Abstract
The oxidation resistance of irradiated ultra-high molecular weight polyethylene (UHMWPE) components used in total joint arthroplasty can be improved by adding a-tocopherol (vitamin E) through diffusion. To ensure long-term oxidative stability, a minimum a-tocopherol concentration needs to be maintained throughout these components. Migration of a-tocopherol out of the components is one mechanism that could compromise long-term oxidative stability. We hypothesized that a-tocopherol could elute out during standard implant fabrication steps such as cleaning as well as during in vivo use. We doped 85 kGy irradiated UHMWPE with a-tocopherol at 120°C and homogenized them at 120°C. We determined the extent of elution of a-tocopherol or its effect on oxidative stability following cleaning in isopropyl alcohol and following five million cycles of simulated normal gait in bovine serum. There was no significant elution of a-tocopherol in repeated and prolonged cleaning in isopropyl alcohol as measured by average surface and bulk a-tocopherol concentrations. There was no change in the oxidative stability following five million cycles of hip simulator testing, indicating minimal elution during simulated normal gait.
Introduction
Ultra-high molecular weight polyethylene (UHMWPE) is the material of choice for load-bearing articular components used in total joint arthroplasty. One of the major factors that limit long-term performance of total joints is the peri-prosthetic osteolysis secondary to wear of UHMWPE components. Radiation cross-linking is used to improve the wear properties of UHMWPE. Subsequent melting is used to eliminate the residual free radicals and to make radiation cross-linked UHMWPE oxidation resistant [1-6]. The disadvantage of melting is that it reduces the crystallinity of irradiated UHMWPE and thereby decreases the mechanical properties and fatigue resistance [7].
A highly cross-linked UHMWPE with low wear and high oxidation resistance has been developed by diffusing the antioxidant a-tocopherol into irradiated UHMWPE [8]. a-Tocopherol provided oxidation resistance by stabilizing the residual free radicals trapped in the crystalline domains of the semi-crystalline UHMWPE as a result of irradiation. By eliminating the need for post-irradiation melting, the fatigue resistance of a 100 kGy irradiated UHMWPE was increased by 32% compared to that of 100 kGy irradiated and melted UHMWPE [8, 9].
a-Tocopherol is the most abundant and effective chain breaking antioxidant in the body [10], whose major role is to react with free radicals in cell membranes and protect polyunsaturated fatty acids from degradation due to oxidation [11]. Oxidation of polyunsaturated fatty acids results in active free radicals (LOO•, LO•), which are then stabilized by a-tocopherol through hydrogen abstraction from the OH group on the chroman ring (Fig 1). Oxidation in polyethylene also takes place by the same mechanism as in fatty acids; therefore, a-tocopherol can prevent peroxy free radicals from attacking other chains and producing more free radicals [12-14]. This would prevent the oxidative embrittlement and deterioration of mechanical properties observed in irradiated and unstabilized UHMWPE.
Fig 1.
a-Tocopherol.
The efficacy of a-tocopherol in protecting irradiated UHMWPE against oxidation has been shown [8, 15]; however, these studies did not take into account possible elution of a-tocopherol out of irradiated finished total joint arthroplasty components. We hypothesized that a-tocopherol migration could compromise oxidative stability of a-tocopherol doped, irradiated UHMWPE components. Elution of the a-tocopherol may occur during the fabrication process when components would be subjected to cleaning before sterilization or during in vivo use under load and motion.
We investigated the migration stability of a-tocopherol in irradiated UHMWPE components after regular cleaning, after multiple cleanings, and after a long soak in isopropyl alcohol before terminal sterilization. We also determined the oxidative stability of irradiated, a-tocopherol-stabilized and sterilized acetabular liners which had been subjected to 5 million cycles of simulated normal gait. To accurately determine the concentration of a-tocopherol weight percentage, we created a calibration curve to convert the a-tocopherol index, calculated by our infrared spectroscopy detection method [16], into an a-tocopherol weight percentage.
