Abstract
Multiple single-unit recording has become one of the most powerful in vivo electro-physiological techniques for studying neural circuits. The demand has been increasing for small and lightweight chronic recording devices that allow fine adjustments to be made over large numbers of electrodes across multiple brain regions. To achieve this, we developed precision motorized microdrive arrays that use a novel motor multiplexing headstage to dramatically reduce wiring while preserving precision of the microdrive control. Versions of the microdrive array were chronically implanted on both rats (21 microdrives) and mice (7 microdrives), and relatively long-term recordings were taken.
INTRODUCTION
Electrophysiological recordings taken from freely behaving animals with chronically implanted microelectrodes have played a critical role in our understanding of neuronal activity at the population level (Fujii and Graybiel 2005; McCasland 1987; O'Keefe and Dostrovsky 1971; Ranck 1973; Taube et al. 1990). The recording devices used in these experiments use various kinds of manually adjustable microdrives, which rely on miniature screws or threaded rods to advance electrodes (Gray et al. 1995; McNaughton et al. 1983; Wilson and McNaughton 1993). These microdrives must be small and light in weight, because of the physical limitations of the animal.
For songbirds, a miniature headstage with three micromotors was developed to adjust electrodes while the bird sang in a soundproof box (Fee and Leonardo 2001). This technical advancement allowed adjustment during active behavior and eliminated the mechanical stress introduced by holding the animal's head during adjustment. A modified version of this motorized microdrive was used to record from mice (Luo et al. 2003). Although this motorized microdrive represented a significant technical improvement over conventional manually adjustable drives, several aspects of its design made it difficult to apply to recordings from multiple sites with independent control over a large number of motorized microdrives.
Another small-scale motorized microdrive (Venkateswaran et al. 2005) used four DC motors controlled by short pulses to displace the electrode. This method was sensitive to mechanical friction and did not address the technical problem of manipulating a large number of motors.
In addition, several larger motorized microdrives were designed for nonhuman primate experiments (Cham et al. 2005; Gray et al. 2007). These microdrives used either Piezoelectric actuators or relatively large stepping motors to realize electrode displacement. Neither of these approaches is appropriate for use in smaller animals with large numbers of recording electrodes.
Here we sought to develop microdrive arrays for smaller animals that allow flexibility with regard to the number and location of the recording sites and the number of microdrives themselves, while minimizing electrical wire connections and overall weight. We developed a new type of motorized microdrive array that satisfies these requirements for both rats and mice. These microdrives have been extensively tested on multiple animals over the past 3 yr. Long-term stable recording of multiple single units from multiple brain regions has been successfully achieved using these devices.
METHODS
In this section, we describe the design and implementation of the electro-mechanical system used to achieve precision control in the large-scale microdrive array.
Individual motorized microdrives
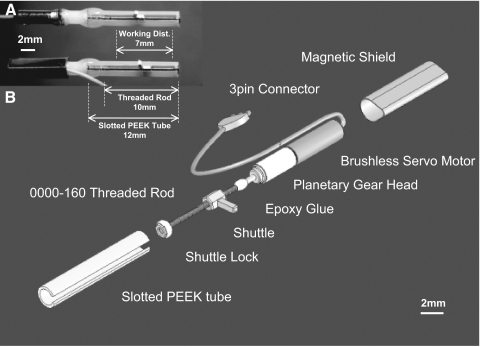
Our motorized microdrive (Fig. 1, A and B) has five basic components: the micromotor, threaded rod (0000160BTR, J.I Morris), slotted PEEK (polyetheretherketone) tube (PKT-062077, Small Parts), shuttle lock, and a shuttle made with brass nuts (N0000160B, J.I Morris). The entire microdrive weighs 300 mg, including 50 mg of magnetic shielding for the rat microdrive array. The exploded view is shown in Fig. 1B. We used the smallest commercially available DC brushless servomotor with a three-stage 47:1 planetary gearbox (0206H05B +02/1 47:1, 1.9 × 9.0 mm, 110 mg, Faulhaber) (Fee and Leonardo 2001). The shuttle was made with two brass nuts and a gold plated electrical pin (0.6 × 0.6 × 2.5 mm, WM17481-ND, Digikey) that were soldered together on a stainless steel threaded rod (0000160CETR, J. I. Morris). First, two brass nuts were placed about a half turn apart on the stainless threaded rod. Second, the brass nuts were preheated by a soldering iron tip for a second. Third, solder wire was applied at the gap of the brass nuts to let the solder infiltrate into the gap as well as their threads to fill excessive mechanical play. The gold plated pin was also soldered at the same time. Finally, the shuttle was threaded back and forth on the threaded rod until it rotated smoothly on the threaded rod. The solder minimizes the gap between the threaded rod and nut so that the shuttle can precisely move along the threaded rod. This step is necessary to minimize the hysteresis or positional lag that occurs whenever a change in the direction of electrode movement is required. The soldered shuttle was cleaned with 100% ethanol bath in a sonicator to remove rosin. Finally, the smoothness and mechanical play of the assembled shuttle was checked under a microscope.
FIG. 1.
