Abstract
More than 30 Curcuma species (Zingiberaceae) are found in Asia, where the rhizomes of these plants are used as both food and medicine, such as in traditional Chinese medicine. The plants are usually aromatic and carminative, and are used to treat indigestion, hepatitis, jaundice, diabetes, atherosclerosis and bacterial infections. Among the Curcuma species, C. longa, C. aromatica and C. xanthorrhiza are popular. The main constituents of Curcuma species are curcuminoids and bisabolane-type sesquiterpenes. Curcumin is the most important constituent among natural curcuminoids found in these plants. Published research has described the biological effects and chemistry of curcumin. Curcumin derivatives have been evaluated for bioactivity and structure-activity relationships (SAR). In this article, we review the literature between 1976 and mid-2008 on the anti-inflammatory, anti-oxidant, anti-HIV, chemopreventive and anti-prostate cancer effects of curcuminoids. Recent studies on curcuminoids, particularly on curcumin, have discovered not only much on the therapeutic activities, but also on mechanisms of molecular biological action and major genomic effects.
Background
Curcuma species
In Asia zingiberaceous plants have been used since ancient times as both spices and medicines, such as in traditional Chinese medicine. Within this plant family, various Curcuma species, particularly C. longa (turmeric), C. aromatica (wild turmeric), and C. xanthorrhiza (Javanese turmeric), have been used. The rhizomes of these plants are usually aromatic and carminative, and are used to treat indigestion, hepatitis, jaundice, diabetes, atherosclerosis and bacterial infections [1,2].
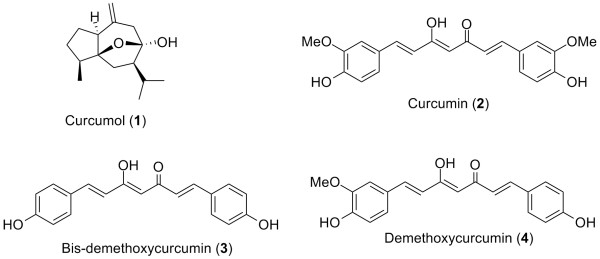
Isolated from Curcuma plants, various bioactive compounds are useful medicines. For example, curcumol (1) (Figure 1), a sesquiterpene isolated from C. aromatica, is useful in treating cervical cancer [3].
Figure 1.
Structures of curcumol and curcuminoids in Curcuma species.
The rhizomes of C. longa, commonly known as turmeric, are used worldwide as spices (e.g. curry), flavoring agents, food preservatives and coloring agents. They are also used as medicines to treat inflammation and sprains in India, China and other Asian countries. Curcuminoids, the main components in Curcuma species, share a common unsaturated alkyl-linked biphenyl structural feature and are responsible for their major pharmacological effects. The biological and chemical properties of curcuminoids were reported [4-9].
Curcuminoids in C. longa and other Curcuma species are mainly curcumin (2), bis-demethoxycurcumin (3) and demethoxycurcumin (4) (Figure 1), among which curcumin is the most studied and shows a broad range of biological activities. This article highlights some of the important biological properties of curcumin and its derivatives, as well as their structure-activity relationships (SAR).
C. xanthorrhiza is used as a tonic in Indonesia and a choleric drug in Europe. Apart from curcuminoids, this species contains bioactive bisabolane-type compounds, such as α-curcumen (5), ar-turmerone (6) and xanthorrhizol (7) (Figure 2). These three compounds demonstrated strong anti-cancer activities against Sarcoma 180 ascites in mice [10-15]. In addition, xanthorrhizol (7) exhibited antibacterial activity [16].
Figure 2.
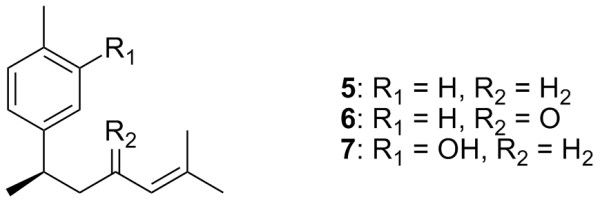
Structure of bisabolane-type compounds in Curcuma species.
Curcumin and its biological activities
Curcumin (2) [diferuloylmethane, 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is the main yellow constituent isolated from C. longa and other Curcuma species. It was first isolated in 1870, but its chemical structure had not been elucidated until 1910 [17] and was subsequently confirmed by synthesis. Curcumin has a unique conjugated structure including two methylated phenols linked by the enol form of a heptadiene-3,5-diketone that gives the compound a bright yellow color.
