Abstract
Epidemiological and in vitro studies have suggested that hyperlipidemia/oxidized phospholipids adversely affect bone. We recently found that oxidized phospholipids attenuate PTH-induced cAMP and immediate-early gene (IEG) expression in MC3T3-E1 cells, raising concerns that clinical hyperlipidemia may attenuate osteoanabolic effects of PTH in vivo. Thus, we studied whether intermittent PTH treatment has differential osteoanabolic effects in wildtype (C57BL/6) and hyperlipidemic (LDLR−/−) mice. Consistent with our previous in vitro studies, induction of IEGs in calvarial tissue, 45 min after a single dose of recombinant hPTH(1-34), was attenuated in LDLR−/− mice compared with C57BL/6 mice. Daily hPTH(1-34) injections for 5 wk significantly increased total and cortical BMD and BMC, assessed by pQCT, in C57BL/6 mice. However, this induction was completely abrogated in LDLR−/− mice. Similarly, PTH(1-34) failed to increase BMD in another hyperlipidemic mouse model, ApoE−/− mice. Histomorphometric analysis showed that trabecular bone of both mice responded similarly to PTH(1-34). Structural parameters improved significantly in response to PTH(1-34) in both mouse strains, although to a lesser degree in LDLR−/− mice. With PTH(1-34) treatment, osteoblast surface trended toward an increase in C57BL/6 mice and increased significantly in LDLR−/− mice. PTH(1-34) did not alter resorption parameters significantly, except for the eroded surface (ES/BS), which was reduced in the C57BL/6 but not in the LDLR−/− mice. These results show that PTH(1-34) has adverse effects on cortical bones of the hyperlipidemic mice, suggesting that the therapeutic effects of PTH may be compromised in the presence of hyperlipidemia.
Key words: PTH, hyperlipidemia, BMD, osteoanabolism, mice
INTRODUCTION
Although traditionally viewed as separate disease entities, epidemiological evidence suggests that hyperlipidemia is particularly common in patients with osteoporosis; the National Health and Nutrition Examination Survey (NHANES III) estimated that 63% of osteoporotic patients have hyperlipidemia (cholesterol > 200 mg/dl). In animal models, diet-induced hyperlipidemia has been associated with a reduction in bone density parameters, including BMD and BMC in both mice and dogs.(1–3) In epidemiological studies, serum lipid levels negatively correlate with whole body BMC,(4) bone mass,(5,6) and BMD.(5) Furthermore, high plasma-low-density lipoprotein (LDL) concentration is a known risk factor for osteopenia in postmenopausal women.(7) Observational studies have suggested that statin use reduces bone loss and fracture risk(8,9); however, this result was not confirmed in subsequent randomized trials.(10–12)
In the pathogenesis of atherosclerosis, LDL particles accumulate in the subendothelial matrix of arteries under hyperlipidemic conditions and undergo nonenzymatic oxidative modification to inflammatory lipids, which act, for example, by increasing production of reactive oxygen species and expression of pro-inflammatory cytokines and chemokines.(13,14) Mice deficient in LDL receptor or apolipoprotein E (ApoE), a constituent protein of LDL, have an elevated serum cholesterol level, two to four times the normal level.(15) This hyperlipidemia is the primary phenotype of both mice; they also, consequently, develop spontaneous atherosclerotic lesions that increase with age. Both strains are routinely used in studies of hyperlipidemia and atherosclerosis.
We previously found histochemical evidence for lipid deposits in the subendothelial matrix of bone haversian canals and adjacent to osteoprogenitor cells in human osteoporotic femurs, and mass spectrophotometric evidence for oxidatively modified inflammatory lipids in the bone marrow of fat-fed C57BL6 mice.(16) Recently, Brodeur et al.(17) showed oxidation of LDL particles by osteoblasts. Thus, osteoblasts are likely to be directly exposed to lipid oxidation products in hyperlipidemia. These lipid oxidation products have been shown to impair bone density,(2) inhibit in vitro osteoblastic differentiation via oxidative stress,(18,19) and enhance in vitro osteoclastic differentiation.(16,20) Almeida et al.(21) recently showed that oxidative stress inhibits osteoblast differentiation by antagonizing the Wnt signaling pathway through squelching of β-catenin. In addition, atherogenic oxidized lipids have been shown to decrease osteoblast viability(17) and increase osteoblast apoptosis,(1) showing that osteoblast function and viability are directly regulated.
Intermittent injection of polypeptides derived from PTH [PTH(1-34); teriparatide] markedly increases bone mass, microarchitecture, and strength in humans and animal models, including both young and old mice.(22–26) In vivo studies with an N-terminal truncated PTH fragment suggests that the anabolic actions of PTH are mediated primarily by the protein kinase A (PKA) pathway.(27–29) The kinetics of PTH clearance suggest that the immediate downstream effects resulting from short-term PTH exposure, such as stimulation of the PKA pathway leading to induction of immediate early genes (IEGs), may be involved in anabolic effects of PTH. In mouse calvarial cells, interleukin-6 (IL-6) and neuron-derived orphan receptor-1 (NOR-1), a member of the nerve growth factor-I (NGF-I) family, are established as IEGs, because both are induced within 2 h of PTH treatment and are involved in osteogenic differentiation.(30–33) We recently showed that oxidized phospholipids significantly attenuated PTH induction of IEGs and BMP-2 induction of osteoblastic differentiation in vitro.(30) Given the adverse effects of oxidized lipids and hyperlipidemia on bone metabolism, we hypothesize that they may also impair the anabolic effects of PTH on bone. In this study, we chose mouse strains that differ in plasma LDL levels (normolipemic, C57BL/6; hyperlipidemic, LDLR−/−, ApoE−/− mice) to investigate PTH-induced gene expression and anabolic effects on bone parameters.