Real and exploded view of individual motorized microdrive. A: actual individual motorized microdrives (rat version) with and without the magnetic shielding. Actual working distance and the relative length of each component are also shown. The connection between the micromotor and the gearbox is reinforced with epoxy glue. B: an exploded view of rat motorized microdrive. A small piece of thermo plastic tubing was used to align the threaded rod and the motor output shaft. The 3-pin connector was made with gold-plated pins and epoxy glue.
Before assembling individual microdrives, we calculated component dimensions based on the working distance required to reach the recording target of interest. The length of the 0000-160 threaded rod should be 2–3 mm longer than the maximum desired working distance. The PEEK tube is 2.0 mm longer than the threaded rod. As shown in Fig. 1A, 12.0 mm of the PEEK tube and 10.0 mm of a 0000-160 threaded rod were needed for a working distance of ∼7.0 mm. Similarly, 8.0 mm of PEEK tube and 6.0 mm of threaded rod were used in the mouse drive for ∼4.0 mm working distance. Next, the slotted PEEK tube was made by using a rotary cutting wheel (#409, Dremel) with a linear guide, sliding the PEEK tube along the linear guide while the wheel cut the wall. The gap width of the slotted part was 0.8 mm wide. Deburring the slotted part is critical for the smooth movement as well as the positional lag of the shuttle. If the width of the slotted part is too large relative to the thickness of the shuttle's pin (0.6 mm), positional lag may increase.
The first step of the assembly process for the individual microdrives is to attach the 0000-160 threaded rod to the output shaft of the micromotor. All of the following components were assembled using ultrafast curing epoxy glue (EPOXY 90 s, Super Glue/Pacer Technology). The motor was kept running at a slower speed (∼1 turn/s) to obtain a straight connection while the glue cured (∼90 s). The shuttle was threaded into the 0000-160 rod with an additional brass 0000-160 nut to lock the other end of the 0000-160 rod. The additional nut was covered with a small amount of epoxy to fill the gaps between the nut and PEEK tube's inner wall. Once the epoxy cured, the slotted PEEK was attached to the micromotor. Finally, the assembled motorized microdrives were tested under the appropriate current and voltage conditions (∼120 mA, 0.45 Veff). Excessive current or voltage (>150 mA, 0.55 Veff) could heat the motor and give fatal damage to an internal stator (coil). The motor has to be operated below +60°C (+140°F) at all times. A simple way to monitor the temperature is to touch the motor with a bare finger during test operation. Another issue with this motor is magnetic flux leakage from the motor itself. We noticed that the output torque of the motor reduces whenever adjacent motor becomes closer than 5 mm. Although the motor itself has a thin layer of Nickel magnetic shielding, there is still an intensive magnetic flux leakage from the motor. To counter this problem, additional magnetic shielding (Fig. 1; 50 mg/motor, NETIC S3-6, Magnetic Shielding) was necessary for individual motors in the higher-density rat microdrive array but not in the mouse microdrive array.
DC motor multiplexing headstage
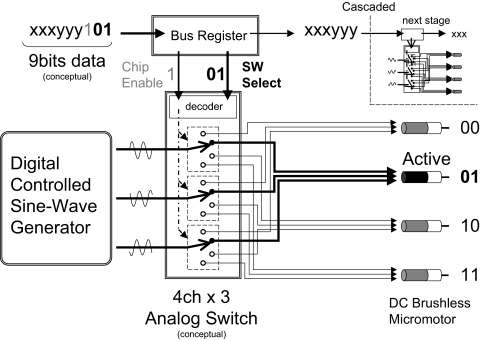
To manage the significant amount of wiring required (e.g., 60 connections for 20 motors), we developed a lightweight high current capacity DC motor multiplexing headstage with serial data communication technology. The core structure of the motor multiplexing circuit is realized by multi-stage shift-and-store bus registers and low on-resistance high-current-output multi-channel analog switches with serial data addressing of individual motors. This headstage consists of multi-channel analog switches (PI5V331Q, Pericom) and multi-stage bus registers (CD4094B, Texas Instruments). The simplified diagram of the headstage is shown in Fig. 2. The micromotor is driven by three sine waves that are shifted by 120° in phase. These waves are provided by a digital sine wave generator (MCBL05002, Faulhaber). The amplitude of these sine waves determines the torque of the motor, whereas the frequency controls the rotation speed of the motor's shaft. The rotation speed of the shaft can be set between ∼3 and 750 deg/s by the sine wave generator. The three sine waves are evenly distributed into the multi-channel analog switches. The analog switches are controlled by the multi-stage bus registers. The outputs of the bus register are encoded into a serial bit stream. In this conceptual figure, if nine bits of data ending with the binary code “101” are sent from the headstage controller, the first “1” bit (indicated as gray in the Fig. 2) will enable the first analog switch. Next, the decoder inside of the analog switch will interpret rest of the code “01” (indicated as bold in the Fig. 2) as the second motor. The rest of the serial data, which is not used to control the first stage of the analog switch, can be used as a control data to initiate another set of micromotors. Thus a large number of motors can be controlled by simply cascading these analog switches and bus registers. In our current design, the eight-channel DC motor multiplexing circuit for mouse drive is a fundamental unit. By cascading the three stages serially, the device becomes a 24-channel multiplexing headstage for rats. Theoretically, hundreds of microdrives can be controlled by the above method with only a few wires, including the power and motor signals. The multiplexing a large number of microdrives can be accomplished by increasing the length of serial data bits instead of increasing the number of wire connections to the animal. As shown in Fig. 3, the multiplexing headstage (Fig. 3A) receives serial data from the custom designed USB Headstage Control Microprocessor board (Fig. 3B) that is controlled through a graphical user interface on the host PC GUI (Fig. 3C). The headstages and the headstage control microprocessor board were designed on a computer-aided design (CAD) software package, OrCAD Capture and OrCAD Layout Plus (Cadence). The headstages were printed as a high-density six-layered circuit board (PCB) at a local factory. The overall size was 37.0 × 38.3 × 5.3 mm and weighed 8.7 g for rat drive. The mouse version was 25.0 × 19.0 × 4.0 mm and weighed 1.8 g (Fig. 4A). The numbers of motors, which are supported by these headstages, are 24 and 8 channels, respectively. This technical solution allowed us to reduce the number of motor connections to only nine fine wires (AWG38: CZ1187, Cooner Wire: 3 analog sine waves plus 6 digital control lines including 2 power lines), regardless of the number of motors (Fig. 3, A and B).