In addition to its well known anti-inflammatory effects, curcumin also possesses other therapeutic effects on numerous biological targets [18]. Other activities of curcumin include reduction of blood cholesterol level, prevention of low density lipoprotein (LDL) oxidation, inhibition of platelet aggregation, suppression of thrombosis and myocardial infarction, suppression of symptoms associated with type II diabetes, rheumatoid arthritis, multiple sclerosis and Alzheimer's disease, inhibition of human immunodeficiency virus (HIV) replication, enhancement of wound healing, increase of bile secretion, protection from liver injury, cataract formation and pulmonary toxicity and fibrosis, exhibition of anti-leishmaniasis and anti-atherosclerotic properties, as well as prevention and treatment of cancer [18]. Curcumin is non-toxic even at high dosages, and has been classified as 'generally recognized as safe' (GRAS) by the National Cancer Institute [19]. There were also studies focusing on the biology and action mechanisms of curcumin [18,20].
Synthetic bioactive curcumin analogs were developed from the natural compound based on the structure-activity relationship (SAR) studies and optimization of compounds as drug candidates in their relations to different activities, including anti-inflammatory, anti-oxidant, anti-HIV, chemopreventive and anti-cancer (prostate cancer), as well as possible action mechanisms.
Anti-inflammation
Anti-inflammatory activity
Curcumin inhibits the metabolism of arachidonic acid, activities of cyclooxygenase, lipoxygenase, cytokines (interleukins and tumor necrosis factor), nuclear factor-κB (NF-κB) and release of steroids [21]. Curcumin stabilizes lysosomal membranes and causes uncoupling of oxidative phosphorylation. It also possesses strong oxygen radical scavenging activity, which confers anti-inflammatory properties. In various animal studies, a dose of curcumin at 100–200 mg per kilogram of body weight exhibited anti-inflammatory activity. The same dose did not have obvious adverse effects on human systems. Oral median lethal dose (LD50) in mice is higher than 2.0 g/kg of body weight [21].
Pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), play key roles in the pathogenesis of osteoarthritis (OA). Anti-inflammatory agents that can suppress the production and catabolic actions of these cytokines may have therapeutic effects on OA and some other osteoarticular disorders. Accordingly, curcumin was examined for its effects on IL-1β and TNF-α signaling pathways in human articular chondrocytes in vitro [22]. Expression of collagen type II, integrin β1, cyclo-oxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9) genes was monitored by Western blotting. The effects of curcumin on the expression, phosphorylation, and nuclear translocation of protein components of the NF-κB system were studied with Western blotting and immunofluorescence respectively. The results indicated that curcumin suppressed IL-1β-induced NF-κB activation via inhibition of inhibitory protein κBα (IκBα) phosphorylation, IκBα degradation, p65 phosphorylation and p65 nuclear translocation. Curcumin also inhibited IL-1β-induced stimulation of up-stream protein kinase B Akt. These events correlated with the down-regulation of NF-κB targets, including COX-2 and MMP-9. Similar data were obtained when chondrocytes were stimulated with TNF-α. Curcumin also reversed the IL-1β-induced down-regulation of collagen type II and β1-integrin receptor expression. These results indicate that curcumin may be a naturally occurring anti-inflammatory nutritional agent for treating OA via suppression of NF-κB mediated IL-β/TNF-α catabolic signaling pathways in chondrocytes [22]. Curcumin was found to act by diverse anti-inflammatory mechanisms at several sites along the inflammation pathway [23].
Anti-inflammatory SAR
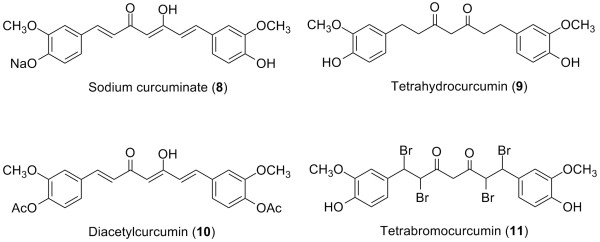
The active constituents of C. longa are curcuminoids, including curcumin (2), demethoxycurcumin (3) and bisdemethoxycurcumin (4) [24] (Figure 1), among which curcumin is the most potent anti-inflammatory agent [25]. In addition to these natural curcuminoids, sodium curcuminate (8) and tetrahydrocurcumin (9) (Figure 3) showed potent anti-inflammatory activity at low doses in carrageenin-induced rat paw edema and cotton pellet granuloma assays [26]. Other semi-synthetic analogs of curcumin were screened for anti-inflammatory activity in the same assays; diacetylcurcumin (10) and tetrabromocurcumin (11) (Figure 3) were the most potent [27,28]. The presence of the β-diketone moiety as a linker between the two phenyl groups was deemed important for the anti-inflammatory activity.
