Abstract
The suggestion that NMDA receptor (NMDAR)-dependent plasticity is subunit specific, with NR2B-types required for long-term depression (LTD) and NR2A-types critical for the induction of long-term potentiation (LTP), has generated much attention and considerable debate. By investigating the suggested subunit-specific roles of NMDARs in the mouse primary visual cortex over development, we report several important findings that clarify the roles of NMDAR subtypes in synaptic plasticity. We observed that LTD was not attenuated by application of ifenprodil, an NR2B-type antagonist, or NVP-AAM007, a less selective NR2A-type antagonist. However, we were surprised that NVP-AAM007 completely blocked adult LTP (postnatal day (P) 45–90), while only modestly affecting juvenile LTP (P21-28). To assess whether this developmental transition reflected an increasing role for NR2A-type receptors with maturity, we characterized the specificity of NVP-AAM007. We found not only that NVP-AAM007 lacks discernable subunit specificity but also that the effects of NVP-AAM077 on LTP could be mimicked using subsaturating concentrations of APV, a global NMDAR antagonist. These results indicate that the effects of NVP-AAM077 on synaptic plasticity are largely explained by nonspecific blockade of NMDARs. Moreover our findings are the first to reveal a developmental increase in the sensitivity of LTP to NMDAR antagonism. We suggest that discrepant reports describing the effect of NVP-AAM077 on LTP may be partially explained by this developmental shift in the properties of LTP. These results indicate that the degree of NMDAR activation required for LTP increases with development, providing insight into a novel underlying mechanism governing the properties of synaptic plasticity.
INTRODUCTION
N-methyl-d-aspartate receptor (NMDAR)-mediated synaptic plasticity is critical for learning and memory (Morris 1989; Moser et al. 1998) as well as experience-dependent modifications that have been particularly well defined in the visual cortex, such as ocular dominance plasticity and orientation selectivity (Bear et al. 1990; Ramoa et al. 2001; Roberts et al. 1998). Because of the heterogeneity of NMDAR subtypes (Cull-Candy et al. 2001; Dingledine et al. 1999), it has been suggested that different subpopulations of NMDARs may mediate unique aspects of synaptic plasticity. The primary visual cortex is an ideal model system to delineate the roles of NMDAR subunits because it undergoes a natural developmental transition in NMDAR expression (Quinlan et al. 1999a). These changes in NMDAR composition occur across a period of developmental (Jiang et al. 2007; Kirkwood et al. 1997) and experience-dependent (Kirkwood et al. 1996; Philpot et al. 2003, 2007) modifications in the properties of synaptic plasticity.
NMDAR composition is modulated over development in an experience-dependent manner (Quinlan et al. 1999b). While all NMDARs contain two obligatory NR1 subunits, these must dimerize with a combination of two NR2A-D or NR3A-B subunits to form a functional receptor (Laube et al. 1998; Mayer and Westbrook 1987; McBain and Mayer 1994; Perez-Otano and Ehlers 2004). These secondary subunits confer distinct functional properties onto NMDARs by influencing current kinetics, glutamate affinity, and the milieu of intracellular signaling proteins proximal to the synapse (Barria and Malinow 2005; Chatterton et al. 2002; Flint et al. 1997; Vicini et al. 1998). The predominant NMDAR subtypes in the postnatal neocortex are NR2A and NR2B (Flint et al. 1997; Monyer et al. 1994; Watanabe et al. 1994). Over development, neocortical NMDARs transition from being primarily NR2B-containing (NR2B-type) to primarily NR2A-containing (NR2A-type) in an experience-dependent manner (Quinlan et al. 1999b). Because NR2B-type NMDARs have slower current kinetics than NR2A-type receptors, NMDAR currents become progressively faster with age (Carmignoto and Vicini 1992; Flint et al. 1997; Hestrin 1992; Vicini et al. 1998). Changes in the composition and function of NMDARs have been tied to changes in the properties of synaptic plasticity (Carmignoto and Vicini 1992; Nase et al. 1999; Philpot et al. 2007), adding to the speculation that different NMDAR subunits contribute to distinct aspects of synaptic plasticity.
The suggestion that the induction of long-term potentiation and depression (LTP and LTD) were mediated, respectively, by NR2A and NR2B subtypes generated great excitement (Liu et al. 2004; Massey et al. 2004). However, while subsequent studies, using a variety of different stimulation protocols, have uncovered a similar subunit-specific trend (Fox et al. 2006; Gerkin et al. 2007), other studies have failed to do so (Bartlett et al. 2007; Berberich et al. 2005; Morishita et al. 2007; Toyoda et al. 2006; Weitlauf et al. 2005). Although the possibility of NR2-specific plasticity is appealing, we suggest that it must be considered in the context of several important factors. First, it is important to assess the specificity of subunit-specific antagonists used in these plasticity studies, as the selectivity of these drugs may change between brain regions due to differences in synaptic cleft glutamate (Frizelle et al. 2006), the composition of NMDARs, or even the proportion of triheteromeric (NR1-NR2A-NR2B) NMDARs at the synapse (Kew et al. 1998; Neyton and Paoletti 2006). Because most regions of the brain, including the visual cortex, display a profound developmental shift in NMDAR subunit composition (Chen et al. 2000; Hestrin 1992; Quinlan et al. 1999a; Ramoa and McCormick 1994), characterizing the efficacy of an antagonist over development (within 1 region) can be a helpful way of discerning its specificity. Second, the effect of a subunit-specific antagonist should be compared with the effect of a global NMDAR antagonist that similarly attenuates NMDAR currents. Such an approach will help delineate whether deficiencies in plasticity can be attributed to a particular NMDAR subunit or to an overall reduction in NMDAR-mediated currents.
In an effort to elucidate whether visual cortical plasticity depends on NMDAR subunit-specific functions, our data reveal that the induction of LTD and LTP is not directed by distinct NMDAR subtypes. Instead our findings unexpectedly provide compelling evidence to suggest a developmental increase in the sensitivity of LTP to disruption by NMDAR antagonism. This developmental change in plasticity could also reconcile the wide range of results that have been reported using NVP-AAM007, a purported NR2A-selective antagonist.
METHODS
Animals
C57BL/6 mice (Charles River, Wilmington, MA) of both genders were used between postnatal days (P) 8–12 (young), P21-28 (juvenile), or P45-90 (adult). These age groups represent periods before, during, and after the classically defined critical period for ocular dominance plasticity in mice (Gordon and Stryker 1996) and are within a developmental period characterized by NR2A upregulation (Quinlan et al. 1999a). NR2A knockout mice were generously supplied by Dr. S. Nakanishi (Kadotani et al. 1996). These mice were rederived on a C57BL/6 background by Charles River Laboratories. All mice were raised on a 12-h light/dark cycle.
Cortical slice preparation
Mice were anesthetized with pentobarbital (40 mg/kg ip) and decapitated on disappearance of corneal reflexes, in compliance with the U.S. Department of Health and Human Services and the University of North Carolina guidelines. Brains were rapidly removed and immersed in ice-cold dissection buffer (composition in mM: 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 75 sucrose, 10 dextrose, 1.3 ascorbic acid, 7 MgCl2, and 0.5 CaCl2) bubbled with 95% O2-5% CO2. The visual cortices were dissected, and 350- to 400-μm coronal slices were prepared using a vibrating microtome (Leica VT1000S). Slices were allowed to recover for 15 min in a 35°C submersion chamber filled with oxygenated artificial cerebrospinal fluid (ACSF) (composition in mM: 124 NaCl, 3 KCl, 1.25 Na2PO4, 26 NaHCO3, 1 MgCl2, 2 CaCl2, and 20 dextrose) and then kept at room temperature for ≥40 min until use (Philpot et al. 2003).
Extracellular field potential recordings
The recording chambers were maintained at 31°C and perfused with ACSF at a rate of 2 ml/min. A stimulation electrode (concentric bipolar tungsten) was positioned in layer (L) 4, and a glass recording electrode (∼1 MΩ) was placed in L2/3. Because the L4-2/3 pathway is thought to be the initial site for receptive field plasticity (Trachtenberg et al. 2000), we focused our study on this pathway. The magnitude of responses evoked by a 200-μs pulse was monitored by the amplitude of the field potential response. Stimulation intensity was adjusted to elicit half the maximal response, and responses were evoked every 30 s. The resulting signals were filtered between 0.1 and 3 kHz, amplified 1,000 times, captured at 10 kHz, and analyzed using pCLAMP 9.2 software (Molecular Devices, Sunnyvale, CA). After achieving a stable baseline (<5% drift in amplitude between the 1st 5 min and the last 5 min of the baseline period), slices were stimulated to induce LTP or LTD. To induce LTP, slices were stimulated with three 1-s 100-Hz stimulations with a 5-min interval between tetanizations. Amplitudes of the field excitatory postsynaptic potentials (fEPSPs) were recorded every 30 s for 45 min after the cessation of the stimulation protocol. To synaptically induce LTD, slices were stimulated with low-frequency stimulation (LFS; 1 Hz for 15 min) (Dudek and Bear 1993). We elicited a chemical form of LTD (chem-LTD) by infusing NMDA (20 μM NMDA) for 3 min (Lee et al. 1998). For both LTD induction protocols, the amplitude of the fEPSP was recorded every 30 s throughout the 80-min experiment. Control and experimental groups were run in an interleaved manner. Changes in synaptic strength were measured by comparing the average response amplitude of the last 15 min following the conditioning stimulation to the preconditioned baseline response. When examining the effect of different NMDAR antagonists, the slices were bath perfused with drug over the duration of the recording.