FIG. 2.
Simplified DC servomotor multiplexer headstage diagram. The DC servomotor multiplexing headstage is realized by numbers of multi-stage bus registers and a multi-channel analog switch. The actual analog switch (Pericom, PI5V331Q) contains dual 4-channel switches.
FIG. 3.
Simplified motorized microdrive system diagram. The motorized microdrive system consists of 3 functional blocks: (A) motor multiplexer headstage with a large numbers of motorized microdrives, (B) headstage control microprocessor board with a sine-wave generator, and (C) host PC motor control GUI software. Blocks A and B are connected by a set of custom-made fine wires, whereas B and C are connected by a regular USB cable. The fine wires do not need ground shielding, whereas the USB cable's shielding needs to be connected to the recording system ground. In normal mode, the motorized drive is remotely operated by custom-made host PC motor control software. During microdrive construction or preparation mode, the embedded microprocessor takes over the sine generator control as stand-alone mode.
FIG. 4.
Custom-designed printed circuit boards. A: 2 scales of the headstage components: The rat version of the multiplexing board has the cluster of motor wirings in the middle, whereas tetrode connectors are isolated at the edge of the board to minimize the electrical interference. The preamp board sits on top of the multiplexing board and the buffered tetrode signal comes out from the triangular connector in the middle of the board. The mouse version has an additional interface board (not shown) that vertically connects both multiplexer and preamp board. B: the top view of the headstage controller. The board was designed as a prototype using non–surface-mount type components, such as 1/8W carbon resisters or electrolytic capacitors for the ease of assembly. The switches and knobs at the right edge of the board are for manual control during microdrive array assembly.
Headstage control board and control GUI software
The headstage control microprocessor board (Figs. 3B and 4B) consists of a three-channel sine wave generator (MCBL05002, Faulhaber) and an embedded microprocessor (BS2SX, Parallax) with USB interface LSI (FT2232C, Future Technologies Device International). The embedded microprocessor interfaces between the host computer and the sine wave generator through USB 1.1 compliant protocol. It computes motor commands such as number of rotations or speed and generates encoded serial data bit streams to the headstage to multiplex the DC servomotors. As a system, the multiplexing headstage receives serial data bit streams from the headstage control board over the fine wires. In our design, 12 bits of serial data are required to multiplex 24 individual microdrives. It takes <1 ms to accomplish the multiplexing function. In addition, the microprocessor serves as a stand-alone controller during microdrive construction and electrode loading. The headstage control board is equipped with an on-board information display so that the user can control individual microdrives in the workshop without a host computer. The host PC software was developed on Microsoft Visual C# 2005 (Microsoft), which communicates with the headstage control board through a USB interface. The software has a GUI-based interface to control the microdrives (Fig. 3C). The motor status is updated every 200 ms through continuous communication between the software and the interface board. The GUI tool was compiled into a Windows XP (Microsoft) executable to have stable continuous USB communication and faster GUI response and update.
Stereolithography
To configure arrays of individual microdrives efficiently in a limited space, we designed inner supports (Fig. 5) using a three-dimensional (3-D) CAD software package (Solidworks, Solidworks). The supports keep individual microdrives in line with the electrodes so that the shuttle can drive the electrode smoothly into the base structure of the microdrive array. We designed two versions of the inner supports for rats and mice with 21 and 7 microdrives, respectively. Changing the number of microdrives simply involves redesigning the inner support. The inner supports were constructed by sending a 3-D CAD data file (STL file) to a stereolithography production company (American Precision Prototyping). The inner support was printed in a high-resolution, acrylonitrile butadiene styrene (ABS)-like material (DSM14120) that is rigid and light in weight.
FIG. 5.
