope. Monkey basket cells had somata that were comparable with those from rats (Table 1). The number of primary dendrites and the total dendritic length did not differ between species (Table 1, Fig. 1C). Dendrites of both monkey and rat basket cells branched up to the fifth-order and had very similar branching patterns. Sholl analysis did not reveal any significant differences in dendritic distribution between monkey and rat basket cells (Fig. 1D). The only features that differed between species were axonal length and number of axonal nodes, both of which were greater in rat than in monkey basket cells (Table 1).
PV immunoreactivity was identified in a subset of FS basket interneurons from monkey (14 of 21; 67%) and rat (11 of 14; 78%) PFC (Fig. 1E). Reasons for the absence of PV-labeling in some cells could be 1) washout of low-weight molecules, such as PV, from the cytosol during the whole cell recording (Baimbridge et al. 1992), or 2) a general reduction in immunoreactivity in brain slices used in physiological experiments. None of the monkey cells (n = 9) that were also tested for calretinin and/or calbindin was immunoreactive for these proteins.
Comparison of the membrane properties of FS basket interneurons containing PV and those that did not undergo immunohistochemistry revealed that the membrane properties of PV-positive interneurons and the rest of the basket cells were similar in both species; the scatterplot of adaptation ratio (AR) coefficient versus spike duration (Fig. 1F) shows substantial overlap of PV-positive and other basket cells with FS phenotype. Based on these results, we pooled data from PV-positive basket cells and all other FS basket cells.
Subthreshold membrane properties of basket cells in adult rat and monkey
Although the RMP of basket cells was comparable in both species (Table 2), most of the other subthreshold membrane properties appeared to be different. Input resistance was significantly larger in basket cells from monkey than from rat (Table 2, Fig. 2, A and B), indicating their greater excitability. Hyperpolarizing current steps of the same strength generated larger voltage responses in basket cells from monkey than from rats. Accordingly, current–voltage (I–V) curves obtained from the subthreshold responses to hyperpolarizing and depolarizing current pulses from monkey cells were much steeper than those from rat (Fig. 2B). The time constant was also longer in monkey basket cells (Table 2).
TABLE 2.
Intrinsic subthreshold and firing physiological properties of basket cells in monkeys and rats
| Property | Monkey (n = 39) | Rat (n = 31) |
|---|---|---|
| Resting membrane potential, mV | −68 ± 8 | −67 ± 6 |
| Spike threshold, mV | −41 ± 5*** | −34 ± 3 |
| Spike threshold, pA | 74 ± 50*** | 132 ± 69 |
| Spike amplitude, mV | 55 ± 11 | 52 ± 7 |
| Spike half-duration, ms | 0.34 ± 0.06* | 0.38 ± 0.08 |
| Spike 10–90% rise time, ms | 0.15 ± 0.03*** | 0.21 ± 0.04 |
| Spike 10–90% fall time, ms | 0.23 ± 0.06 | 0.25 ± 0.03 |
| First spike latency, ms | 71 ± 69*** | 220 ± 159 |
| Afterhyperpolarization amplitude, mV | 23 ± 8 | 24 ± 5 |
| Adaptation ratio coefficient | 0.90 ± 0.13 | 0.85 ± 0.12 |
| Number of spikes (20 pA above threshold) | 16 ± 5** | 11 ± 5 |
| CV of interspike intervals (20 pA above threshold) | 5.1 ± 2.2*** | 27 ± 26 |
| Input resistance, MΩ | 251 ± 130* | 182 ± 83 |
| Time constant, ms | 9.5 ± 2.8* | 7.7 ± 3.8 |
Values are means ± SD. Significantly different between the two species:
P < 0.05;
P < 0.01;
P < 0.001.
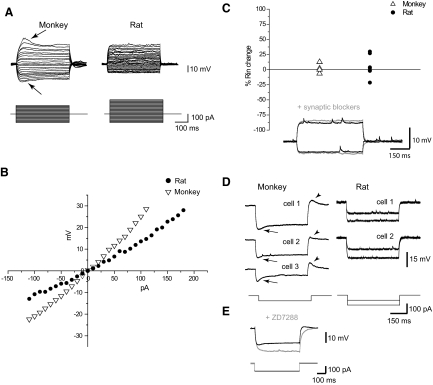
FIG. 2.
Subthreshold membrane properties of basket cells in monkey and rat. A: voltage responses to the hyperpolarizing and depolarizing current steps (representative examples from monkey and rat). Note the depolarizing sag on hyperpolarizing responses and the hump on depolarizing responses in monkey basket cells (arrows). B: current–voltage plots for traces shown in A. C: synaptic blockers (CNQX, 20 μM; APV, 50 μM; gabazine, 4.5–6 μM) did not produce any significant changes in input resistance in both monkey and rat basket cells. D: different examples of depolarizing sags (arrows) and rebound depolarizations (arrowheads) in monkey basket cells. Traces from rat basket cells lack both depolarizing sag and rebound depolarization. E: ZD7288 (20 μM) eliminated depolarizing sag in monkey basket cells (n = 4).