MATERIALS AND METHODS
Animals
Wildtype, LDLR−/−, and ApoE−/− mice (all C57BL/6 background) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of California at Los Angeles. Animals were given a standard Purina Chow diet.
Treatment and analysis of PTH effects on IEG expression
A single hPTH(1-34) (Sigma; 40 μg/kg) or vehicle injection (intraperitoneally) was performed in 15-wk-old female C57BL/6 and LDLR−/− mice (n = 5/group), as previously described.(33) Forty-five minutes after the injection, total RNA was isolated from the calvaria and tibia, and real-time RT-qPCR was performed using the One-Step qRT-PCR SuperMix Kit (BioChain) and Mx3005P Real-Time PCR System (Stratagene). Sequences for the primers are as follows: IL-6 (sense) 5′-TGTATGAACAACGATGAT-GCACTT-3′, (antisense) 5′-GGTACTCCAGAAGACC AGAGGAAAT-3′; NOR-1 (sense) 5′-AGCCCAGTATAGCCCTTC-3′, (antisense) 5′-ATGATTTCTGTGGTGTATTCC-3′; β-actin (sense) 5′-AGAGGGAAATCGTGCGT GAC- 3′; (antisense) 5′-CAATAGTGATGACCTGGCCGT- 3′.
Treatment and analysis of PTH effects on bone
Female C57BL/6 or LDLR−/− mice (20 wk old; 10 mice/treatment group) were injected (subcutaneously) with vehicle or hPTH(1-34) (40 μg/kg/d) 5 d/wk for 5 wk, as previously described.(34) PTH dose was adjusted weekly according to body weight. For dynamic histomorphometric analysis, mice were also injected (intraperitoneally) with calcein and demeclocycline (each at 20 mg/kg body weight; Sigma) at 10 and 2 days before the end of the study, respectively. Female ApoE−/− mice (52 wk old; 5 mice/group) were injected with vehicle or hPTH(1-34) (5 d/wk) for 6 wk. Animals were killed 20–24 h after the last injection, and blood was collected by cardiac puncture. Plasma was stored at −80°C until use for lipid analysis, kindly performed by the UCLA Atherosclerosis Research Unit core laboratory. Hearts including the aortic roots were isolated and embedded in OCT compound and frozen at −80°C. The sections containing the aortic roots were stained with oil Red O for atherosclerotic lesions.
pQCT analysis
Femurs and tibias were carefully harvested, gently cleaned of surrounding tissues, protected from light, and stored in 70% ethanol at 4°C. Mice with weights >1 SD from the mean and broken bones during harvest were excluded from data analysis. The numbers of bones included in the analysis are indicated in each data table. The right femur and tibia were analyzed for BMD parameters by pQCT, using a Stratec XCT-Research M (Norland Medical Systems, Ft Atkinson, WI, USA) and associated software (version 5.4; Stratec Medizintechnik, Pforzheim, Germany). Calibration was performed at the beginning of each session using a manufacturer-provided phantom. For the trabecular bone analysis, one metaphyseal slice was obtained at a site ∼2.45 mm from the distal end of each femur. For the cortical bone analysis, each bone was scanned at eight evenly spaced (1.15 mm apart) slices, beginning 3.2 mm from the distal end of the femur. The distal-most slice may extend slightly into the metaphysis. For each mouse, the data were averaged over the eight slices, and these values were averaged over the number of mice in each group (stated as “n”).
Trabecular bone histomorphometric analysis
Eight left femurs, randomly chosen from each group, were subjected to static and dynamic histomorphometry. Bones were fixed in 70% ethanol, dehydrated, and embedded undecalcified in methyl methacrylate. Longitudinal sections, 5 μm thick, were cut on a Microm microtome (Microm, Richards-Allan Scientific, Kalamazoo, MI, USA) and stained with toluidine blue, pH 6.4. Static parameters of bone formation and resorption were measured in a defined area between 181 and 725 μm from the growth plate, using an OsteoMeasure morphometry system (Osteometrics, Atlanta, GA, USA). For dynamic histomorphometry, mineralizing surface per bone surface and mineral apposition rate were measured in unstained sections under UV light, using a B-2A set long pass filter consisting of an excitation filter ranging from 450 to 490 nm, a barrier filter at 515 nm, and a dichroic mirror at 500 nm. Bone formation rate (BFR) was calculated based on the dual fluorescence labeling, as mentioned above. The terminology and units used are those recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research. Two bones from the vehicle-treated C57BL/6 group were excluded because of damage during processing.
Statistical analysis
PTH induction of bone parameters was compared between vehicle and PTH-treated groups in each mouse strain by the Student's t-test. Comparisons across all groups were performed with two-way ANOVA, followed by the Fisher's PLSD test using StatView (v4.5; Abacus). Values are expressed as means ± SE. A value of p ≤ 0.05 is considered significant.
