Abstract
Background
Idiopathic neuropathy patients are at high risk of impaired glucose tolerance (IGT). Hyperglycemia, low high density lipoprotein (HDL), elevated triglycerides (TRG), hypertension and central obesity co-associate and constitute the metabolic syndrome. Patients with hyperglycemia are at high risk of having the syndrome and each of its features. Our null hypothesis was that patients with neuropathy and IGT would have a higher prevalence of other metabolic syndrome features than those without hyperglycemia. The primary objective was to determine if metabolic syndrome features other than hyperglycemia increase neuropathy risk.
Methods
The prevalence of metabolic syndrome features was determined among 219 sequential patients with idiopathic peripheral neuropathy. Subjects were classified as having IGT or normoglycemia. The prevalence of metabolic syndrome was compared to published population prevalence data. To compensate for potential referral bias, data were also compared for175 diabetic subjects without neuropathy, given the well-recognized risk of metabolic syndrome among diabetic individuals.
Results
Contrary to our hypothesis, neuropathy patients with normoglycemia and IGT shared a similarly elevated prevalence of metabolic syndrome features compared to published normal populations. Compared to diabetic subjects without neuropathy, the normoglycemic neuropathy patients had significantly higher total and LDL cholesterol, and a higher prevalence of abnormal HDL and triglycerides. The prevalence of obesity and hypertension were similar among patient groups. Normoglycemic neuropathy subjects had significantly more features of metabolic syndrome (other than hyperglycemia) than diabetics.
Conclusions
These findings demonstrate an association between neuropathy and metabolic syndrome features other than hyperglycemia. Lipid abnormalities are particularly prevalent among neuropathy subjects.
Keywords: peripheral neuropathy, diabetes, impaired glucose tolerance, metabolic syndrome
Introduction
Peripheral neuropathy is a common clinical problem confronting the practicing neurologist. Several groups have demonstrated a 30-45% prevalence of impaired glucose tolerance (IGT) in patients with otherwise idiopathic neuropathy 1. IGT (“prediabetes”) is one aspect of an overall metabolic syndrome that is also characterized by hyperlipidemia, hypertension, and central obesity. Patients with IGT and diabetes are highly likely to have other features of metabolic syndrome. The relationship between neuropathy and features of metabolic syndrome other than IGT is unknown. We hypothesized that patients with IGT and neuropathy would have a higher prevalence of metabolic syndrome and its components than those with normal glucose tolerance (NGT) and neuropathy.
Research Design and Methods
All patients with suspected peripheral neuropathy seen in the University of Utah neuromuscular clinic undergo a standardized evaluation that includes a recommendation for an oral glucose tolerance test (OGTT). Between 1997 and 2006, 491 sequential patients with suspected idiopathic peripheral neuropathy were seen. Those with primary demyelinating and motor neuropathies, sensory ganglionopathies and mononeuritis multiplex were excluded. A diagnosis of distal symmetric peripheral neuropathy was made by one of 3 neuromuscular experts. A clinically based diagnosis of neuropathy was made by the neuromuscular attending physician at the time of the clinical encounter. All subjects had symptoms and signs of neuropathy. When deemed necessary by the attending physician, confirmatory tests such as nerve conduction studies and skin biopsy for measurement of epidermal nerve fiber density were performed, although these tests were not required. Electrodiagnostic studies were used to exclude polyradiculopathy and inflammatory demyelinating neuropathies. Subjects were carefully questioned regarding potential neuropathy etiologies including toxic exposures, vitamin deficiencies and connective tissue disease and a thorough family history for neuropathy symptoms or foot problems was taken. An alternative cause for neuropathy was identified in 194 patients (e.g. inflammatory neuropathy, positive family history of non-diabetic neuropathy, heavy alcohol use, hepatitis C or other disease known to cause neuropathy). Vitamin B12, TSH, and serum protein electrophoresis and immunofixation were measured in each of the remaining 297 subjects, and additional laboratory evaluation was ordered when clinically indicated. Those with B12 deficiency, hypothyroidism, paraprotienemia or other relevant laboratory abnormality (e.g. positive antinuclear antibody) were excluded. Of this group, 219 (74%) underwent the recommended oral glucose tolerance test and had an otherwise normal laboratory evaluation.