Figure 3.
Structures of semi-synthetic analogs tested for anti-inflammatory activity.
Nurfina et al. designed and synthesized 13 symmetrical curcumin analogs (12–24) [29]. Anti-inflammatory activity was evaluated by inhibition of carrageenin-induced swelling of rat paw (Table 1); and the following SAR conclusions were drawn: (a) appropriate substituents on the phenyl rings were found necessary for anti-inflammatory activity. Unsubstituted compound 12, ortho-methoxy, substituted analog 18, and meta-methoxy substituted analog 13 showed no inhibitory activity; (b) proper substituents at the para-positions of the phenyl rings were also crucial. A para-phenolic group leads to the most potent anti-inflammatory activity [compare 3 (p-OH), 21 (p-CH3), 20 (p-OCH3), 19 (p-Cl) as well as 2 with 22 and 24 with 14]; and (c) size of the substituents adjacent to a para-phenol was found to be important for potency. Dimethyl substitution (15) at R2 and R4 enhanced the activity most, followed by diethyl (16) and dimethoxy (24). Compound 21 with two isopropyl moieties showed weaker activity, while 23 with bulky tetrabutyl substitution at both positions showed no anti-inflammatory activity.
Table 1.
Anti-inflammatory activity data of curcumin derivatives 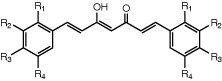
| Compound | R1 | R2 | R3 | R4 | ED50 (mg/kg) |
| 2 | H | OCH3 | OH | H | 38 ± 4 |
| 3 | H | H | OH | H | 73 ± 5 |
| 12 | H | H | H | H | NA |
| 13 | H | OCH3 | H | H | NA |
| 14 | H | OCH3 | OCH3 | OCH3 | NA |
| 15 | H | CH3 | OH | CH3 | 13 ± 2 |
| 16 | H | C2H5 | OH | C2H5 | 22 ± 6 |
| 17 | H | i-C3H7 | OH | i-C3H7 | 58 ± 21 |
| 18 | OCH3 | H | H | H | NA |
| 19 | H | H | Cl | H | NA |
| 20 | H | H | OCH3 | H | 82 ± 7 |
| 21 | H | H | CH3 | H | 80 ± 18 |
| 22 | H | OCH3 | OCH3 | H | 50 ± 22 |
| 23 | H | t-C4H9 | OH | t-C4H9 | NA |
| 24 | H | OCH3 | OH | OCH3 | 28 ± 5 |
NA: not active
ED50 values are expressed as 'means ± standard deviations'.
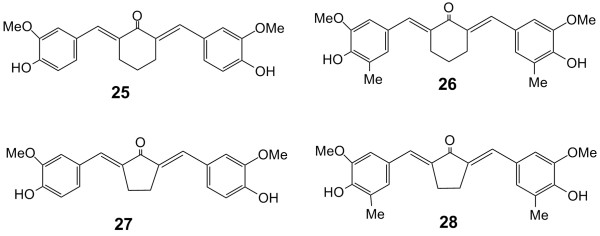
Cyclovalone (25) and three analogs (26–28) (Figure 4) having a cyclohexanone or cyclopentanone in the linker between the two phenyl rings showed anti-inflammatory activity to inhibit cyclooxygenase [30]. Compounds 26–28 were more potent than curcumin (2) which was used as a reference standard. The dimethylated 28 and 26 were more potent than 27 and 25 respectively, and thus, the addition of methyl groups on the phenyl rings enhanced anti-inflammatory activity. The increased size of the cycloalkanone ring, by replacing the cyclopentanone in 27 with a cyclohexanone in 25, increased inhibitory potency. However, this effect was not seen in the dimethylated compounds 28 and 26 respectively, both of which were comparably potent.
Figure 4.
Structures of cyclovalone (25) and three related analogs.
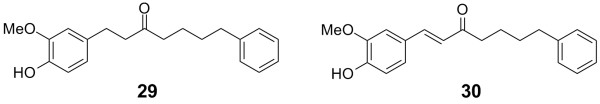
Besides curcumin, other structurally related constituents of plants in the Zingiberaceae family possess anti-inflammatory activity [31]. Examples are the phenolic yakuchinones A and B (29 and 30) isolated from Alpinia oxyphylla [32-34] (Figure 5).