RESULTS
Effects of PTH induction on IEG expression in wildtype and LDLR−/− mice
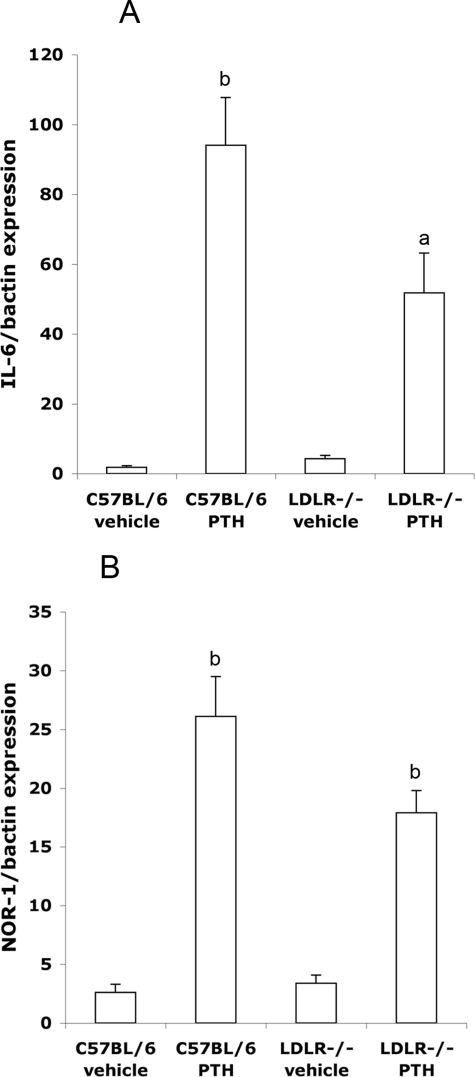
We previously found that, in MC3T3-E1 cells, lipid oxidation products attenuated PTH signaling by attenuating cAMP induction and expression of IEGs.(30) To test whether PTH-induced IEG expression was altered by hyperlipidemia in vivo, a single injection of hPTH(1-34) was administered into 15-wk-old normolipemic (C57BL/6) and hyperlipidemic (LDLR−/−) mice, and expression of IL-6 and NOR-1 in calvarial tissue was assessed 45 min after the injection. Results showed that, consistent with our in vitro findings, PTH(1-34) treatment induced IL-6 and NOR-1 gene expression by 52-fold and 10-fold, respectively, in C57BL/6 mice, but only 12-fold and 5-fold, respectively, in LDLR−/− mice (Figs. 1A and 1B).
FIG. 1.
Effect of a single PTH(1-34) injection on IEG expression in calvaria of normolipemic (C57BL/6) and hyperlipidemic (LDLR−/−) mice. Real-time RT-qPCR analysis of (A) IL-6 and (B) NOR-1 expression 45 min after injection with vehicle or hPTH(1-34). Data are mean ± SE. a p ≤ 0.05 and b p ≤ 0.005, all relative to the corresponding vehicle-treated control (n = 5/group).
Effects of anabolic PTH(1-34) treatment on LDLR−/− mice
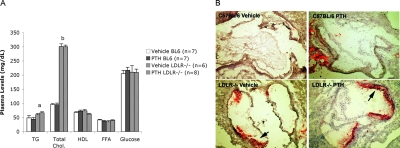
Because the PTH-induced IEG expression was attenuated in calvaria of the hyperlipidemic mice, we next assessed the effects of hyperlipidemia on the bone anabolic actions of intermittent PTH treatment. C57BL/6 and LDLR−/− mice were injected daily with vehicle or hPTH(1-34) for 5 wk. Terminal plasma lipid levels showed ∼3-fold greater total cholesterol levels in LDLR−/− mice than C57BL/6 mice, confirming the hyperlipidemic condition (Fig. 2A). As expected, triglyceride levels were also significantly higher (∼30%) in LDLR−/− than C57BL/6 mice, whereas glucose, free fatty acid, and high-density lipoprotein (HDL)-cholesterol levels were similar between the two strains (Fig. 2A). Oil-red O staining of aortic root sections confirmed the presence of early atherosclerotic lesions in LDLR−/− mice but not in C57BL/6 mice (Fig. 2B). PTH(1-34) treatment did not significantly alter lipid levels or atherosclerotic lesions in either mouse strain (Fig. 2).
FIG. 2.
Plasma lipoprotein levels and atherosclerotic lesions in C57BL/6 and LDLR−/− mice. (A) Plasma triglycerides (TGs), total cholesterol (Chol), HDL, free fatty acid (FFA), and glucose levels of vehicle- or PTH(1-34)–injected mice. Numbers of mice (n) are as indicated. Data are mean ± SE. (B) Oil red O staining of the aortic root of vehicle- or PTH(1-34)-injected C57BL/6 and LDLR−/− mice. Arrows indicate oil red O–stained atherosclerotic lesions. a p ≤ 0.05 and b p ≤ 0.001, all relative to C57BL/6.