The demographic features of the 219 patients who underwent OGTT were collected. Their blood pressure, height and weight were recorded, and body mass index was calculated. Use of antihypertensive agents or lipid lower agents were noted. These data were available for each subject. Because a lipid profile was not part of the standard diagnostic evaluation for idiopathic neuropathy, this data was not available for each patient visit and was collected from the charts retrospectively when necessary. If the data were not available, the patient and their primary care physician were contacted. Complete lipid data was available for 154 (70%) of patients. In the remaining 30%, only partial lipid data was available, no lipid profile had been measured or the subject was lost to follow up. The prevalence of each metabolic syndrome feature among those with complete lipid data was compared between those found to have NGT versus IGT. Diagnosis of metabolic syndrome was made using the Adult Treatment Panel III (ATPIII) criteria which require 3 of the following: IGT or diabetes, systolic blood pressure >130 or diastolic > 85 mm Hg, obesity (BMI > 30 kg/m2), HDL < 40 mg/dl for women and < 50 mg/dl for men, and triglycerides > 150 mg/dl 2. If a patient was taking an antihypertensive agent without an alternate indication, they were considered to have hypertension even if the BP was normal. Subjects who were on a lipid lower agent were granted one point towards a diagnosis of metabolic syndrome if both HDL and triglycerides were normal. These results were compared to prevalence rates for metabolic syndrome features from large epidemiological studies 3-6. Results were also compared to those of a group of 175 subjects with diabetes but no neuropathy participating in a longitudinal natural history study (The Utah Diabetic Neuropathy Study). All subjects underwent clinical history and examination, nerve conduction studies, quantitative sensory testing for cold and vibration, quantitative sudomotor axon reflex testing and skin biopsy at the ankle and distal and proximal thigh. Neuropathy diagnosis was based on the presence of an abnormality of more than two measures. The mean duration of diabetes was 86 +/- 79 months. These subjects have been recruited from a large community based network of primary care clinics (the University of Utah Heath Network, UUHN). A random selection of UUHN patients with diabetes have been contacted regarding participation in the study, thus minimizing referral bias. Neuropathy was excluded based on signs, symptoms and electrophysiologic testing.
Student’s t tests were used to compare mean values for each parameter and contingency table analysis was used to compare the proportion of each group with an abnormality for each parameter. In order to evaluate the possibility that only patients deemed likely to have diabetes or IGT underwent OGTT or lipid measurement, BMI was compared between the 219 who underwent OGTT and the 78 who did not as well as between subjects with and without complete lipid data.
Results
By OGTT, IGT was found in 97/219 (44%) and previously unrecognized diabetes was found in 16 (7.3%). Complete lipid data were available for 154 (68 with IGT, 9 with diabetes, and 77 with NGT. There was a similar prevalence among patients who received a complete lipid evaluation (IGT 68/154, 44% and diabetes 9/154, 5.8%). The IGT and newly diagnosed diabetic patients were similar in all other respects and for purposes of comparison to normoglycemic patients the two groups were combined. Contrary to our hypothesis, there was no difference in any aspect of metabolic syndrome between IGT and NGT neuropathy patients (table 1). Both groups had a similarly high prevalence of abnormal HDL cholesterol and triglycerides, obesity and hypertension. Indeed, each aspect of the metabolic syndrome was more prevalent among neuropathy subjects as a group than hyperglycemia. Metabolic syndrome was found in 86% of IGT neuropathy patients and in 54% of NGT neuropathy patients. This difference is not unexpected given that the IGT patients, by definition, fulfill one criterion. The number of metabolic syndrome criteria met other than IGT did not differ between the two groups. BMI did not differ between subjects who underwent OGTT or lipid measurement and those who did not, arguing against a referral bias for these tests.
Table 1.