Figure 5.
Structures of yakuchinones A (29) and B (30).
Anti-oxidation
Anti-oxidant activity
Most natural anti-oxidants can be classified into two types of compounds, namely phenolic and β-diketone [35]. Sesaminol isolated from sesame belongs to the former, while n-triacontane-16,18-dione isolated from the leaf wax of Eucalyptus belongs to the latter. Curcumin (2) is one of the few anti-oxidants that possess both phenolic hydroxy and β-diketone groups in one molecule. Its unique conjugated structure includes two phenols and an enol form of a β-diketone. Therefore, it may have a typical radical trapping ability and a chain-breaking anti-oxidant activity.
Curcumin is a potent anti-oxidant whose action mechanism is not well understood. However, the nonenzymatic anti-oxidant process of a phenolic compound is generally thought to have two stages as follows:
| S-OO• + AH ↔ SOOH + A• |
| A• + X• → nonradical materials |
Where S is the oxidized substance; AH is the phenolic anti-oxidant; A• is the anti-oxidant radical; and X• is another radical species or the same species as A• [35]. While the first stage is reversible, the second stage is irreversible and must produce stable radical terminated compounds. Structural elucidation of the terminated compounds may contribute significantly to understanding the mechanism of the phenolic anti-oxidant. It has recently been shown that dimerization is a main termination process of the radical reaction of curcumin itself. In food, the anti-oxidant coexists with large amounts of oxidizable biomolecules, such as polyunsaturated lipids. These biomolecules were found to produce reactive peroxy radicals during their oxidation, which may act as X• and couple with the anti-oxidant radical (A•) in the second step of the above anti-oxidation scheme [36].
Anti-oxidant SAR
Curcumin showed both anti-oxidant and pro-oxidant effects in oxygen radical reactions. Depending on the experimental conditions, it may act as a scavenger of hydroxy radicals or a catalyst in the formation of hydroxy radicals [37-39]. The anti-oxidant effect of curcumin presumably arises from scavenging of biological free radicals.
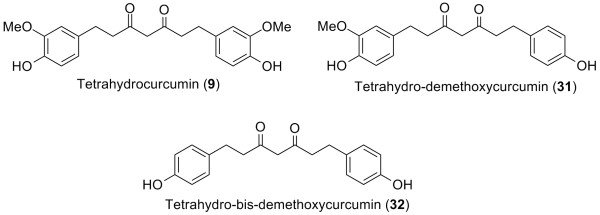
The anti-oxidant activities of three natural curcuminoids (2–4) and their hydrogenated analogs (9, 31, 32) (Figure 6) were examined in three bioassay models, i.e. the linoleic acid auto-oxidation model, rabbit erythrocyte membrane ghost system, and rat liver microsome system. The results obtained from the three models were consistent. Curcumin (2) and tetrahydrocurcumin (9) had the strongest anti-oxidant activity among the natural and hydrogenated curcuminoids respectively [35]. Among all six compounds, tetrahydrocurcumin (9) showed the highest potency, implying that hydrogenation of curcuminoids increased their anti-oxidant ability. Absence of one or both methoxy groups resulted in decreased anti-oxidant activity in both natural curcuminoids and tetrahydrocurcuminoids. In contrast, Sharma et al. reported that the presence of methoxy groups in the phenyl rings of curcumin enhanced anti-oxidant activity [40].
Figure 6.
Structures of tetrahydrocurcuminoids.
Venkatessan et al. [41] used three models to investigate the importance of the phenolic hydroxy groups, as well as other substituents on the phenyl rings of curcuminoids, to anti-oxidant activity. The three anti-oxidant bioassays were inhibition of lipid peroxidation, free radical scavenging activity by the DPPH method, and free radical scavenging activity by the ABTS method. The data and compound structures are shown in Table 2. Generally, curcumin analogs with a phenolic moiety were more potent than non-phenolic analogs, and thus, phenolic substitution is important for anti-oxidant activity. Compound 15, a 4'-hydroxy-3',5'-dimethyl substituted analog, showed potency in all three bioassays. However, compound 23, a 4'-hydroxy-3',5'-di-t-butyl analog, was ten-fold less potent in the lipid peroxidation assay, indicating that steric hindrance at the positions flanking the hydroxyl group decreased anti-oxidative activity. Changing the 3'-methoxy group in curcumin (2) to an ethoxy group in 33 had little effect on anti-oxidant activity, but both compounds were more potent than 3, which does not have an alkoxy group at the 3'-position. In all three systems, tetrahydrocurcumin (9) and curcumin (2) showed comparable activity. This result suggests that enhanced electron delocalization of the double bonds may not be essential to anti-oxidant activity of curcuminoids.