pQCT analysis of femoral and tibial bone parameters
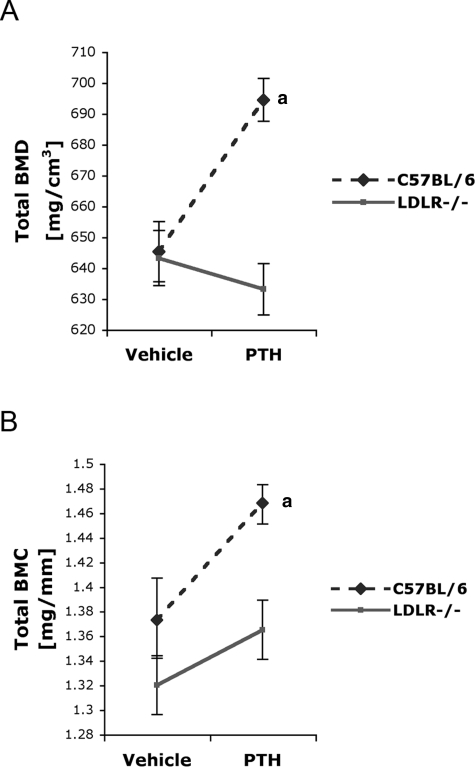
Bone parameters, assessed by pQCT on the right femur, are presented in Fig. 3 and Table 1. In C57BL/6 mice, intermittent PTH(1-34) treatment significantly increased total BMD and BMC compared with vehicle controls (Figs. 3A and 3B). In contrast, PTH(1-34) did not increase total BMD and BMC in LDLR−/− mice (Figs. 3A and 3B). PTH(1-34) also failed to increase femoral cortical BMD and BMC in the hyperlipidemic mice (Table 1). Similar results were obtained from pQCT analysis of right tibias, where PTH(1-34) treatment had no significant effects in LDLR−/− mice (Table 2).
FIG. 3.
Effect of intermittent PTH(1-34) injection on femoral total BMD of C57BL/6 and LDLR−/− mice. (A) Total BMD and (B) total BMC of vehicle-treated or PTH(1-34)–treated C57BL/6 and LDLR−/− mice was determined by pQCT. For “n” values, see Table 1. Data expressed as mean ± SE. a p ≤ 0.05 relative to vehicle-treated control.
Table 1.
Effect of Intermittent PTH(1-34) on Femoral Bone
|
C57BL/6 |
LDLR-/- |
||||||
| Vehicle (n = 6) | PTH (n = 6) | Percent change | Vehicle (n = 7) | PTH (n = 9) | Percent change | ||
| BMD (mg/cm3) | Cortical | 1116 ± 10 | 1158 ± 7* | +3.8 | 1099 ± 9 | 1097 ± 8 | |
| Trabecular | 94.9 ± 12.0 | 117.4 ± 5.0 | 102.2 ± 12.5 | 120.7 ± 2.2 | |||
| BMC (mg/mm) | Cortical | 1.153 ± 0.034 | 1.268 ± 0.015* | +10.4 | 1.102 ± 0.026 | 1.137 ± 0.028 | |
| Trabecular | 0.147 ± 0.018 | 0.147 ± 0.009 | 0.146 ± 0.021 | 0.152 ± 0.007 | |||
| Area (mm2) | Total | 2.131 ± 0.039 | 2.120 ± 0.057 | 2.059 ± 0.041 | 2.165 ± 0.088† | +5.2 | |
| Cortical | 1.032 ± 0.023 | 1.094 ± 0.013* | +6.0 | 1.001 ± 0.014 | 1.035 ± 0.018 | ||
| Trabecular | 1.548 ± 0.040 | 1.243 ± 0.077* | −19.7 | 1.404 ± 0.042 | 1.264 ± 0.05† | −9.9 | |
| PERI_C (mm) | 5.171 ± 0.028 | 5.155 ± 0.060 | 5.080 ± 0.037 | 5.207 ± 0.047† | +2.5 | ||
| ENDO_C (mm) | 3.709 ± 0.043 | 3.579 ± 0.070 | 3.632 ± 0.046 | 3.752 ± 0.047 | |||
| CRT_THK_C (mm) | 0.233 ± 0.005 | 0.251 ± 0.003* | +7.8 | 0.230 ± 0.004 | 0.232 ± 0.003 | ||
* p ≤ 0.05 vs. vehicle C57BL/6.
† p ≤ 0.05 vs. vehicle LDLR-/-.
Table 2.
Effect of Intermittent PTH(1-34) on Tibial Bone
|
C57BL/6 |
LDLR-/- |
||||||
| Vehicle (n = 7) | PTH (n = 7) | Percent change | Vehicle (n = 7) | PTH (n = 9) | Percent change | ||
| BMD (mg/cm3) | Total | 729 ± 7.9 | 779 ± 14* | +6.8 | 729 ± 13 | 729 ± 7.6 | |
| Cortical | 1073 ± 6.4 | 1117 ± 9.5† | +4.1 | 1073 ± 7.2 | 1079 ± 6.7 | ||
| Trabecular | 76.2 ± 6.8 | 88.6 ± 5.3 | 85.6 ± 6.3 | 85.6 ± 6.7 | |||
| BMC (mg/mm) | Total | 1.05 ± 0.025 | 1.15 ± 0.028* | +9.6 | 1.02 ± 0.027 | 1.03 ± 0.022 | |
| Cortical | 0.870 ± 0.024 | 0.985 ± 0.030* | +13 | 0.849 ± 0.026 | 0.860 ± 0.021 | ||
| Trabecular | 0.033 ± 0.003 | 0.031 ± 0.003 | 0.031 ± 0.003 | 0.031 ± 0.03 | |||
| Area (mm2) | Total | 1.46 ± 0.022 | 1.49 ± 0.026 | 1.42 ± 0.023 | 1.43 ± 0.022 | ||
| Cortical | 0.814 ± 0.019 | 0.883 ± 0.021* | +8.5 | 0.794 ± 0.021 | 0.800 ± 0.015 | ||
| Trabecular | 0.445 ± 0.034 | 0.356 ± 0.019* | −20 | 0.391 ± 0.015 | 0.379 ± 0.014 | ||
| PERI_C (mm) | 4.26 ± 0.033 | 4.31 ± 0.038 | 4.21 ± 0.035 | 4.22 ± 0.033 | |||
| ENDO_C (mm) | 2.82 ± 0.024 | 2.74 ± 0.043 | 2.77 ± 0.039 | 2.78 ± 0.028 | |||
| CRT_THK_C (mm) | 0.230 ± 0.004 | 0.251 ± 0.006* | +8.9 | 0.228 ± 0.006 | 0.229 ± 0.003 | ||
* p ≤ 0.05 and † p ≤ 0.005 (both compared with vehicle-treated C57BL/6).