Voltage-clamp recordings
To pharmacologically isolate NMDAR-mediated currents, slices were placed in a submersion chamber, maintained at 30°C, and perfused at 2 ml/min with oxygenated ACSF modified to contain (in mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose, 4 MgCl2, 4 CaCl2, 0.001 glycine, 0.05 picrotoxin, and 0.02 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) or 6,7-dinitroquinoxaline-2,3-dione (DNQX). Cells were visualized using a Nikon E600FN microscope (Tokyo, Japan) equipped with infrared differential interference contrast (IR-DIC) optics. Patch pipettes were pulled from thick-walled borosilicate glass (P97, Sutter Instrument, Novato, CA). Open tip resistances were 3–6 MΩ when pipettes were filled with the internal solution containing (in mM): 102 cesium gluconate, 5 TEA-chloride, 3.7 NaCl, 20 HEPES, 0.3 sodium guanosine triphosphate, 4 magnesium adenosine triphosphate, 0.2 EGTA, 10 bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (BAPTA), and 5 QX-314 chloride (Alomone Labs, Israel) with pH adjusted to 7.2 and osmolarity adjusted to ∼300 mmol/kg by addition of sucrose. Voltage-clamp recordings were performed at +40 mV in the whole cell configuration using a patch-clamp amplifier (Multiclamp 700A, Molecular Devices), and data were acquired and analyzed using pCLAMP 9.2 software (Molecular Devices). Cells were held at a depolarized potential to remove Mg2+ block, as opposed to recording in nominal Mg2+ at a resting membrane potential, to avoid polysynaptic responses. Pipette seal resistances were >1 GΩ, and pipette capacitive transients were minimized prior to breakthrough. Changes in series resistance were monitored throughout the experiment by giving a test pulse and measuring the amplitude of the capacitive current. However, this method is susceptible to filtering error and overestimates series resistance by >30%. Therefore we used off-line measurements, employing a previously published approach that is highly insensitive to filtering (Santos-Sacchi 1993), to more accurately estimate series resistance, cell capacitance, and the fast membrane time constant. Drugs were infused into the recording chamber at a final concentration of 50 nM NVP-AAM077, 3 μM ifenprodil, or 1 μM APV and were applied for 20 min, with the last 10 min serving as the comparison to the pre-drug baseline. The concentrations for NVP-AAM077 and ifenprodil were chosen because previous reports demonstrate that they provide the highest degree of specificity (Feng et al. 2004; Frizelle et al. 2006; Williams 1993). The concentration of APV was empirically determined, using whole cell recordings, which is further described in results. NMDAR amplitudes and weighted time constants (τw) of the receptor decay kinetics were compared before and after drug application. NMDAR excitatory postsynaptic current (EPSC) decays were well fit with a double exponential, as described previously (Rumbaugh and Vicini 1999), using the following equation: I(t) = Ifexp(−t/τf) + Isexp(−t/τs), where I is the current amplitude, t is time, If and Is are the peak amplitudes of the fast and slow components, respectively, and τf and τs are their respective time constants. NMDAR decay kinetics were quantified by a weighted time constant, which was calculated in milliseconds as: τw = τf *[If/(If + Is)] + τs *[Is/(If + Is)]. For cells to be included for analysis Rinput, Rseries, and Iholding had to fluctuate by <30%. The recorded series resistance averaged 25.9 ± 1.5 MΩ. Input resistances recorded at +40 mV did not significantly differ between age groups (P12-P18, 182.1 ± 32.0 MΩ; P21-P28, 152.2 ± 13.1 MΩ; and P45-P90, 129.8 ± 6.3) or drug applications (baseline, 151.2 ± 10.0 MΩ; 50 nM NVP-AAM077, 122.1 ± 11.1 MΩ; 3 μM ifenprodil, 162.7 ± 18.9 MΩ; and 1 μM APV, 128.3 ± 14.9 MΩ). EPSCs were evoked from a stimulating electrode (concentric bipolar; 200 μM tip separation) placed in L4, and stimulation was given for 200 μs every 15 s. EPSCs were recorded in L2/3 pyramidal cells.
Biochemical fractions
Each biochemical fraction was prepared using visual cortices pooled from 3–10 brains (brains per pooled sample; P8, n = 10; P16, n = 5; P26, n = 5; P62, n = 3), as previously described (Yashiro et al. 2005). Samples were homogenized in HEPES-buffered sucrose (4 mM HEPES, 0.32 M sucrose, pH 7.4) using a motor-driven dounce homogenizer. Postnuclear supernatant (PNS) fractions were prepared by centrifuging homogenates twice at 1,000 × g for 10 min to eliminate nuclei. PNS fractions were centrifuged at 10,000 × g for 20 min yielding crude synaptic pellets, which were then suspended in HEPES-buffered sucrose and centrifuged. The resulting pellets were lysed in a hypoosmotic buffer (4 mM HEPES, pH 7.4) using a motor-driven dounce homogenizer and mixed constantly for 30 min. Lysates were centrifuged at 25,000 × g for 20 min and pellets were suspended in HEPES-buffered sucrose to obtain lysed synaptosomal membrane (LSM) fractions. LSM fractions were subjected to density centrifugation (150,000 x g, 2 h) using a gradient consisting of 0.8, 1.0, and 1.2 M sucrose in 4 mM HEPES (pH 7.4). Synaptic plasma membrane (SPM) fractions were collected at the 1.0–1.2 M interface, diluted with 4 mM HEPES, and pelleted (150,000 × g, 30 min). These pellets were resuspended in 50 mM HEPES (pH 7.4) containing 0.5% Triton X-100, rotated for 15 min, and centrifuged at 32,000 × g for 20 min. The resulting pellets were resuspended in a 0.5% Triton-containing buffer, rotated for 15 min, and centrifuged at 200,000 × g for 20 min to obtain postsynaptic density (PSD) fractions, which were suspended in 50 mM HEPES containing 0.2% SDS. Complete protease inhibitor cocktail tablets (Roche), pepstatin 10 μg/ml, and phosphatase inhibitor cocktail 1 and 2 were added to all buffers. The preceding procedures were carried out on ice or in a cold room and fractions were stored at −80°C. Protein concentrations were measured using Coomassie Plus reagent (Pierce, IL).
Immunoblot analysis
PSD fractions (5 μg) were resolved by 7.5% SDS-PAGE and transferred to nitrocellulose membranes. Both blotting and imaging with the Odyssey system (LI-COR, NE) were carried out following the manufacturer's protocols. The primary antibodies used were rabbit anti-NR2A (1:500, sc-9056, Santa Cruz, CA) and goat anti-NR2B (1:10,000, sc-1469, Santa Cruz, CA). The employed secondary antibodies were Alexa Fluor 680-labeled anti-goat IgG (1:5,000, Molecular Probes, OR) and IRDye 800-labeled anti-rabbit IgG (1:3,000, Rockland, PA). All protein quantification was based off the average of three separately loaded lanes. Because the PSD fraction is enriched for synaptic proteins, commonly employed loading controls that remain constant over the course of development are not available.
Pharmacological agents
Unless otherwise noted, all chemicals were purchased from Sigma (St. Louis, MO).
Statistics
Data were expressed as means ± SE. Statistical comparisons were performed using InStat3 software (GraphPad Software, San Diego, CA). For multiple group comparisons, analyses of variance (ANOVAs) were first performed, followed by between-group comparisons with Student-Newman-Keuls (SNK) tests. Significance was placed at P < 0.05. All reported levels of statistical significance represent two-tailed values.
