Abstract
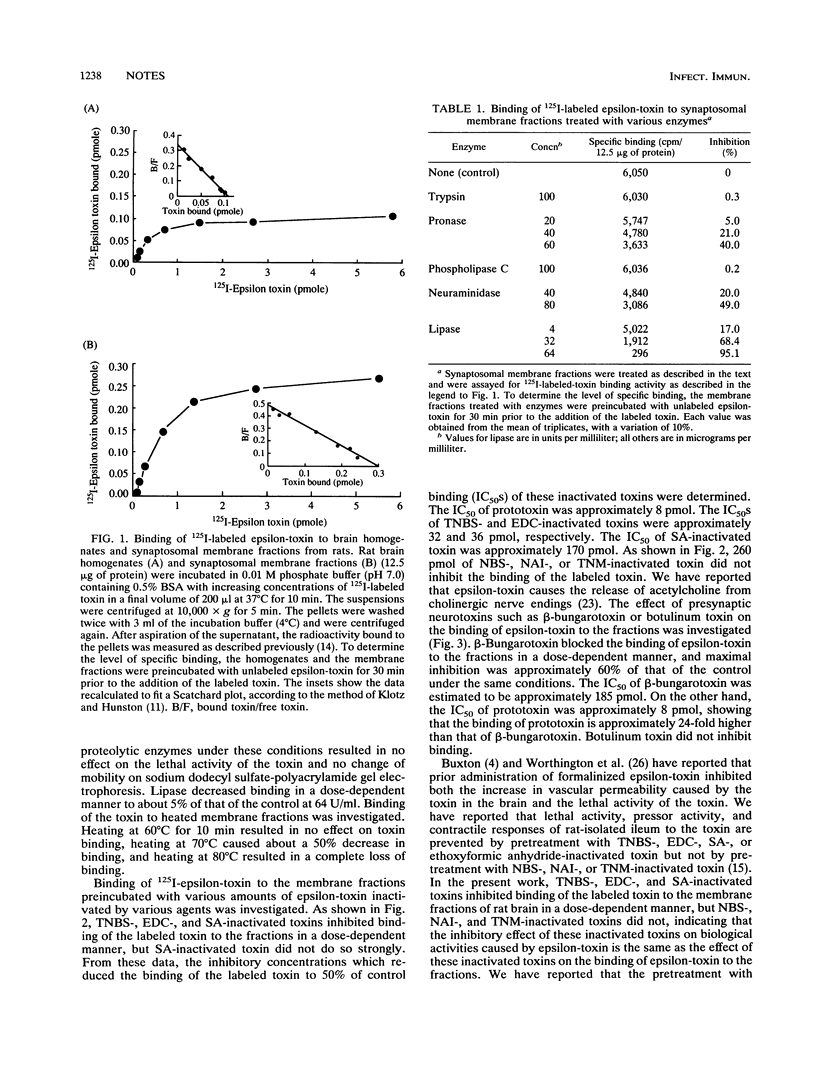
125I-epsilon-toxin showed high affinity to rat brain homogenates and synaptosomal membrane fractions, having single binding phases with dissociation constants (Kds) of 2.5 and 3.3 nM, respectively. Treatment of synaptosomal membrane fractions with pronase and neuraminidase lowered the binding of the labeled toxin, whereas treatment with trypsin and phospholipase C did not. Heating of the fractions resulted in a decrease in the binding of the toxin. These data suggest that interaction of epsilon-toxin with cell membranes in the brain is facilitated by a sialoglycoprotein. On the other hand, treatment of the membrane fractions with lipase resulted in complete loss of binding, suggesting that the interaction may require an appropriate lipid environment. These data suggest the presence of specific binding sites in brain tissue for epsilon-toxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTY I., BULLEN J. J. The effect of Clostridium welchii type D culture filtrates on the permeability of the mouse intestine. J Pathol Bacteriol. 1956 Apr;71(2):311–323. doi: 10.1002/path.1700710206. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. Use of horseradish peroxidase to study the antagonism of Clostridium welchii (Cl. perfringens) type D epsilon toxin in mice by the formalinized epsilon prototoxin. J Comp Pathol. 1976 Jan;86(1):67–72. doi: 10.1016/0021-9975(76)90029-3. [DOI] [PubMed] [Google Scholar]
- Cotman C. W. Isolation of synaptosomal and synaptic plasma membrane fractions. Methods Enzymol. 1974;31:445–452. doi: 10.1016/0076-6879(74)31050-6. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Nomura S., Oshita Y., Sakurai J. Excitatory effect of Clostridium perfringens alpha toxin on the rat isolated aorta. Br J Pharmacol. 1986 Jul;88(3):531–539. doi: 10.1111/j.1476-5381.1986.tb10233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRINER L. A., CARLSON W. D. Enterotoxemia of sheep. II. Distribution of I-131 radioiodinated serum albumin in brains of Clostridium perfringens type D intoxicated lambs. Am J Vet Res. 1961 May;22:443–446. [PubMed] [Google Scholar]
- GRINER L. A. Enterotoxemi of sheep. I. Effects of Clostridium perfringens type D toxin on the brains of sheep and mice. Am J Vet Res. 1961 May;22:429–442. [PubMed] [Google Scholar]
- Klotz I. M., Hunston D. L. Properties of graphical representations of multiple classes of binding sites. Biochemistry. 1971 Aug 3;10(16):3065–3069. doi: 10.1021/bi00792a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagahama M., Sakurai J. Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon. 1991;29(2):211–217. doi: 10.1016/0041-0101(91)90105-z. [DOI] [PubMed] [Google Scholar]