Consistent with previous reports,(35,36) PTH(1-34) significantly increased cortical thickness in both femoral and tibial bones of C57BL/6 mice (Tables 1 and 2). Cortical area was also significantly increased in response to PTH(1-34) without a significant increase in the total cross-sectional area or periosteal circumference (Tables 1 and 2). There was a concomitant, significant decrease in the trabecular area and a trend toward decrease in the endosteal circumference (Table 1 and 2). In contrast, in LDLR−/− mice, PTH(1-34) significantly increased total cross-sectional area and periosteal circumference in femurs, without any significant change or trend in these parameters in tibial bones. In femurs of the LDLR−/− mice, there was a concomitant trend toward increase in cortical area and endosteal circumference (Tables 1 and 2).
Two-way ANOVA showed significant interactions (p < 0.05) between PTH(1-34) treatment and mouse strains in both femoral and tibial total BMD and cortical BMD, as well as cortical thickness and area, indicating a significant effect of hyperlipidemia on PTH response. There were no significant differences in these bone parameters between vehicle-treated C57BL/6 and LDLR−/− mice. The pQCT analysis also showed a trend toward increase in trabecular density in response to intermittent PTH(1-34) in both mouse strains (Tables 1 and 2). Therefore, trabecular bone parameters of the left femoral metaphysis were further analyzed by the static and dynamic histomorphometry.
Histomorphometric analysis of trabecular bone
Histomorphometric data of the left femurs is presented in Table 3. The intermittent PTH(1-34) treatment significantly increased structural parameters in both mouse strains, including trabecular bone volume (BV/TV), trabecular number (Tb.N), and trabecular thickness (Tb.Th) (Table 3), but the increase in these parameters was somewhat less in LDLR−/− mice. As reported previously in wildtype mice,(34) trabecular separation (Tb.Sp) was significantly reduced by the PTH(1-34) treatment in both strains (34% in the C57BL/6 and 22% in LDLR−/− mice; Table 3). PTH(1-34) significantly increased osteoblast numbers (N.Ob/T.Ar) similarly in both strains. PTH(1-34) also increased mineral apposition rate (MAR) in both strains, but interestingly, the induction was somewhat higher in LDLR−/− mice (Table 3). With PTH(1-34) treatment, osteoblast surface (ObS/BS) trended toward an increase in C57BL/6 mice and increased significantly in LDLR−/− mice. PTH(1-34) did not alter resorption parameters significantly, except for the eroded surface (ES/BS), which was significantly reduced in the C57BL/6 but not in the LDLR−/− mice.
Table 3.
Effect of Intermittent PTH(1-34) on Trabecular Histomorphormetric Parameters
|
C57BL/6 |
LDLR-/- |
|||||
| Vehicle (n = 6) | PTH (n =8) | Percent change | Vehicle (n = 7) | PTH (n = 8) | Percent change | |
| BV/TV (%) | 4.61 ± 0.76 | 7.84 ± 0.58* | +70 | 5.24 ± 0.44 | 7.53 ± 0.34† | +44 |
| Tb.Th (mm) | 25.22 ± 1.42 | 31.61 ± 0.74† | +25 | 24.28 ± 0.97 | 28.59 ± 0.89* | +18 |
| Tb.N (/mm) | 1.78 ± 0.21 | 2.47 ± 0.14‡ | +38 | 2.14 ± 0.11 | 2.63 ± 0.09* | +23 |
| Tb.Sp (mm) | 581.60 ± 78.85 | 382.71 ± 23.15‡ | −34 | 451.20 ± 28.30 | 354.17 ± 13.44* | −22 |
| O.Th (μm) | 1.04 ± 0.50 | 1.47 ± 0.77 | 1.21 ± 0.51 | 1.24 ± 0.61 | ||
| OS/BS (%) | 1.29 ± 0.48 | 1.05 ± 0.14 | 1.60 ± 0.45 | 1.80 ± 0.46 | ||
| OV/BV (%) | 0.08 ± 0.04 | 0.07 ± 0.08 | 0.17 ± 0.08 | 0.22 ± 0.15 | ||
| ObS/BS (%) | 7.29 ± 1.53 | 11.26 ± 1.33 | 5.49 ± 1.61 | 12.48 ± 2.27‡ | +128 | |
| N.Ob/T.Ar (/mm2) | 14.91 ± 4.11 | 29.96 ± 4.99‡ | +101 | 14.29 ± 4.06 | 29.11 ± 3.98‡ | +104 |
| MS/BS (%) | 9.16 ± 1.09 | 8.28 ± 1.31 | 6.58 ± 1.06 | 8.12 ± 1.39 | ||
| MAR (μm/d) | 1.40 ± 0.10 | 1.66 ± 0.08‡ | +19 | 1.20 ± 0.19 | 1.60 ± 0.09‡ | +33 |
| BFR/BS (μm3/μm2/yr) | 45.82 ± 5.25 | 51.13 ± 8.61 | 27.33 ± 3.54§ | 49.65 ± 10.23 | ||
| ES/BS (%) | 8.10 ± 1.49 | 4.08 ± 0.84‡ | −50 | 5.09 ± 0.80 | 4.19 ± 0.74 | |
| OcS/BS (%) | 2.21 ± 0.46 | 1.31 ± 0.33 | 0.77 ± 0.18§ | 0.79 ± 0.17 | ||
| N.Oc/T.Ar (/mm2) | 2.75 ± 0.66 | 2.17 ± 0.49 | 1.15 ± 0.3§ | 1.37 ± 0.28 | ||
Data are mean ± SE.