RESULTS
Developmental changes in NMDAR subunit expression and function in mouse visual cortex
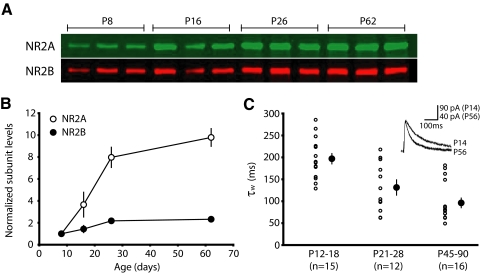
To understand the roles of different NMDAR subunits in plasticity, we focused our studies on the primary visual cortex, a region that exhibits a developmental upregulation of NR2A-type relative to NR2B-type receptors. While this subunit transition in NMDARs has been well-described in several species (Chen et al. 2000; Quinlan et al. 1999a; Roberts and Ramoa 1999; Sheng et al. 1994), we wanted to characterize the specific nature of this trend in murine visual cortex. By enriching for proteins associated with the postsynaptic density (PSD), over a range of developmental time points, we were able to temporally reveal the graded expression of postsynaptic NMDAR subunits. Because this preparation enriches for postsynaptic proteins, it is well-suited to discern the composition of receptors mediating synaptically-evoked currents. Pools of visual cortex samples from P8, P16, P26, and P62 animals were run in triplicate, averaged, and compared. Consistent with observations in other species, we found a dramatic developmental increase in NR2A between P8 and P62, with only a modest upregulation of NR2B (Fig. 1, A and B; NR2A subunit levels relative to P8: P16, 3.66 ± 1.13; P26, 7.97 ± 0.92; P62, 9.79 ± 0.81; P < 0.001. NR2B subunit levels relative to P8: P16, 1.43 ± 0.33; P26, 2.18 ± 0.15; P62, 2.33 ± 0.07; P < 0.01).
FIG. 1.
The functional expression of synaptic NR2A/NR2B increases over development in the mouse visual cortex. A: postsynaptic density fractions, enriched for synaptic proteins, show the relative expression of NR2A and NR2B at 4 developmental time points. Samples were obtained by pooling together visual cortices from 3 to 10 brains (see methods). B: N-methyl-d-aspartate receptor (NMDAR) subunit expression normalized to P8 values. NR2A expression increases dramatically over development, whereas there is only a modest developmental increase in NR2B expression. Note that some error bars are obscured by the overlying symbol. C: shortening of NMDAR current durations over development reflects an increase in the relative level of NR2A expression. Using whole cell voltage-clamp recordings, NMDAR currents were isolated at 3 age groups from L2/3 pyramidal cells following extracellular L4 stimulation. Cells were held at +40 mV in the presence of 6,7-dinitroquinoxaline-2,3-dione (DNQX; see methods). ○, averages of individual cells; •, the age group's average ± SE. A double-exponential fit of the deactivation current revealed a decrease in τw, corresponding to faster current kinetics over development. Stimulation artifacts were blanked in this and all subsequent figures for clarity.
To further characterize the nature of this developmental subunit transition, we pharmacologically isolated whole cell NMDAR currents in layer (L) 2/3 pyramidal cells over the same age range. Because NR2A-type receptors have faster decay kinetics than NR2B-type receptors (Carmignoto and Vicini 1992; Flint et al. 1997; Hestrin 1992; Monyer et al. 1992; Stocca and Vicini 1998; Vicini et al. 1998), this allowed us to corroborate our biochemical data with a physiological measure. As expected, an ANOVA revealed a significant decrease in τw over development (Fig. 1C), demonstrating that NMDAR currents become progressively faster with age (average: P12-18, 196.9 ± 11.8, n = 15; P21-28, 131.3 ± 16.0, n = 12; P45-90, 96.2 ± 11.0, n = 16; P < 0.0001).
By measuring NR2 expression and NMDAR current kinetics at different ages, we show 1) the expression of NR2A relative to NR2B increases with development; 2) the most rapid upregulation of synaptic NR2A occurs prior to P30; and 3) there is a functional correlate to changes in subunit expression at the synapse. With this information, we were able to evaluate synaptic plasticity at discrete developmental stages, using the natural process of NR2A upregulation as a mechanism to address the potentially unique roles of NR2A and NR2B-type NMDARs in synaptic plasticity.
LFS-LTD is developmentally sensitive to NMDAR antagonism
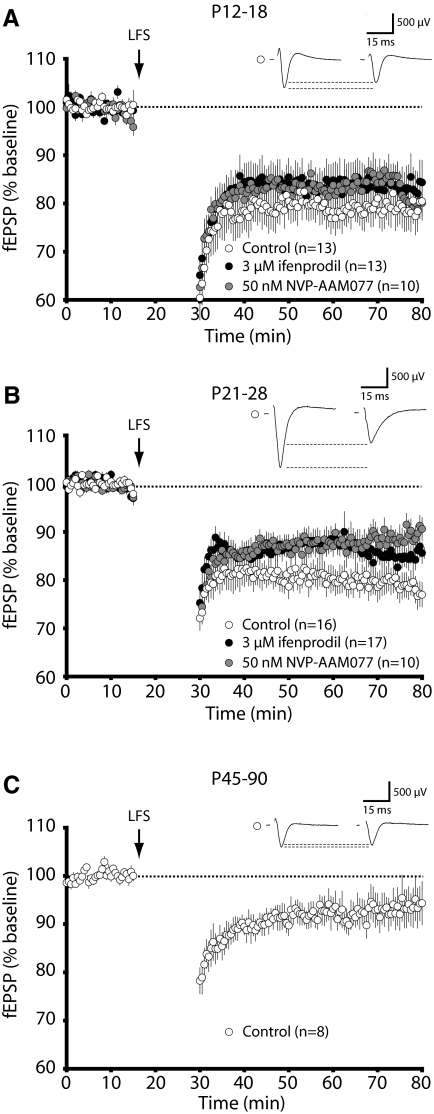
We began by evaluating whether LFS-LTD in the primary visual cortex was NR2B dependent, as had been reported previously in other regions of the brain (Liu et al. 2004; Massey et al. 2004). To isolate the roles of different NMDAR subunits, we conducted these experiments in the presence of ifenprodil, an NR2B-type antagonist (Williams et al. 1993), or NVP-AAM007, a low-affinity NR2A-type antagonist (Auberson et al. 2002; Feng et al. 2004). In young animals (P12-18), we found that neither ifenprodil nor NVP-AAM007 had an appreciable effect on the magnitude of LFS-LTD (Fig. 2A; average: control, 79.0 ± 3.5% of baseline response, n = 13; 50 nM NVP-AAM077, 81.8 ± 2.0% n = 10; 3 μM ifenprodil, 83.8 ± 4.2%, n = 13; P = 0.6334). However, in juvenile (P21-28) animals, both antagonists modestly attenuated the level of LFS-LTD (Fig. 2B; average: control, 79.1 ± 2.7%, n = 15; 50 nM NVP-AAM077, 88.7 ± 2.1% n = 16; 3 μM ifenprodil, 85.3 ± 1.8%, n = 17; P < 0.05). Taken together, it appears that both subpopulations of pharmacologically sensitive NMDARs can contribute to LFS-LTD expression, but neither is absolutely required for its induction. This is similar to previous observations using subsaturating concentrations of APV, a pan-NMDAR antagonist, to attenuate the magnitude of LTD induced by 1-Hz stimulation (Philpot et al. 2003). Consistent with previous observations that 1-Hz LFS produces less robust LTD with age (Jiang et al. 2003, 2007; Kirkwood et al. 1997), we observed that the magnitude of 1-Hz LFS-LTD decreases substantially with age (Fig. 2C). Therefore, in adult (P45-90) animals, the small degree of plasticity evoked with 1-Hz stimulation (average: control, 95.4 ± 4.5%) precluded us from investigating the effect of antagonist application on this form of LTD. However, we do not suggest that LTD is absent in adult mice, as previous studies have used different stimulation protocols, such as a paired-pulse LFS stimulation, to induce LTD in the adult cortex (Jiang et al. 2003; Lee et al. 2003).
FIG. 2.
NVP-AAM077 and ifenprodil have developmentally specific effects on the expression of low-frequency stimulation long-term depression (LFS-LTD) in the visual cortex. LTD was induced with 1-Hz stimulation (15 min) of L4 (↓). Data represent averages ± SE. Representative waveforms show field potentials before and after 1-Hz stimulation under control conditions. A: in young animals (P12-18), LTD is insensitive to either 3 μM ifenprodil or 50 nM NVP-AAM077. B: in juvenile animals (P21-28), LTD is modestly attenuated by either 3 μM ifenprodil or 50 nM NVP-AAM077. C: in adult animals (P45-90), only a small level of LTD could be induced by 1-Hz stimulation under control conditions.