Materials and Methods
Correlation of a-Tocopherol Concentration (as Measured by FTIR) to a-Tocopherol Content
Cubes (30×30×30 mm) were machined from annealed GUR1050 UHMWPE, packaged under argon gas with an oxygen scavenger (Fresh Pax™, Multisorb Technologies, Inc., Buffalo, NY), and gamma irradiated to 85 kGy. Thin sections (∼150 μm) were cut from these cubes using a sledge microtome (Model 90-91-1177, LKB-Produkter AB, Bromma Sweden) and immersed in a-tocopherol (DL-a-tocopherol, C29H50O2 - 430.72 g/mol, F. Hoffmann-La Roche AG, Basel Switzerland) at 120°C for varying durations. The thin sections were then homogenized at 120°C in argon gas for 24 hours. The thin sections were weighed before and after a-tocopherol doping and homogenization, and the weight increase was used to calculate an a-tocopherol weight percent for each section. The thin sections were analyzed via Fourier Transform Infrared Spectroscopy (FTIR), described below, and five spectra were collected at random since the a-tocopherol concentration was expected to be uniform throughout the sample. The a-tocopherol weight percents and the a-tocopherol index values from the individual thin sections were used to create a correlation curve.
Elution During Cleaning with Isopropyl Alcohol (IPA)
Test samples (30×30×10 mm blocks) were machined from annealed GUR1050 UHMWPE stock. These blocks were packaged under argon gas with an oxygen scavenger and gamma irradiated to 85 kGy. The blocks were subsequently doped in a-tocopherol at 120°C for 5 hours and homogenized at 120°C for 64 hours under argon gas.
Four groups of samples (n=3 each) were prepared: (1) Control samples, which were not subjected to any cleaning, (2) regular cleaning samples, which were soaked in IPA for 15-30 minutes and subsequently wiped, (3) multiple cleaning samples, which were soaked three consecutive times in IPA for 15-30 minutes each time and subsequently wiped, and (4) long soaked samples, which were soaked in IPA for 8 hours and subsequently wiped with IPA. The cleaning method used to create group (2) was a common protocol used by major orthopaedics manufacturers. The cleaning methods used to create groups (3) and (4) represented worst-case scenarios (i.e. if a component were accidentally subjected to multiple cleanings, or if a component were accidentally left soaking over an entire work shift). All four groups were analyzed using FTIR, described below, and the average bulk and surface tocopherol index values were converted to an a-tocopherol content by using the linear regression obtained from the correlation curve.
Oxidative Stability of a-Tocopherol Doped, Irradiated UHMWPE After Hip Simulator Testing
Acetabular liners (inner diameters of 28 and 36 mm) were machined from GUR1050 UHMWPE stock. The thickness of these liners was 4.9 mm. Each liner was individually packaged under argon gas with an oxygen scavenger and gamma irradiated to 85 kGy. Due to the reduced thickness of these liners compared to the 10 mm-thick blocks used for cleaning in IPA, the doping and homogenization times were shortened to ensure that the a-tocopherol concentration profiles were similar in all samples. Each liner was taken out of its packaging and doped in a-tocopherol at 120°C for 2 hours and subsequently homogenized at 120°C for 24 hours in argon gas. They were cooled to room temperature under argon gas and wiped clean with ethyl alcohol. These liners were further cleaned by soaking in IPA at room temperature for 15-30 minutes. The liners were subsequently packaged under argon gas with an oxygen scavenger were gamma sterilized. Conventional acetabular liners were also tested as a control set. These liners had a 28 mm ID and were also 4.9 mm thick. The acetabular liners were machined from GUR1050 UHMWPE stock, packaged in argon gas, and gamma sterilized.
Hip simulator testing was done in 100% bovine serum stabilized with 10.7 millimoles of ethylenediamine tetraacetate (EDTA, Fisher Scientific, Pittsburgh, PA) and 33 mL of penicillin-streptomycin solution (Sigma-Aldrich, St. Louis, MO) per 500 mL of serum. All testing was performed on the 12-station Boston hip simulator (AMTI, Watertown, MA) at 2 Hz for a total of 5 million cycles (MC). The kinematics used was a standard walking gait cycle with a peak load of 3000 N. All stations were temperature controlled at 37°C with circulating bovine serum. After hip simulator testing, the liners (n=2 of each group) were accelerated aged at 80°C in air for 5 weeks. Oxidation was then quantified by FTIR, as described below, at the region of the articular surface that was subjected to the maximum load during hip simulator testing.
