Abstract
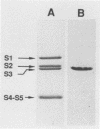
A panel of human monoclonal antibodies reactive with pertussis toxin has been generated by means of Epstein-Barr virus infection. One of these, the 3F11 monoclonal antibody, showed the ability to neutralize in vitro and in vivo the toxic effects of the toxin. Western blot (immunoblot) analysis located the 3F11 epitope on the S3 subunit.
Full text
PDF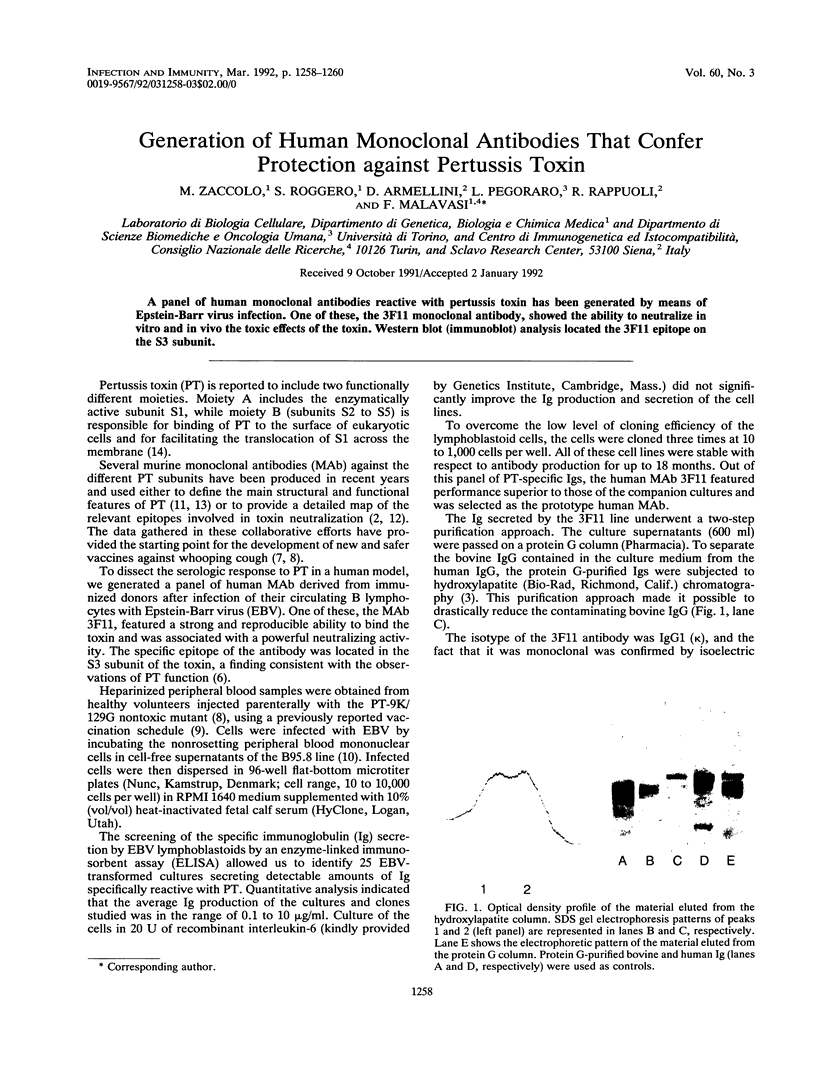
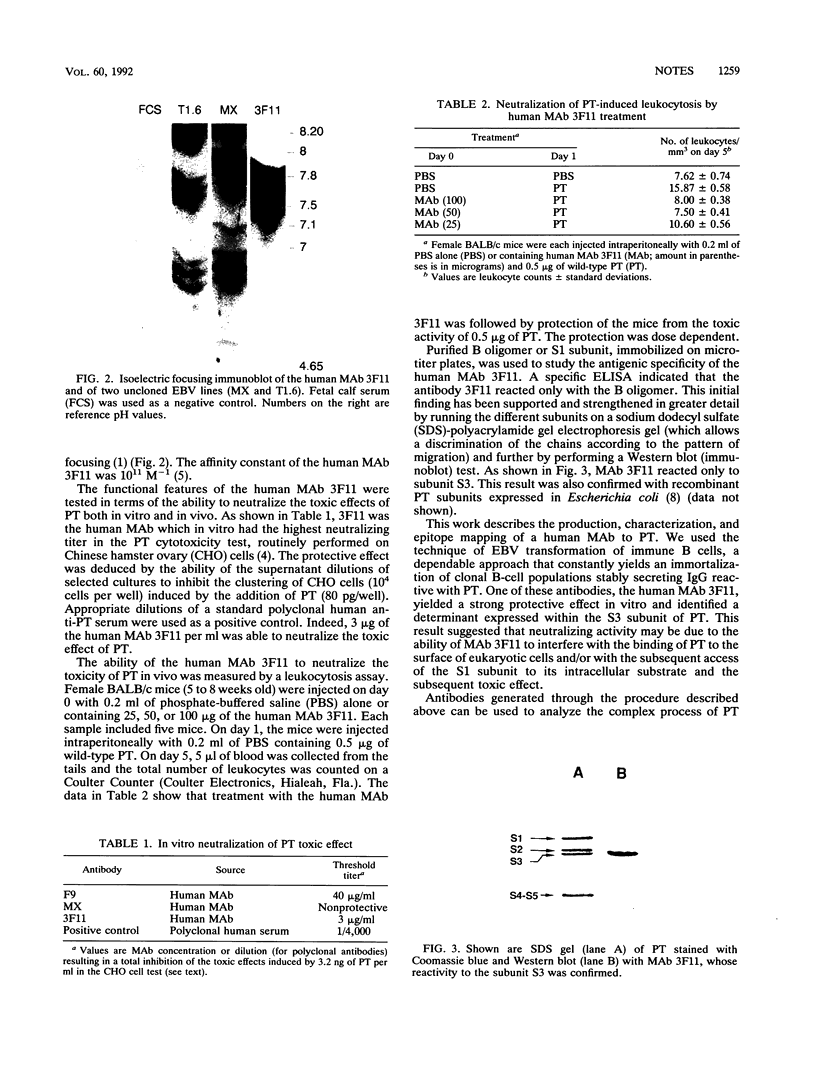

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessio M., Roggero S., Funaro A., De Monte L. B., Peruzzi L., Geuna M., Malavasi F. CD38 molecule: structural and biochemical analysis on human T lymphocytes, thymocytes, and plasma cells. J Immunol. 1990 Aug 1;145(3):878–884. [PubMed] [Google Scholar]
- DeMonte L. B., Nistico P., Tecce R., Dellabona P., Momo M., Anichini A., Mariani M., Natali P. G., Malavasi F. Gene transfer by retrovirus-derived shuttle vectors in the generation of murine bispecific monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2941–2945. doi: 10.1073/pnas.87.8.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani M., Bracci L., Presentini R., Nucci D., Neri P., Antoni G. Immunogenicity of a free synthetic peptide: carrier-conjugation enhances antibody affinity for the native protein. Mol Immunol. 