Structure of three-dimensional (3-D) printed inner support. A: vertical cross-section of the rat inner support: The thickness of the sidewall is ∼2 mm in the rat support, whereas the mouse inner support has 1 mm of thickness. The current 3-D printing technology does not have enough resolution to make holes for the 30-Ga tubes, so that one has to drill holes before the assembly. B: actual inner support. The material is called ABS (acrylonitrile butadiene styrene)-like material. It is durable enough to hold microdrives with the epoxy glue even after 2 yr of use.
Microdrive array assembly
The microdrive array assembly process (Fig. 6 : rat version) was as follows: first, several bundles of 30-Ga hypodermic tubes (HTX-30R, Small Parts) were cut to length and molded into a dental acrylic base. The length of each 30-Ga tube at this point was ∼50 mm. The mold was made from a piece of Delrin sheet (SDE-0500, Small Parts) with hole locations corresponding to desired recording sites. Next, the 30-Ga bundles were inserted into the holes on the Delrin sheet. The bottom part of the bundle was wrapped by a piece of scotch tape to make a mold (Fig. 6C1). A small amount of dental acrylic was poured into the mold to fix the 30-Ga bundles into one piece (Fig. 6C2). Second, three double-row L-shaped connectors (ED90268-ND, Digikey) for the tetrodes were placed on top of the inner support and fixed with dental acrylic resin (Fig. 6C3). Once the resin was cured, each 30-Ga tube was carefully threaded through the bottom part of the inner support by slightly bending the tubes (Fig. 6C4). The distance between the bottom part of the molded acrylic resin and the bottom part of the printed inner support was ∼15 mm. The gap between the inner support and the bundle was filled with additional dental acrylic resin (Fig. 6C5). The 30-Ga tubes were trimmed with a cutting wheel so that the tips were barely sticking out from the inner support (Fig. 6C6). Third, the individual microdrives were attached to the inner support using ultrafast curing epoxy glue (EPOXY 90 s, Super Glue/Pacer Technology). Proper alignment of these components is critical for smooth movement of the electrodes. We used 5.5 mil stainless wire that was inserted in the 30-Ga tube to align the microdrive until the epoxy was cured (Fig. 6C7). Once every part was cured, individual motors were connected to the DC motor multiplexer. Finally, a protective plastic cone was attached at the bottom of the microdrive array.
FIG. 6.
The rat microdrive array. A: an exploded view of the rat microdrive array: the tetrode connectors are fixed to the inner support with the dental acrylic. The shielded protection cone is attached to the bottom part of the microdrive array with 5 miniature screws. B: an actual view of the rat microdrive array. To make connection between the bundles of 30-Ga tubes and the bottom part of the inner support is to vertically align and hold both parts. Apply relatively thick dental acrylic to the 30-Ga tubes with a syringe. The relationships of the 30-Ga tube, polyimide tube, and the working distance are shown in the right panel. C: overall steps of the rat microdrive array construction. Red arrow indicates major work procedure in each step.
The mouse version (Fig. 7) was almost identical in terms of microdrive array structure and differed only in scale and the way in which tetrodes and motor wirings were connected. The mouse version used another small interface printed circuit board that allowed the preamp and motor control board to be detached while the animal was in their home cage.
FIG. 7.
The mouse microdrive array. A: an exploded view of the mouse microdrive array. Basically, the overall structure is identical to the rat version except for the scale and the additional interface board. This way of connection is to reduce overall weight while the animal is in the home cage. B: an actual view of the mouse microdrive array. The bottom cannula has intentionally made angled tip to the right. In addition, the red-colored tubings are polyimide 30-Ga tubes for testing. Working distance and relative length of the 30-Ga tube and polyimide tube are shown.
Tetrode loading and preparation for the surgery
Tetrodes made with four twisted 12.7-μm polyimide-coated nichrome wires (California Fine Wire) were used to make unit recordings. They were inserted into supporting 5.5 mil OD polyimide tubes (Phelps Dodge) and attached to the shuttle with epoxy. Individual electrode wires were stripped of insulation at the end and connected to L-shaped terminal posts on the inner support using heat-shrink tubing (Ployester, Advanced Polymer) and silver paint (Silver Print II, GC Electronics) for the rat drive. For the mouse drive, the tetrodes were connected to a custom-made interface board using gold electrical pins. The tetrodes were cut to their final length with sharp serrated scissors and electroplated with a gold solution to impedance between 400 kΩ and 600 kΩ at 1 kHz. After checking the electrical connectivity of the tetrodes, a small amount of mineral oil (M8410, Sigma-Adlrich) was applied to the tip of the microdrive array. This procedure prevents the tetrodes clogging in the 30-Ga bundle with postsurgical fluids such as blood or cerebrospinal fluid (CSF). Visual inspection under the microscope suggests that the minimum amount that a microdrive can be reliably advanced is 3–4 μm. The motor does not provide positional feedback (e.g., rotor position provided by hall-effect sensors) to the motor controller and therefore relies on open loop control. However, the motor can work robustly as long as adequate voltage (0.5 Veff) and current (50–60 mA) is provided under the appropriate mechanical loads.