Spontaneous synaptic activity in vivo can directly affect a cell's input resistance (Steriade et al. 2001) and therefore could potentially influence the observed differences between monkey and rat PFC basket cells. To assess the direct effects of spontaneous synaptic activity in our brain slice conditions, we recorded the responses to subthreshold hyperpolarizing current pulses after blocking excitatory and inhibitory synaptic transmission with gabazine (4.5–6 μM), CNQX (20 μM), and APV (50 μM). Blockade of spontaneous synaptic activity did not result in a significant change of input resistance in any of the species (rat: P = 0.79, n = 8; monkey: P = 0.75, n = 4, paired comparison) (Fig. 2C). We therefore conclude that the difference in the input resistance observed between the two species can be attributed to the intrinsic membrane properties, but not to the shunting associated with the presence of synaptic conductances.
Although the I–V plot revealed time-independent inward rectification in both monkey and rat basket cells (Fig. 2, A and B), the majority of monkey basket cells exhibited time-dependent changes of responses to the hyperpolarizing current pulses. This characteristic depolarizing sag was estimated to be 19 ± 9% with a stimulation current of −50 pA (n = 32) in monkey basket cells. The majority (69%) of monkey basket cells exhibited sag that was ≥15%. At the same level of stimulation current, and also with the increase of the stimulation current up to −100 pA (Fig. 2D), we failed to observe any depolarizing sag in rat basket cells. Depolarizing sag in monkey basket cells was blocked by bath application of Ih channel blocker (Fig. 2E). When subthreshold depolarizing current steps were applied, monkey basket cells generated voltage responses with an initial hump followed by a hyperpolarizing sag (Fig. 2A). We estimated the hump to be 29 ± 10% (n = 31) on the subthreshold sweeps preceding generation of AP in monkey basket cells. The hump was not observed in rat basket cells.
Firing properties of basket cells in adult monkey and rat
SINGLE SPIKE PROPERTIES.
Single spikes of monkey and rat basket cells were comparable in some parameters, including spike amplitude and afterhyperpolarization (AHP) amplitude (Table 2, Fig. 3A). However, interspecies differences in other single-spike parameters were observed. Monkey basket neurons had a shorter spike duration than that in rats. A difference between species was observed in spike rise time but not in decay time, indicating different activation properties or density of Na+ channels in the two species.
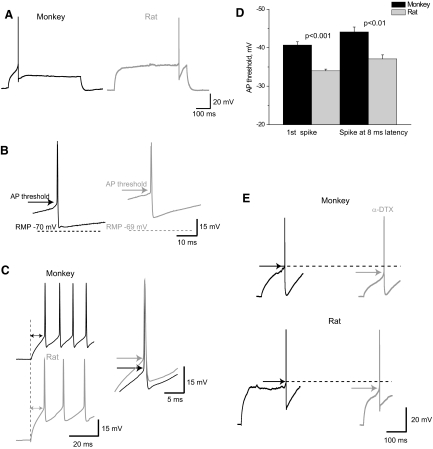
FIG. 3.
Properties of the first evoked spike in monkey and rat basket cells. A: first suprathreshold response to the depolarizing current was significantly delayed in rat, but not in monkey basket cells. B: spike threshold was considerably lower in monkey basket cells than that in rat (APs are truncated). C: spikes evoked by rectangular current pulses with the latency of 8 ms still had more negative threshold in monkey (n = 15) basket cells than that in rat (n = 14). D: threshold of the 1st spikes and 8-ms-latency spikes is lower in monkeys than that in rats. Error bars represent SE. E: α-dendrotoxin (α-DTX) decreased spike threshold for the 1st spikes evoked in monkey (n = 4) and rat basket cells (n = 5).
The majority of basket cells from rat PFC demonstrated the delayed firing pattern with near-threshold current intensities (Table 2, Fig. 3A). This delay of the first spike progressively shortened with the subsequently stronger current stimulation. At the same time, monkey basket cells generated a first spike with a considerably shorter delay at all ranges of stimulating current (Figs. 3A and 4A).
Action potential threshold was considerably lower in basket cells from monkeys than that from rats (Table 2, Fig. 3B), which could be associated with the higher Rin in monkey basket cells (Prescott et al. 2006; Segev and London 1999), as well as with the other mechanisms and, again, could be indicative of greater excitability of monkey basket cells.