Comparison of metabolic syndrome features among study groups. The demographic and metabolic characteristics of patients with NGT and IGT neuropathy are compared with characteristics of diabetic subjects without neuropathy. Subjects with NGT and IGT neuropathy were significantly more likely to have reduced HDL or elevated TRG (p<0.001) and had lower HDL and higher TRG (p<0.001) than diabetic subjects. Diabetic subjects were younger and had higher BMI (p<0.001). Contingency table analysis indicated a significantly higher number of metabolic syndrome features other than hyperglycemia among NGT neuropathy compared with diabetic subjects (p<0.05). (FPG - fasting plasma glucose, OGTT - glucose 2 hours following oral glucose tolerance test, CHOL - total cholesterol, LDL - low density lipoprotein, HDL - high density lipoprotein. TRIG - triglycerides, SBP - systolic blood pressure, DBP - diastolic blood pressure, all laboratory values in mg% and blood pressure in mm Hg)
| NGT | IGT | Diabetes | ||
|---|---|---|---|---|
| Number | 77 | 77 | 175 | |
| Gender | Male | 48% (n=37) | 43% (n=33) | 48% (n=84) |
| Female | 52% (n=40) | 57% (n=44) | 51% (n=91) | |
| Age | 62.9 +/- 12 | 61.2 +/- 8.7 | 53.4 +/- 9.5 (p<.001) | |
| Months of known diabetes | 0 | 0 | 81 +/- 80 | |
| Metabolic Features | FPG | 82.2 +/-8.4 | 102 +/- 22 | ---- |
| OGTT | 97.2 +/- 28 | 164 +/- 49 | ---- | |
| CHOL | 201 +/-34 | 193.7 +/- 42 | 173 +/-48.1 (p<.001) | |
| LDL | 113 +/-28 | 104 +/-34 | 90.1 +/- 34.7 (p<.001) | |
| HDL | 51.2 +/- 18 | 48.4 +/- 15 | 51.1 +/- 15.9 | |
| TRIG | 203 +/-130 | 225 +/- 131 | 196+/- 350 | |
| BMI | 29.7 +/- 5.7 | 30.8 +/- 5.2 | 32.5 +/- 7.5 (p<.001) | |
| Blood Pressure | SBP | 131 +/-19 | 140 +/-17 | 132 +/- 17 |
| DBP | 76.7 +/- 11 | 79.3 +/- 11 | 75.9 +/- 9.8 | |
| Percent with: | Hypertension | 74% | 88% | 66% |
| Reduced HDL | 65% | 59% | 35% (p<.001) | |
| Hypertriglyceridemia | 74% | 78% | 38% (p<.001) | |
| Obesity | 51% | 49% | 55% | |
| Statin Use | 26% | 25% | 58% (p<0.001) | |
| Metabolic Syndrome | Total # of Criteria Met | 2.5 | 2.7 | 2 |
| % Meeting 0 Criterion | 3% | 2% | 11 | |
| % Meeting 1 Criterion | 97% | 98% | 89 | |
| % Meeting 2 Criterion | 81% | 86% | 70 | |
| % Meeting 3 Criterion | 54% | 65% | 41 | |
| % Meeting 4 Criterion | 19% | 22% | 13 | |
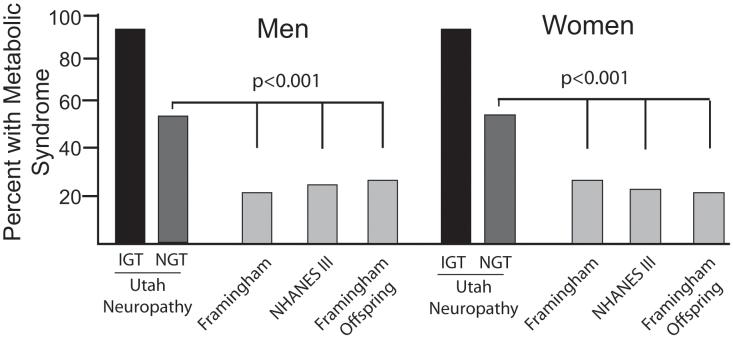
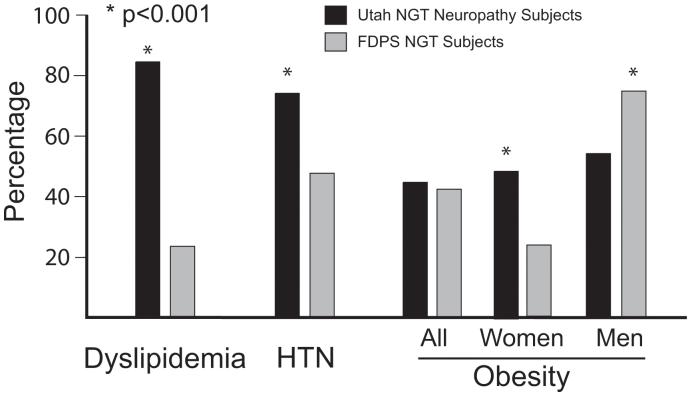
There are several large epidemiologic studies from the United States that permit comparison of the prevalence of metabolic syndrome between our neuropathy patients and the normal population 3-5. In each instance, the prevalence among NGT neuropathy patients was significantly higher, nearly twice that among the normal gender matched population (figure 1). These studies do not provide separate data on metabolic syndrome prevalence for patients with NGT. The Finnish Diabetes Prevention Study (FDPS) has reported prevalence data for dyslipidemia, hypertension and obesity among the general population, segregated into those with NGT or IGT 6. Our NGT neuropathy patients had a significantly higher rate of a cholesterol or lipid abnormality (83% s. 33% p<0.001) and hypertension (74% vs 56% p<0.001) than did the FDPS normoglycemic subjects. The rate of obesity did not differ between the populations when considered as a whole, although the rate was significantly higher among women with neuropathy (p<0.001) (figure 2).
