Abstract
Whereas Kvβ2 subunits modulate potassium current properties carried by Kv1 channel complexes in heterologous systems, little is known about the contributions of Kvβ2 subunits to native potassium channel function. Using antisense approaches and in situ recordings from Xenopus embryo spinal cord neurons, we tested the in vivo roles of Kvβ2 subunits in modulation of voltage-dependent potassium current (IKv). We focused on 1) two different populations of dorsal spinal neurons that express both Kvβ2 and Kv1 α-subunit genes and 2) the 24- and 48-h developmental period, during which IKv undergoes developmental regulation. At both 24 and 48 h, antisense methods produced efficient knock-down of both Kvβ2 protein and IKv. At both times, dominant negative suppression of Kv1 channels also eliminated IKv, indicating that Kv1 channels require Kvβ2 subunits to function in dorsal spinal neurons. Even though Kv1 channels determined the IKv values of both dorsal neuron types, comparisons of their IKv properties revealed important differences at both developmental stages. The latter results support the notion that different Kv1 α-subunits and/or posttranslational modifications underlie the IKv values of the two dorsal neuron types. Overall, the results demonstrate that Kvβ2 subunits function in vivo as obligatory subunits of Kv1 channels in at least two neuron types and two different developmental stages.
INTRODUCTION
Voltage-gated potassium (Kv) channels play important roles in excitable cells by regulating resting membrane potential, action potential duration, firing frequency, and neurotransmitter release (Hille 2001). Functional Kv channel protein complexes contain pore-forming (α) and auxiliary (e.g., β) subunits (Li et al. 2006; Pongs et al. 1999; Torres et al. 2007; Trimmer 1998). The pore-forming α-subunits comprise members of several different Kv subfamilies (Kv1–12). When expressed in heterologous systems, Kv1, Kv2, Kv3, Kv4, Kv7, Kv10, Kv11, and Kv12 α-subunits form functional channels even in the absence of auxiliary subunits (Coetzee et al. 1999; Gutman et al. 2003).
In vivo biochemical and immunohistochemical studies have revealed that the majority of Kv1 channel complexes contain auxiliary subunits that belong to the Kvβ gene family (Kvβ1–Kvβ3; Heinemann et al. 1995, 1996; Nakahira et al. 1996;Parcej et al. 1992; Rettig et al. 1994; Rhodes et al. 1995, 1996, 1997; Scott et al. 1994; Shamotienko et al. 1997; Shi et al. 1996). Kvβ subunits also interact with Kv4 and Kv12 α-subunits (Chen et al. 1996, 2000; Wilson et al. 1998; Yang et al. 2001; but see Tang et al. 1998). Despite the widespread presence of Kvβ2 subunits in native Kv channel complexes, little is known about their physiological roles in vivo.
The limited available information about in vivo Kvβ2 subunit function has been obtained from studies of the Drosophila mutant, hyperkinetic (Hk; Chouinard et al. 1995; Ikeda and Kaplan 1970; Kaplan and Trout 1974), and a genetically engineered Kvβ2 mouse knock-out (McCormack et al. 2002). Drosophila has a single gene, Hk, that is orthologous to mammalian Kvβ1, Kvβ2, and Kvβ3 genes. Hk mutants display an abnormal motor phenotype consisting of rhythmic leg shaking. Interestingly, Kv1 (Shaker) currents have reduced amplitudes and altered kinetic properties (Wang and Wu 1996; Yao and Wu 1999). The Kvβ2 knock-out mouse displays seizures, cold swimming-induced tremors, and reduced life spans (McCormack et al. 2002). Despite work from heterologous systems suggesting chaperone-like functions for Kvβ2 genes on Kv1 complexes, the mouse knock-out provided no evidence for abnormal Kv1 channel biosynthesis or trafficking on elimination of Kvβ2 protein. These results highlight that the in vivo roles of Kvβ subunits are poorly understood.
Studies of Kvβ2 subunits expressed heterologously have provided information regarding their interactions and functional effects on α-subunits. Xu et al. (1998) analyzed subunit stoichiometry and found four Kvβ2 subunits per channel; that is, equal numbers of Kvβ2 and Kv1 α-subunits exist in a single Kv1 channel complex. In vitro, Kvβ2 subunits produce diverse functional consequences, including effects on voltage dependence of activation, activation and inactivation kinetics, channel surface membrane expression, and current density (Heinemann et al. 1996; Manganas and Trimmer 2000; Morales et al. 1996; Rettig et al. 1994). Because Kvβ2 subunits increase channel complex stability and cell surface insertion of Kv1 α-subunits in heterologous systems, chaperone-like roles have been proposed for Kvβ2 subunits (Accili et al. 1997; Campomanes et al. 2002; Nagaya and Papazian 1997; Shi et al. 1996).
Because effects seen in heterologous systems do not always recapitulate mechanisms in vivo, we took advantage of unique features of Xenopus embryonic spinal cord neurons to test the in vivo function of Kvβ2 subunits. Voltage-gated potassium currents (IKv) have been studied in vitro as well as in vivo in spinal neurons of the Xenopus embryo (Desarmenien et al. 1993; O'Dowd et al. 1988; Pineda and Ribera 2008). The Xenopus embryo expresses Kvβ2 mRNA in spinal cord neurons during the same developmental period during which extensive regulation of voltage-gated potassium current occurs (Lazaroff et al. 1999). Further, the developmental changes in IKv consist of increases in current density and acceleration of activation kinetics (Barish et al. 1986; Lockery and Spitzer 1992; O'Dowd et al. 1988), potassium current properties that are modulated by Kvβ2 subunits when coexpressed heterologously with Kv1 α-subunits. Moreover, dorsal spinal neurons express Kv1.1 α-subunit and Kvβ2 transcripts, providing an experimentally accessible and relevant neuronal population for the study of the in vivo roles of Kvβ2 subunits (Lazaroff et al. 1999; Ribera and Nguyen 1993).
To test the in vivo roles of Kvβ2 subunits, we used two different antisense (AS) strategies to knock down Kvβ2 protein. Western blot analysis indicated that morpholino oligonucelotides (MOs) produced effective knock-down of Kvβ2 protein. We focused on two different populations of neurons in the dorsal spinal cord: Rohon–Beard (RB) primary mechanosensory neurons and immediately adjacent dorsal non-Rohon–Beard cells (non-RBs). In the absence of Kvβ2 protein, specific populations of dorsal spinal cord neurons that express the gene displayed a near total elimination of IKv. This was true for both dorsal neuron types at two different developmental stages. However, IKv properties differed substantially between RBs and non-RBs and as a function of development. With respect to Kvβ2 knock-down, the Kv1 dominant negative efficiently suppressed IKv channel in the two different neuron types regardless of developmental stage. Thus irrespective of developmental stage, Kvβ2 protein knock-down reduced IKv density in spinal neurons as effectively as did dominant negative suppression of Kv1 channel function. Taken together, the results support the view that Kvβ2 subunits play an essential role in Kv1 channel function. Moreover, because Kvβ2 knock-down effectively suppressed IKv in both 24- and 48-h neurons, the essential role of Kvβ2 in Kv1 channel function is constant and not developmentally regulated during a period when IKv properties undergo substantial changes.
METHODS
Xenopus embryos and microinjection
All experimental procedures were approved by the Animal Care and Use Committees of the Center for Comparative Medicine at the University of Colorado Denver at the Anschutz Medical Campus. In vitro fertilization and RNA microinjection were performed as described previously (Jones and Ribera 1994). Embryos were staged on the basis of external morphology (Nieuwkoop and Faber 1967).
Working MO (1–150 pg/nl) or RNA (120–200 pg/nl) solutions were prepared by dilution of stock aliquots with RNAse-free water containing as a lineage tracer RNA encoding green fluorescent protein (GFP; 6 ng/nl, a kind gift of Dr. Michael Klymkowsky, University of Colorado, Boulder; Blaine and Ribera 2001). A total volume of 10 nl was injected into one cell of two-cell stage embryos using a gas-driven injection apparatus (2–3 psi for 2 s; PLI-100, Medical System, Greenvale, NY) with fine-drawn micropipettes (∼1- to 2-μm tip diameter; Sutter P-87 Puller, Sutter Instruments, Novato, CA). The day after injection, embryos were examined with epifluorescent illumination and those expressing GFP within the neural tube were kept at room temperature until the desired developmental stage.
KNOCK-DOWN OF KVβ2.
Kvβ2 function was knocked down by injection of either morpholino (MO) or antisense RNA. MOs were designed and synthesized by GeneTools (Philomath, OR). The Xenopus Kvβ2(Kvβ2MO) targeted the predicted translation start methionine and had the following sequence: 5′-AgT CTg Tgg TCg ATT CTg gAT ACAT-3′. The control Kvβ2MO (CtlMO) was designed by inverting the Kvβ2MO sequence: (5′-TAC ATA ggT CTT AgC Tgg TgT CTgA-3′). Aliquots of MO stock solutions were prepared by resuspending the oligonucleotides in RNAse-free water at a final concentration of 12.5 μg/μl (1.5 mM) and stored at −80°C. For both Kvβ2MO and antisense Kvβ2, dose–response curves were determined to assess specificity of the knock-down.