Notably, the first spike that we used for the measurements of the voltage threshold was significantly more delayed in rat than in monkey basket cells. Delayed first spike is known to be associated with the activation of low-threshold K+ channels (Banks et al. 1996) that can also affect spike threshold and thus influence the observed interspecies difference. Would the difference between the species persist for the APs occurring with equal and much shorter latency, which would also be physiologically more meaningful? To address this issue we measured the threshold of the APs that were generated with a latency of about 8 ms, which is similar to the EPSP spike latency reported for the FS interneurons (Fricker and Miles 2000; Maccaferri and Dingledine 2002). Voltage threshold of the spikes occurring with a latency of about 8 ms was still more negative in monkey than in rat basket cells (Fig. 3, C and D).
A more delayed first spike in rat basket cells can have a higher AP threshold due either to inactivation of Na+ currents or to activation of low-threshold K+ currents. We evaluated the second of these possibilities by the application of the Kv1 channel blocker α-dendrotoxin (α-DTX). α-DTX decreased the first-spike latency by 47% (137 ± 86 and 72 ± 44 ms, P < 0.05, n = 5, paired comparison) in rat basket cells, but it did not significantly affect the latency in monkey basket cells. Also, we found that after blocking Kv1 channels, the threshold of first spikes became more negative in rat (by 16%: −33 ± 3.5 mV before and −39.5 ± 2.5 mV after, n = 5, P = 0.01), as well as in monkey basket cells (by 13%: −41.5 ± 5 and −47 ± 5 mV accordingly, n = 4, P < 0.05; Fig. 3E). Similar effects of α-DTX on the spike threshold in the two species might indicate that the Kv1 current that contributes to the longer first-spike delay in rat basket cells does not seem to be responsible for the observed interspecies difference in AP threshold.
FIRING PATTERN PROPERTIES.
Both rat and monkey basket cells fired without, or with very little, adaptation. Representative examples of the nonadapting firing pattern in monkey and rat basket cells are shown in Fig. 4A. With the highest intensities of stimulation current, the temporal structure of firing was similar in monkey and rat basket cells, but rat cells exhibited quiescent periods at near-threshold levels of stimulating current (Fig. 4A), which made their firing more irregular. This irregularity was evaluated by the CV of ISIs, which was significantly higher in rat basket cells (n = 23) than that in monkey (n = 28) at near-threshold levels of stimulating currents (0–60 pA above threshold) according to the ANOVA test (F = 32.5; P < 0.001) and Fisher post hoc test (Fig. 4B). The same type of analysis was performed exclusively for the population of PV-positive basket cells in both species. Similarly to the combined populations of basket cells, PV-positive basket cells in rat (n = 9) have a more irregular firing pattern than that from monkey (F = 16.3; P < 0.001, n = 10). Post hoc Fisher test revealed differences at the levels of stimulating current of 10–30 and 50 pA (data not shown).
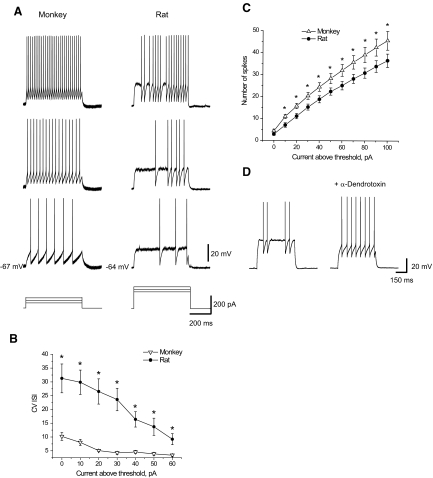
FIG. 4.
Firing pattern in basket cells from monkeys and rats. A: firing patterns produced by monkey and rat basket cells in response to the current steps of different intensities. Note that the firing pattern in rats was characterized by the presence of quiescent periods. B: plot of coefficient of variation of interspike intervals (CV ISIs) vs. current intensity demonstrates more variability in ISIs in rat basket cells (n = 28) than that in monkey basket cells (n = 31). The difference was more substantial for the responses evoked by the near-threshold current intensities. Error bars represent SE. Asterisks indicate P < 0.05. C: number of spikes evoked in monkeys (n = 20) was considerably larger than that in rats (n = 18) for the near-threshold current intensities. Error bars represent SE. Asterisks indicate P < 0.05. D: application of α-DTX (150–200 nM) eliminated quiescent periods in rat firing pattern (n = 5).
To further compare the excitability of monkey and rat basket cells, we looked at the number of spikes produced in these cells by different stimulating currents. Relative to rats, monkey basket cells generated more spikes at levels of 10–100 pA above firing threshold according to the ANOVA test (F = 4.15; P < 0.05, n = 20) and post hoc Fisher test (Fig. 4C, Table 2). Fewer spikes were associated with the irregular firing in rat basket cells interrupted by quiescent periods. Quiescent periods and irregularity of firing were eliminated after the blockade of Kv1 channels by α-DTX (Fig. 4D).