Figure 1.
Prevalence of metabolic syndrome among evaluated cohorts. Both male and female IGT and NGT neuropathy patients had a significantly higher prevalence of metabolic syndrome that several large population based cohorts (p<0.001) 3-5.
Figure 2.
Prevalence of features of metabolic syndrome in neuropathy patients compared to published population controls. NGT neuropathy patients had a significantly higher rate of dyslipidemia and hypertension when compared to NGT patients participating in the Finnish Diabetes Prevention Study (p<0.001). Female neuropathy patients were significantly more obese (p<0.001) although there was no difference between men 6.
Metabolic syndrome features were also compared between neuropathy subjects and a cohort of 175 diabetic subjects without neuropathy. The diabetic subjects were younger and more obese than the patients in either neuropathy group. However, both the NGT and IGT neuropathy subjects had significantly higher total and LDL cholesterol, and similar mean blood pressure, HDL cholesterol and triglycerides. Abnormal HDL and TRG were more common among NGT and IGT neuropathy patients than diabetic subjects (p<0.001). NGT neuropathy subjects fulfilled more non-hyperglycemia metabolic syndrome criteria than diabetic subjects (p<0.05, table 1). Significantly more diabetic subjects were taking a statin (58% vs 25%, p<0.001). This finding is not unexpected given the routine use of statin medications in diabetes.
Conclusions
Individuals with IGT and diabetes are more likely to have metabolic syndrome and each of its component features than those with NGT. Therefore our findings of an indistinguishable prevalence of non-hyperglycemia metabolic syndrome features between idiopathic neuropathy patients with IGT and NGT are unexpected, and suggest features of metabolic syndrome other than IGT may represent independent risk factors for peripheral neuropathy. It has been suggested the neuropathy associated with IGT may represent the earliest stage of diabetic nerve injury. IGT and early diabetic neuropathy both cause preferential small fiber injury and both share similar physiologic abnormalities in microvascular reactivity 7. While the high prevalence of IGT in neuropathy cohorts has proven a reproducible finding, only one study has used a case control design to determine if this prevalence is truly elevated relative to non-neuropathy patients. The prevalence of IGT or newly diagnosed diabetes in neuropathy patients was twice that in controls (16/49 vs 7/49) but when age, gender and BMI were factored in this difference was not statistically significant. However, serum triglycerides were significantly higher in neuropathy patients 8. The non-neuropathy controls were chosen from non-blood relatives or friends, so they might be expected to have a similar rate of obesity and its complications as the neuropathy patients 9. Indeed, BMI did not differ between groups. Interestingly, the mean BMI of the neuropathy cohort was 27.1, much lower than in our larger neuropathy cohort (30 kg/m2), suggesting the populations may be meaningfully different in other aspects. Regardless, the finding of higher serum triglycerides in the neuropathy cohort is consistent with our findings, and supports a role for dyslipidemia in neuropathy development.