NR2B-type NMDARs are not required for the expression of chemically induced LTD
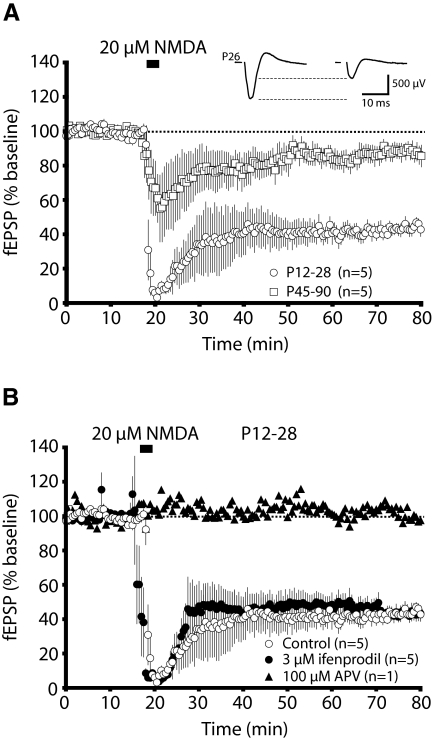
Because extrasynaptic NR2B-type NMDARs have been suggested to play a critical role in the induction of LTD (Massey et al. 2004), we chose to globally stimulate both synaptic and extrasynaptic NMDARs by bath application of NMDA. This protocol has been shown to induce a chemical form of LTD (chem-LTD) that is mediated by rapid dephosphorylation of AMPA receptors and is occluded by homosynaptic LTD, suggesting that chem-LTD and LFS-LTD share common underlying mechanisms (Lee et al. 1998). We found chem-LTD to decrease in magnitude with age, mirroring the developmental profile observed with LFS-LTD (Fig. 3A; average: P12-28, 44.7 ± 2.5%, n = 5; P45-90, 86.4 ± 3.9% n = 5; P < 0.0001). We also confirmed that this form of LTD is NMDAR-dependent by completely blocking it with 100 μM D,L-APV (Fig. 3B; APV, 105%, n = 1). Yet chem-LTD is not attenuated by antagonizing ifenprodil-sensitive, presumably NR2B-type, NMDARs (Fig. 3B; average: control, 44.7 ± 2.5%, n = 5; 3 μM ifenprodil, 44.6 ± 4.5%, n = 5). Thus both our synaptic and extrasynaptic stimulation paradigms fail to suggest a critical role for NR2B-type NMDARs in the induction of LTD in the mouse visual cortex.
FIG. 3.
Chemically induced LTD (chem-LTD) is insensitive to ifenprodil application. A: the magnitude of chem-LTD induced by brief application of NMDA (20 μM) is greater in the visual cortex of young (P12-18) animals than adult (P45-90) animals. Representative traces are shown from a P26 recording. B: ifenprodil (3 μM) fails to reduce the magnitude of chem-LTD, whereas 2-amino-5-phosphonovaleric acid (APV, 100 μM) prevents its induction.
NVP-AAM077 completely blocks LTP in adult, but not juvenile, animals
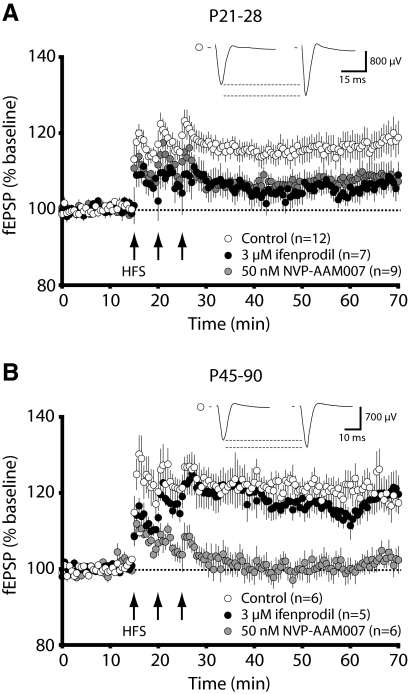
In an attempt to investigate the discrete roles of NR2A- and NR2B-type NMDARs over development, we bath applied ifenprodil and NVP-AAM077 at different developmental time points. We observed that juvenile LTP was significantly attenuated, but not blocked, in the presence of either antagonist (Fig. 4A; average: control, 116.8 ± 3.1%, n = 12; 50 nM NVP, 108.0 ± 2.5%, n = 9; 3 μM ifenprodil, 105.7 ± 2.7% n = 7; P < 0.05). However, we were surprised that the induction of adult LTP was completely prevented by NVP-AAM077, while entirely unaffected by ifenprodil (Fig. 4B; average: control, 120.5 ± 3.7%, n = 6; 50 nM NVP, 101.4 ± 2.7%, n = 6; 3 μM ifenprodil, 116.9 ± 3.9%, n = 5; P < 0.01). To further examine why ifenprodil and NVP-AAM077 had different effects on synaptic plasticity at different developmental time points, we characterized the fidelity of these NMDAR antagonists in our slice preparation.
FIG. 4.
The ability of ifenprodil or NVP-AAM077 to block LTP in the visual cortex is age-dependent. A: in juvenile animals (P21-28), LTP is significantly attenuated by either ifenprodil or NVP-AAM077. B: in adult animals (P45-90), 50 nM NVP-AAM077 completely blocks LTP, whereas 3 μM ifenprodil has no effect on its magnitude. In both panels, the traces shown above each plot are averages of the 15 min baseline and the last 15 min poststimulation of a representative recording under control conditions.
Developmental effect of NVP-AAM077 is not the result of NR2A-type specificity
Pharmacological antagonists, with varying degrees of subunit specificity, have been used to parse the role of different NMDAR subtypes in synaptic plasticity (Bartlett et al. 2007; Berberich et al. 2005, 2007; Fox et al. 2006; Hrabetova et al. 2000; Massey et al. 2004; Morishita et al. 2007; Sheng et al. 1994; Toyoda et al. 2006; Weitlauf et al. 2005). While the NR2B-specific antagonist, ifenprodil, has been well characterized (Williams et al. 1993), the recently developed NR2A-selective antagonist NVP-AAM077 has been the subject of controversy. Initial reports assessing the specificity of NVP-AAM077 for human NR2A-containing NMDARs indicated that NVP-AAM077 had a 110-fold preference for human NR2A over NR2B (Auberson et al. 2002). To date, more than 100 studies have been performed and interpreted under the assumption that NVP-AAM077 is highly selective for NR2A-containing NMDARs in rodents. However, several recent studies examining the specificity of NVP-AAM077 suggest that the drug has a much lower subunit preference in rodent compared with human NMDARs and that the drug cannot reliably distinguish between NR2A- and NR2B-type receptors (Feng et al. 2004; Frizelle et al. 2006; Neyton and Paoletti 2006).
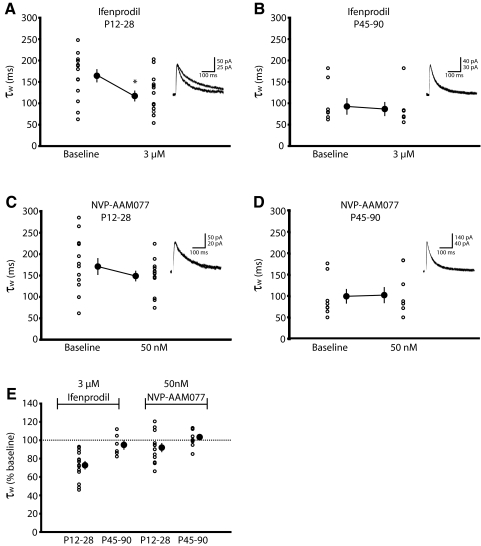
Although NVP-AAM077 is reported to have only modest selectivity for rodent NR2A-type receptors (Feng et al. 2004; Frizelle et al. 2006), we wanted to test whether it was sensitive enough to discriminate the developmental upregulation of NR2A relative to NR2B that we had established both biochemically and electrophysiologically (Fig. 1, A–C). To probe the specificity of these antagonists, NMDAR current durations were quantified by a weighted time constant (see methods) and measured before and after drug application. Because NR2B-type receptors have slower decay kinetics than NR2A-types, we reasoned that selective antagonism of NR2B-type receptors with ifenprodil should increase NMDAR decay kinetics and cause a decrease in current decay time (e.g., a decrease in τw) relative to the pre-drug condition. We expected this effect would be most pronounced in young animals that express high levels of NR2B. Similarly, blockade of faster NR2A subtypes would be expected to prolong NMDAR decay kinetics, and this effect would be most profound during adulthood when NR2A-containing NMDARs predominate at the synapse.
As predicted, ifenprodil significantly shortened NMDAR decay kinetics in young but not adult animals [average (in ms): P12-28 baseline, 164.5 ± 14.3; P12-28 3 μM ifenprodil, 117.2 ± 11.6, n = 14; P < 0.05; P45-90 baseline, 92.4 ± 18.2; P45-90 3 μM ifenprodil 86.4 ± 15.4, n = 6], validating that the developmental change in relative NR2 expression can be pharmacologically discerned with a reliable subunit-specific antagonist (Fig. 5, A and B). However, we observed that NVP-AAM077 did not alter the duration of isolated NMDAR current kinetics at either developmental stage (Fig. 5, C and D; average in ms: P12-28 baseline, 171.2 ± 18.6; P12-28 50 nM NVP, 149.1 ± 11.3, n = 13; P45-90 baseline, 98.4 ± 14.4; P45-90 50 nM NVP 101.1 ± 14.9, n = 10). Thus NVP-AAM077 failed to selectively antagonize the fast NR2A component of the NMDAR current, even in adulthood when the relative levels of NR2A are highest. These data are summarized in Fig. 5E.
FIG. 5.