ANTISENSE KVβ2 RNA (ASβ2).
ASβ2 was synthesized as described previously (Lazaroff et al. 2002). Briefly, the plasmid containing Kvβ2 (pCS2+) was linearized with Hin dIII and cRNA was synthesized by in vitro transcription with T7 RNA polymerase (Promega, Madison, WI) in the presence of ribonucleotide triphosphates (Pharmacia Biotech, Piscataway, NY). As a control for the antisense, an irrelevant RNA (GFP) was used. We found no differences between IKv in neurons derived from uninjected or GFP-injected blastomeres at either 24 or 48 h (not shown). In addition, previous work (Lazaroff et al. 2002) demonstrated ASβ2 selectively eliminated effects of Kvβ2 but not Kvβ4 RNA injection into Xenopus oocytes.
DOMINANT NEGATIVE KV1 α-SUBUNIT.
The Kv1 α-subunit dominant negative (Kv1DN) was generated as described previously (Ribera 1996). cRNA was synthesized by linearizing the plasmid with Xba I and in vitro transcription with SP6 RNA polymerase in the presence of ribonucleotide triphosphates (Pharmacia Biotech) and cap analogue (Boehringer Manheim, Indianapolis, IN). RNA concentrations were determined spectrophotometrically (Nanodrop N-1000, NanoDrop Technologies, Wilmington, DE). RNA integrity was assessed by agarose-formaldehyde gel electrophoresis.
Protein extraction
St 34/35 Xenopus embryos were homogenized in MK lysis buffer (in mM: 50 Tris pH 8.0, 150 NaCl, 0.5% NP40, 0.5% Triton-X100, 1 EGTA, pH 7.4; Klymkowsky Lab On-line Methods; http://spot.colorado.edu/∼klym/) containing 1× protease inhibitor (Halt Protease Inhibitor Cocktail Kit; Pierce, Rockford, IL) or 2% SDS in 50 mM Tris (pH 7.5). Homogenates were centrifuged and embryo supernatants were treated to remove excess lipid with PHM-L Liposorb absorbent according to the manufacturer's instructions (Calbiochem, San Diego, CA). Protein extract aliquots were stored at −80°C until use.
Western blots
Whole embryo protein extracts (20 μg) were resolved using SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon P; Millipore, Billerica, MA) by wet electrotransfer (Towbin et al. 1992). Prior to incubation with antibody, membranes were blocked for 2 h in Tris-buffered saline (TBS; in mM: 136 NaCl, 2.6 KCl, 24.7 Tris; pH 7.4) with 5% nonfat evaporated milk and 0.1% Tween 20. Blots were then incubated overnight at 4°C in blocking buffer containing the primary antibody, either anti-Kvβ2 (1:50, Clone 17/70, NeuroMab, Davis, CA; http://www.neuromab.org; Bekele-Arcuri et al. 1996) or anti-Kv1.1 (1:50, clone K20/78, NeuroMab). After being rinsed in TBST (TBS containing 0.5% Tween 20), blots were incubated with secondary antibody. For standard Western blot analysis, a horseradish peroxidase-conjugated anti-mouse secondary antibody was used (1:2,000; Bio-Rad Laboratories, Hercules, CA). Blots were then incubated in a chemiluminescent substrate at room temperature for 1–5 min (Pierce) and imaged using a Kodak Image Station 440 CF and Molecular Imaging Software (Carestream Health, Rochester, NY). These experiments were repeated at least three times.
For quantitative measurements, blots were incubated with an Alexa 647-conjugated anti-mouse secondary antibody (1:2,000; Invitrogen, Carlsbad, CA) for 2 h at room temperature (20–22°C) and then scanned using a Typhoon 9400 multimode imager (GE Healthcare; Little Chalfont, Buckinghamshire, UK). Gels were analyzed using ImageQuant Densitometer software (Molecular Dynamics, GE Healthcare, Pittsburgh, PA). An image of a representative assay is shown as well as average data for the total of three experiments.
Semiintact preparations of Xenopus embryos
St 22/23 and St 35/36 Xenopus embryos were dissected using slight modifications of methods previously described for semiintact preparations of zebrafish embryos (Pineda et al. 2005; Ribera and Nüsslein-Volhard 1998). Briefly, in the presence of Ringer solution (in mM: 145.0 NaCl, 3.0 KCl, 1.8 CaCl2, 10.0 HEPES; pH 7.2) containing 0.02% Tricaine (ethyl 3-aminobenzoate methanesulfonate salt; Sigma–Aldrich, St. Louis, MO), the yolky endoderm was removed and embryos were mounted ventral-side down onto glass coverslips using Vetbond Tissue Adhesive (3M Animal Care Products, St. Paul, MN). Embryos were then killed in the presence of anesthesia by transection at the level of the hindbrain. Removing the skin and dorsal fin fold exposed the spinal cord. Tricaine was removed by washing the preparation with ≥40 ml of recording solution over the course of 15 min. Preparations were viewed with differential interference contrast optics on an Axioskop FS2 microscope (Carl Zeiss MicroImaging, Hamburg, Germany) at a magnification of ×640. RB cells were identified on the basis of: 1) superficial location at the dorsal surface of the spinal cord, 2) large soma diameter (∼20 μm), and 3) position relative to the midline (Baccaglini and Spitzer 1977). On the basis of the absence or presence of GFP, cells were identified as internal control (GFP−) or MO, AS, or Kv1DN (GFP+) neurons, as done previously (Blaine and Ribera 2001).
Electrophysiological methods
Conventional whole cell patch-clamp techniques (Hamill et al. 1981) were used in voltage-clamp mode. Experiments were conducted at room temperature (20–22°C) using an Axopatch 200B amplifier and a Digidata 1440A analog to digital interface in conjunction with the pClamp 10.0 software recording package (Molecular Devices, Sunnyvale, CA).
Unpolished electrodes were fabricated from borosilicate glass (Microcaps; Drummond Scientific, Broomall, PA) with tip resistances ranging between 2.0 and 3.5 MΩ when filled with pipette solution (in mM: 100 KCl, 10 mM EGTA, 10 HEPES; pH 7.4 with NaOH). After establishment of the whole cell configuration, monoexponential capacitative transients were indicative of adequate spatial control of membrane voltage. Additional criteria were used to assess the quality of the recordings: 1) membrane resistance >120 MΩ, 2) holding current <250 pA, and 3) stable access resistance of <12 MΩ.
For recording of the outward potassium currents, the bath solution contained (in mM): 80 NaCl, 3 KCl, 5 MgCl2, 10 CoCl2, 5 HEPES, and 0.003 tetrodotoxin; pH 7.4 with NaOH. Currents were elicited by 60-ms depolarizing voltage steps to test potentials ranging between −60 and +100 mV in 10-mV increments from a holding potential of −80 mV; tail currents were recorded at −40 mV after the activating steps. Series resistance was routinely compensated by 75–85% with a lag of 10 μs. Currents were filtered at 5 kHz and digitized at 25 kHz. Passive leak and capacitative transients were subtracted on-line using the P/8 algorithm of the software.
Data analysis
Data analysis was accomplished using AxoGraph 10 (Axograph Scientific, Sidney, Australia), Excel (Microsoft, Redmond, WA), and Origin (OriginLab, Northampton, MA) software. Variability in cell size was accounted for by dividing current amplitudes by the membrane capacitance, which serves as an indicator of membrane surface area (1 pF/cm2; Marty and Neher 1983). Thus current data are presented as current densities (pA/μm2). For current density–voltage (I–V) plots, steady-state currents were measured by averaging values during a 10-ms interval at the end of each pulse (from 45 to 55 ms). Conductance–voltage (G–V) relationships were constructed by dividing current density by driving force, using the calculated potassium equilibrium potential of −88.3 mV. The Boltzmann equation (G = Gmax/{1 = exp[(V1/2 − V)/k]}, where Gmax is the maximal conductance, V1/2 is the voltage of half-maximal activation, and k is the slope factor) was fitted to the data. G–V plots were not corrected for the small voltage errors (<4 mV) introduced by the uncompensated fraction of the series resistance.
Data presentation
Results are presented as means ± SE. Statistical analysis was performed using GraphPad InStat software (GraphPad Software, San Diego, CA). Statistical comparisons were done using the Student's t-test or ANOVA, for comparisons of two or multiple groups, respectively. ANOVA analysis was followed by Bonferroni correction to consider multiple comparisons. A threshold of P ≤ 0.05 was used to determine statistical significance.