Table 2.
Anti-oxidant activity data of curcumin derivatives 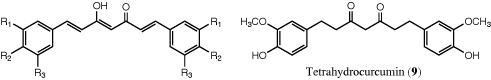
| Compound | R1 | R2 | R3 | Lipid peroxidation inhibition IC50 (μM) | DPPH scavenging IC50 (μM) | ABTS scavenging TEAC | ||
| 3 min | 9 min | 15 min | ||||||
| 2 | OCH3 | OH | H | 1.30 | 20.02 | 2.61 | 3.09 | 3.37 |
| 3 | H | OH | H | 2.19 | 32.08 | 3.04 | 4.31 | 4.96 |
| 9 | structure formula 9 above | 1.83 | 18.22 | 2.08 | 2.37 | 2.52 | ||
| 10 | OCH3 | OAc | H | 1.85 | NA | 1.33 | 2.01 | 2.33 |
| 12 | H | H | H | NA | >250 | 1.57 | 2.78 | 3.36 |
| 14 | OCH3 | OCH3 | OCH3 | 15.32 | NA | 1.90 | 2.98 | 3.43 |
| 15 | CH3 | OH | CH3 | 0.63 | 21.75 | 0.89 | 1.13 | 1.28 |
| 20 | H | OCH3 | H | NA | >250 | 2.05 | 2.04 | 2.14 |
| 21 | H | CH3 | H | NA | >250 | 0.67 | 1.52 | 1.96 |
| 22 | OCH3 | OCH3 | H | NA | >250 | 1.86 | 2.49 | 2.67 |
| 23 | t-C4H9 | OH | t-C4H9 | 6.48 | 23.72 | 0.81 | 0.96 | 1.07 |
| 33 | OC2H5 | OH | H | 1.11 | 30.32 | 2.36 | 3.07 | 3.32 |
| 34 | H | SCH3 | H | NA | NA below 90 | 1.09 | ND | ND |
IC50 is the concentration required for 50% inhibition of lipid peroxidation or scavenging of DPPH radical. TEAC is the trolox equivalent anti-oxidation capacity, which is defined as the mM concentration of a trolox solution having the antioxidant capacity equivalent to a 1.0 mM solution of the substance under investigation.
NA: not active
ND: not determined.
The anti-oxidant mechanisms of curcumin have been investigated. The salient finding is that curcumin is a phenolic chain-breaking anti-oxidant, which donates H atoms from the phenolic groups [42-47]. However, some contrasting results suggest that H atom donation takes place at the active methylene group in the diketone moiety [48,49]. Ligeret et al. evaluated the effects of curcumin and numerous derivatives on the mitochondrial permeability transition pore (PTP), which can release apoptogenic factors from mitochondria to induce apoptosis [50]. The authors postulated that PTP opening is closely related to the anti-oxidant property of curcumin. Based on the data on mitochondria swelling, O2• and HO• production, thiol oxidation and DPPH• reduction, the authors concluded that phenolic groups in curcuminoids are essential for activity, and are more effective at the para position than at the ortho position. In addition, an electron donating group at the ortho position relative to the phenolic group is also required for activity, while t-butyl and bulky substituents are not favorable. In contrast, electron-withdrawing substitution, such as NO2, reduced activity. Although ferulic acid does not show anti-oxidant effects, replacing the β-diketone moiety of curcumin with a cyclohexanone ring attenuated anti-oxidant activity. Thus, the authors concluded that the β-diketone contributed to, but could not induce, the activity of curcumin derivatives. The conclusions agree with the prevailing SAR for anti-oxidant activity.