* p ≤ 0.005, † p ≤ 0.001, and ‡ p ≤ 0.05 compared with respective controls.
§ p ≤ 0.05 compared with control-treated C57BL/6.
Effects of anabolic PTH on ApoE−/− mice
To determine whether the effect of hyperlipidemia extends to older mice and other mouse models of hyperlipidemia, we tested the effects of PTH in older mice of the hyperlipidemic strain, ApoE−/−, on the same BL/6 background. Fifty-two-week-old ApoE−/− mice were injected with either vehicle or hPTH(1-34) daily (5 d/wk) for 6 wks, and BMD was assessed by pQCT. Knopp et al.(26) have previously shown that 4-wk daily PTH treatment of 18-mo-old C57BL/6 mice significantly induced total BMD by 4.2%. As shown in Table 4, PTH(1-34) treatment did not significantly induce either femoral or tibial BMD in ApoE−/− mice. BMC was significantly increased by PTH(1-34) in the tibia but not in the femur (Table 4).
Table 4.
Effect of Intermittent PTH(1-34) in ApoE-/- Mice
| Total BMD (mg/cm3) | Cortical BMD (mg/cm3) | Total BMC (mg/mm) | Cortical BMC (mg/mm) | |
| Femoral bone | ||||
| Vehicle | 564 ± 16 | 1057 ± 21 | 1.28 ± 0.04 | 1.03 ± 0.04 |
| PTH | 596 ± 16 | 1088 ± 15 | 1.37 ± 0.04 | 1.12 ± 0.05 |
| p | 0.19 | 0.26 | 0.13 | 0.18 |
| Tibial bone | ||||
| Vehicle | 704 ± 24 | 1070 ± 19 | 0.89 ± 0.04 | 0.73 ± 0.03 |
| PTH | 680 ± 6 | 1045 ± 8 | 1.02 ± 0.03 | 0.83 ± 0.03 |
| p | 0.39 | 0.28 | <0.05 | <0.05 |
DISCUSSION
Although the importance of bioactive, oxidized lipids is well recognized in the pathogenesis of atherosclerosis, their effects on bone are less known. Previously, our studies showed that lipid oxidation products have opposite effects on the function of osteoblasts and osteoclasts: inhibiting osteoblastic potential,(2,19) whereas promoting osteoclastic potential.(16,20) Our recent in vitro findings suggest that bioactive lipid oxidation products also have adverse effects on PTH signaling, suggesting that they may inhibit anabolic effects of PTH in vivo.(30) In this study, the results suggest that PTH responses, especially in cortical bone, are also adversely affected in hyperlipidemic mice.
As expected, the pQCT results showed that intermittent PTH(1-34) treatment increased total and cortical BMD in the wildtype mice. However, these effects were abrogated in the hyperlipidemic mice. Consistent with reports by other investigators,(35,36) the pQCT results suggest a predominantly inward growth (endosteal apposition) in response to PTH(1-34) in C57BL/6 mice, based on increased cortical cross-sectional area with a concomitant decrease in the trabecular (medullary) cross-sectional area without altering total cross-sectional area and periosteal circumference. In contrast, in LDLR−/− mice, the pQCT results suggest a predominantly outward growth (periosteal apposition) in response to PTH(1-34), based on increased total area with a concomitant increase in periosteal circumference and endosteal circumference.
Interestingly, histomorphometric analysis showed that trabecular bone of C57BL/6 and LDLR−/− mice responded similarly to PTH(1-34). Several trabecular bone parameters improved significantly in response to PTH(1-34) in both mouse strains, suggesting that PTH anabolism is preserved in trabecular bone even in the presence of hyperlipidemia. The basis for this differential effect of hyperlipidemia on cortical versus trabecular bone is not known. We speculate that the high turnover nature of trabecular bone may protect it from hyperlipidemic effects because it allows a shorter exposure time to bioactive, oxidized lipids. Alternatively, the protective effect may be caused by antioxidant enzymes produced by dendritic cells in the trabecular marrow space.(37)
Histomorphometric analysis of the trabecular bone also showed that, at baseline, hyperlipidemic mice have a lower basal bone formation rate than wildtype mice. This is consistent with our previous findings that lipid oxidation products have adverse effects on osteoblastic differentiation.(2,17,19) In addition, at baseline, hyperlipidemic mice had less osteoclast surface and fewer osteoclast numbers than wildtype mice, suggesting a lower bone turnover state. Although we previously found that bone marrow pre-osteoclasts isolated from hyperlipidemic mice have greater in vitro resorptive activity than pre-osteoclasts from wildtype mice, those experiments were performed in the presence of exogenous RANKL and macrophage-colony stimulating factor (M-CSF) and in the absence of osteoblasts, autologous or otherwise.(16) The difference is likely because of the fact that in vivo osteoclastogenesis is coupled to osteoblasts that are also impaired by hyperlipidemia.(38)
Some indices of resorption are affected by PTH in wildtype mice, such as a significant decrease in eroded surface and a downward trend in osteoclast surface. These effects were not observed in the hyperlipidemic mice. Inhibitory effects of PTH on resorption have been previously reported in in vitro studies, where osteoclast numbers decline in response to PTH(1-34) in bone marrow culture,(39) in rodent models, where eroded surface was reduced,(40,41) and in clinical studies by Dempster et al.(42) using daily intermittent PTH(1-34).