RESULTS
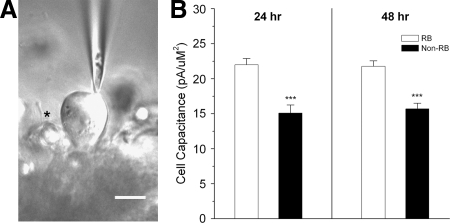
The known molecular determinants of potassium current in the dorsal spinal cord of Xenopus embryos consist of Kv1 α-subunits and Kvβ2 subunits (Lazaroff et al. 1999; Ribera and Nguyen 1993). To investigate the functional role of Kvβ2 subunits in vivo, we developed a semiintact preparation that allowed us to identify two different types of dorsal spinal cord neurons. Primary sensory RB and neighboring dorsal non-RB neurons were identified during an experiment on the basis of position and soma diameter (Fig. 1A). RBs had larger soma membrane area, as assessed by cell capacitance, than that of non-RB neurons (Fig. 1B). The difference in cell size between RBs and non-RBs permitted their reliable identification in situ.
FIG. 1.
Semiintact preparation of the Xenopus spinal cord allowed electrophysiological study of 2 different neuronal populations. A: using differential interference contrast optics, we identified neurons in semiintact spinal cord preparations of the St 35/36 Xenopus embryos. Rohon–Beard (RB) cells were identifiable on the basis of their characteristic position and large diameter (∼20 μm). In the photo, a patch pipette contacts an RB. A non-RB neuron is present to the left of the RB and out of focus (asterisk). B: at both 24 and 48 h, RBs had significantly larger cell surface membrane area than neighboring non-RBs (P ≤ 0.001) as assessed by cell membrane capacitance.
Knock-down of Kvβ2 protein reduced IKv density
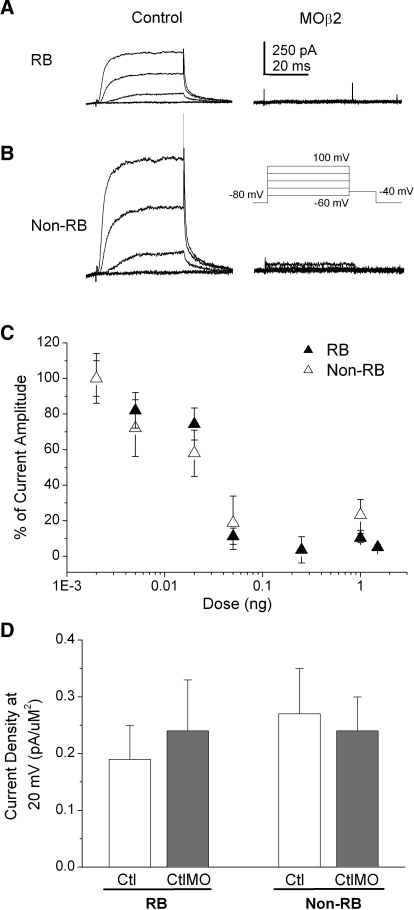
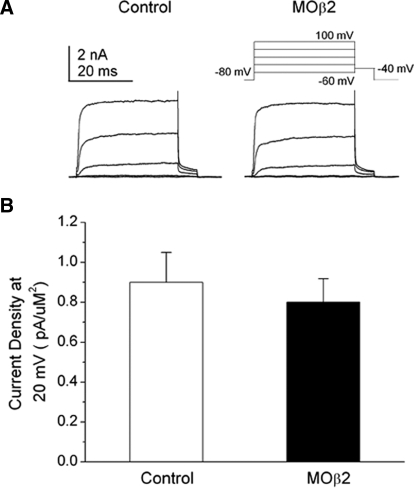
We tested the contribution of the Kvβ2 auxiliary subunit to IKv of RB and non-RB dorsal neurons by using both morpholino and standard antisense RNA strategies to knock down the Kvβ2 protein in the Xenopus embryo. In 24-h embryos injected unilaterally with 1 ng Kvβ2MO and GFP RNA, we recorded from internal control GFP− RB neurons and GFP+ neurons (see methods). GFP− RB neurons displayed substantial IKv (Fig. 2A). In contrast, in GFP+ RB neurons, we found effective suppression of IKv (Fig. 2A). Similar effects of Kvβ2MO on IKv were observed in non-RB neurons (Fig. 2B). We also determined the dose–response relationship for the Kvβ2 morpholino (Kvβ2MO) on IKv density (Fig. 2C). Morpholino injection led to a steep dose-dependent decrease in IKv amplitude in both RB and non-RB cells. For injected doses <0.02 ng, the Kvβ2MO blocked between 20 and 30% of the recorded total IKv amplitude. Using a dose ≥0.05 ng resulted in a surprising, almost complete knock-down of IKv. In contrast, injection of the CtlMO at a dose of 1 ng had no effect on IKv density in either RB or non-RB neurons (Fig. 2D).
FIG. 2.
Injection of Kvβ2MO reduced voltage-dependent potassium current (IKv) current density in RB and non-RB neurons in a dose-dependent manner. A: in 24 h Kvβ2MO-injected (1 ng MO) embryos, internal control GFP− RB neurons (left) displayed normal IKv amplitudes. In contrast, GFP+ RB neurons (right) displayed no or small IKv amplitudes. B: in Kvβ2MO-injected (1 ng MO) embryos, internal control GFP− non-RB neurons (left) displayed normal IKv amplitudes. In contrast, GFP+ non-RB neurons (right) displayed no or small IKv amplitudes. C: Kvβ2MO doses ranging between 0.001 and 1.5 ng were injected into Xenopus embryos and IKv was recorded from RB (black triangles) and non-RB (white triangles neurons) at 24 h. The percentage of remaining, unblocked current is shown as normalized current amplitude. n values ranged between 3 and 7. D: in contrast to the Kvβ2MO, the CtlMO (1.0 ng) had no effect on IKv density in either RB or non-RB cells. n values ranged between 9 and 16.
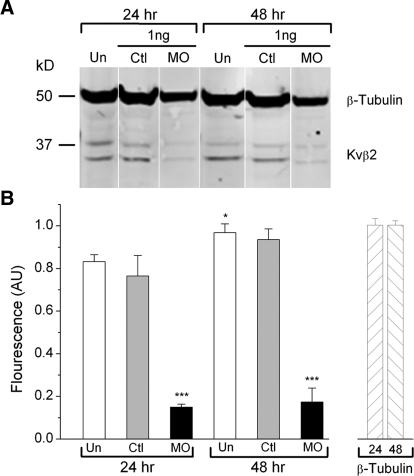
In heterologous systems, Kvβ2 subunits increase surface membrane expression and current density produced by Kv1 α-subunits; however, they are not required for formation of functional Kv1 channels (Coetzee et al. 1999; Gutman et al. 2003). Given this precedent, we were surprised by the complete knock-down of IKv produced by Kvβ2MO and concerned about nonspecific effects. To confirm that the Kvβ2MO acted as expected by knocking down Kvβ2 protein, we used Western blot analysis to determine Kvβ2 protein levels in uninjected, CtlMO- and Kvβ2MO-injected embryos (Fig. 3). We used a dose of MO that led to complete knock-down of IKv (1.0 ng; Fig. 2C). In uninjected embryos, the levels of Kvβ2 protein increased slightly, but significantly, between 24 and 48 h (Fig. 3, A and B; P < 0.01). Furthermore, embryos injected with CtlMO had Kvβ2 protein levels similar to those of uninjected embryos, suggesting that the injection itself or MOs in general did not produce nonspecific effects on IKv. In contrast, embryos injected with Kvβ2MO had significantly reduced levels of Kvβ2 protein at both 24 and 48 h (P ≤ 0.001). Kvβ2MO had no effect on levels of the internal control, β-tubulin (Fig. 3B). These results suggest that the Kvβ2MO led to a specific knock-down of Kvβ2 protein.
FIG. 3.
Kvβ2MO injection produced efficient and selective knock-down of Kvβ2 protein. A: embryos were either uninjected or injected with 1.0 ng of Kvβ2MO or CtlMO. Total protein was extracted from 24- and 48-h embryos. Approximately 20 μg of total protein was loaded in each lane. Kvβ2 protein was detected as a doublet at 35 at 37 kDa. β-Tubulin (50 kDa) was used as an internal control to test for nonspecific effects of Kvβ2MO on protein levels. B: the relative amounts of immunoreactivity in each fraction were determined on the basis of fluorescence intensity (see methods). Kvβ2 fluorescence levels in each lane were normalized to β-tubulin levels and the values are presented in terms of arbitrary units (AUs). At both 24 and 48 h, Kvβ2MO injection (black bars) led to efficient knock-down of Kvβ2 protein vs. either uninjected (white bars) or CtlMO-injected embryos (gray bars). Values represent the mean of 3 independent experiments ± SE (P < 0.0001, for uninjected or CtlMO-injected vs. Kvβ2MO-injected embryos).