Surgery and adjusting
Because the physical structure of the microdrive array is almost identical to the conventional manual microdrive array, surgical procedure for drive implantation remained unchanged. Animals were initially anesthetized with a cocktail of ketamine (50 mg/kg) and xylazine (6 mg/kg) and maintained on isoflourane gas with oxygen carrier (1.5–2.5%). The motorized microdrive array was attached to the skull with miniature anchor screws (8–10 screws) and dental acrylic. Similar to the conventional microdrive arrays, the electrodes were kept inside their guide cannulae (∼200 μm from the tip) during surgery, and the dura was removed. All implanted animals lifted their heads immediately after recovering from the anesthesia. Because the rat's body weight was ∼500 g, it carried the 29-g microdrive easily. Surprisingly, a 35-g mouse could carry a 4.8-g drive without any difficulty. We started adjusting the electrodes after several days of recovery. Each microdrive could be moved at a speed between 1.3 and 330 μm/s. The theoretical minimum step at which the tip can be moved is ∼0.62 μm at all speed ranges. We used faster speed (∼50 μm/s) with larger incremental steps (∼20 μm) at the beginning of adjusting. However, as we came closer to the recording target, slower speed (∼2 μm/s) with finer steps (<5 μm) was used to obtain optimal unit isolation. All surgical procedures were carried out following the Massachusetts Institute of Technology and National Institutes of Health animal care guidelines.
Data acquisition and unit isolation
Multiple single unit recordings were made using a setup similar to that used in previous work (Wilson and McNaughton 1993). Electrical signals from the tetrodes were first preamplified at the microdrive with a custom-designed source follower 75-channel preamplifier (Fig. 4A, right column). The signal was amplified between 5,000- and 30,000-fold at the main amplifier (Neuralynx) and digitized with a set of 12 synchronized PCs with a custom-made acquisition program (M. Wilson and L. Frank). At the main amplifier, the signals were split and band-pass filtered in two ways to record local field potentials (LFPs) or single unit activity. To record spike activity, the original signals were filtered between 300 Hz and 9 kHz and sampled at 31.25 kHz. To record LFPs, the signal was filtered between 0.1 and 475 Hz and sampled continuously at 2 kHz. In addition, the animal head position was recorded using two LEDs mounted to the array. Each LED was sampled at 30 Hz. The digitized spike signals were manually isolated into individual unit activities on the basis of spike amplitudes using a custom software package (Xclust2, M. Wilson).
Postmortem cleaning and reusing the microdrive array
The microdrive array tip was immediately dipped into stabilized hydrogen peroxide (3%) for at least 1 day to soften residue at the tip. All tetrodes were cut at the bottom part of the polyimide tube and removed from the bottom of the microdrive array to minimize biological residue in the 30-Ga tube. The remaining polyimide tubes were carefully detached from the shuttle. The tetrodes were removed from the connecting posts with a fine-tip tweezers. Finally, the microdrive array tip was dipped into 100% ethanol bath using a sonicator to clean the tip. All microdrive movements were carefully tested under the binocular microscope.
RESULTS
Chronically implanted microdrive arrays
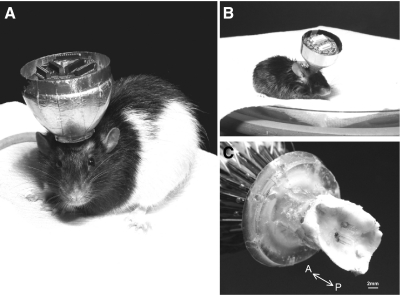
A total of seven male rats (450–500 g; Fig. 8A), and five male mice (30–35 g, Fig. 8B) were tested. During these tests, two sets of rat microdrive arrays and another two sets of mouse microdrive arrays were used to test the electromechanical reliability and the reusability of the microdrive array. The duration of the experiments ranged from 2 to 5 mo in both the rat and mouse. Typical duration was ∼3 mo. On average, we took at least 2–3 wk to reach desired recording target before we started neuronal recordings. To test whether the device could be used in recordings from both shallow and deep brain structures, we recorded from several different brain areas, including the somatosensory cortex, hippocampus, and anterior part of thalamus. Figure 8C shows a bottom-angled view of the postmortem cranium with the motorized microdrive after 3 mo of recording. In this particular animal, a total of four recording sites (2 different brain areas with bilateral configuration) using 21 tetrodes can be seen.
FIG. 8.
Chronically implanted large-scale motorized microdrive array. A: a male Long-Evans rat (6 mo old, 500 g) with the 21 motorized microdrive array (26 g net, 29 g with a protection cone). The drive was implanted for >3 mo and yielded acceptable multi-unit recordings throughout the recording period (∼6 wk). The outer metallic cone provides protection for the microdrive and Faraday cage-like electrical shielding. B: a C57BL/6 mouse (10 mo old, 35 g) with the 7 motorized microdrive array (4.2 g net, 4.8 g with a cone). C: electrode tips of the rat motorized microdrive array under the rat cranium. The figure shows successful insertion of the electrodes into the brain using fine adjustments over several weeks.