There are other data that support a role for metabolic syndrome features as modifying factors for diabetic neuropathy. The EURODIAB study showed that obesity, dyslipidemia and hypertension each constituted an independent neuropathy risk factor among diabetic subjects over an average of seven years of follow-up 10. Several smaller studies have shown an increased prevalence of neuropathy among obese patients compared to lean controls 11-13. Among 30 metabolic syndrome subjects, 20 of whom had diabetes, mean dendritic length of epidermal nerve fibers negatively correlated with HDL among those with isolated metabolic syndrome and positively correlated with hemoglobin A1c among those with diabetes 14. The Steno 2 study demonstrated that aggressive treatment targeted at each aspect of metabolic syndrome reduced the risk of both macrovascular and microvascular outcomes, including autonomic neuropathy 15.
Cellular and molecular consequences of metabolic syndrome features closely mesh with recognized pathogenic mechanisms in hyperglycemic neuropathy, especially nitric oxide inhibition, vascular dysregulation and oxidative injury 16. Insulin resistance and dyslipidemia are intertwined and self-reinforcing processes that are intimately associated with obesity and distribution of lipid deposition 17. Enlarged adipocytes are endocrinologically active cells that mediate vascular lipotoxicity through release of free fatty acids (FFAs) and adipokines. FFAs potently inhibit endothelial nitric oxide synthesis and vasodilation, driving microvascular ischemia 18, 19. Enlarged adipocytes reduce expression of the vasodilatory and vasoprotective agent adiponectin, accelerating endothelial cell proliferation and macrophage mediated atherosclerotic injury 20. Finally, very engorged adipocytes lyse, releasing FFAs, adipokines, and inducing an inflammatory response mediated primarily by macrophages. Activated macrophages release cytokines that themselves accelerate endothelial injury. These processes provide a mechanism for linking obesity, adiposity, insulin resistance and neuropathy pathogenesis.
The findings of this study add to the evidence in the literature linking lipid abnormalities, obesity and hypertension to neuropathy risk. Dyslipidemia and hypertension were more prevalent in our neuropathy cohort than IGT suggesting they may be equally important factors in development of otherwise idiopathic neuropathy. Our study has several limitations. It is possible our neuropathy cohort may be relatively obese because of a referral bias. The principal impact of this potential bias is the possibility that only subjects who were likely to have IGT or metabolic syndrome underwent OGTT or lipid measurement, thereby biasing the results. However, the finding of similar BMI between groups that did and did not undergo these tests argues against this bias. The use of published epidemiologic studies to determine the prevalence of metabolic syndrome in the general population is not ideal, despite the very large size and methodological rigor of these studies. Collection of valid normative data to which the data from neuropathy subjects could be compared would require a large case control study. Given this was not practical, neuropathy subjects were compared to a group of 175 diabetic subjects for whom BMI, blood pressure and lipid panels were available. It is well recognized that diabetic individuals have a higher risk of obesity, hypertension, dyslipidemia and metabolic syndrome than their normoglycemic counterparts 6. NGT neuropathy subjects had more frequent and severe lipid abnormalities and were similar in other respects to diabetic subjects. This finding supports the conclusion that NGT neuropathy subjects are more likely to have underlying metabolic syndrome than the non-diabetic control population. However, the comparison to the diabetic cohort must be interpreted with some caution. More diabetic subjects were taking statin medications compared to neuropathy subjects. This finding might partially explain the higher total and LDL cholesterol levels in the NGT and IGT neuropathy cohorts. It also suggests statin use is not a risk factor for neuropathy development. The mean age of the diabetic cohort was 8.7 years less than the neuropathy cohort which also may bias to more metabolic syndrome features in the later group. Overall, however, the data from this study associating lipid abnormalities with neuropathy are more compelling that those linking IGT to neuropathy.