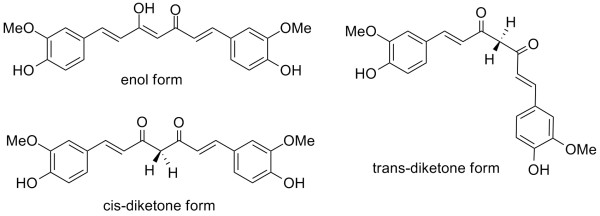
However, in one study, a curcumin analog without phenolic and methoxy groups was found to be as potent as curcumin in terms of scavenging hydroxy radicals and other redox properties [51]. Wright employed theoretical chemistry to interpret the controversy [52]; taking into account the diversity of test free radicals, solvents, and pH ranges used in the literature. First, he explored the stabilities of curcumin conformers, pointing out that the enol form is the most stable, followed by the trans-diketo form, and then the cis-diketo form (Figure 7). Calculations showed that the phenolic O-H is the weakest bond in curcuminoids. This theoretical approach favors the necessity of a phenolic OH group for the anti-oxidant activity of curcumin and its analogs. However, the C-H bond of the methylene group becomes active when radicals with high bond dissociation enthalpy, such as methyl and t-butoxy radicals, are used. Thus, differences among experimental results can be possibly due to the differences in the attacking radicals used in different bioassay systems.
Figure 7.
Structures of curcumin conformers.
Anti-HIV
Anti-HIV activity
Oxidative stress is implicated in HIV-infection. It was suggested that plant anti-oxidants may offer protection from viral replication and cell death associated with oxidative stress in patients with HIV/acquired immune deficiency syndrome (AIDS) [53]. Curcumin (2) can inhibit purified HIV type 1 integrase, HIV-1 and HIV-2 protease, and HIV-1 long terminal repeat-directed gene expression of acutely or chronically infected HIV-1 cells. Curcumin can also inhibit lipopolysaccharide-induced activation of NF-κB, a factor involved in the activation and replication of HIV-1. However, curcumin did not show significant efficacy in clinical trials.
In addition to the lipid soluble component curcumin, turmeric also contains the water-soluble extract turmerin (molecular weight: 24000 Daltons). Neither turmeric nor turmerin has been studied for anti-HIV activity. In a limited number of studies, cell viability and p24 antigen release by CEMss-T cells infected with HIV-IIIB strain (acute infection model) and proliferative responses of human mononuclear cells derived from HIV patients (chronic infection model) stimulated with phytohematoglutinin, concanavalin A, and pokeweed mitogen were examined in the presence of AZT, curcumin, and turmerin. In infective assays, neither turmerin nor curcumin individually reduced p24 antigen release or improved cell viability [53]. However, AZT (5 μM) plus turmerin (800 ng/ml) inhibited infection by 37% and increased cell numbers by 30%. In the proliferation assay, lymphocytes from HIV-infected patients showed better inhibition of mitogen responsiveness to turmerin (800 ng/ml) than that of AZT at 5 μM or turmerin at 80 ng/ml. Turmerin inhibited HIV-infected T-cell proliferation and, in combination with AZT, decreased T-cell infection and increased cell viability. These data suggest that effective anti-HIV therapy may be possible using lower, less toxic doses of AZT in the presence of turmerin [53].
Anti-HIV SAR
In addition to reverse transcriptase and protease, HIV-1 integrase is being explored as a new target for the discovery of effective AIDS treatments. HIV-1 integrase is the enzyme that catalyzes the integration of the double-strained DNA of HIV into the host chromosome [54]. Curcumin inhibited this activity of HIV-1 integrase [54]. Other classes of compounds inhibited HIV-1 integrase in enzyme assays, but few showed specificity against HIV-1 integrase and even fewer were active in cell-based assays [55]. Curcumin was reported to have moderate activity in cell-based assays, in addition to its activity in enzyme assays [56].
Therefore, modified curcumin analogs were developed for anti-HIV potency as well as action mechanism studies [54,57]. Mazumder et al. [57] synthesized curcumin analogs (Table 3) as probes to study the mechanism of anti-HIV-1 integrase. Evidence suggests that curcumin does not bind to HIV-1 integrase at either the DNA-binding domain [58] or the binding site of another HIV-1 integrase inhibitor, i.e. NSC 158393 [59]. Compounds without a hydroxy group on the phenyl ring (12, 20) did not inhibit HIV-1 integrase. Therefore, hydroxy groups on the phenyl rings are apparently essential for inhibitory activity. Compounds 35 and 36, which contain two and one catechol ring respectively, exhibited much greater activity than curcumin (2), indicating that replacing one or both methoxy groups on curcumin with hydroxy groups increased anti-HIV activity. Tetrahydrocurcumin (9), with a saturated linker between the phenyl groups, did not show inhibitory activity in this assay, suggesting that an unsaturated linking group also contributed to activity. In addition, compound 37, with a unique linker bridging two catechol rings, showed potency comparable to that of 35 and 36, and greater than that of 2.