If the results obtained with the Kvβ2MO were directly due to Kvβ2 knock-down rather than possible off-target effects, other methods that result in Kvβ2 knock-down would be expected to produce similar effects (Eisen and Smith 2008). As an alternative to MO-mediated knock-down of Kvβ2 protein, we used traditional RNA antisense (AS) methods to block translation of Kvβ2 subunits. We previously used this method to knock down Kvβ2 subunits and found that it effectively eliminated endogenous Kvβ2 mRNA in Xenopus embryos and Kv1 currents in embryonic myocytes (Lazaroff et al. 2002).
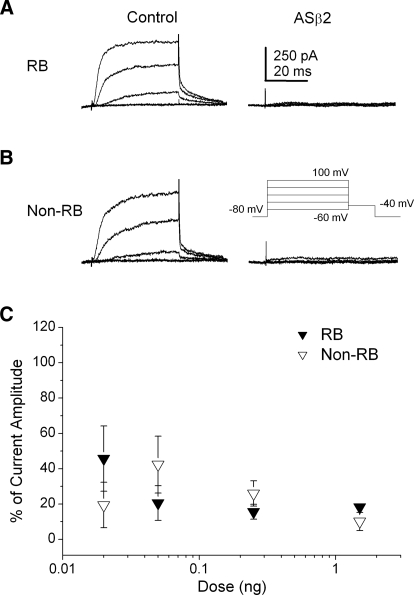
As found for the Kvβ2MO, antisense Kvβ2 (ASβ2) led to an efficient knock-down of IKv in a dose-dependent manner in both RB and non-RB neurons (Fig. 4). In 24-h embryos injected unilaterally with 1 ng ASβ2, internal control RB neurons displayed substantial IKv. In contrast, in GFP+ RB we found effective suppression of IKv (Fig. 4A). Similar effects of ASβ2 on IKv were observed in non-RB neurons (Fig. 4B). In comparison to the Kvβ2MO dose–response curve, the ASβ2 dose–response curve was less steep (Fig. 4C). However, at doses of ASβ2 ≥0.25 ng, IKv was effectively eliminated in both RB and non-RB neurons. Therefore two different strategies for blocking Kvβ2 protein translation produced similar and efficient reductions in IKv density.
FIG. 4.
Injection of ASβ2 reduced IKv density in RB and non-RB neurons in a dose-dependent manner. A: in 24 h ASβ2-injected (1 ng AS) embryos, internal control GFP− RB neurons (left) displayed normal IKv amplitudes. In contrast, GFP+ RB neurons (right) displayed no or small IKv amplitudes. B: in Kvβ2MO-injected (1 ng AS) embryos, internal control GFP− non-RB neurons (left) displayed normal IKv amplitudes. In contrast, GFP+ non-RB neurons (right) displayed no or small IKv amplitudes. C: ASβ2 doses ranging between 0.02 and 1.5 ng were injected into Xenopus embryos and IKv was recorded from RB (black triangles) and non-RB (white triangles) neurons at 24 h. The percentage of remaining, unblocked current is shown as normalized current amplitude. n values ranged between 9 and 19.
Because Kvβ2MO and ASβ2 produced a surprisingly efficient reduction of IKv amplitude, we used several different tests to assay the specificities of the knock-down methods. As mentioned, both Kvβ2MO and ASβ2 had effects on IKv that were dose dependent, as expected for a specific action of an antisense agent (Figs. 2 and 4). Moreover, on injection of subsaturating doses of either Kvβ2MO or ASβ2, IKv decreased in amplitude (Figs. 2 and 4) but did not show changes in kinetic or voltage-dependent properties (Table 1). The latter result is consistent with normal function of the Kvβ2 subunit in the remaining Kv channels that contribute to the residual IKv, rather than nonspecific actions of either Kvβ2MO or ASβ2 on membrane currents.
TABLE 1.
Partial knock-down of Kvβ2 has no effect on kinetic or voltage-dependent IKv properties
| IKv Property | t1/2, ms | V1/2, mV | k |
|---|---|---|---|
| RB | |||
| Control (n = 6) | 2.5 ± 0.2 | 20.7 ± 1.1 | 12.3 ± 1.9 |
| ASβ2, 0.2 ng (n = 3) | 2.9 ± 1.3 | 22.7 ± 2.8 | 12.3 ± 1.0 |
| MOβ2, 0.02 ng (n = 4) | ND | 23.8 ± 1.1 | 11.4 ± 1.2 |
| Non-RB | |||
| Control (n = 4) | |||
| ASβ2, 0.2 ng (n = 3) | ND | 18.6 ± 2.4 | 13.7 ± 1.0 |
| MOβ2, 0.02 ng (n = 3) | ND | 20.5 ± 2.0 | 14.0 ± 2.0 |
Values are means ± SE; n values are in parentheses. t1/2 was evaluated at +20 mV. ND, not determined (small current amplitudes prevented reliable measurements).
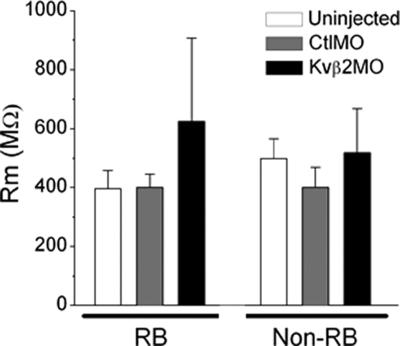
In addition, the CtlMO did not affect IKv, indicating that MO injection per se did not produce nonspecific effects on IKv (Fig. 2D). Further, as assessed by Western blot analysis, the Kv1.1 protein showed no apparent reduction in mobility or amount in extracts prepared from 48-h-old embryos injected with 1 ng of either MOβ2 or ASβ2 (Fig. 5), demonstrating that the antisense agents did not target other Kv proteins. We also tested whether the Kvβ2MO or the ASβ2 had nonspecific effects on general membrane properties. Uninjected, CtlMO-, Kvβ2MO-, and ASβ2-treated neurons had similar membrane input resistances (Fig. 6).
FIG. 5.
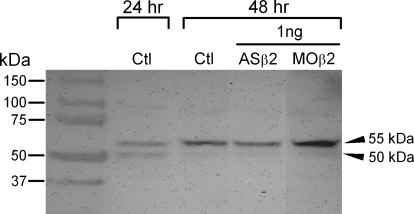
Injection of Kvβ2MO or ASβ2 did not affect Kv1.1 protein. Total protein extracts were obtained from 24- and 48-h uninjected (Ctl) and 48-h embryos injected with 1.0 ng of either ASβ2 or Kvβ2MO. Protein (20 μg) was loaded in each lane. In Ctl 24-h embryos, Kv1.1 protein appeared as a prominent doublet at about 50 and 55 kDa. Arrows indicate the approximately 50-kDa immature and the 55-kDa mature forms of the Kv1 protein. In contrast, at 48 h, control embryos contained much less of the 50-kDa form and comparatively more of the 55-kDa form. Protein extracted from ASβ2 or Kvβ2MO injected embryos was similar to 48-h controls and showed a prominent Kv1.1 band at 55 kDa.
FIG. 6.
Kvβ2MO and ASβ2 did not affect other membrane properties. Neither Kvβ2MO nor ASβ2 injections had effects on RB or non-RB input resistance (MΩ) compared with control neurons. n values ranged between 9 and 19.
An additional test of Kvβ2MO specificity entailed recording IKv from ventral spinal neurons, a population that does not express the Kvβ2 gene (Lazaroff et al. 1999). As expected, if the morpholino targeted Kvβ2 mRNA specifically, Kvβ2MO injection had no effect on IKv density of ventral neurons (Fig. 7).
FIG. 7.
Kvβ2 knock-down had no effect on IKv recorded from ventral neurons, a population that does not express the Kvβ2 gene. A: IKv did not differ between ventral neurons of Kvβ2MO (right) vs. CtlMO (left) injected embryos. The voltage command protocol used is shown at the top of the left panel. For illustrative proposes, only current traces elicited by depolarizations to −60, −30, 0, +30, and +60 mV are shown. B: mean ventral neuron IKv density did not differ between Kvβ2MO- and CtlMO-injected embryos. Kvβ2MO and CtlMO data are represented by white (n = 5) and black (n = 4) triangles, respectively.
Overall, the results indicated that both Kvβ2MO and ASβ2 specifically targeted Kvβ2. Moreover, the MO and antisense studies suggest that, in contrast to results obtained from heterologous systems, Kvβ2 subunits are required in vivo for formation of functional Kv channels in both RB and non-RB dorsal cells.
Dominant negative suppression of Kv1α function also abolished IKv in RB and non-RB dorsal cells
The preceding data suggest that Kvβ2 subunits are required for the formation of functional Kv1 potassium channel complexes in dorsal spinal neurons. In heterologous systems, Kvβ2 subunits associate with Kv1, Kv4, and Kv12 α-subunits. Whereas expression of Kv4 or Kv12 α-subunits in Xenopus embryos has not been reported, evidence for Kv1 α-subunit expression in the spinal cord exists. Kv1.2 α-subunit mRNA is expressed in the dorsal ectoderm at the time of neural induction (Ribera 1990). Kv1.1 α-subunit transcripts localize to dorsal regions of the Xenopus spinal cord, similarly to Kvβ2 mRNA (Lazaroff et al. 1999; Ribera and Nguyen 1993). On this basis, Kv1 α-subunits are good candidates for targets of Kvβ2 association and modulation.