Behavioral performance with the microdrive arrays
The total weight of our entire headstage system including microdrive array, motor controller, preamp, and head tracking LEDs was 40 (rat) and 8.5 g (mouse). To reduce the weight of the recording system during behavioral testing, we used a counter-balancing pulley system consisting of a set of lightweight pulleys on the ceiling with fishing line attached to balancing weights. The bundle of fine wire is vertically supported by the balancing weights, which can freely move over the behavioral arena. Rats were tested on a circular track (110 cm diam, 10 cm wide; Fig. 9). The top left panel shows the top view of the behavioral test environment. The middle and right panels show the trajectories of the rat during the task. The middle panel shows only a portion (150 s) of the task, whereas the right panel shows the entire trajectory of the task (∼1,500 s). The rat had already trained with this spatial alteration task to obtain food rewards for ∼1 wk after the surgery. The bottom left panel shows the instantaneous velocity over time. The average velocity during the task was ∼38 cm/s (a dashed line in the Fig. 9, bottom left), whereas the maximum velocity was close to 120 cm/s. The bottom right panel shows the distribution of its velocity of the entire task. It shows that the animal's behavior was consistent through out the task for ∼100 trials, close to 55 m in total travel distance.
FIG. 9.
Behavioral performance of a rat. Top panels: representative trajectories of a rat carrying the motorized microdrive array during a circular track task. Green and red trajectories were plotted by the front and back infrared LEDs attached to the microdrive array, respectively. Bottom panels: instantaneous velocity over time and its velocity histogram during the behavior. The dashed line in the bottom left is the averaged velocity during the task. Top middle figure: trajectory of the animal during the period marked by * in the bottom left figure.
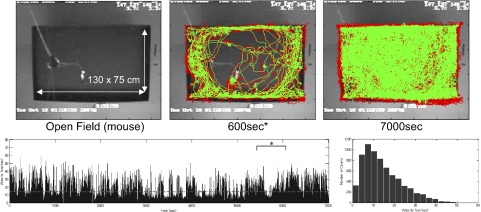
Implanted mice were tested in an open field (130 × 75 cm) task (Fig. 10). The top middle panel shows the trajectory of the mouse during free exploration for 600 s. The mouse continued exploration for ∼2 h (Fig. 10, bottom). The average velocity during this test was ∼14 cm/s (a dashed line in Fig. 10, bottom left), whereas the maximum velocity was close to 60 cm/s. The total travel distance was ∼100 m. We did not observe obvious differences in behavior with our microdrive arrays compared with conventional microdrive arrays.
FIG. 10.
Behavioral performance of a mouse. Top panels: representative trajectories of a mouse carrying the motorized microdrive array during a large open field exploration task. Red and green trajectories were plotted by the front and back infrared LEDs attached to the microdrive array, respectively. Bottom panels: instantaneous velocity over time and its velocity histogram during the behavior. The dashed line in the bottom left figure is the averaged velocity during the task. Top middle figure: trajectory of the animal during the period marked by * in the bottom left figure.
Electromagnetic noise level
To assess electrical noise, we recorded neuronal signals in the following typical recording conditions: 10,000 gain, low cut-off: 300 Hz, high cut-off: 9 kHz, sampled at 31.25 kHz. In Fig. 11, the first three waveforms (A–C) were recorded when the tetrode was in the white matter (corpus callosum) to check the background noise level. Waveform A was under condition of only the preamplifier but without connection of the motor control circuit board. The background noise level was 92 ± 0.46 (SE) μV peak-to-peak [33 ± 0.16 μV root mean square (RMS)]. Waveform B was obtained when the motor control circuit was connected to the microdrive array with the power supplied. The noise level was 96 ± 0.33 μV peak-to-peak (34 ± 0.12 μV RMS). Waveform C shows the slight interference of the sine wave during the adjacent motor was driven at slower speed. The noise level was 321 ± 4.84 μV peak-to-peak (113 ± 1.71 μV RMS). The last two waveforms were recorded when the electrode was in either (D) the hippocampus CA1 area or (E) somatosensory cortex deep layer (the spike polarities are inverted in this figure). The signal magnitude of the largest spike in these areas was 1,830 and 2,350 μV peak-to-peak, respectively. As we showed in this figure, there is no significant increase in baseline noise level regardless of the presence of the motor multiplexing controller. The major interference that we noticed was the artifact while the micromotor was in motion. This is presumably caused by alternating magnetic flux leakage from adjacent micromotor, because this phenomenon was not observed on all tetrodes. We found the magnitude of the artifact depends on how closely the electrodes run from the adjacent micromotors. The tetrodes that pass closer to the motor housing tend to pick up some of the sine waves that originate from the motor. However, the magnitude of the artifact was small enough as well as the time duration of each movement was short enough (usually completes in a few seconds) to continue monitoring neuronal signals. In practice, this would not pose a serious problem because data would not typically be acquired during adjustment.
FIG. 11.
Electrical interference and artifacts under various conditions. Signal magnitudes of (A) white matter (corpus callosum) without the multiplexer board; (B) white matter (corpus callosum) with the multiplexer board on; (C) white matter (corpus callosum) with the multiplexer board on, motor in motion; (D) dorsal hippocampus CA1 area with the multiplexer board on; and (E) somatosensory area with the multiplexer board on.