Miniature EPSPs of monkey and rat basket cells
Although we showed that blockade of spontaneous synaptic activity did not produce immediate changes in membrane properties (Fig. 2C), it is known that decreasing the levels of excitatory synaptic input in a long-term fashion can increase the firing response elicited by current injection, reflecting involvement of homeostatic mechanisms (Desai et al. 1999; Turrigiano and Nelson 2000). Differential properties of excitatory responses in the two species, if revealed, could provide potential explanatory mechanisms for the observed interspecies differences in membrane properties. Thus we analyzed the properties of excitatory postsynaptic inputs received by interneurons, which were shown to be cell-type specific (Cossart et al. 2006).
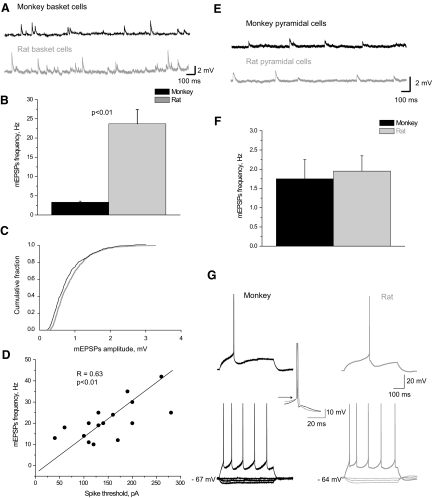
To characterize the excitatory inputs to FS basket cells, we recorded mEPSPs, which provide a measure of number and strength of excitatory inputs onto an individual neuron. As shown on the representative sweeps (Fig. 5A) and on the bar graph (Fig. 5B), the frequency of mEPSPs was considerably higher in rat than that in monkey basket cells. At the same time, the average mEPSP amplitude was 0.92 ± 0.24 mV in monkey basket cells (n = 6) and 0.95 ± 0.19 mV in rat basket cells (n = 16). Cumulative histograms of mEPSP amplitude showed that the amplitude distribution in monkey basket cells was similar to that from rat basket cells (Fig. 5C). The kinetics of events was also comparable in the two species (rise times: monkey 1.9 ± 0.2 ms; rat: 1.8 ± 0.2 ms), although we observed a tendency toward a longer decay time in monkey EPSPs (12.9 ± 5 ms) than that in rats (11.5 ± 3 ms), which can be associated with their longer membrane time constant.
FIG. 5.
Miniature excitatory postsynaptic potentials (mEPSPs) in monkey and rat basket cells. A: series of mEPSPs recorded from monkey and rat basket cells. B: mEPSPs were more frequent in rats (n = 16) than in monkeys (n = 6). Error bars represent SE. C: cumulative frequency distribution histograms of mEPSP amplitudes were similar in monkey (n = 6) and rat basket cells (n = 16). D: frequency of mEPSPs positively correlated with the spike threshold (pA) in rat basket cells (n = 16). E: series of mEPSPs recorded from monkey and rat pyramidal cells. F: mEPSP frequencies were similar in rat (n = 11) and in monkey (n = 7) pyramidal cells. Error bars represent SE. G: membrane properties of pyramidal cells were comparable in both species.
To address the potential mechanism that connects membrane properties and an excitatory drive expressed as a frequency of quantal synaptic inputs, we estimated the correlation between mEPSP frequency and spike threshold in rat basket cells. A significant positive correlation between these two parameters was found (Fig. 5D), providing evidence for the potential long-term association of synaptic inputs and membrane properties.
To exclude the possibility that differences in mEPSP frequencies can be caused by varying qualities of slices, we also estimated mEPSP frequencies in monkey and rat pyramidal cells. In contrast to basket cells, mEPSP frequency of pyramidal cells was comparable in monkey and rat PFC (Fig. 5, E and F). These results suggest that the differences in mEPSP frequency reported here are cell-type and species specific.
Interestingly, unlike in FS basket cells, physiological membrane properties of pyramidal cells were very similar in monkey (n = 27) and rat (n = 21) (Fig. 5G). Specifically, they did not show any significant difference in input resistance, spike threshold, firing frequency, or first-spike latency.
DISCUSSION
The results of this study revealed important differences between monkeys and rats in the intrinsic physiological characteristics of PFC basket cells. In contrast to rat basket cells, monkey basket cells were more excitable, as indicated by their lower spike threshold, higher input resistance and the number of spikes they could generate at near-threshold current intensities. Furthermore, basket cells in monkeys produced a less-delayed first spike at near-threshold currents than that in rats. On the contrary, the morphological characteristics of basket cells were very similar in both species. Our findings suggest that interspecies differences in FS basket cells can be detected at the level of intrinsic cellular properties and provide some insights into the different spiking behavior of FS units during working-memory tasks in monkey and rat (Constantinidis and Goldman-Rakic 2002; Jung et al. 1998;Wilson et al. 1994).
Differential membrane properties of basket cells from monkey and rat
A number of physiological properties of basket cells were highly comparable across species. The similarities included single spike properties such as spike amplitude, spike decay time, and the AHP amplitude, as well as firing pattern properties, such as high firing frequency and lack of frequency adaptation. Still, some critical electrophysiological properties differed significantly between monkey and rat basket cells.