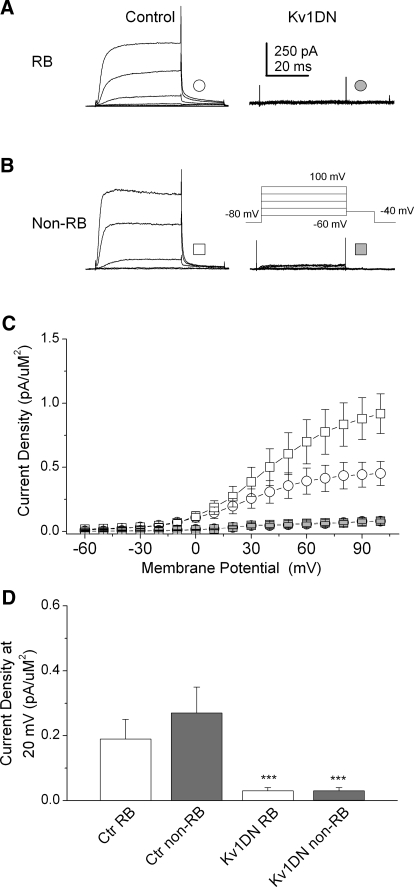
Overexpression of a dominant negative Kv1 α-subunit (Kv1DN) has served previously as an efficient method for blocking the contribution of the Kv1α subunit containing channels to IKv (Lazaroff et al. 2002; Ribera 1996). We used this approach to block functional Kv1 channels in situ. We found that dominant negative suppression of Kv1 α-subunit-containing channels produced a nearly total reduction in IKv amplitude in both RB and non-RB neurons (Fig. 8), suggesting that Kv1 channels underlie the IKv of dorsal spinal neurons. Moreover, dominant negative suppression of Kv1 channels reduced IKv amplitudes as effectively as did Kvβ2MO or ASβ2, supporting the view that both β- and α-subunits are obligate members of functional Kv1 channel complexes.
FIG. 8.
Kv1DN reduced IKv density in RB and non-RB neurons to the same extent as did Kvβ2MO or ASβ2. A and B: in 24-h Kv1DN injected (1 ng) embryos, internal control GFP− RB neurons displayed normal IKv amplitudes, whereas GFP+ RB neurons displayed small IKv amplitudes. Similar effects were found in non-RB neurons (B). C: at all voltages, IKv amplitudes in RB and non-RB were reduced by injection of the Kv1DN (symbols are indicated in A and B). D: Kv1DN and ASβ2 efficiently reduced IKv density (*P < 0.001 vs. Ctl), to the same extent as did MO or AS knock-down of Kvβ2 (cf. Figs. 2 and 4).
RB IKv showed developmental changes in density but not the apparent rate of activation
On the basis of previous studies of spinal neurons developing in culture, the IKv values of RB and non-RB neurons should undergo dramatic changes in density and activation kinetics between 24 and 48 h (Barish 1986; O'Dowd et al. 1988). To test this prediction, we determined how IKv changed in vivo between 24 and 48 h in RB and non-RB neurons.
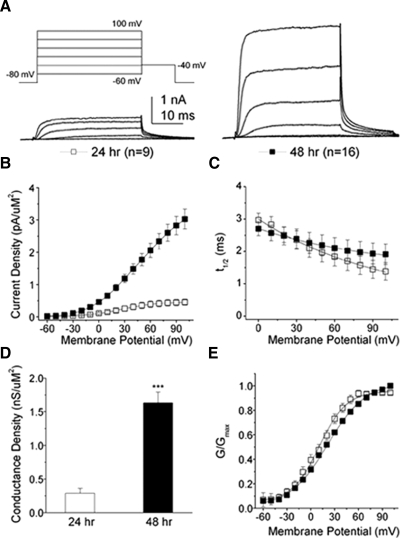
RB IKv density increased about fivefold between 24 and 48 h (Fig. 9, A and B; 0.20 ± 0.06 to 0.90 ± 0.10 pA/μm2, respectively, at +20 mV; P < 0.0001). In contrast, during the same period, the rate of activation, evaluated as the time to half-maximum current (t1/2), did not change (Fig. 9C; 2.50 ± 0.23 vs. 2.48 ± 0.28 ms for 24 and 48 h, respectively, at +20 mV). Compared with the average developmental changes noted for IKv of spinal neurons in culture (e.g., O'Dowd et al. 1988), RB IKv in vivo showed larger increases in current density but no decrease in t1/2.
FIG. 9.
Between 24 and 48 h, RB IKv increased 5-fold in density without changes in activation rate. A: IKv from Xenopus RB neurons increased in amplitude between 24 and 48 h. The voltage command protocol used is shown at the top of the left panel. For illustrative proposes, only current traces elicited by depolarizations to −60, −30, 0, +30, and +60 mV are shown. B: mean RB IKv density increased about 5-fold between 24 and 48 h (P < 0.0001, for membrane potentials between 0 and +100 mV). For B, C, and E, 24- and 48-h data are represented by white (n = 9) and black (n = 16) squares, respectively. C: the rate of activation of IKv was assessed by measuring the time to half-maximum current (t1/2). No statistically significant differences between 24- and 48-h data were found. D: between 24 (white bar) and 48 (black bar) h, RB potassium maximal conductance (Gmax) increased about 6-fold, from 0.28 ± 0.07 to 1.63 ± 0.16 pS/μm2. Values represent the means ± SE (P < 0.0001, 24 vs. 48 h data). E: the steady-state voltage-dependent properties of activation for RB potassium conductance did change between 24 and 48 h. Curves were fit to a single Boltzmann equation and V1/2 and k values were calculated (Table 2).
Consistent with the changes in current density, conductance density increased about sixfold between 24 and 48 h (Fig. 9D; 0.30 ± 0.07 to 1.60 ± 0.20 pS/μm2, respectively, P < 0.0001). To examine voltage-dependent properties of activation, we determined the maximal conductance (Gmax) and then plotted normalized conductance as a function of voltage. The Boltzmann equation was fitted to the normalized conductance plots (Fig. 9E) to calculate the voltage of half-activation (V1/2) and the slope constant (k) for each developmental stage. Although no differences were found for V1/2 (Table 2), a small but significant decrease in voltage sensitivity, as reflected by an increase in the slope factor k, was found (Table 2; P < 0.0001). The small increase in k has no obvious physiological significance and mostly likely reflects distortions in the normalized curve due to nonsaturation of the conductance. Overall, these results reveal developmental changes in the maximal conductance of RB IKv.
TABLE 2.
Steady-state–dependent properties of activation
|
24 h |
48 h
|
|||
|---|---|---|---|---|
| V1/2, mV | k | V1/2, mV | k | |
| RBs | 20.7 ± 1.2 (9) | 12.3 ± 1.9 (9) | 20.3 ± 2.0 (16) | 25.3 ± 1.8 (16)* |
| Non-RBs | 21.0 ± 2.2 (6) | 11.8 ± 0.4 (6) | 2.1 ± 0.9 (7)** | 23.6 ± 2.5 (7)*** |
P < 0.0001 vs. 24-h RB k;
P < 0.001 vs. 24-h non-RB V1/2;
P < 0.001 vs. 24-h non-RB k.
Non-RB IKv showed different developmental changes
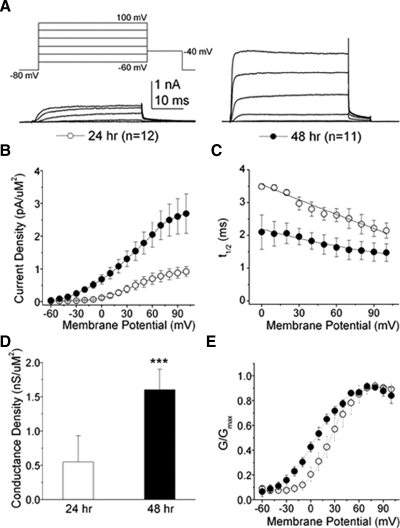
Between 24 and 48 h, non-RB IKv density increased about fourfold from 0.30 ± 0.10 to 1.10 ± 0.20 pA/μm2 (Fig. 10, A and B; +20 mV; P < 0.0001). However, no statistically significant differences were found between RB and non-RB IKv densities when compared at the same developmental stage (cf. Figs. 9, A and B and 10, A and B).
FIG. 10.