Quantification of a-Tocopherol Concentration and Oxidation via FTIR
Fourier Transform Infrared Spectroscopy (FTIR, Bio-Rad FTS155/UMA500, Natick MA) was done on thin (∼150 μm) sections cut using a sledge microtome. Infrared spectra were collected from one edge of the sample to the other in 100 μm and 500 μm intervals, with each spectrum recorded as an average of 32 individual infrared scans. The infrared spectra were analyzed to calculate an a-tocopherol index as the ratio of the areas under the a-tocopherol absorbance at 1262 cm−1 and the polyethylene skeletal absorbance at 1895 cm−1. The limits of integration for the a-tocopherol absorbance were adjusted to 1245 and 1275 cm−1 and the limits of integration for the polyethylene skeletal absorbance at 1895 cm−1 were adjusted to 1850 and 1985 cm−1. The data are also reported as average surface and bulk a-tocopherol indices. The a-tocopherol indices measured in the first 500 μm from the surface and within a 1000 μm region in the center of the sample were averaged and reported as average surface and bulk a-tocopherol indices, STI and BTI respectively. Similarly, the oxidation index was obtained by calculating the area under the peak at 1740 cm−1 and normalizing to the methylene vibration at 1370 cm−1 for each infrared spectrum, as outlined in ASTM Standard F2102-01e1.
Results
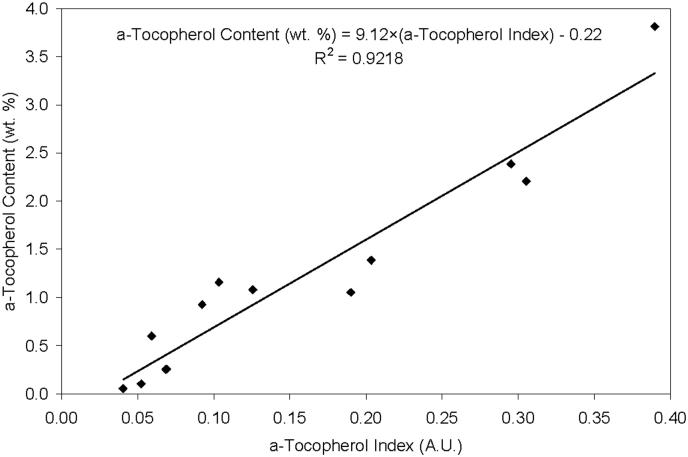
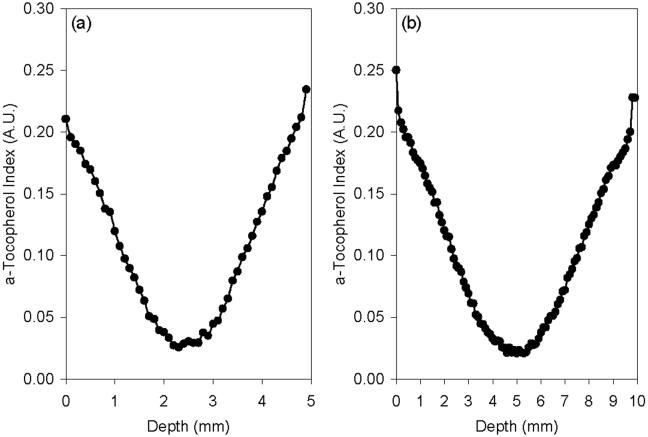
The a-tocopherol content scaled linearly with the a-tocopherol concentration index measured by infrared spectroscopy (Fig 2). Extrapolation of this regression line to zero vitamin E concentration gave a minimum a-tocopherol index of 0.024 as indicated by the x-intercept of the graph in Fig 2. This value represents the minimum a-tocopherol index that could be measured in polyethylene containing a-tocopherol by infrared spectroscopy. a-Tocopherol penetrated through the entire thickness of the 4.9 mm thick acetabular liners (Fig 3a) and 10 mm-thick samples used for cleaning in IPA (Fig 3b) following doping and homogenization at 120°C. The resulting a-tocopherol profiles for both the 4.9 mm and 10 mm samples were similar with a surface index value of 0.2-0.25 and a bulk index above 0.024 (Fig 3). The surface and bulk a-tocopherol content achieved were approximately 1.61 ± 0.18 and 0.04 ± 0.03 wt. %, respectively, in both sets of samples.