Monkey basket cells were more excitable than those in rats, displaying higher input resistance, lower threshold of AP, and higher firing frequency. Excitability in general and input resistance in particular are shown to be defined by the density of K+ currents, which are mostly leak currents but also inward rectifiers (Gorelova et al. 2002; Nichols and Lopatin 1997; Rudy 1988). We showed that AP threshold was more negative in monkey basket cells than that in rat because spikes occurred at different latencies. More negative spike thresholds can be associated with the differential expression of voltage-gated channels that are open at the near-threshold level of membrane potential and can be responsible for the shunting of Na+ currents (the only mechanism of an ideal isopotential structure). Thus association between Kv1 channels and excitability was previously demonstrated (Glazebrook et al. 2002; Guan et al. 2006). In this study, we showed that Kv1 channel blocker decreased AP threshold in both monkey and rat basket cells. Properties of the inward Na+ and low-threshold T-type Ca2+ currents could also contribute to the observed differences in firing threshold (Yang et al. 1996). Ih currents are also shown to contribute to neuronal excitability (Pape 1996; Robinson and Sigelbaum 2003). Aponte et al. (2006) demonstrated their presence in FS interneurons in the hippocampus of rats. In our study we showed the presence of Ih currents in monkey FS interneurons, although we could not resolve the differences in expression between the two species. Dissimilarities in AP threshold could also be connected with the differences in input resistance between the species (Prescott et al. 2006; Segev and London 1999); higher input resistance in monkey basket cells can produce more negative AP threshold. The role of the passive membrane properties in the sculpting of the spike threshold was shown in modeling studies that demonstrated that the electrotonic structure of the cable affects voltage, as well as current, spike threshold (Segev and London 1999). Recently, Prescott et al. (2006) showed that changes in input resistance (or membrane conductance) produced by shunting could influence voltage spike threshold in a phase-plane model and also in dynamic-clamp experiments in real CA1 pyramidal neurons. The possible mechanism suggested by the authors is that with a larger outward leak current (in high conductance states), AP generation requires a greater activation of the sodium current.
Also, we demonstrated that the first spikes in monkey basket cells were less delayed than in rat basket cells. Mainly, they generated the first spike shortly after stimulation onset. More delayed first spikes in rat basket cells could be associated with the low-threshold outward K+ current (Banks et al. 1996).
Unlike firing in monkey basket cells, the firing pattern in rat PFC basket cells was often interrupted by quiescent periods with the near-threshold current intensities, so the firing pattern appeared irregular or “stuttering” (Markram et al. 2004): with stronger current intensities, the irregular firing pattern became typical fast-spiking nonadapting. This feature of FS basket cells was previously reported in rat neocortex (Descalzo et al. 2005; Kawaguchi 1995). The irregular firing of rat basket cells resulted in fewer spikes at the near-threshold current levels. Mechanisms producing irregular firing in rat basket cells could involve activation of the low-threshold Kv1 channels since we showed that application of Kv1 channel blocker eliminated quiescent periods in their firing pattern (Fig. 4D). The same effect was also demonstrated in other studies on rat neocortex when α-DTX blocked “stuttering” in interneurons (Toledo-Rodriguez et al. 2004).
The connectivity pattern of the dendritic tree can determine the firing properties of neurons (Mainen and Sejnowski 1996; van Ooyen et al. 2002). Quantitative morphological analysis of basket cells from monkeys and rats undertaken in our study did not reveal any significant interspecies differences and thus could not provide an explanatory mechanism for the observed differences in physiological membrane properties between the species.
The firing activity of neurons is largely driven by the properties of their synaptic inputs (Fuchs et al. 2001; Pouille and Scanziani 2004; Traub et al. 1993). First, spontaneous synaptic activity can directly affect input resistance, as was previously shown through in vivo studies (Steriade et al. 2001), and therefore can influence the observed difference between monkey and rat basket cells. This influence can be executed as a direct shunt of applied current. To test this possibility, we measured input resistance of basket cells before and after application of synaptic blockers. No significant difference was found in either species (Fig. 2C). However, it should be noted here, that the concentration of gabazine used in this study is most probably not enough to block tonic GABA current, which can also influence input resistance (Semyanov et al. 2003; Stell and Mody 2002).