Between 24 and 48 h, non-RB IKv displayed increases in density and decreases in t1/2. A: non-RB IKv amplitudes increased between 24 (white circles; n = 12) and 48 h (black circles; n = 11). For illustrative proposes, only current traces elicited by depolarizations to −60, −30, 0, +30, and +60 mV are shown. B: the current density–voltage (I–V) relationships for IKv recorded from non-RBs showed that IKv density increased about 4-fold between 24 and 48 h. C: in contrast to RB IKv, the t1/2 for non-RB IKv showed a developmental decrease, reflecting the increased rate of apparent activation (P < 0.001 for voltages between 0 and 80 mV). D: between 24 (white bar) and 48 (black bar) h, non-RB potassium maximal conductance (Gmax) increased about 3-fold, from 0.55 ± 0.11 to 1.60 ± 0.30 pS/μm2. Values represent the means ± SE (P < 0.005, 24 vs. 48 h data). E: the steady-state voltage-dependent properties of activation for non-RB potassium conductance changed between 24 and 48 h. Curves were fit to a single Boltzmann equation and V1/2 and k values were calculated (Table 2).
In contrast to RB cells, the t1/2 of non-RB IKv decreased significantly between 24 and 48 h (Fig. 10C; at +20 mV: 3.30 ± 0.07 to 2.00 ± 0.40 ms, respectively; P < 0.03), reflecting the more rapid apparent activation of non-RB IKv at the later developmental stage. The developmental decrease in t1/2 of non-RB IKv is consistent with the average changes in t1/2 reported for neurons in culture or dorsolateral neurons in situ (Desarmenien et al. 1993; O'Dowd et al. 1988).
Consistent with the changes in current density, non-RB conductance density increased significantly about threefold between 24 and 48 h (Fig. 10D; 0.55 ± 0.10 to 1.60 ± 0.30 pS/μm2, respectively, P < 0.03). Contrary to RB IKv, however, non-RB IKv showed a negative shift in the V1/2 for steady-state activation between 24 and 48 h (Fig. 10E; Table 2), an effect that would lead to increased current amplitudes at more depolarized potentials. In addition, voltage sensitivity, as assessed by the slope factor k, decreased between 24 and 48 h (Table 2; P < 0.001). These results indicate that potassium conductance increased and altered its voltage-dependent properties of activation between 24 and 48 h.
As predicted on the basis of study of spinal neurons in culture (Barish 1986; O'Dowd et al. 1988), the IKv values of RBs and non-RBs showed substantial developmental regulation between 24 and 48 h of embryonic development (Figs. 9 and 10). However, the changes in each neuron type differed. The IKv of RB neurons increased in fivefold in density without any apparent changes in activation kinetics. In non-RB neurons, IKv also increased in density, about fourfold. In contrast to RBs, IKv of non-RB neurons also showed an apparent increase in activation kinetics, as assessed by a decrease in t1/2.
Kv1 α-subunits underlie developmental changes in IKv of both RB and non-RB neurons
Knock-down of either Kvβ2 or dominant negative suppression of Kv1 channels effectively eliminated the IKv values of RB and non-RB neurons at 24 h (Figs. 2, 4, and 8). Previous results suggest that developmental changes in IKv in some spinal neurons developing in culture rely on Kv1 channels (Gurantz et al. 1996; Ribera 1996). Accordingly, we next determined whether Kv1 channels account not only for the initial IKv recorded at 24 h from RB and non-RB neurons, but also for the different developmental changes in IKv in each neuron type.
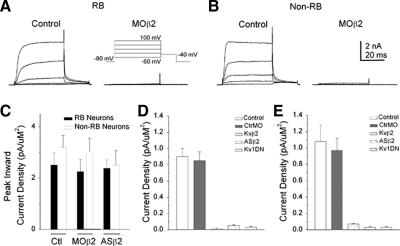
Similar to results obtained at 24 h (Fig. 2), Kvβ2MO injection led to efficient elimination of IKv at 48 h (Fig. 11A). Kvβ2MO also led to efficient knock-down of IKv in non-RB neurons at 48 h (Fig. 11B). However, inward current densities did not differ between uninjected, CtlMO, or Kvβ2MO neurons at 48 h (Fig. 11C). Moreover, injection of ASβ2 produced results for RB (Fig. 11D) and non-RB (Fig. 11E) that were similar to those obtained by injection of Kvβ2MO. These results indicate that at both 24 and 48 h, knock-down of Kvβ2 subunits eliminated IKv.
FIG. 11.
Kv1 channels underlay IKv of both RB and non-RB neurons of 48-h Xenopus embryos. A: in 48-h Kvβ2MO-injected (1 ng MO) embryos, internal control GFP− RB neurons displayed normal IKv amplitudes. In contrast, GFP+ RB neurons displayed no or small IKv amplitudes. B: in 48-h Kvβ2MO-injected (1 ng MO) embryos, internal control GFP− non-RB neurons displayed normal IKv amplitudes. In contrast, GFP+ non-RB neurons displayed no or small IKv amplitudes. C: CtlMO, Kvβ2MO, and ASβ2 injected Xenopus embryos did not show effects over peak inward current density (pA/pF). n values ranged between 6 and 19. D: Kvβ2MO, ASβ2, and Kv1DN all led to effective suppression of IKv in RB neurons from 48-h embryos. n values ranged between 6 and 19. E: Kvβ2MO, ASβ2, and Kv1DN all led to effective suppression of IKv in non-RB neurons from 48-h embryos. n values ranged between 6 and 19.
We next tested whether Kv1 channels determined IKv in dorsal spinal neurons at 48 as well as at 24 h. Kv1 dominant negative suppression of Kv1 channels reduced IKv amplitudes of RB and non-RB neurons at 48 h as efficiently as at 24 h (Fig. 11 D, and E). These findings support the view that Kv1 channels underlie IKv of dorsal spinal neurons at both developmental stages. Taken together, the results indicate that, at both 24 and 48 h, Kvβ2MO effects were due to elimination of Kv1 channel function.
DISCUSSION
Typically, the effects of Kvβ subunits on Kv1 channel function entail modulation of the kinetics and amplitude of current carried by the pore-forming α-subunit. Our results point to a yet more fundamental role in vivo, whereby Kv1 channel function has an essential requirement for the Kvβ2 subunit in dorsal spinal neurons. Knock-down of Kvβ2 protein, by either MO or RNA antisense methods, produced a nearly total reduction in IKv amplitude in dorsal spinal cord neurons. Further, partial knock-down of Kvβ2 protein reduced only the amplitude but not the properties (e.g., t1/2, V1/2) of IKv, consistent with normal function of Kvβ2 subunits in channels underlying the residual current (Fig. 3, Table 1). Moreover, Kvβ2 knock-down produced effects similar to those of dominant negative suppression of Kv1 channel complexes, as expected if Kvβ2 subunits function as obligatory components of Kv1 channels.
In heterologous systems, Kvβ2 subunits modulate properties of potassium currents (Accili et al. 1997; England et al. 1995a,b; Heinemann et al. 1994, 1995, 1996; Majumder et al. 1995; Morales et al. 1996; Rettig et al. 1994; Shi et al. 1996) that are developmentally regulated in embryonic spinal neurons, e.g., current density and activation kinetics (Barish 1986; Desarmenien et al. 1993; O'Dowd et al. 1988). However, we found that at both 24 and 48 h, Kvβ2 knock-down led to efficient elimination of IKv. These findings indicate that rather than play a role in developmental regulation of Kv1 channels, Kvβ2 subunits are always required for Kv1 channel function in two different dorsal spinal cord neurons.
The essential in vivo requirement for Kvβ2 subunits differs from conclusions obtained from study of heterologously expressed subunits. When expressed in Xenopus oocytes or cell lines (e.g., HEK293), Kvβ2 subunits can increase the surface and functional density of Kv1 channels. However, in heterologous systems, Kv1 channel formation occurs in the absence of the Kvβ2 subunit. It is possible that when Kv1 α-subunits are expressed at physiological levels, the role of the Kvβ2 subunit becomes essential. In contrast, under the aphysiological and high expression levels associated with heterologous systems, the essential requirement may be masked. Further, we focused on two dorsal neuron types that are known to express Kvβ2 subunits but not other Kvβ subunits. It is likely that, for neurons that express multiple Kvβ subunits, another Kvβ may replace Kvβ2 when the latter is eliminated. Thus the essential role of Kvβ2 subunits may be evident only when it is absent in cells that express no other Kvβ subunit.
Heterologous system results would predict a reduction in Kv1 α-subunit protein levels, maturation, and/or surface expression in the absence of Kvβ2. However, McCormack et al. (2002) found that the Kv1.2 α-subunit underwent normal glycosylation to its mature form in a mouse Kvβ2 knock-out. In addition, Kv1.2 α-subunit protein levels, as assessed by Western blot, were unchanged by knock-out of Kvβ2. Similarly, our results did not show any apparent changes in Kv1.1 protein expression as determined by Western blot analysis in ASβ2- or Kvβ2MO-injected embryos (Fig. 5). In the murine model, immunofluorescent examination of Kv1.1 and Kv1.2 α-subunits in brain sections also showed no differences between wild-type and knock-out with respect to either localization or amount (McCormack et al. 2002).