Unlike ifenprodil, NVP-AAM077 fails to alter NMDAR decay kinetics in a manner consistent with subtype specificity. A: perfusion of ifenprodil results in faster NMDAR current kinetics early in development (P21–P28), reflected as a decrease in τw, suggesting ifenprodil selectively blocks long-duration currents from NR2B-containing NMDARs. ○, raw data points; •, means ± SE before and after drug application. Right: traces are representative of NMDAR currents before and after ifenprodil application. *P < 0.05 from baseline. B: ifenprodil does not alter NMDAR decay kinetics in pyramidal cells from older mice (P45–P90), likely reflecting the small proportion of NR2B-containing NMDARs at this age. C: NVP-AAM077 fails to alter NMDAR current decay kinetics in cells from young mice. D: NVP-AAM077 also fails to alter NMDAR decay kinetics in older mice (P45–P90), when NR2A-containing NMDARs are expected to predominate at the synapse, suggesting that the drug lacks specificity for NR2A-containing NMDARs. E: data are normalized and summarized.
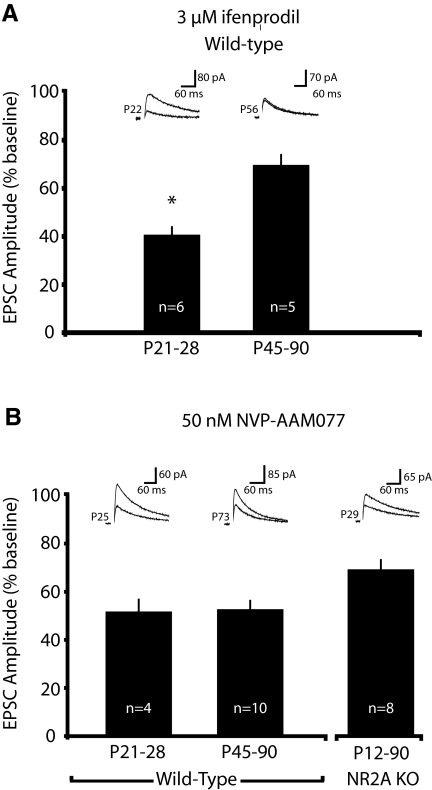
To provide an additional gauge of the subunit specificity of these drugs, we evaluated their degree of antagonism with consideration to the amount of subunit expression over development (Fig. 1, A and B). As shown previously, we expected ifenprodil-induced antagonism of NMDAR currents to be greatest in young animals that express relatively high levels of NR2B-type receptors (Vicini et al. 1998; Yoshimura et al. 2003). Conversely, assuming a modest degree of selectivity, NVP-AAM077 should attenuate a progressively greater proportion of NMDAR currents over development, as expression of the NR2A subunit increases. To evaluate the ability of NMDAR antagonists to block NMDAR-mediated currents, we measured their effects on current amplitude. As predicted, ifenprodil discriminated the developmental transition in synaptic NMDAR subunit expression by antagonizing significantly less NMDAR current in adult animals (Fig. 6A; % baseline amplitude: P21-28, 40.4 ± 3.5% n = 6; P45-90, 69.5 ± 4.5%, n = 5; P < 0.001). However, NVP-AAM077 failed to exhibit selectivity for NR2A subtypes, as it attenuates NMDAR EPSCs to a similar extent across development (Fig. 6B; % baseline amplitude: P21-28, 50.6 ± 5.3% n = 4; P45-90, 52.0 ± 3.9%, n = 10; similar effects were seen on charge transfer, data not shown), suggesting that NVP-AAM077 largely lacks specificity for NR2A subtypes in mice. To additionally test whether NVP-AAM077 lacked selectivity for NR2A subtypes in the mouse visual cortex, we tested the effect of the drug on NMDAR currents from mice lacking NR2A. Further verifying that NVP-AAM077 lacks specificity for NR2A subtypes, the drug significantly blocked NMDAR currents in the visual cortex of NR2A knockout mice, across a broad developmental spectrum. (Fig. 6B; % baseline: NR2A KO P12-90, 68.6 ± 4.3%, n = 8; P < 0.001).
FIG. 6.
Block of NMDAR currents by ifenprodil, but not NVP-AAM077, correlates to developmental changes in NR2A/NR2B expression. A: antagonism of NMDAR currents by ifenprodil is reduced over development, reflecting the developmental decrease in relative NR2B expression. B: NVP-AAM077 similarly antagonized NMDAR currents over the same developmental time frame as the expression of NR2A increased. NVP-AAM077 also blocked a significant proportion of the NMDAR current in mice lacking NR2A. Taken together these data indicate that NVP-AAM077 lacks the ability to discriminate between NR2A and NR2B types in the mouse visual cortex.
In summary, we found evidence that NVP-AAM077 has limited specificity for NR2A-type receptors in mice. However, it is difficult to reconcile how a nonselective NMDAR antagonist can have discrete effects on plasticity at different developmental time points. The developmental effect of NVP-AAM077 cannot be attributed to increased NMDAR current antagonism because the drug blocks a comparable amount of NMDAR current at both ages (Fig. 6B). Therefore it seemed reasonable to postulate that NVP-AAM077 has a greater affect on adult LTP, not due to its action on NR2A-type receptors, but due to a developmental increase in the amount of NMDAR activation required for plasticity.
Developmental differences in the requirements for LTP can explain why NVP-AAM077 has a more profound effect on adult LTP
An age-dependent shift in the amount of NMDAR activation needed to induce LTP could underlie the developmental change in response to NVP-AAM077. That is, the induction of LTP in adult cortex may be more sensitive to disruption by a partial block of NMDAR currents than juvenile cortex.
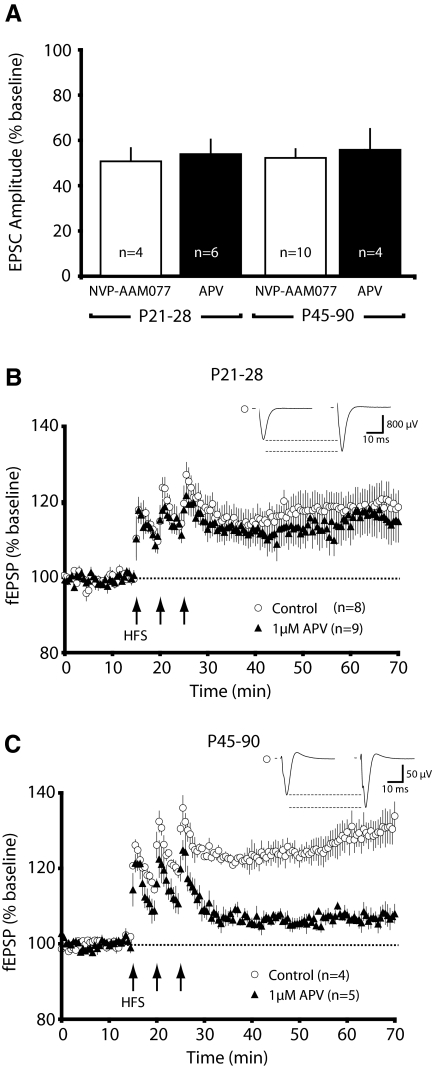
To measure whether there are age-dependent differences in the ability to disrupt LTP with NMDAR antagonists, we first compared the amount of NMDAR current blocked by NVP-AAM077 during juvenile and adult stages of development (Fig. 6B). It is clear that the total amount of NMDAR current remaining after NVP-AAM077 application is comparable at both developmental stages. However, the same degree of NMDAR antagonism at these two developmental time points caused distinct effects on LTP (Fig. 4, A and B). These data encouraged us to test whether other relatively nonsubunit selective NMDAR antagonists had similar age-dependent consequences on LTP induction.
Because the threshold for plasticity has been shown to vary with experience-dependent regulation of NMDAR current (Philpot et al. 2003), we postulated that there might also be age-dependent changes in NMDAR requirements for the induction of LTP. To investigate this possibility, we used a subsaturating concentration of APV, a pan-NMDAR blocker. While there are some indications that APV has a slight preference for recombinant NR2A-type receptors (Buller and Monaghan 1997), this has not been demonstrated in endogenously expressed receptors (Christie et al. 2000). In our preparation, 1 μM APV failed to attenuate more current with maturity and did not prolong NMDAR current kinetics (data not shown), as would have been expected if the drug had a higher affinity for NR2A-type receptors. These data are consistent with previous observations that APV cannot distinguish differences in the relative expression of NMDAR subtypes in rodent visual cortex (Quinlan et al. 1999b).
If the developmental effect of NVP-AAM077 on LTP could be attributed to a shift in NMDAR requirements needed to induce plasticity and not a subunit-specific effect, subsaturating concentrations of APV should mimic the age-dependent effects of NVP-AAM077. Through empirical observations, we found that 1 μM APV and 50 nM NVP-AAM077 antagonized a similar amount of NMDAR current in juvenile and adult animals (Fig. 7A; amplitude % baseline: P21-28: NVP, 50.6 ± 5.3, n = 4; APV, 53.9 ± 6.4, n = 6; P45-90: NVP, 52.0 ± 3.9, n = 10; APV 55.8 ± 9.2, n = 4). Excitingly, 1 μM APV failed to antagonize LTP in the juvenile visual cortex, whereas it strongly attenuated LTP in adults [Fig. 7, B and C; average (P21-28): control, 119.1 ± 4.1%, n = 8; 1 μM APV, 115.8 ± 5.5% n = 9; average (P45-90): control, 128.9 ± 3.6%, n = 4; 1 μM APV, 107.2 ± 1.5% n = 5; P = 0.001]. Thus, much like NVP-AAM077, APV blocked a similar magnitude of the NMDAR current in juvenile and adult animals, yet differentially blocked the magnitude of LTP.