These findings suggest that hyperlipidemic mice have reduced cortical bone responses to intermittent PTH(1-34). Other models of skeletal PTH resistance have been developed, such as the SAMP6 mice (P6 substrain of senescence-accelerated mice), which develop osteoporosis early in life and are used to model accelerated skeletal aging.(43) Jilka et al.(43) have shown that high-dose PTH can overcome skeletal PTH resistance in these mice. Thus, one may speculate that high-dose PTH may similarly overcome the skeletal resistance to PTH in these hyperlipidemic mice. Indeed, Shao et al.,(44) in a study addressing effects of high-dose PTH(1-34) on vascular calcification in hyperlipidemic mice, noted an increase in BMD along with a dramatic reduction in aortic mineral deposition.
It is notable that bone metabolism may differ in mice and humans. For example, PTH effects are most prominent in the tibia and femur in mice but are first detected in the spine in humans,(34,45,46) possibly because of evolutionary differences in posture. Nevertheless, because our findings are consistent with prior epidemiological and in vitro studies, they may be applicable to human disease. Further studies are warranted to investigate the efficacy of PTH treatment in patients with disorders of lipid metabolism.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (DK076009-01 to YT and HL081202 to LLD). The authors thank Dr L Castellani for lipid analysis, X Wang for expert advise on aortic root sectioning, and Dr R Pereira (UCLA Bone Histomorphometric Laboratory) for bone histomorphometry and for valuable suggestions.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Hirasawa H, Tanaka S, Sakai A, Tsutsui M, Shimokawa H, Miyata H, Moriwaki S, Niida S, Ito M, Nakamura T. ApoE gene deficiency enhances the reduction of bone formation induced by a high-fat diet through the stimulation of p53-mediated apoptosis in osteoblastic cells. J Bone Miner Res. 2007;22:1020–1030. doi: 10.1359/jbmr.070330. [DOI] [PubMed] [Google Scholar]
- 2.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 3.Turek JJ, Watkins BA, Schoenlein IA, Allen KG, Hayek MG, Aldrich CG. Oxidized lipid depresses canine growth, immune function, and bone formation. J Nutr Biochem. 2003;14:24–31. doi: 10.1016/s0955-2863(02)00221-8. [DOI] [PubMed] [Google Scholar]
- 4.Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 5.Tanko LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women. Bone. 2003;32:8–14. doi: 10.1016/s8756-3282(02)00918-3. [DOI] [PubMed] [Google Scholar]
- 6.Wu LY, Yang TC, Kuo SW, Hsiao CF, Hung YJ, Hsieh CH, Tseng HC, Hsieh AT, Chen TW, Chang JB, Pei D. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29:317–325. doi: 10.1081/erc-120025039. [DOI] [PubMed] [Google Scholar]
- 7.Poli A, Bruschi F, Cesana B, Rossi M, Paoletti R, Crosignani PG. Plasma low-density lipoprotein cholesterol and bone mass densitometry in postmenopausal women. Obstet Gynecol. 2003;102:922–926. doi: 10.1016/j.obstetgynecol.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355:2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 9.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283:3205–3210. doi: 10.1001/jama.283.24.3205. [DOI] [PubMed] [Google Scholar]
- 10.Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC. Statin use, bone mineral density, and fracture risk: Geelong Osteoporosis Study. Arch Intern Med. 2002;162:537–540. doi: 10.1001/archinte.162.5.537. [DOI] [PubMed] [Google Scholar]
- 11.Reid IR, Hague W, Emberson J, Baker J, Tonkin A, Hunt D, MacMahon S, Sharpe N. Effect of pravastatin on frequency of fracture in the LIPID study: Secondary analysis of a randomised controlled trial. Long-term Intervention with Pravastatin in Ischaemic Disease. Lancet. 2001;357:509–512. doi: 10.1016/s0140-6736(00)04042-3. [DOI] [PubMed] [Google Scholar]
- 12.van Staa TP, Wegman S, de Vries F, Leufkens B, Cooper C. Use of statins and risk of fractures. JAMA. 2001;285:1850–1855. doi: 10.1001/jama.285.14.1850. [DOI] [PubMed] [Google Scholar]
- 13.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- 15.Moghadasian MH, McManus BM, Nguyen LB, Shefer S, Nadji M, Godin DV, Green TJ, Hill J, Yang Y, Scudamore CH, Frohlich JJ. Pathophysiology of apolipoprotein E deficiency in mice: Relevance to apo E-related disorders in humans. FASEB J. 2001;15:2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 16.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24:e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 17.Brodeur MR, Brissetteb L, Falstraultb L, Ouelletb P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2007;44:506–517. doi: 10.1016/j.freeradbiomed.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 19.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 20.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 21.Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- 22.Reeve J. Teriparatide and future anabolic treatments for osteoporosis. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 5th ed. Washington, DC, USA: Amer Soc Bone & Mineral Res.; 2003. pp. 1–8. [Google Scholar]