- Nagahama M., Takahashi T., Sakurai J. Effect of prior treatment with Clostridium perfringens epsilon toxin inactivated by various agents on lethal, pressor and contractile activities of the toxin. FEMS Microbiol Lett. 1990 Oct;60(1-2):59–62. doi: 10.1016/0378-1097(90)90345-q. [DOI] [PubMed] [Google Scholar]
- Othman I. B., Spokes J. W., Dolly J. O. Preparation of neurotoxic 3H-beta-bungarotoxin: demonstration of saturable binding to brain synapses and its inhibition by toxin I. Eur J Biochem. 1982 Nov;128(1):267–276. doi: 10.1111/j.1432-1033.1982.tb06961.x. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Amino groups in Clostridium perfringens epsilon prototoxin and epsilon toxin. Microb Pathog. 1986 Oct;1(5):417–423. doi: 10.1016/0882-4010(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Carboxyl groups in Clostridium perfringens epsilon toxin. Microb Pathog. 1987 Dec;3(6):469–474. doi: 10.1016/0882-4010(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M., Fujii Y. Effect of Clostridium perfringens epsilon toxin on the cardiovascular system of rats. Infect Immun. 1983 Dec;42(3):1183–1186. doi: 10.1128/iai.42.3.1183-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Role of one tryptophan residue in the lethal activity of Clostridium perfringens epsilon toxin. Biochem Biophys Res Commun. 1985 Apr 30;128(2):760–766. doi: 10.1016/0006-291x(85)90112-3. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M., Takahashi T. Contraction induced by Clostridium perfringens epsilon toxin in the isolated rat ileum. FEMS Microbiol Lett. 1989 Apr;49(2-3):269–272. doi: 10.1016/0378-1097(89)90051-7. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. The inactivation of Clostridium perfringens epsilon toxin by treatment with tetranitromethane and N-acetylimidazole. Toxicon. 1987;25(3):279–284. doi: 10.1016/0041-0101(87)90256-x. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Nagahama M. Tryptophan content of Clostridium perfringens epsilon toxin. Infect Immun. 1985 Jan;47(1):260–263. doi: 10.1128/iai.47.1.260-263.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Tse C. K., Dolly J. O., Hambleton P., Melling J. Radioiodination of botulinum neurotoxin type A with retention of biological activity and its binding to brain synaptosomes. Eur J Biochem. 1983 Mar 15;131(2):437–445. doi: 10.1111/j.1432-1033.1983.tb07282.x. [DOI] [PubMed] [Google Scholar]