Fig 2.
Correlation between the a-tocopherol index (measured by FTIR) and the a-tocopherol content (measured gravimetrically) of a-tocopherol doped and homogenized UHMWPE.
Fig 3.
Representative a-tocopherol concentration profiles in (a) 4.9 mm thick and (b) 10 mm thick 85 kGy irradiated, a-tocopherol doped and homogenized UHMWPE.
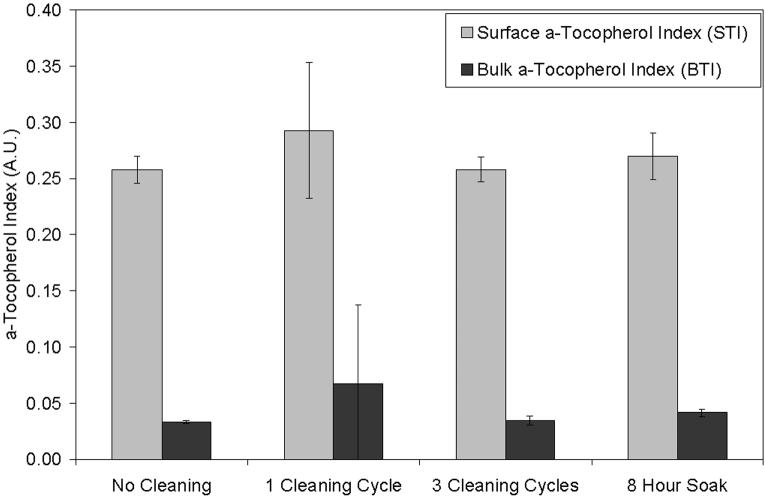
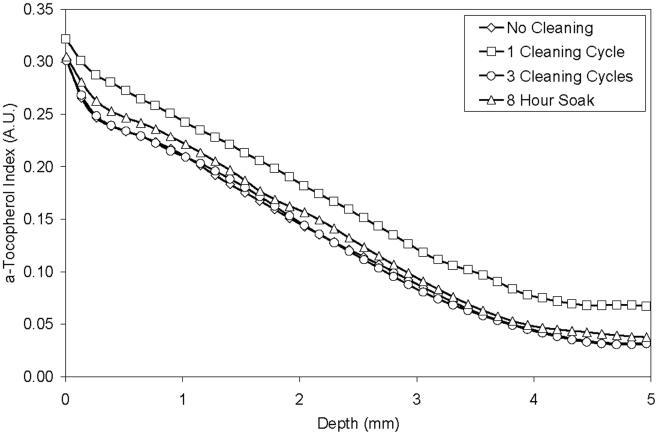
There was no substantial change in the average surface or bulk a-tocopherol indices (Fig 4) and corresponding concentrations (Table 1) of 85 kGy irradiated, a-tocopherol doped blocks after 1 cycle of cleaning in IPA, after 3 cycles of cleaning in IPA, or after an 8 hour soak in IPA. There were no substantial changes in the a-tocopherol concentration profiles of individual samples after cleaning in IPA (Fig 5).
Fig 4.
Average surface a-tocopherol index (STI) and bulk a-tocopherol index (BTI) of 85 kGy irradiated, a-tocopherol doped and homogenized UHMWPE samples (1) without cleaning, (2) after 1 regular cleaning cycle, (3) after 3 regular cleaning cycles, and (4) after an 8 hour soak in IPA (n=3 at least).
Table 1.
The average surface and bulk a-tocopherol indices (STI and BTI, respectively) for 85 kGy irradiated, a-tocopherol doped UHMWPE cleaned by different methods.
| STI | Calculated a-Tocopherol Content (wt. %) | BTI | Calculated a-Tocopherol Content (wt. %) | |
|---|---|---|---|---|
| Cleaning in Isopropyl Alcohol | ||||
| No Cleaning | 0.258 ± 0.012 | 2.13 ± 0.11 | 0.033 ± 0.001 | 0.08 ± 0.01 |
| 1 Cleaning Cycle | 0.293 ± 0.060 | 2.45 ± 0.55 | 0.060 ± 0.070 | 0.33 ± 0.44 |
| 3 Cleaning Cycles | 0.258 ± 0.011 | 2.13 ± 0.10 | 0.034 ± 0.004 | 0.09 ± 0.03 |
| 8 Hour Soak | 0.270 ± 0.021 | 2.24 ± 0.19 | 0.042 ± 0.003 | 0.16 ± 0.03 |
Fig 5.