1987 Mar;24(3):297–303. doi: 10.1016/0161-5890(87)90148-9. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Tomasi M., Schiavo G., Rappuoli R. Hydrophobic photolabelling of pertussis toxin subunits interacting with lipids. FEBS Lett. 1986 Jan 6;194(2):301–304. doi: 10.1016/0014-5793(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Podda A., Nencioni L., De Magistris M. T., Di Tommaso A., Bossù P., Nuti S., Pileri P., Peppoloni S., Bugnoli M., Ruggiero P. Metabolic, humoral, and cellular responses in adult volunteers immunized with the genetically inactivated pertussis toxin mutant PT-9K/129G. J Exp Med. 1990 Sep 1;172(3):861–868. doi: 10.1084/jem.172.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Ito A., Chiba J., Sato Y. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect Immun. 1984 Nov;46(2):422–428. doi: 10.1128/iai.46.2.422-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y., Ito A., Ohishi I. Effect of monoclonal antibody to pertussis toxin on toxin activity. Infect Immun. 1987 Apr;55(4):909–915. doi: 10.1128/iai.55.4.909-915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y. Protective activities in mice of monoclonal antibodies against pertussis toxin. Infect Immun. 1990 Oct;58(10):3369–3374. doi: 10.1128/iai.58.10.3369-3374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]