Another interpretation of our findings is that neuropathy patients may be more prone to have metabolic syndrome or IGT because of a sedentary lifestyle due to pain. The fact that BMI was lower among NGT neuropathy subjects than the diabetic group and the recent observation that diet and exercise intervention results in short term improvement in IGT-associated neuropathy argue against this possibility 21. Further study is necessary to confirm these findings and further examine the pathogenic role of obesity and dyslipidemia. A large well powered prospective case control study of idiopathic neuropathy patients, as well as neuropathy screening studies among obese and non-obese populations are desirable.
Acknowledgements
This work was supported by NIH grants NS40458, DK064814 (AGS, JRS) and the University of Utah General Clinical Research Center Grant M01RR0064. AGS and JRS had full access to all of the data and take responsibility for data integrity and accuracy of analysis. This work was presented in part at the 2006 American Academy of Neurology annual meeting in San Diego, CA.
The authors wish to thank the staff at the University of Utah General Clinical Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singleton JR, Smith AG, Bromberg MB. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care. 2001;24:1448–1453. doi: 10.2337/diacare.24.8.1448. [DOI] [PubMed] [Google Scholar]
- 2.The Expert Panel on Detection E. Treatment of High Blood Cholesterol in Adults . The Third Report of the ATP III. National Institutes of Health National Heart Lung and Blood Institute; Bethesda Maryland: 2001. NIH publication 01-3670: http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm. [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Meigs JB, D’Agostino RB, Sr., Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. The Framingham Offspring Study. Diabetes. 1997;46:1594–600. doi: 10.2337/diacare.46.10.1594. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 6.Ilanne-Parikka P, Eriksson JG, Lindstrom J, et al. Prevalence of the metabolic syndrome and its components: findings from a Finnish general population sample and the Diabetes Prevention Study cohort. Diabetes Care. 2004;27:2135–40. doi: 10.2337/diacare.27.9.2135. [DOI] [PubMed] [Google Scholar]
- 7.Smith AG, Robinson Singleton J. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci. 2006;242:9–14. doi: 10.1016/j.jns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Hughes RA, Umapathi T, Gray IA, et al. A controlled investigation of the cause of chronic idiopathic axonal polyneuropathy. Brain. 2004;127:1723–30. doi: 10.1093/brain/awh192. [DOI] [PubMed] [Google Scholar]
- 9.Christakis N, Fowler J. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 10.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–50. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 11.Tentolouris N, Grapsas E, Stambulis E, Papageorgiou K, Katsilambros N. Impact of body mass on autonomic function in persons with type 2 diabetes. Diabetes Res Clin Pract. 1999;46:29–33. doi: 10.1016/s0168-8227(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 12.Herman RM, Brower JB, Stoddard DG, et al. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes (Lond) 2006 doi: 10.1038/sj.ijo.0803418. [DOI] [PubMed] [Google Scholar]
- 13.Straub RH, Thum M, Hollerbach C, Palitzsch KD, Scholmerich J. Impact of obesity on neuropathic late complications in NIDDM. Diabetes Care. 1994;17:1290–4. doi: 10.2337/diacare.17.11.1290. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger GL, Mehrabyan A, Simmons K, et al. Small fiber neuropathy is associated with the metabolic syndrome. Metab Syndr Relat Disord. 2005;3:113–21. doi: 10.1089/met.2005.3.113. [DOI] [PubMed] [Google Scholar]
- 15.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 16.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–73. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- 17.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 18.Esenabhalu VE, Schaeffer G, Graier WF. Free fatty acid overload attenuates Ca2+ signaling and NO production in endothelial cells. Antioxid Redox Signal. 2003;5:147–53. doi: 10.1089/152308603764816505. [DOI] [PubMed] [Google Scholar]
- 19.Pleiner J, Schaller G, Mittermayer F, Bayerle-Eder M, Roden M, Wolzt M. FFA-induced endothelial dysfunction can be corrected by vitamin C. J Clin Endocrinol Metab. 2002;87:2913–7. doi: 10.1210/jcem.87.6.8596. [DOI] [PubMed] [Google Scholar]
- 20.Ukkol O, Santaniemi M. Adiponectin: a link between excess adiposity and associated comorbidities? J Mol Med. 2002;80:696–702. doi: 10.1007/s00109-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 21.Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–9. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]