a-Tocopherol concentration profiles of 85 kGy irradiated, a-tocopherol doped and homogenized UHMWPE samples (1) without cleaning, (2) after 1 regular cleaning cycle, (3) after 3 regular cleaning cycles, and (4) after an 8 hour soak in IPA. Each line represents a splined average of at least n=3 samples.
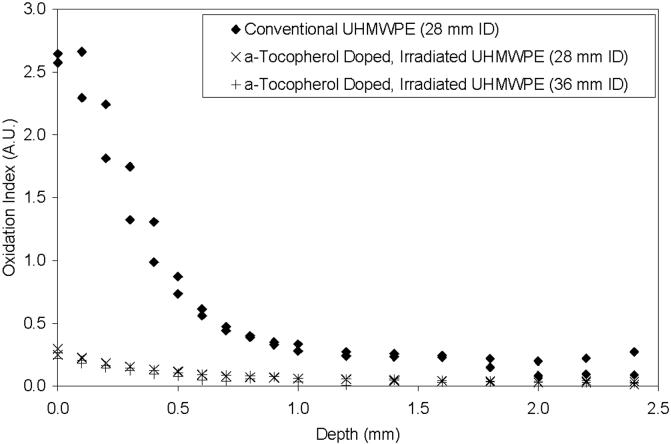
The conventional UHMWPE acetabular liners that were tested in the hip simulator were accelerated aged subsequently. These conventional liners showed very high levels of surface oxidation, which decreased towards the bulk of the sample, but nevertheless was detectable to approximately 2 mm below the articular surface (Fig 6). In contrast, the 85 kGy irradiated, a-tocopherol stabilized UHMWPE acetabular liners that were tested for 5-million cycles of simulated gait showed only baseline oxidation levels following subsequent accelerated aging (Fig 6).
Fig 6.
Oxidation profiles of acetabular liners after 5 million cycles of simulated gait and subsequent aging at 80°C in air for 5 weeks. Two samples from each group were analyzed.
Discussion
The aim of the present study was to determine the effect of probable manufacturing conditions and simulated in vivo conditions on the migration stability of a-tocopherol in irradiated UHMWPE. The hypothesis was that these conditions would lead to the extraction of a-tocopherol out of irradiated UHMWPE, which might lead to loss of oxidative stability. Our hypothesis tested negative. We found that the migration of a-tocopherol is negligible and we also found no adverse effects on the oxidative stability of a-tocopherol stabilized, irradiated UHMWPE acetabular liners following long-term simulated normal gait.
One of our aims was to investigate the differences in a-tocopherol concentration profile due solely to the cleaning procedure commonly used in manufacturing of joint implants. IPA is a polar and hydrophilic solvent but it can also dissolve a-tocopherol. Therefore we expected that the cleaning procedure involving IPA would cause significant migration of a-tocopherol, especially under adverse conditions with repeated application or exposure for a prolonged period of time; yet we could not detect any a-tocopherol migration under these conditions. This was presumably because of the low solubility of a-tocopherol in alcohol at room temperature and low diffusion rate of a-tocopherol in UHMWPE at room temperature [17, 18].