Table 3.
Anti-HIV integrase activity data of curcumin derivatives 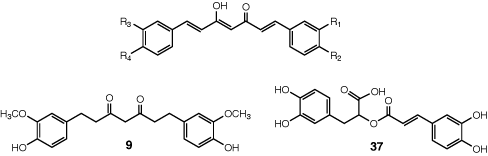
| Compound | R1 | R2 | R3 | R4 | 3-processing IC50 (μM) | Strand transfer IC50 (μM) |
| 2 | OCH3 | OH | OCH3 | OH | 150 | 140 |
| 3 | H | OH | H | OH | 120 | 80 ± 20 |
| 4 | H | OH | OCH3 | OH | 140 | 120 |
| 9 | structure formula 9 above | >300 | >300 | |||
| 12 | H | H | H | H | >300 | >300 |
| 20 | H | OCH3 | H | OCH3 | >300 | >300 |
| 35 | OH | OH | OH | OH | 6.0 ± 1.5 | 3.1 ± 0.12 |
| 36 | OCH3 | OH | OH | OH | 18.0 ± 9.0 | 9.0 ± 3.0 |
| 37 | structure formula 37 above | 9 ± 7 | 4.0 ± 1.5 | |||
IC50 values are expressed as 'means ± standard deviations'.
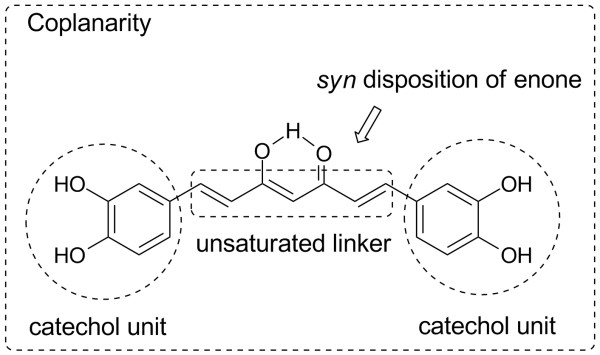
In the further SAR investigation of curcumin analogs as inhibitors of HIV-1 integrase, a syn disposition of the C=C=C=O moiety in the linker and a coplanar structure were found to be important to the integrase inhibitory activity of curcumin analogs [55]. The experimental results are consistent with the quantitative structure-activity relationships (QSAR) computed with MOE (Chemical Computing Group, Canada) and Cerius2 (Molecular Simulations, USA) programs [60]. Figure 8 summarizes the anti-HIV-1 integrase SAR of curcumin analogs. However, no therapeutic indices were reported for the tested compounds.
Figure 8.
Schematic diagram of structural features favoring anti-HIV-1 integrase activity.
Chemoprevention
Chemoprevention is a relatively new concept. It attempts to intervene at early stages of cancer before the invasive stage begins [61]. Nontoxic agents are administered to otherwise healthy individuals who may be at increased risk for cancer. Some potential diet-derived chemopreventive agents include epigallocatechin gallate in green tea, curcumin in curry and genistein in soya. Curcumin demonstrated a wide-range of chemopreventive activities in preclinical carcinogenic models of colon, duodenum, fore-stomach, mammary, oral and sebaceous/skin cancers. The National Cancer Institute is conducting Phase I clinical trials of curcumin as a chemopreventive agent for colon cancer [62]. Curcumin's chemopreventive mechanisms are pleiotropic. It enhanced the activities of Phase 2 detoxification enzymes of xenobotic metabolism, including glutathione transferase [63] and NADPH:quinone reductase [64]. It also inhibited pro-carcinogen activating Phase 1 enzymes such as cytochrome P450 1A1 [65]. As regards its mode of chemopreventive action in colon cancer, curcumin exhibited diverse metabolic, cellular and molecular activities including inhibition of arachidonic acid formation and its further metabolism to eicosanoids [66].
Anti-prostate cancer
Prostate cancer is the most common cancer among males in the West [67] and is a complex heterogeneous disease that affects different men differently. The cause of prostate cancer is largely unknown. However, androgen and the androgen receptor (AR) are postulated to play crucial roles in the development of prostate cancer [68].