FIG. 7.
There is a developmental increase in the ability to disrupt LTP with a nonsubunit-selective NMDAR antagonist. A: in juvenile (P21-28) and adult (P45-P90) mice, subsaturating concentrations of APV (1 μM) and NVP-AAM077 (50 nM) block a similar degree of NMDAR current amplitude. NVP-AAM077 data are replotted from Fig. 6B for the purposes of comparison. B: subsaturating APV fails to attenuate the induction of LTP in the visual cortex of young mice (P21-28). C: subsaturating APV dramatically reduces the magnitude of LTP in adult mice (P45-90).
While the effect of NVP-AAM077 (Fig. 4, A and B) and APV (Fig. 7, B and C) are not identical, they both demonstrate the same developmental trend. This strongly suggests that the degree of NMDAR activation required for LTP increases with development. Although the induction of plasticity has been shown to be sensitive to partial NMDAR blockade (Berberich et al. 2007; Cummings et al. 1996; Nishiyama et al. 2000; Philpot et al. 2003), our study provides the first indication that LTP sensitivity to NMDAR antagonism changes with development.
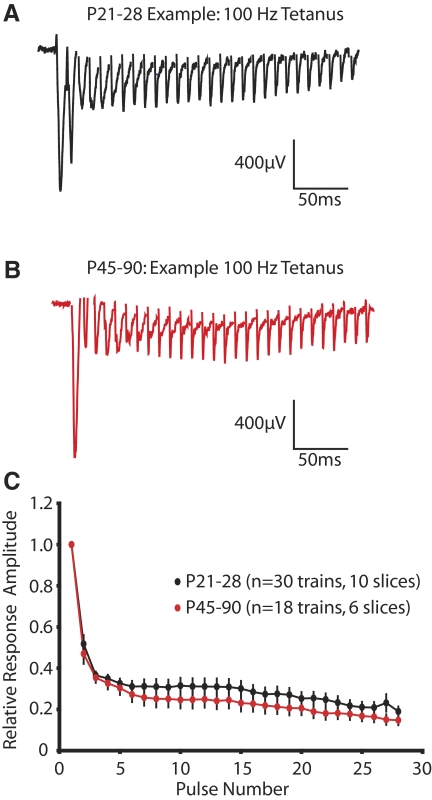
The developmental effect of NMDAR antagonism on LTP could reflect a developmental shift in the postsynaptic response to the tetanizing LTP stimulus. This is particularly relevant because the neocortex undergoes a significant postnatal maturation of inhibitory networks (Hensch 2005; Rozas et al. 2001) and presynaptic release properties (Reyes and Sakmann 1999) that could influence short-term synaptic dynamics. To begin to address this, the relative field potential amplitude of the first 28 pulses in the 100-Hz tetanus were analyzed (Fig. 8, A–D). The rate of decay of the field response was quantified by fitting the normalized data with a triple exponential defined as I(t) = I1*exp(−t/τ1) + I2*exp(−t/τ2) + I3*exp(−t/τ3) + C, where Ix is the peak amplitude of the field response, τx is the corresponding decay time constant, and C is the plateau potential of the response. For quantification, the three decay constants were combined into a weighted time constant as follows: τw = τ1 * [I1/(I1 + I2 + I3)] + τ2 * [I2/(I1 + I2 + I3)] + τ3 * [I3/(I1 + I2 + I3)]. No significant differences were observed between the two ages of interest as both the weighted time constant and the plateau potential of the response decay during the 100-Hz tetanus were similar at both developmental stages (P21–P28 τw = 13.0 ± 1.4 ms, P45–P90 τw = 15.6 ± 1.5 ms, P = 0.22; P21–P28 C = 0.26 ± 0.03, P45–P90 C = 0.20 ± 0.03, P = 0.21). These findings are consistent with previous observations showing that there are minimal developmental changes in the L4-L2/3 paired-pulse response (Rozas et al. 2001), although there are much greater age-dependent effects on the L2/3 paired-pulse response when stimulation is evoked from white-matter (Ramoa and Sur 1996; Rozas et al. 2001). In addition to observing a similar decay in the field potential response during the 100-Hz stimulation, we also observed that the half-maximal amplitude of the postsynaptic response was similar at both developmental ages (average fEPSP amplitude: P21-28, 1023.7 ± 118.4 μV, n = 10; P45-90, 1121.8 ± 149.0 μV, n = 6; P = 0.6166). In addition, using immunogold electron microscopy, it appears that the size of the postsynaptic density and the number of synaptic NMDARs is similar between juvenile and adult stages of development (R. J. Corlew and R. J. Weinberg, personal communications). These results, taken together, suggest that the increasing developmental sensitivity to NMDAR antagonism is not a reflection of a developmental change in our ability to drive postsynaptic L2/3 activity with L4 stimulation. Although our data suggest that the degree of NMDAR activation required for the expression of 100-Hz LTP is increased in adulthood at the L4-L2/3 synapse, we cannot rule out the possibility that age-dependent differences in the susceptibility to NMDAR antagonists might also arise from changes in the way extracellular stimulation recruits neocortical microcircuits.
FIG. 8.
There are no apparent differences between juveniles (P21-28) and adults (P45-90) in the L4-L2/3 field potential responses generated by 100-Hz stimulation. Example field potential traces from juvenile (A) and adult (B) stages of development. The first 28 pulses of a 100-Hz stimulus are depicted, and stimulus artifacts are clipped for clarity. C: the normalized field potential amplitude of the first 28 pulses of a 100-Hz stimulus does not differ between juvenile and adult animals.
DISCUSSION
The work described here uses a pharmacological approach, over the course of development, to determine whether NMDAR subtypes have specific roles in synaptic plasticity. A major finding of our study is that ifenprodil-sensitive and NVP-AAM077-sensitive NMDARs have overlapping roles in synaptic plasticity. Both populations can contribute to the expression of LTD and LTP. Using the same NMDAR antagonists, our conclusions are in striking contrast to previous studies that suggest opposing roles for NR2A and NR2B in synaptic plasticity. We help resolve this controversy by showing it can be explained, at least in part, by the nonspecific effects of a purported NR2A-selective antagonist and by the age-dependent differences in the properties of synaptic plasticity. Specifically, we demonstrate that NVP-AAM077, a commonly employed antagonist, lacks strong selectivity for NR2A-containing NMDARs in rodents. This is highlighted by showing that the age-dependent effects of NVP-AAM077 on LTP can be largely replicated using a nonselective NMDAR antagonist (APV) at subsaturating concentrations. These studies also demonstrate that expression of synaptic plasticity is more easily disrupted by NMDAR antagonists in the adult, compared with the juvenile, cortex. The implications of this are not yet known, although we speculate that the more robust synaptic plasticity in early development is important for shaping cortical networks during early critical periods.
Activation of NMDARs is required for the induction of LTD in the visual cortex (Kirkwood and Bear 1994). However, this LTD is not critically mediated by synaptic or extrasynaptic NR2B-type receptors that are sensitive to ifenprodil. While these findings contrast with previous reports in the hippocampus and perirhinal cortex (Liu et al. 2004; Massey et al. 2004), our results are in agreement with a recent report in the hippocampus conducted across three separate laboratories (Morishita et al. 2007). Interestingly, we did observe a subtle, but significant, developmental increase in the disruption of LTD by NVP-AAM077 and ifenprodil. This suggests that there is an age-dependent increase in the degree of NMDAR activation required for the full expression of LTD. This observation may help explain why it is more difficult to induce LTD, if it can be induced at all, in many parts of the adult brain (Kirkwood et al. 1996, 1997) with a low frequency stimulation protocol.
NMDARs are also required for the induction of LTP in the visual cortex (Kirkwood and Bear 1995). Similar to what we observed for LTD, we show that neither NVP-AAM077 nor ifenprodil-sensitive populations of NMDARs are required for the induction of LTP. Interestingly, ifenprodil has no effect on adult LTP, although it attenuates LTP in juvenile mice. We suggest that this maturational change may simply be a consequence of the fact that ifenprodil blocks a greater proportion of the total NMDAR current in juvenile mice. In contrast to our observations using ifenprodil, we observed that NVP-AAM077 more effectively blocks LTP in adult cortex compared with juvenile cortex. This observation is in agreement with recent findings, at the single-cell level, in cortical neurons (Le Roux et al. 2007). While we initially hypothesized that the increasing ability of NVP-AAM077 to block LTP with age was due to an increasing role for NR2A in LTP with development, we discarded this notion for two reasons. First, we used convergent approaches to demonstrate that NVP-AAM077 lacks appreciable specificity for blocking NR2A-containing NMDARs. Second, because NVP-AAM077 blocks the same amount of NMDAR current at both developmental stages studied, we reasoned that a more plausible interpretation of our data is that the degree of NMDAR activation required for plasticity increases with age. In support of this idea, we show that a sub-saturating concentration of APV, a nonsubunit selective NMDAR antagonist, mimics the age-dependent consequences of NVP-AAM077 on LTP. Taken together, our data provide convincing evidence that the developmental effect observed with the NMDAR antagonists NVP-AAM077 and APV reflects an increase in the degree of NMDAR activation required for LTP.