Reflected in spontaneous activity excitatory inputs received by neurons are also known to produce long-term homeostatic changes in ion channels and thus in membrane properties of neurons, as was previously demonstrated (Desai et al. 1999; Pratt and Aizenman 2007; Turrigiano and Nelson 2000). In this study, we found that the frequency of mEPSPs is much lower in monkey than that in rat basket cells. The higher frequency of mEPSPs in rat basket cells can indicate more abundant excitatory synapses impinging onto rat basket cells than onto those in monkeys. Two studies using electron microscopy reported on the number of asymmetric synapses in different types of interneurons. Although they used different methodologies, thus not allowing for direct comparison between species, indirectly the studies suggest that the density of excitatory synapses is lower in monkey than that in rat basket cells (Gulyas et al. 1999; Melchitzky and Lewis 2003). Given the positive correlation between the spike threshold and mEPSP frequency, this finding might represent a homeostatic mechanism by which neurons with more abundant excitatory inputs reduce their excitability to normalize the effects of greater synaptic drive and thereby maintain a constant functional output (Hansel et al. 2001; Marder and Goaillard 2006;Turrigiano and Nelson 2000). Importantly, because the properties of dendrites did not differ between the FS basket cells recorded from monkey and rat PFC (Table 1, Fig. 1), it is unlikely that the differences in mEPSP frequency are due to a less well preserved FS basket cell dendritic tree in slices of monkey PFC.
Notably, the frequency, amplitude, and kinetics of miniature events were comparable in pyramidal cells from both monkeys and rats. Interestingly, physiological membrane properties of pyramidal cells in the hippocampus were also very similar in both monkeys and rats (Altemus et al. 2005).
Contribution to species differences in PFC functioning
PV-positive basket cells appear to be responsible for generation of gamma-oscillations in the hippocampus, since interneurons providing perisomatic inhibition were found to fire with gamma-frequency discharge rate and to be strongly phase locked to the different rhythms of network oscillations (Buzsáki and Draguhn 2004; Hajos et al. 2004; Tukker et al. 2007). Irregularity in firing of rat basket cells (due to the presence of quiescent periods in their firing pattern), as well as delayed firing, can contribute to the differences in shaping of neuronal oscillations in the two species (Buzsáki and Draguhn 2004).
The different physiological properties of basket FS interneurons from monkey and rat PFC may further inform our understanding of the nature of the functional differences between primate and rodent PFC during the performance of WM tasks (Brown and Bowman 2002; Preuss 1995; Uylings et al. 2003). Although “memory cells” that are active during the delay period of working-memory tasks are described in both monkey and rat PFC, their spiking behavior is different in the two species—both excitatory and inhibitory “memory cells” fire at considerably lower frequencies in rats (Constantinidis and Goldman-Rakic 2002; Jung et al. 1998;Wilson et al. 1994).
In conclusion, the results of this study help clarify the translation of data from rodents to primates and is critical for studies focused on understanding the pathophysiological mechanisms of mental illnesses, such as schizophrenia, that are associated with dysfunction of PFC interneurons (Lewis et al. 2005).
GRANTS
This work was supported by National Institute of Mental Health Grants R01MH-067963 and R01MH-051234. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Acknowledgments
We thank O. A. Krimer and J. Kosakowski for excellent technical assistance.
Present addresses: N. V. Povysheva, University of Pittsburgh, Department of Neuroscience, Pittsburgh, PA 15260; A. V. Zaitsev, Trinity College, Department of Physiology, Dublin, Ireland.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Altemus et al. 2005.Altemus KL, Lavenex P, Ishizuka N, Amaral DG. Morphological characteristics and electrophysiological properties of CA1 pyramidal neurons in macaque monkeys. Neuroscience 136: 741–756, 2005. [DOI] [PubMed] [Google Scholar]
- Aponte et al. 2006.Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574: 229–243, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge et al. 1992.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci 15: 303–308, 1992. [DOI] [PubMed] [Google Scholar]
- Banks et al. 1996.Banks MI, Haberly LB, Jackson MB. Layer-specific properties of the transient K current (IA) in piriform cortex. J Neurosci 16: 3862–3876, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu 1993.Beaulieu C Numerical data on neocortical neurons in adult rat, with special reference to the GABA population. Brain Res 609: 284–292, 1993. [DOI] [PubMed] [Google Scholar]
- Brown and Bowman 2002.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci 25: 340–343, 2002. [DOI] [PubMed] [Google Scholar]
- Butt et al. 2005.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48: 591–604, 2005. [DOI] [PubMed] [Google Scholar]
- Buzsáki and Draguhn 2004.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science 304: 1926–1929, 2004. [DOI] [PubMed] [Google Scholar]
- Conde et al. 1994.Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 341: 95–116, 1994. [DOI] [PubMed] [Google Scholar]
- Constantinidis and Goldman-Rakic 2002.Constantinidis C, Goldman-Rakic PS. Correlated discharges among putative pyramidal neurons and interneurons in the primate prefrontal cortex. J Neurophysiol 88: 3487–3497, 2002. [DOI] [PubMed] [Google Scholar]
- Cossart et al. 2006.Cossart R, Petanjek Z, Dumitriu D, Hirsch JC, Ben-Ari Y, Esclapez M, Bernard C. Interneurons targeting similar layers receive synaptic inputs with similar kinetics. Hippocampus 16: 408–420, 2006. [DOI] [PubMed] [Google Scholar]