Fine step adjustments
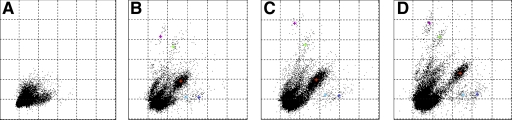
One key advantage of the motorized microdrive array is ability to adjust electrodes at finer displacement steps to optimize unit isolation. To examine the potential effect of finer positional control, we tried to find a minimum adjusting step in which a systematic change could be seen in spike cluster distributions. Each panel in Fig. 12 shows the recording of spikes from multiple neurons on a single tetrode. Each data point represents a single spike event. These points are plotted at the peak amplitude of that spike on the first versus the second channels of a four-channel tetrode. The figures show that tetrodes advanced in small steps at the slowest speed (1.3 μm/s) showed systematic cluster movement. Figure 12A was the initial state as the electrode approached the hippocampal CA1 pyramidal cell layer. The tetrode was advanced over a period of 3 wk to reach this point and was left untouched for at least a day. Then the tetrode was advanced by 80 μm from the initial state. The cluster plot began to change over the following 2 h and finally settled down to the clusters shown in the Fig. 12B. At this point, we changed adjusting strategy to much finer steps. The electrode was advanced by 8 μm, and Fig. 12C was obtained. After an hour, the electrode was advanced by another 8 μm, and the unit isolation became clearer than the previous two adjustments (some reference + icons are shown). Another 8-μm displacement made the unit isolation worse than Fig. 12D. We also tried much finer displacement steps such as 4 or 2 μm, which had produced observable movement on the test bench. However, we could not confirm systematic cluster changes with these smaller displacements.
FIG. 12.
Improved isolated unit activity with small-step adjustments. A: initial state. B: after a large electrode displacement (80 μm) from the initial state. C: slight improvement isolation following an 8-μm positive advancement. D: improvement following another 8-μm advancement.
Isolated unit activities in multiple areas
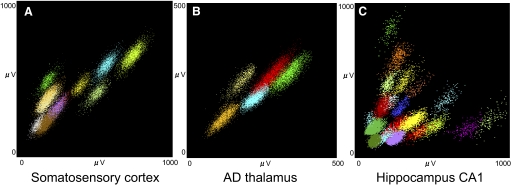
Examples of data are shown in Fig. 13. Colors represent the postrecording classification of the spikes into clusters. All tetrodes were remotely advanced by 16-μm (1/10th of a turn) increments in the beginning and by 8–4 μm (1/20th–1/40th) during the later fine adjustment phase. The data shown in Fig. 13A were recorded from the rat somatosensory cortex (∼1,500 μm deep from the surface of the dura). A total of 11 individual units can be clearly isolated from this tetrode. Although we do not have sufficient data to make a quantitative comparison with our comparable manual microdrive arrays, these results suggest a superior yield with the motorized drives. Improvements were observed in rat thalamic recordings as well (Fig. 13B). A total of five units were clearly isolated from an anterior dorsal (AD) thalamus tetrode (∼4,000 μm deep from the surface). Our conventional yield is typically one to three units per tetrode in this region. Hippocampal recordings (Fig. 13C) also provided improved higher yields relative to conventional techniques. In Fig. 13C, a total of 20 single units were isolated from one tetrode in hippocampal CA1 area of the mouse.
FIG. 13.
Multiple single-unit activities yielded by motorized microdrive array. The individual cell clusters plotted as channel 1 vs. channel 2 spike amplitude from the 4 channels of a tetrode. Examples are from different recording sites across different animals: (A) rat somatosensory cortex, (B) rat anterior dorsal thalamus, and (C) mouse hippocampal CA1.
Stability across multiple days
In addition to number of isolated units, the stability of isolated units across multiple days is an important issue. Figure 14 shows a total of 11 individual units in rat somatosensory cortex that were stable across 4 consecutive days. These isolated units were obtained from deep layers (∼1,350 μm from the start) of the somatosensory cortex. The tetrode was advanced over a period of 3 wk (50–100 μm/d) at slower speeds (2–5 μm/s) and smaller steps (4 μm) and was left untouched for at least 2 days at this position before the first day of recording. As is often the case with independently adjustable multiple electrodes, any attempts to move one electrode may influence the stability or isolation of units on adjacent electrodes. We found that the slower speed of advancement and the smaller steps of displacement over a given time could greatly minimize this effect. We also tested performance with a range of rotational speeds (>100 μm/s) and incremental steps (>30 μm). Although we could hold well-isolated unit activities for at most couple of hours, we could not obtain long-term stable recordings with these adjustment parameters. Based on these tests, we concluded that slower movements and smaller steps are essential factors for high quality recording. Overall, our experience suggests that the motorized microdrive array has advantages in the precision of the electrode positioning as well as isolation, yield, and stability of recorded units compared with conventional manual microdrive arrays.
FIG. 14.
Unit cluster stability over 4 consecutive days. Example of multiple units recorded from the rat somatosensory cortex across multiple days. The 1st day shown here is ∼3 wk after surgery. The larger amplitude units, which means closer to the electrode tip, tend to be more sensitive to movement.