The underlying mechanism regulating this developmental shift in the properties of LTP are likely multifold and are currently unknown. Although we demonstrate that NR2A and NR2B subtypes do not have polarized roles in synaptic plasticity, developmental changes in the properties of synaptic plasticity are likely to still be mediated, at least in part, by differences in the NMDAR signaling complex. For example, a developmental change in the relative proportion of diheteromeric (e.g., NR1-NR2B) and triheteromeric (NR1-NR2A-NR2B) NMDARs could alter the requirements for synaptic plasticity. In fact, our pharmacological data suggest that the relative amount of triheteromeric receptors may increase between juvenile and adult stages of development. While our findings show that the NR2A/NR2B ratio changes only modestly between P26 and P62, the NR2B antagonist ifenprodil blocks significantly more NMDAR current at the younger developmental time point. This is revealing because several studies have shown that ifenprodil can better antagonize diheteromeric NR2B-containing NMDARs than triheteromeric receptors, containing both NR2A and NR2B subunits (Kew et al. 1998; Tovar and Westbrook 1999). Therefore we postulate that the developmental decrease in ifenprodil sensitivity may reflect an increased presence of triheteromeric NMDARs, which have been documented in both the cortex and hippocampus (Al-Hallaq et al. 2007; Luo et al. 1997; Sheng et al. 1994). While a developmental change in the relative amount of triheteromeric receptors has not been demonstrated in hippocampus (Al-Hallaq et al. 2007), our data, and that of others (Kew et al. 1998), hint that there may be a developmental increase in cortical triheteromeric NMDARs. While the decay kinetics of triheteromeric NMDARs are intermediate to diheteromeric (NR1/NR2A and NR1/NR2B) NMDARs that contain only one type of NR2 subunit, it is unclear how these triheteromeric NMDARs influence plasticity. The intracellular signaling of triheteromeric receptors may differ from that of diheteromeric receptors because signaling cascades, linked to specific NR2 subunit, may be affected by oligomerization. However, the NR2B subunit, which is more likely to be in a diheteromeric form during juvenile stages of development, has several attributes that favor its involvement in the expression of LTP. First, NR2B-type receptors have been shown to allow more calcium influx per unit charge than NR2A-type receptors (Sobczyk et al. 2005). In addition, NR2B subunits recruit CaMKII, a critical modulator of LTP, to the synapse (Barria and Malinow 2005; Lisman et al. 2002). Taken together, a developmental decrease in NR2B-like properties, which may parallel the increase in triheteromeric NMDARs, would be less permissive for calcium influx and NR2B-associated signaling cascades at the synapse. As such, LTP may be more easily induced, and less easily disrupted, when diheteromeric NR2B-type NMDAR expression is high (Philpot et al. 2007). Thus a developmental change in NMDAR subunit composition may be one factor, in addition to other developmental changes in the synaptic milieu (Berardi et al. 2004; Hensch 2005), that increases the sensitivity of LTP to NMDAR antagonism.
If the composition of NMDARs at young synapses allows LTP to occur at a lower threshold, thereby making LTP less easily disrupted by partial NMDAR blockade, it might be expected that the amplitude of LTP should be greater in young animals. However, previous findings indicate that LTP induction and expression may be regulated by separate mechanisms. Indeed synapses may compete for “plasticity factors” that limit the expression of LTP (Fonseca et al. 2004). Further support for this hypothesis comes from observations of plasticity in NR2A knockout animals, where the threshold for inducing LTP is lowered without an appreciable change in the magnitude of the LTP expressed (Philpot et al. 2007). Thus one set of “plasticity factors” might control the threshold for modifying synapses, whereas a second, likely overlapping, set of molecules might regulate the magnitude of synaptic modifications.
Parsing out the mechanisms underlying the developmental increase in the degree of NMDAR activation required for plasticity will require further investigation. For example, it is already clear that the maturation of inhibitory circuitry is of profound importance (Corlew et al. 2007; Hensch and Fagiolini 2005; Huang et al. 1999; Maffei et al. 2006; Steele and Mauk 1999; Yoshimura et al. 2003), while its role in regulating the properties of synaptic plasticity continues to be more fully defined. Although we provide evidence that our ability to drive L4-L2/3 activity is similar between juvenile and adult stages of development, we cannot preclude the possibility that enhanced cortical inhibition, changes in presynaptic release probability, or differences in synaptic NMDAR content may play a role in shifting the ability of NMDAR antagonists to disrupt LTP over development.
Although the properties underlying the mechanisms of synaptic plasticity are complex, our work contributes several important findings that increase our understanding of developmentally regulated plasticity and distinct NMDAR subunit functions. First, our data indicate that ifenprodil-sensitive NR2B-type NMDARs are not critical for the induction of either LTD or LTP. Second, NVP-AAM077 is an unreliable NR2A-type antagonist in the mouse visual cortex. Thus the myriad of studies that have been interpreted with the assumption that NVP-AAM077 is a highly subunit-selective antagonist must be re-evaluated. Third, we reveal a developmental increase in the degree of NMDAR activation required for the induction of LTP. These observations not only elucidate an important mechanism that regulates plasticity over development, they may also reconcile discrepant results concerning NR2 subunit-specific roles in plasticity. The roles of NR2A and NR2B may be better understood in the future through careful consideration of the limitations of subunit-selective antagonists, the age of the animal, the contribution of triheteromeric NMDARs, and the brain region and species being examined.
GRANTS
This work was funded by a Helen Lyng White Fellowship and National Institutes of Health Grant T32-HD-40127 to A. C. Roberts and the Whitehall Foundation and National Institutes of Health Grant RO1 EY-018323 B. D. Philpot.