The knock-out mice were also examined for behavioral deficits but not for defects that would be evident at the level of cellular electrophysiology. Our results suggest that the increased seizure incidence and shorter life spans of Kvβ2 knock-out mice might be a direct consequence of severe loss of Kv1 channel function. Consistent with this view, Kv1.1 α-subunit knock-out mice also have seizures (Smart et al. 1998; Wenzel et al. 2007). Similarly, Kv1.2 α-subunt knock-out mice have seizures and reduced life spans (Brew et al. 2007).
Our results are consistent with those obtained by study of knock-out mice. We found that Kvβ2 subunits were not required for maturation of Kv1.1 α-subunit-containing channels in the endoplasmic reticulum and Golgi because no increase in the immature form was detected after knock-down of Kvβ2 (Fig. 5). On the basis of our data, we conclude that Kvβ2 subunits affect trafficking of Kv1 channels to the surface membrane or the function of Kv1 channels in the surface membrane.
The finding that either Kvβ2 knock-down or dominant negative suppression of Kv1 channel function eliminated IKv leads to two additional conclusions. Our results provide evidence for Kvβ2 participation in Kv1 but not other Kv channels (e.g., Kv4, Kv12; Wilson et al. 1998; Yang et al. 2001). Previously, we found that Kvβ2 knock-down and Kv1 dominant negative suppression reduced IKv to the same extent in embryonic myocytes (Lazaroff et al. 2002). Although the identity of the current that persisted on Kvβ2 knock-down and Kv1 dominant negative suppression was not determined, Kv2 channels are likely candidates because myocytes express the gene (Burger and Ribera 1996). Because neither previous studies nor the present study examined cell types that are known to express either Kv4 or Kv12 α-subunits, our results do not exclude the possibility that Kvβ2 subunits might affect function of Kv4 or Kv12 channels in vivo.
Second, recent work has indicated that the IKv properties of dorsal (type I) and ventral (type II) spinal cord neurons differ with respect to biophysical and pharmacological properties (Pineda and Ribera 2008). We interpret these data as evidence for Kv1 channel complexes serving as the molecular determinants of IKv in dorsal type I but not ventral type II spinal neurons. Interestingly, even though Kv1 channels underlie the IKvs of two different dorsal spinal neuron types, our results demonstrated functional differences between the IKv values of RB and non-RB neurons. The developmental increases in potassium conductance were similar in RBs and non-RBs (five- and fourfold, respectively; Figs. 9 and 10). However, the developmental changes in activation kinetics differed (Figs. 9 and 10). Between 24 and 48 h, the t1/2 did not change for RB IKv but did for that of non-RBs. From study of spinal neurons in culture, a decrease, rather than no change, in t1/2 was expected (O'Dowd et al. 1988).
The simplest interpretation of these data is that 1) RBs and non-RBs express different Kv1 α-subunit isotypes and 2) Kvβ2 subunits are obligate members of a diverse range of Kv1 channel complexes at the developmental stages relevant to our studies. An alternative, more complex, interpretation is that RBs and non-RBs express the same Kv1 α-subunit isotype but then apply different posttranslational mechanisms to sculpt functional properties of the native current during development. The latter possibility is especially intriguing given the findings of Desarmenien and Spitzer (1991). These authors found that the developmental change in activation kinetics of IKv was partially accounted for by a calcium/protein kinase C–dependent mechanism. Desarmenien and Spitzer (1991) studied spinal neurons in a culture of unknown identity. Our data suggest that the partial dependence on calcium and protein kinase C was a consequence of the necessary population analysis of the heterogeneous spinal cord neuron types that exist in culture, rather than study of specific cell types that is possible in the in situ preparations. For example, we found that the IKv of non-RBs, but not RBs, showed a developmental change in activation kinetics. In addition, it is possible that the calcium/protein kinase C mechanism could target some potassium channel subunit isotypes but not others. The in situ preparation that we have developed will allow future testing of these possibilities.
Overall, the results indicate that the “auxiliary” Kvβ2 subunit plays an essential role for Kv1 channel function in dorsal spinal neurons. In addition, Kv1 channel function shows the same absolute requirement for Kvβ2 subunits at both 24 and 48 h. Thus Kvβ2 subunits do not mediate the changes in IKv that occur during this period. Rather, developmental changes in Kv1 α-subunit isoform expression or posttranslational modification underlie maturation of IKv in dorsal spinal neurons.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-25217.
Acknowledgments
We thank members of the Ribera laboratory for discussion.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Accili et al. 1997.Accili EA, Kiehn J, Yang Q, Wang Z, Brown AM, Wible BA. Separable Kvβ subunit domains alter expression and gating of potassium channels. J Biol Chem 272: 25824–25831, 1997. [DOI] [PubMed] [Google Scholar]
- Baccaglini and Spitzer 1977.Baccaglini PI, Spitzer NC. Developmental changes in the inward current of the action potential of Rohon–Beard neurones. J Physiol 271: 93–117, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish 1986.Barish ME Differentiation of voltage-gated potassium current and modulation of excitability in cultured amphibian spinal neurones. J Physiol 375: 229–250, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekele-Arcuri et al. 1996.Bekele-Arcuri Z, Matos MF, Manganas L, Strassle BW, Monaghan MM, Rhodes KJ, Trimmer JS. Generation and characterization of subtype-specific monoclonal antibodies to K+ channel α- and β-subunit polypeptides. Neuropharmacology 35: 851–865, 1996. [DOI] [PubMed] [Google Scholar]
- Blaine and Ribera 2001.Blaine JT, Ribera AB. Kv2 channels form delayed-rectifier potassium channels in situ. J Neurosci 21: 1473–1480, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew et al. 2007.Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 98: 1501–1525, 2007. [DOI] [PubMed] [Google Scholar]
- Burger and Ribera 1996.Burger C, Ribera AB. Xenopus spinal neurons express Kv2 potassium channel transcripts during embryonic development. J Neurosci 16: 1412–1421, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campomanes et al. 2002.Campomanes CR, Carroll KI, Manganas LN, Hershberger ME, Gong B, Antonucci DE, Rhodes KJ, Trimmer JS. Kv beta subunit oxidoreductase activity and Kv1 potassium channel trafficking. J Biol Chem 277: 8298–8305, 2002. [DOI] [PubMed] [Google Scholar]
- Chen et al. 1996.Chen ML, Hoshi T, Wu CF. Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes. Neuron 17: 535–542, 1996. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2000.Chen ML, Hoshi T, Wu CF. Sh and eag K+ channel subunit interaction in frog oocytes depends on level and time of expression. Biophys J 79: 1358–1368, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard et al. 1995.Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci USA 92: 6763–6767, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee et al. 1999.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999. [DOI] [PubMed] [Google Scholar]
- Desarmenien et al. 1993.Desarmenien MG, Clendening B, Spitzer NC. In vivo development of voltage-dependent ionic currents in embryonic Xenopus spinal neurons. J Neurosci 13: 2575–2581, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desarmenien and Spitzer 1991.Desarmenien MG, Spitzer NC. Role of calcium and protein kinase C in development of the delayed rectifier potassium current in Xenopus spinal neurons. Neuron 7: 797–805, 1991. [DOI] [PubMed] [Google Scholar]
- Eisen and Smith 2008.Eisen JS, Smith JC. Controlling morpholino experiments: don't stop making antisense. Development 135: 1735–1743, 2008. [DOI] [PubMed] [Google Scholar]
- England et al. 1995a.England SK, Uebele VN, Kodali J, Bennet PB, Tamkun MM. A novel K+ channel beta subunit (hKv beta 1.3) is produced via alternative mRNA splicing. J Biol Chem 270: 28531–28534, 1995a. [DOI] [PubMed] [Google Scholar]
- England et al. 1995b.England SK, Uebele VN, Shear H, Kodali J, Bennet PB, Tamkun MM. Characterization of a voltage gated K+ channel beta subunit expressed in human heart. Proc Natl Acad Sci USA 92: 6309–6313, 1995b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurantz et al. 1996.Gurantz D, Ribera AB, Spitzer NC. Temporal regulation of Shaker and Shab-like potassium potassium channel gene expression in single embryonic spinal neurons during K+ current development. J Neurosci 16: 3287–3295, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman et al. 2003.Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O'Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev 55: 583–586, 2003. [DOI] [PubMed] [Google Scholar]
- Hamill et al. 1981.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch 391: 85–100, 1981. [DOI] [PubMed] [Google Scholar]
- Heinemann et al. 1994.Heinemann S, Rettig J, Scott V, Parcej DN, Lorra C, Dolly J, Pongs O. The inactivation behaviour of voltage-gated K-channels may be determined by association of alpha- and beta-subunits. J Physiol (Paris) 88: 173–180, 1994. [DOI] [PubMed] [Google Scholar]
- Heinemann et al. 1996.Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel β-subunits from rat brain. J Physiol 493: 625–633, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann et al. 1995.Heinemann SH, Rettig J, Wunder F, Pongs O. Molecular and functional characterization of a rat brain Kvβ3 potassium channel subunit. FEBS Lett 377: 383–389, 1995. [DOI] [PubMed] [Google Scholar]
- Hille 2001.Hille B Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001.