DISCUSSION
We designed and tested two versions of large-scale motorized microdrive arrays for chronic recording in smaller animals, based on a modular and flexible design concept. The results showed that our novel motorized microdrive array allowed us to obtain long-term recordings with quality comparable to, or exceeding that of, conventional manual microdrive arrays (Gray et al. 1995; Wilson and McNaughton 1993). The drive design is relatively simple and flexible, allowing it to support recordings from brain structures at a variety of locations and depths. Most of the mechanical components, such as the brushless motor, planetary gearhead, shuttle, and shuttle block nut are reusable and easy to overhaul as described in the previous section. This is also true for the electrical components, such as the motor multiplexing headstage, the headstage controller, or the preamplifier. Only tetrodes and the supporting polyimide tubings require replacement. We have been routinely reusing the microdrive arrays over the past 3 yr.
The major advantage of our new motorized microdrive array over existing techniques (Fee and Leonardo 2001; Luo et al. 2003; Venkateswaran et al. 2005) is the ability to remotely control a large number of microdrives slowly and precisely while maintaining the overall size and weight of conventional microdrive arrays (Gray et al. 1995; Wilson and McNaughton 1993). Slower speed and smaller steps in the adjustments seem to enhance quality and stability of chronic recordings.
Known issues and future improvement
Although the current design met our overall performance objectives, there are still additional improvements that could be made.
Although we tried minimizing the overall weight, the electrical printed circuit board, which is the heaviest part of the microdrive array, can be optimized in terms of weight and size. The current version uses discrete design, which consists of multiple packages of integrated circuits. If these discrete integrated circuits can be integrated into a single large-scale-integrated (LSI) circuit package, the weight and the size of the headstage can be dramatically decreased. This is also the case with the preamplifier board. Because both circuits are simple in terms of complexity, this could easily be done for future versions. Use of thinner printed circuit boards (thickness: 0.031 in, current: 0.062 in), which are widely used in portable electronics, would also reduce weight. Individual motors could be directly connected to the printed circuit board rather than using electrical pins and receptacles for better reliability, because we found that we rarely needed to remove the motor from the printed circuit board once they were assembled in to a microdrive array.
In summary, the recording device that we described here offers significant advantages compared with conventional recording techniques (Gray et al. 1995; Wilson and McNaughton 1993) in terms of higher quality and stability of isolated unit activities by supporting a large number of motorized microdrives that precisely control electrodes placed in multiple brain regions. Our modular design concept of the motorized microdrive array can be adapted easily to other paradigms, especially to behaviorally sensitive long-term recordings.
GRANTS
This work was supported in part by a RIKEN-MIT Neuroscience Research Center grant and a National Institutes of Health grant to M. A. Wilson.
Acknowledgments
We thank J. Tani and RIKEN-Institute of Physical and Chemical Research Brain Science Institute of Japan for supporting J. Yamamoto's early stage of his stay at Massachusetts Institute of Technology. We thank M. Fee and K. Nakazawa for discussion and comments; D. Ji and T. Davidson for reading an early version of this manuscript; and F. Kloosterman and J. Jung for technical advice for the mouse surgery.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Cham 2005.Cham JG, Branchaud EA, Nenadic Z, Greger B, Andersen RA, Burdick JW. Semi-chronic motorized microdrive and control algorithm for autonomously isolating and maintaining optimal extracellular action potentials. J Neurophysiol 93: 570–579, 2005. [DOI] [PubMed] [Google Scholar]
- Fee 2001.Fee MS, Leonardo A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J Neurosci Methods 112: 83–94, 2001. [DOI] [PubMed] [Google Scholar]
- Fujii 2005.Fujii N, Graybiel AM. Time-varying covariance of neural activities recorded in striatum and frontal cortex as monkeys perform sequential-saccade tasks. Proc Natl Acad Sci USA 102: 9032–9037, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray 2007.Gray CM, Goodell B, Lear A. Multichannel micromanipulator and chamber system for recording multineuronal activity in alert, non-human primates. J Neurophysiol 98: 527–536, 2007. [DOI] [PubMed] [Google Scholar]
- Gray 1995.Gray CM, Maldonado PE, Wilson MA, McNaughton BL. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods 63: 43–54, 1995. [DOI] [PubMed] [Google Scholar]
- Luo 2003.Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science 299: 1196–1201, 2003. [DOI] [PubMed] [Google Scholar]
- McCasland 1987.McCasland JS Neuronal control of bird song production. J Neurosci 7: 23–39, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton 1983.McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods 8: 391–397, 1983. [DOI] [PubMed] [Google Scholar]
- O'Keefe 1971.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971. [DOI] [PubMed] [Google Scholar]
- Ranck 1973.Ranck JB Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 41: 461–531, 1973. [DOI] [PubMed] [Google Scholar]
- Taube 1990.Taube JS, Muller RU, Ranck JB Jr. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci 10: 420–435, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran 2005.Venkateswaran R, Boldt C, Parthasarathy J, Ziaie B, Erdman AG, Redish AD. A motorized microdrive for recording of neural ensembles in awake behaving rats. J Biomech Eng 127: 1035–1040, 2005. [DOI] [PubMed] [Google Scholar]
- Wilson 1993.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science 261: 1055–1058, 1993. [DOI] [PubMed] [Google Scholar]