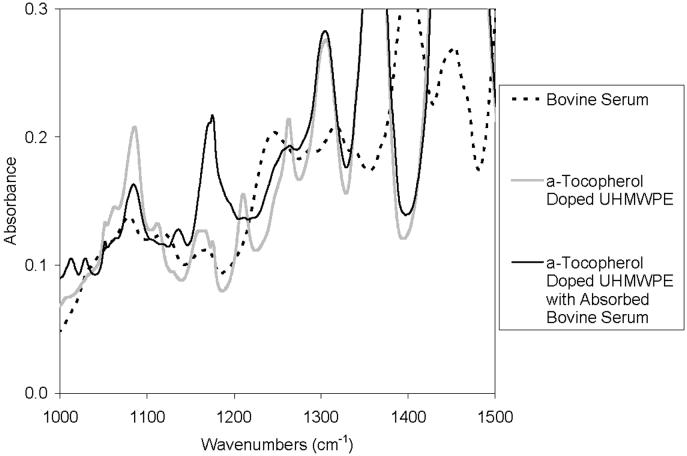
Another possible environment that could cause migration of a-tocopherol is the synovial fluid at body temperature. In addition to being in synovial fluid, the liners would be subjected to in vivo load and kinematics, the effect of which on a-tocopherol diffusion are not known. Synovial fluid is a hydrophilic environment; therefore, the driving force for the hydrophobic molecules of the a-tocopherol out of the polyethylene would be expected to be very small. However, the dissolved lipids and lipophilic components in synovial fluid could change the driving force of the fluid environment, enhancing the elution of a-tocopherol out of the liners. The typical testing environment used as an analogue to synovial fluid is bovine serum. We first attempted to measure the migration in a-tocopherol doped, irradiated UHMWPE acetabular liners after hip simulator testing in bovine serum. However, the a-tocopherol concentration profile of the acetabular liners tested in the hip simulator could not be determined accurately. This was caused by the diffusion of serum lipids into the components [19, 20], which created a broad absorbance peak at 1262 cm−1 when viewed under infrared spectroscopy. This broad peak convoluted the characteristic a-tocopherol absorbance peak that we used to determine the a-tocopherol concentration (Fig 7). We also attempted to remove the absorbed serum lipids by boiling the parts in hexane; however this also removed the a-tocopherol from the parts.
Fig 7.
Comparison of the fingerprint region in FTIR spectra of Bovine Serum, a-Tocopherol Doped UHMWPE, and a-Tocopherol Doped UHMWPE with Absorbed Bovine Serum. Note the broad peak in the bovine serum spectrum at approximately 1250 cm-1, interfering with the peak used to calculate the a-tocopherol index.
The alternative approach we used was to determine the oxidative stability of these acetabular liners after in vitro testing. We postulated that, with the substantial migration of a-tocopherol during simulated gait, the liners would be prone to oxidation. Following accelerated aging of the liners after in vitro testing (Fig 6), we found that the oxidative stability of 85 kGy irradiated, a-tocopherol doped and gamma sterilized UHMWPE was much higher than that of conventional, gamma-sterilized UHMWPE. This indicated that the a-tocopherol elution, if any, did not compromise the oxidative stability of the material.
Although a-tocopherol concentration profiles could not be directly determined, the weight loss of the a-tocopherol doped liners was approximately 5 mg in 5 million cycles of testing [9]. It has been shown that an a-tocopherol concentration as low as 0.1 wt% could protect 100 kGy irradiated UHMWPE against oxidation [8, 16, 21]. The acetabular liners used in this study contained approximately 100 mg of a-tocopherol (∼1 wt. %). If the entire weight loss that occurred during 5 million cycles of hip simulator testing were to be migration of a-tocopherol, it would take approximately 90 million cycles to bring the a-tocopherol concentration to 0.1 wt. %. This would approximately translate to 90 years of in vivo service to reduce the a-tocopherol concentration to levels that can still protect irradiated UHMWPE against oxidation. Even this extreme case would not be expected to lead to loss of enough a-tocopherol to jeopardize the oxidative stability of a-tocopherol doped, irradiated UHMWPE in vivo.
Conclusion
Our hypothesis tested negative - the elution of a-tocopherol out of 85 kGy irradiated, a-tocopherol doped UHMWPE components during the fabrication process and during in vivo load and kinematics was negligible. We showed that in environments that simulated probable chemical driving forces for elution of a-tocopherol out of the UHMWPE component, very little a-tocopherol elution was observed. Thus, we conclude that a-tocopherol migration in an irradiated, a-tocopherol doped and terminally gamma sterilized UHMWPE is not expected to adversely affect long-term oxidative stability.
Acknowledgements
This work was funded by NIH R01 AR051142-02 and a research grant from Biomet, Inc.
This study was funded by NIH R01 AR051142 and a research grant from Biomet, Inc.