- Ikeda and Kaplan 1970.Ikeda K, Kaplan WD. Patterned neural activity of a mutant Drosophila melanogaster. Proc Natl Acad Sci USA 66: 765–772, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones and Ribera 1994.Jones SM, Ribera AB. Overexpression of a potassium channel gene perturbs neural differentiation. J Neurosci 14: 2789–2799, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan and Trout 1974.Kaplan WD, Trout WE. Genetic manipulation of an abnormal jump response in Drosophila. Genetics 77: 721–739, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroff et al. 1999.Lazaroff MA, Hofmann AD, Ribera AB. Xenopus embryonic spinal neurons express potassium channel Kvβ subunits. J Neurosci 19: 10706–10715, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaroff et al. 2002.Lazaroff MA, Taylor AD, Ribera AB. In vivo analysis of Kvβ2 function in Xenopus embryonic myocytes. J Physiol 541: 673–683, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. 2006.Li Y, Um SY, McDonald TV. Voltage-gated potassium channels: regulation by accessory subunits. Neuroscientist 12: 199–210, 2006. [DOI] [PubMed] [Google Scholar]
- Lockery and Spitzer 1992.Lockery SR, Spitzer NC. Reconstruction of action potential development from whole-cell currents of differentiating spinal neurons. J Neurosci 12: 2268–2287, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder et al. 1995.Majumder K, De Biasi M, Wang Z, Wible BA. Molecular cloning and functional expression of a novel potassium channel beta-subunit from human atrium. FEBS Lett 361: 13–16, 1995. [DOI] [PubMed] [Google Scholar]
- Manganas and Trimmer 2000.Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem 275: 29685–29693, 2000. [DOI] [PubMed] [Google Scholar]
- Marty and Neher 1983.Marty A, Neher E. Tight-seal whole-cell recording. In: Single Channel Recording, edited by Sakmann B, Neher E. New York: Plenum Press, 1983, p. 107–122.
- McCormack et al. 2002.McCormack K, Connor JX, Zhou L, Ho LL, Ganetzky B, Chiu SY, Messing A. Genetic analysis of the mammalian K+ channel beta subunit Kvβ2 (Kcnab2). J Biol Chem 277: 13219–13228, 2002. [DOI] [PubMed] [Google Scholar]
- Morales et al. 1996.Morales MJ, Wee JO, Wang S, Strauss HC, Rasmusson RL. The N-terminal domain of a K+ channel beta subunit increases the rate of C-type inactivation from the cytoplasmic side of the channel. Proc Natl Acad Sci USA 93: 15119–15123, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya and Papazian 1997.Nagaya N, Papazian DM. Potassium channel alpha and beta subunits assemble in the endoplasmic reticulum. J Biol Chem 272: 3022–3027, 1997. [DOI] [PubMed] [Google Scholar]
- Nakahira et al. 1996.Nakahira K, Shi G, Rhodes KJ, Trimmer JS. Selective interaction of voltage-gated K+ channel beta-subunits with alpha-subunits. J Biol Chem 271: 7084–7089, 1996. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop and Faber 1967.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Amsterdam: North-Holland, 1967.
- O'Dowd et al. 1988.O'Dowd DK, Ribera AB, Spitzer NC. Development of voltage-dependent calcium, sodium, and potassium currents in Xenopus spinal neurons. J Neurosci 8: 792–805, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcej et al. 1992.Parcej DN, Scott VE, Dolly JO. Oligomeric properties of alpha-dendrotoxin-sensitive potassium ion channels purified from bovine brain. Biochemistry 31: 11084–11088, 1992. [DOI] [PubMed] [Google Scholar]
- Pineda et al. 2005.Pineda RH, Heiser RA, Ribera AB. Molecular determinants of INa in vivo in embryonic zebrafish sensory neurons. J Neurophysiol 93: 3582–3593, 2005. [DOI] [PubMed] [Google Scholar]
- Pineda and Ribera 2008.Pineda RH, Ribera AB. Dorsal-ventral gradient for neuronal plasticity in the embryonic spinal cord. J Neurosci 28: 3824–3834, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs et al. 1999.Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese KP, Silva AJ, Storm JF. Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann NY Acad Sci 868: 344–355, 1999. [DOI] [PubMed] [Google Scholar]
- Rettig et al. 1994.Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature 369: 289–294, 1994. [DOI] [PubMed] [Google Scholar]
- Rhodes et al. 1995.Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci 15: 5360–5371, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes et al. 1996.Rhodes KJ, Monaghan MM, Barrezueta NX, Nawoschik S, Bekele-Arcuri Z, Matos MF, Nakahira K, Schechter LE, Trimmer JS. Voltage-gated K+ channel beta subunits: expression and distribution of Kvβ1 and Kvβ2 in adult rat brain. J Neurosci 16: 4846–4860, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes et al. 1997.Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci 17: 8246–8258, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera 1990.Ribera AB A potassium channel gene is expressed at neural induction. Neuron 5: 691–701, 1990. [DOI] [PubMed] [Google Scholar]
- Ribera 1996.Ribera AB Homogeneous development of electrical excitability via heterogeneous ion channel expression. J Neurosci 16: 1123–1130, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera and Nguyen 1993.Ribera AB, Nguyen DA. Primary sensory neurons express a Shaker-like potassium channel gene. J Neurosci 13: 4988–4996, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera and Nusslein-Volhard 1998.Ribera AB, Nusslein-Volhard C. Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J Neurosci 18: 9181–9191, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott et al. 1994.Scott VE, Muniz ZM, Sewing S, Lichtinghagen R, Parcej DN, Pongs O, Dolly JO. Antibodies specific for distinct Kv subunits unveil a heterooligomeric basis for subtypes of alpha-dendrotoxin-sensitive K+ channels in bovine brain. Biochemistry 33: 1617–1623, 1994. [DOI] [PubMed] [Google Scholar]
- Shamotienko et al. 1997.Shamotienko OG, Parcej DN, Dolly JO. Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry 36: 8195–8201, 1997. [DOI] [PubMed] [Google Scholar]
- Shi et al. 1996.Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron 16: 843–852, 1996. [DOI] [PubMed] [Google Scholar]
- Smart et al. 1998.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the Kv1.1 potassium channel causes epilepsy in mice. Neuron 20: 809–819, 1998. [DOI] [PubMed] [Google Scholar]
- Tang et al. 1998.Tang CY, Schulteis CT, Jimenez RM, Papazian DM. Shaker and ether-a-go-go K+ channel subunits fail to coassemble in Xenopus oocytes. Biophys J 75: 1263–1270, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres et al. 2007.Torres YP, Morera FJ, Carvacho I, Latorre R. A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem 282: 24485–24489, 2007. [DOI] [PubMed] [Google Scholar]
- Towbin et al. 1992.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24: 145–149, 1992. [PubMed] [Google Scholar]
- Trimmer 1998.Trimmer JS Regulation of ion channel expression by cytoplasmic subunits. Curr Opin Neurobiol 8: 370–374, 1998. [DOI] [PubMed] [Google Scholar]
- Wang and Wu 1996.Wang JW, Wu CF. In vivo functional role of the Drosophila hyperkinetic beta subunit in gating and inactivation of Shaker K+ channels. Biophys J 71: 3167–3176, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel et al. 2007.Wenzel HJ, Vacher H, Clark E, Trimmer JS, Lee AL, Sapolsky RM, Tempel BL, Schwartzkroin PA. Structural consequences of Kcna1 gene deletion and transfer in the mouse hippocampus. Epilepsia 48: 2023–2046, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al. 1998.Wilson GF, Wang Z, Chouinard SW, Griffith LC, Ganetzky B. Interaction of the K channel beta subunit, Hyperkinetic, with eag family members. J Biol Chem 273: 6389–6394, 1998. [DOI] [PubMed] [Google Scholar]
- Xu et al. 1998.Xu J, Yu W, Wright JM, Raab RW, Li M. Distinct functional stoichiometry of potassium channel beta subunits. Proc Natl Acad Sci USA 95: 1846–1851, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. 2001.Yang EK, Alvira MR, Levitan ES, Takimoto K. Kvβ subunits increase expression of Kv4.3 channels by interacting with their C termini. J Biol Chem 276: 4839–4844, 2001. [DOI] [PubMed] [Google Scholar]
- Yao and Wu 1999.Yao WD, Wu CF. Auxiliary Hyperkinetic beta subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J Neurophysiol 81: 2472–2484, 1999. [DOI] [PubMed] [Google Scholar]