- DeFelipe et al. 2002.DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J Neurocytol 31: 299–316, 2002. [DOI] [PubMed] [Google Scholar]
- Desai et al. 1999.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999. [DOI] [PubMed] [Google Scholar]
- Descalzo et al. 2005.Descalzo VF, Nowak LG, Brumberg JC, McCormick DA, Sanchez-Vives MV. Slow adaptation in fast-spiking neurons of visual cortex. J Neurophysiol 93: 1111–1118, 2005. [DOI] [PubMed] [Google Scholar]
- Desgent et al. 2005.Desgent S, Boire D, Ptito M. Distribution of calcium binding proteins in visual and auditory cortices of hamsters. Exp Brain Res 163: 159–172, 2005. [DOI] [PubMed] [Google Scholar]
- Douglas and Martin 2004.Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci 27: 419–451, 2004. [DOI] [PubMed] [Google Scholar]
- Freund and Buzsáki 1996.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. [DOI] [PubMed] [Google Scholar]
- Fricker and Miles 2000.Fricker D, Miles R. EPSP amplification and the precision of spike timing in hippocampal neurons. Neuron 28: 559–569, 2000. [DOI] [PubMed] [Google Scholar]
- Fuchs et al. 2001.Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, Kopell N, Whittington MA, Monyer H. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc Natl Acad Sci USA 98: 3571–3576, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott and Bacon 1996.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol 364: 609–636, 1996. [DOI] [PubMed] [Google Scholar]
- Gabbott et al. 1997.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol 377: 465–499, 1997. [DOI] [PubMed] [Google Scholar]
- Glazebrook et al. 2002.Glazebrook PA, Ramirez AN, Schild JH, Shieh CC, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol 541: 467–482, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer et al. 1998.Glezer II, Hof PR, Morgane PJ. Comparative analysis of calcium-binding protein-immunoreactive neuronal populations in the auditory and visual systems of the bottlenose dolphin (Tursiops truncatus) and the macaque monkey (Macaca fascicularis). J Chem Neuroanat 15: 203–237, 1998. [DOI] [PubMed] [Google Scholar]
- Gonchar and Burkhalter 1997.Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex 7: 347–358, 1997. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos et al. 2000.Gonzalez-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10: 82–92, 2000. [DOI] [PubMed] [Google Scholar]
- Gorelova et al. 2002.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol 88: 3150–3166, 2002. [DOI] [PubMed] [Google Scholar]
- Guan et al. 2006.Guan D, Lee JC, Tkatch T, Surmeier DJ, Armstrong WE, Foehring RC. Expression and biophysical properties of Kv1 channels in supragranular neocortical pyramidal neurones. J Physiol 571: 371–389, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas et al. 1999.Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci 19: 10082–10097, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos et al. 2004.Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 24: 9127–9137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel et al. 2001.Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci 4: 467–475, 2001. [DOI] [PubMed] [Google Scholar]
- Jones and Hendry 1984.Jones EG, Hendry SHC. Basket cells. In: Cerebral Cortex, edited by Peters A, Jones E. New York: Plenum Press, 1984, p. 309–335.
- Jung et al. 1998.Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex 8: 437–450, 1998. [DOI] [PubMed] [Google Scholar]
- Kawaguchi 1995.Kawaguchi Y Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci 15: 2638–2655, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi and Kubota 1997.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7: 476–486, 1997. [DOI] [PubMed] [Google Scholar]
- Krimer et al. 2005.Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol 94: 3009–3022, 2005. [DOI] [PubMed] [Google Scholar]
- Kubota et al. 1994.Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649: 159–173, 1994. [DOI] [PubMed] [Google Scholar]
- Letinic et al. 2002.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature 417: 645–649, 2002. [DOI] [PubMed] [Google Scholar]
- Lewis et al. 2005.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324, 2005. [DOI] [PubMed] [Google Scholar]
- Lund and Lewis 1993.Lund JS, Lewis DA. Local circuit neurons of developing and mature macaque prefrontal cortex: Golgi and immunocytochemical characteristics. J Comp Neurol 328: 282–312, 1993. [DOI] [PubMed] [Google Scholar]
- Maccaferri and Dingledine 2002.Maccaferri G, Dingledine R. Control of feedforward dendritic inhibition by NMDA receptor-dependent spike timing in hippocampal interneurons. J Neurosci 22: 5462–5472, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen and Sejnowski 1996.Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382: 363–366, 1996. [DOI] [PubMed] [Google Scholar]
- Marder and Goaillard 2006.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci 7: 563–574, 2006. [DOI] [PubMed] [Google Scholar]
- Markram et al. 2004.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004. [DOI] [PubMed] [Google Scholar]
- Meinecke and Peters 1987.Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. J Comp Neurol 261: 388–404, 1987. [DOI] [PubMed] [Google Scholar]
- Melchitzky and Lewis 2003.Melchitzky DS, Lewis DA. Pyramidal neuron local axon terminals in monkey prefrontal cortex: differential targeting of subclasses of GABA neurons. Cereb Cortex 13: 452–460, 2003. [DOI] [PubMed] [Google Scholar]
- Nelson et al. 2006.Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci: 29: 339–345, 2006. [DOI] [PubMed] [Google Scholar]
- Nichols and Lopatin 1997.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997. [DOI] [PubMed] [Google Scholar]
- Pape 1996.Pape HC Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327, 1996. [DOI] [PubMed] [Google Scholar]
- Paxinos and Watson 1998.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed.). New York: Academic Press, 1998.