Acknowledgments
We thank Y. Auberson of the Novartis Institutes for Biomedical Research (Basel) for the generous gift of NVP-AAM077, and we are indebted to P. Manis for useful suggestions and for assistance in generating off-line analyses. We also thank R. Corlew, R. Weinberg, and M. Henson for technical support and for editing of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Al-Hallaq et al. 2007.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci 27: 8334–8343, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson et al. 2002.Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett 12: 1099–1102, 2002. [DOI] [PubMed] [Google Scholar]
- Barria and Malinow 2005.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301, 2005. [DOI] [PubMed] [Google Scholar]
- Bartlett et al. 2007.Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52: 60–70, 2007. [DOI] [PubMed] [Google Scholar]
- Bear et al. 1990.Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci 10: 909–925, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi et al. 2004.Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron 44: 905–908, 2004. [DOI] [PubMed] [Google Scholar]
- Berberich et al. 2007.Berberich S, Jensen V, Hvalby O, Seeburg PH, Kohr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology 52: 77–86, 2007. [DOI] [PubMed] [Google Scholar]
- Berberich et al. 2005.Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci 25: 6907–6910, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller and Monaghan 1997.Buller AL, Monaghan DT. Pharmacological heterogeneity of NMDA receptors: characterization of NR1a/NR2D heteromers expressed in Xenopus oocytes. Eur J Pharmacol 320: 87–94, 1997. [DOI] [PubMed] [Google Scholar]
- Carmignoto and Vicini 1992.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258: 1007–1011, 1992. [DOI] [PubMed] [Google Scholar]
- Chatterton et al. 2002.Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415: 793–798, 2002. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2000.Chen L, Cooper NG, Mower GD. Developmental changes in the expression of NMDA receptor subunits (NR1, NR2A, NR2B) in the cat visual cortex and the effects of dark rearing. Brain Res Mol Brain Res 78: 196–200, 2000. [DOI] [PubMed] [Google Scholar]
- Christie et al. 2000.Christie JM, Jane DE, Monaghan DT. Native N-methyl-d-aspartate receptors containing NR2A and NR2B subunits have pharmacologically distinct competitive antagonist binding sites. J Pharmacol Exp Ther 292: 1169–1174, 2000. [PubMed] [Google Scholar]
- Corlew et al. 2007.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci 27: 9835–9845, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy et al. 2001.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11: 327–335, 2001. [DOI] [PubMed] [Google Scholar]
- Cummings et al. 1996.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron 16: 825–833, 1996. [DOI] [PubMed] [Google Scholar]
- Dingledine et al. 1999.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 51: 7–61, 1999. [PubMed] [Google Scholar]
- Dudek and Bear 1993.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13: 2910–2918, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. 2004.Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141: 508–516, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint et al. 1997.Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci 17: 2469–2476, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca et al. 2004.Fonseca R, Nagerl UV, Morris RG, Bonhoeffer T. Competing for memory: hippocampal LTP under regimes of reduced protein synthesis. Neuron 44: 1011–1020, 2004. [DOI] [PubMed] [Google Scholar]
- Fox et al. 2006.Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus 16: 907–915, 2006. [DOI] [PubMed] [Google Scholar]
- Frizelle et al. 2006.Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquino xalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-d-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol 70: 1022–1032, 2006. [DOI] [PubMed] [Google Scholar]
- Gerkin et al. 2007.Gerkin RC, Lau PM, Nauen DW, Wang YT, Bi GQ. modular competition driven by NMDA receptor subtypes in spike-timing-dependent plasticity. J Neurophysiol 97: 2851–2862, 2007. [DOI] [PubMed] [Google Scholar]
- Gordon and Stryker 1996.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci 16: 3274–3286, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch 2005.Hensch TK Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888, 2005. [DOI] [PubMed] [Google Scholar]
- Hensch and Fagiolini 2005.Hensch TK, Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog Brain Res 147: 115–124, 2005. [DOI] [PubMed] [Google Scholar]
- Hestrin 1992.Hestrin S Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 357: 686–689, 1992. [DOI] [PubMed] [Google Scholar]
- Hrabetova et al. 2000.Hrabetova S, Serrano P, Blace N, Tse HW, Skifter DA, Jane DE, Monaghan DT, Sacktor TC. Distinct NMDA receptor subpopulations contribute to long-term potentiation and long-term depression induction. J Neurosci 20: RC81, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. 1999.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755, 1999. [DOI] [PubMed] [Google Scholar]
- Jiang et al. 2003.Jiang B, Akaneya Y, Hata Y, Tsumoto T. Long-term depression is not induced by low-frequency stimulation in rat visual cortex in vivo: a possible preventing role of endogenous brain-derived neurotrophic factor. J Neurosci 23: 3761–3770, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. 2007.Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci 27: 9648–9652, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadotani et al. 1996.Kadotani H, Hirano T, Masugi M, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Motor discoordination results from combined gene disruption of the NMDA receptor NR2A and NR2C subunits, but not from single disruption of the NR2A or NR2C subunit. J Neurosci 16: 7859–7867, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew et al. 1998.Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci 18: 1935–1943, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood and Bear 1994.Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci 14: 3404–3412, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood and Bear 1995.Kirkwood A, Bear MF. Elementary forms of synaptic plasticity in the visual cortex. Biol Res 28: 73–80, 1995. [PubMed] [Google Scholar]
- Kirkwood et al. 1996.Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature 381: 526–528, 1996. [DOI] [PubMed] [Google Scholar]
- Kirkwood et al. 1997.Kirkwood A, Silva A, Bear MF. Age-dependent decrease of synaptic plasticity in the neocortex of alphaCaMKII mutant mice. Proc Natl Acad Sci USA 94: 3380–3383, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube et al. 1998.Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci 18: 2954–2961, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux et al. 2007.Le Roux N, Amar M, Moreau A, Fossier P. Involvement of NR2A- or NR2B-containing N-methyl-d-aspartate receptors in the potentiation of cortical layer 5 pyramidal neuron inputs depends on the developmental stage. Eur J Neurosci 26: 289–301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. 1998.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron 21: 1151–1162, 1998. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2003.Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643, 2003. [DOI] [PubMed] [Google Scholar]
- Lisman et al. 2002.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190, 2002. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2004.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021–1024, 2004. [DOI] [PubMed] [Google Scholar]
- Luo et al. 1997.Luo J, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51: 79–86, 1997. [DOI] [PubMed] [Google Scholar]
- Maffei et al. 2006.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature 443: 81–84, 2006. [DOI] [PubMed] [Google Scholar]
- Massey et al. 2004.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24: 7821–7828, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer and Westbrook 1987.Mayer ML, Westbrook GL. Permeation and block of N-methyl-d-aspartic acid receptor channels by divalent cations in mouse cultured central neurons. J Physiol 394: 501–527, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain and Mayer 1994.McBain CJ, Mayer ML. N-methyl-d-aspartic acid receptor structure and function. Physiol Rev 74: 723–760, 1994. [DOI] [PubMed] [Google Scholar]
- Monyer et al. 1994.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12: 529–540, 1994. [DOI] [PubMed] [Google Scholar]
- Monyer et al. 1992.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256: 1217–1221, 1992. [DOI] [PubMed] [Google Scholar]
- Morishita et al. 2007.Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology 52: 71–76, 2007. [DOI] [PubMed] [Google Scholar]
- Morris 1989.Morris RG Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-d-aspartate receptor antagonist AP5. J Neurosci 9: 3040–3057, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser et al. 1998.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science 281: 2038–2042, 1998. [DOI] [PubMed] [Google Scholar]
- Nase et al. 1999.Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Genetic and epigenetic regulation of NMDA receptor expression in the rat visual cortex. Eur J Neurosci 11: 4320–4326, 1999. [DOI] [PubMed] [Google Scholar]
- Neyton and Paoletti 2006.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci 26: 1331–1333, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama et al. 2000.Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408: 584–588, 2000. [DOI] [PubMed] [Google Scholar]
- Perez-Otano and Ehlers 2004.Perez-Otano I, Ehlers MD. Learning from NMDA receptor trafficking: clues to the development and maturation of glutamatergic synapses. Neurosignals 13: 175–189, 2004. [DOI] [PubMed] [Google Scholar]
- Philpot et al. 2007.Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron 53: 495–502, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot et al. 2003.Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci 23: 5583–5588, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan et al. 1999a.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-d-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA 96: 12876–12880, 1999a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan et al. 1999b.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci 2: 352–357, 1999b. [DOI] [PubMed] [Google Scholar]
- Ramoa and McCormick 1994.Ramoa AS, McCormick DA. Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. J Neurosci 14: 2098–2105, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa et al. 2001.Ramoa AS, Mower AF, Liao D, Jafri SI. Suppression of cortical NMDA receptor function prevents development of orientation selectivity in the primary visual cortex. J Neurosci 21: 4299–4309, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa and Sur 1996.Ramoa AS, Sur M. Short-term synaptic plasticity in the visual cortex during development. Cereb Cortex 6: 640–646, 1996. [DOI] [PubMed] [Google Scholar]
- Reyes and Sakmann 1999.Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci 19: 3827–3835, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts et al. 1998.Roberts EB, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. J Neurophysiol 80: 1021–1032, 1998. [DOI] [PubMed] [Google Scholar]
- Roberts and Ramoa 1999.Roberts EB, Ramoa AS. Enhanced NR2A subunit expression and decreased NMDA receptor decay time at the onset of ocular dominance plasticity in the ferret. J Neurophysiol 81: 2587–2591, 1999. [DOI] [PubMed] [Google Scholar]
- Rozas et al. 2001.Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci 21: 6791–6801, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh and Vicini 1999.Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci 19: 10603–10610, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi 1993.Santos-Sacchi J Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci 13: 3599–3611, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng et al. 1994.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368: 144–147, 1994. [DOI] [PubMed] [Google Scholar]
- Sobczyk et al. 2005.Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci 25: 6037–6046, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele and Mauk 1999.Steele PM, Mauk MD. Inhibitory control of LTP and LTD: stability of synapse strength. J Neurophysiol 81: 1559–1566, 1999. [DOI] [PubMed] [Google Scholar]
- Stocca and Vicini 1998.Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol 507: 13–24, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar and Westbrook 1999.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci 19: 4180–4188, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda et al. 2006.Toyoda H, Zhao MG, Zhuo M. NMDA receptor-dependent long-term depression in the anterior cingulate cortex. Rev Neurosci 17: 403–413, 2006. [DOI] [PubMed] [Google Scholar]
- Trachtenberg et al. 2000.Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science 287: 2029–2032, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini et al. 1998.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J Neurophysiol 79: 555–566, 1998. [DOI] [PubMed] [Google Scholar]
- Watanabe et al. 1994.Watanabe M, Mishina M, Inoue Y. Distinct spatiotemporal distributions of the N-methyl-d-aspartate receptor channel subunit mRNAs in the mouse cervical cord. J Comp Neurol 345: 314–319, 1994. [DOI] [PubMed] [Google Scholar]
- Weitlauf et al. 2005.Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci 25: 8386–8390, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams 1993.Williams K Ifenprodil discriminates subtypes of the N-methyl-d-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44: 851–859, 1993. [PubMed] [Google Scholar]
- Williams et al. 1993.Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 10: 267–278, 1993. [DOI] [PubMed] [Google Scholar]
- Yashiro et al. 2005.Yashiro K, Corlew R, Philpot BD. Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. J Neurosci 25: 11684–11692, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura et al. 2003.Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci 23: 6557–6566, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]