- 23.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT., Jr Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: A multicentre trial. BMJ. 1980;280:1340–1344. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen CJ. What's new with PTH in osteoporosis: Where are we and where are we headed. Trends Endocrinol Metab. 2004;15:229–233. doi: 10.1016/j.tem.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Rosen CJ, Bilezikian JP. Clinical review 123: Anabolic therapy for osteoporosis. J Clin Endocrinol Metab. 2001;86:957–964. doi: 10.1210/jcem.86.3.7366. [DOI] [PubMed] [Google Scholar]
- 26.Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K. The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology. 2005;146:1983–1990. doi: 10.1210/en.2004-0770. [DOI] [PubMed] [Google Scholar]
- 27.Hilliker S, Wergedal JE, Gruber HE, Bettica P, Baylink DJ. Truncation of the amino terminus of PTH alters its anabolic activity on bone in vivo. Bone. 1996;19:469–477. doi: 10.1016/s8756-3282(96)00230-x. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC. Determination of parathyroid hormone's dual effects on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem. 2007;45:33086–33097. doi: 10.1074/jbc.M705194200. [DOI] [PubMed] [Google Scholar]
- 29.Rixon RH, Whitfield JF, Gagnon L, Isaacs RJ, Maclean S, Chakravarthy B, Durkin JP, Neugebauer W, Ross V, Sung W, Willick GE. Parathyroid hormone fragments may stimulate bone growth in ovariectomized rats by activating adenylyl cyclase. J Bone Miner Res. 1994;9:1179–1189. doi: 10.1002/jbmr.5650090807. [DOI] [PubMed] [Google Scholar]
- 30.Huang MS, Morony S, Lu J, Zhang Z, Bezouglaia O, Tseng W, Tetradis S, Demer LL, Tintut Y. Atherogenic Phospholipids Attenuate Osteogenic Signaling by BMP-2 and Parathyroid Hormone in Osteoblasts. J Biol Chem. 2007;282:21237–21243. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YF, Harrison JR, Lorenzo JA, Kream BE. Parathyroid hormone induces interleukin-6 heterogeneous nuclear and messenger RNA expression in murine calvarial organ cultures. Bone. 1998;23:327–332. doi: 10.1016/s8756-3282(98)00115-x. [DOI] [PubMed] [Google Scholar]
- 32.Liang JD, Hock JM, Sandusky GE, Santerre RF, Onyia JE. Immunohistochemical localization of selected early response genes expressed in trabecular bone of young rats given hPTH 1-34. Calcif Tissue Int. 1999;65:369–373. doi: 10.1007/s002239900715. [DOI] [PubMed] [Google Scholar]
- 33.Pirih FQ, Aghaloo TL, Bezouglaia O, Nervina JM, Tetradis S. Parathyroid hormone induces the NR4A family of nuclear orphan receptors in vivo. Biochem Biophys Res Commun. 2005;332:494–503. doi: 10.1016/j.bbrc.2005.04.132. [DOI] [PubMed] [Google Scholar]
- 34.Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, Dempster DW, Nieves J, Lindsay R. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–816. doi: 10.1359/jbmr.2002.17.5.808. [DOI] [PubMed] [Google Scholar]
- 35.Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J Bone Miner Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D, Singh R, Divieti P, Guo J, Bouxsein ML, Bringhurst FR. Contributions of parathyroid hormone (PTH)/PTH-related peptide receptor signaling pathways to the anabolic effect of PTH on bone. Bone. 2007;40:1453–1461. doi: 10.1016/j.bone.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, van Dorsselaer A, Lotteau V, Rabourdin-Combe C, Rabilloud T, Servet-Delprat C. High expression of antioxidant proteins in dendritic cells: Possible implications in atherosclerosis. Mol Cell Proteomics. 2006;5:726–736. doi: 10.1074/mcp.M500262-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Lee SK, Adams DJ, Gronowicz GA, Kream BE. CREM deficiency in mice alters the response of bone to intermittent parathyroid hormone treatment. Bone. 2007;40:1135–1143. doi: 10.1016/j.bone.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukata S, Hagino H, Okano T, Yamane I, Kameyama Y, Teshima R. Effect of intermittent administration of human parathyroid hormone on bone mineral density and arthritis in rats with collagen-induced arthritis. Arthritis Rheum. 2004;50:4060–4069. doi: 10.1002/art.20728. [DOI] [PubMed] [Google Scholar]
- 41.Ma YL, Bryant HU, Zeng Q, Schmidt A, Hoover J, Cole HW, Yao W, Jee WS, Sato M. New bone formation with teriparatide [human parathyroid hormone-(1-34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology. 2003;144:2008–2015. doi: 10.1210/en.2002-221061. [DOI] [PubMed] [Google Scholar]
- 42.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 43.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1-34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 45.Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R. Parathyroid hormone added to established hormone therapy: Effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16:925–931. doi: 10.1359/jbmr.2001.16.5.925. [DOI] [PubMed] [Google Scholar]
- 46.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]