- Petanjek et al. 2004.Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex (May 13, 2008) doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed]
- Pouille and Scanziani 2004.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature 429: 717–723, 2004. [DOI] [PubMed] [Google Scholar]
- Povysheva et al. 2006.Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, Kroner S, Barrionuevo G, Lewis DA, Krimer LS. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex 16: 541–552, 2006. [DOI] [PubMed] [Google Scholar]
- Povysheva et al. 2007.Povysheva NV, Zaitsev AV, Kroner S, Krimer OA, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Electrophysiological differences between neurogliaform cells from monkey and rat prefrontal cortex. J Neurophysiol 97: 1030–1039, 2007. [DOI] [PubMed] [Google Scholar]
- Pratt and Aizenman 2007.Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott et al. 2006.Prescott SA, Ratte S, De Koninck Y, Sejnowski TJ. Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J Neurosci 26: 9084–9097, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss 1995.Preuss TM Do rats have prefrontal cortex? The Rose–Woolsey–Akert Program reconsidered. J Cogn Neurosci 7: 1–24, 1995. [DOI] [PubMed] [Google Scholar]
- Rao et al. 1999.Rao SG, Williams GV, Goldman-Rakic PS. Isodirectional tuning of adjacent interneurons and pyramidal cells during working memory: evidence for microcolumnar organization in PFC. J Neurophysiol 81: 1903–1916, 1999. [DOI] [PubMed] [Google Scholar]
- Rao et al. 2000.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci 20: 485–494, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson and Siegelbaum 2003.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453–480, 2003. [DOI] [PubMed] [Google Scholar]
- Rudy 1988.Rudy B Diversity and ubiquity of K channels. Neuroscience 25: 729–749, 1988. [DOI] [PubMed] [Google Scholar]
- Segev and London 1999.Segev I, London M. A theoretical view of passive and active dendrites. In: Dendrites, edited by Stuart G, Spruston N, Hausser M. New York: Oxford Univ. Press, 1999, p. 205–230.
- Semyanov et al. 2003.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484–490, 2003. [DOI] [PubMed] [Google Scholar]
- Silberberg et al. 2002.Silberberg G, Gupta A, Markram H. Stereotypy in neocortical microcircuits. Trends Neurosci 25: 227–230, 2002. [DOI] [PubMed] [Google Scholar]
- Somogyi et al. 1998.Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev 26: 113–135, 1998. [DOI] [PubMed] [Google Scholar]
- Stell and Mody 2002.Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci 22: RC223, 2002. [DOI] [PMC free article] [PubMed]
- Steriade et al. 2001.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85: 1969–1985, 2001. [DOI] [PubMed] [Google Scholar]
- Tanaka 1999.Tanaka S Architecture and dynamics of the primate prefrontal cortical circuit for spatial working memory. Neural Networks 12: 1007–1020, 1999. [DOI] [PubMed] [Google Scholar]
- Thomson et al. 1996.Thomson AM, West DC, Hahn J, Deuchars J. Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol 496: 81–102, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez et al. 2004.Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, Markram H. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex 14: 1310–1327, 2004. [DOI] [PubMed] [Google Scholar]
- Traub et al. 1993.Traub RD, Miles R, Jefferys JG. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol 461: 525–547, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker et al. 2007.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci 27: 8184–8189, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano and Nelson 2000.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10: 358–364, 2000. [DOI] [PubMed] [Google Scholar]
- Uylings et al. 2003.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res 146: 3–17, 2003. [DOI] [PubMed] [Google Scholar]
- van Ooyen et al. 2002.van Ooyen A, Duijnhouwer J, Remme MW, van Pelt J. The effect of dendritic topology on firing patterns in model neurons. Network 13: 311–325, 2002. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2004.Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al. 1994.Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci USA 91: 4009–4013, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. 2004.Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci 24: 2612–2622, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. 2006.Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499: 144–160, 2006. [DOI] [PubMed] [Google Scholar]
- Yang et al. 1996.Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V–VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci 16: 1904–1921, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev et al. 2005.Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15: 1178–1186, 2005. [DOI] [PubMed] [Google Scholar]