References
- 1.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Unified Wear Model for Highly Crosslinked Ultra-high Molecular Weight Polyethylenes (UHMWPE) Biomaterials. 1999;20(16):1463–1470. doi: 10.1016/s0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH. 1999 HAP Paul Award. A novel method of crosslinking UHMWPE to improve wear, reduce oxidation and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 3.Muratoglu OK, O'Connor DO, Bragdon CR, Delaney J, Jasty M, Harris WH, Merrill EW, Venugopalan P. Gradient crosslinking of UHMWPE using irradiation in molten state for total joint arthroplasty. Biomaterials. 2001;23:717–724. doi: 10.1016/s0142-9612(01)00176-4. [DOI] [PubMed] [Google Scholar]
- 4.Muratoglu OK, Bragdon CR, O'Connor DO, Perinchief RS, Estok DM, Jasty M, Harris WH. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: A new concept. J Arthroplasty. 2001;16(8 suppl):24–30. doi: 10.1054/arth.2001.28376. [DOI] [PubMed] [Google Scholar]
- 5.Muratoglu OK, Merrill EW, Bragdon CR, O'Connor DO, Hoeffel D, Burroughs B, Jasty M, Harris WH. Effect of Radiation, Heat, and Aging on In Vitro Wear Resistance of Polyethylene. Clinical Orthopaedics & Related Research. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 6.McKellop H, Shen F-W, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant ultra-high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 7.Oral E, Malhi A, Muratoglu O. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006 doi: 10.1016/j.biomaterials.2005.06.025. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. α-Tocopherol Doped Irradiated UHMWPE for High Fatigue Resistance and Low Wear. Biomaterials. 2004;25(24):5515–5522. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Oral E, Christensen S, Malhi A, Wannomae K, Muratoglu O. Wear resistance and mechanical properties of highly crosslinked UHMWPE doped with vitamin E. Journal of Arthroplasty. 2005 doi: 10.1016/j.arth.2005.07.009. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 11.Packer L, Kagan VE. Vitamin E: The antioxidant harvesting center of membranes and lipoproteins. In: Packer L, Fuchs J, editors. Vitamin E in Health and Disease. Marcel Dekker, Inc.; New York: 1993. [Google Scholar]
- 12.Burton G, Ingold K. Autoxidation of Biological Molecules. 1. The Antioxidant Activity of Vitamin E and Related Chain-Breaking Phenolic Antioxidants in Vitro. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]
- 13.Burton GW, Traber MG. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annual Reviews in Nutrition. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 14.Kamal-Eldin A, Appelqvist L. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 15.Mori A, Sakuramoto I, Tomita N, Kawano S, Nagata K, Utsumi K, Moriya H. Mechanical behavior of UHMWPE when mixed with vitamin E; 47th Annual Meeting, Orthopaedic Reseach Society; 2001. [Google Scholar]
- 16.Oral E, Greenbaum E, Malhi A, Muratoglu O. Characterization of blends of α-Tocopherol with UHMWPE. Biomaterials. 2005;26:6657–6663. doi: 10.1016/j.biomaterials.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oral E, Wannomae K, Huang A, Rowell S, Muratoglu O. α-Tocopherol diffusion into UHMWPE. I. Diffusion and homogenization in highly cross-linked UHMWPE. Biomaterials. 2006 (submitted) [Google Scholar]
- 18.Wannomae K, Rowell S, Muratoglu O. α-Tocopherol diffusion into UHMWPE. II. Model prediction of α-tocopherol diffusion and homogenization. Biomaterials. 2006 (submitted) [Google Scholar]
- 19.James SP, Blazka S, Merrill EW, Jasty M, Lee KR, Bragdon CR, Harris WH. Challenge to the concept that UHMWPE acetabular components oxidize in vivo. Biomaterials. 1993;14(9):643–7. doi: 10.1016/0142-9612(93)90062-7. [DOI] [PubMed] [Google Scholar]
- 20.Costa L, Bracco P, del Prever EB, Luda MP, Trossarelli L. Analysis of products diffused into UHMWPE prosthetic components in vivo. Biomaterials. 2001;22(4):307–315. doi: 10.1016/s0142-9612(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 21.Oral E, Wannomae K, Muratoglu O. The effect of doping conditions on α-tocopherol stabilized UHMWPE; Transactions, 51st Annual Meeting of the Orthopaedic Research Society; 2005. [Google Scholar]