Prostate cancer is currently treated with a combination of surgery, radiation and chemotherapy. The therapeutic agents used clinically include steroidal anti-androgens, such as cyproterone acetate, and non-steroidal anti-androgens, such as flutamide and bicartamide. The steroidal anti-androgens possess partial agonistic activity and overlapping effects with other hormonal systems, leading to complications such as severe cardiovascular problems, gynecomastia, libido loss and erectile dysfunction [69-71]. Non-steroidal anti-androgens have fewer side effects and higher oral bioavailability than steroidal anti-androgens.
While non-steroidal anti-androgens are advantageous, anti-androgen withdrawal syndrome was found in patients receiving non-steroidal anti-androgens for several months [72,73]. Long-term drug usage would lead to mutation of the AR, and the non-steroidal anti-androgens may exhibit agonistic activity to the mutant AR [74]. In addition, the clinically available anti-androgens are unable to kill prostate cancer cells, and within one to three years of drug administration, the cancer usually develops into an androgen refractory stage [72-74]. Therefore, new classes of anti-prostate cancer drugs are urgently needed.
Prostate cancer occurs much less frequently in Asia than in the West [75], possibly due to dietary differences. Turmeric is much more highly consumed as both spice and medicine in India, Thailand, China and Japan than in the West. Thus, we and other researchers investigated turmeric and its constituent curcumin for anti-prostate cancer effects.
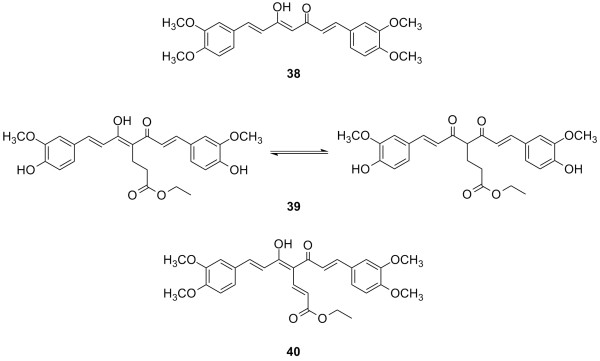
Although curcumin is a well known anti-inflammatory and anti-oxidant agent, its anti-prostate cancer activity has not been extensively explored. Over the last decade, our research group has used curcumin (2) as a lead compound for the design and synthesis of curcumin analogs as a new class of potential anti-androgenic agents for the treatment of prostate cancer as well as for action mechanism studies [76-81]. Certain curcumin analogs including 38 (JC-9), 39 (4-ethoxycarbonyl curcumin, ECECu) and 40 (LL-80) (Figure 9), showed potent in vitro cytotoxic activity against LNCaP and PC-3 human prostate cancer cell lines (Table 4). Among them, compound 40 showed the most potent activity, suggesting that introducing a conjugated side chain in the enol-ketone linker may stabilize the enol-ketone form as the predominant tautomer (Figure 9), which may contribute to the anti-prostate cancer activity. Although the entire structure of the AR has not been fully determined and the mechanism of how curcumin derivatives interact with the AR is still unclear, preliminary studies showed that these curcumin derivatives inhibit AR function via an AR degradation pathway, which plays an important role in the growth of prostate cancer [82,83]. In addition, compound 38 (JC-9) with its potent anti-androgenic activity and stable physiological properties was identified as a lead anti-AR compound. Clinical trials against prostate cancer are being planned.
Figure 9.
Structures of JC-9 (38), ECECur (39) and LL-80 (40) with anti-prostate cancer activity.
Table 4.
Cytotoxic activity data of curcumin derivatives against PC-3 and LNCaP prostate cancer cell lines 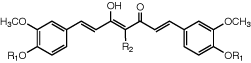
| Compound | R1 | R2 | PC-3 IC50 (μM)* | LNCaP IC50 (μM)* |
| 2 | H | H | 7.7 | 3.8 |
| 38 | CH3 | H | 1.1 | 1.3 |
| 39 | H | CH2CH2COOEt | 5.1 | 1.5 |
| 40 | CH3 | CH=CHCOOEt | 1.0 | 0.2 |
IC50 values are mean concentrations that inhibit cell growth by 50% (variation between replicates was less than 5%).
IC50 values are expressed as 'means'.
We prepared four series of new curcumin analogs [81] including monophenyl curcumin analogs, heterocycle-containing curcumin analogs, curcumin analogs bearing various substituents on the phenyl rings, and curcumin analogs with various linkers, which are being tested for their anti-prostate cancer activity and action mechanism. New curcumin analogs from other research groups [84-86] are also being evaluated for cytotoxic activity against two human prostate cancer cell lines, i.e. LNCaP and PC-3, and inhibitory activity to the AR, with goals to elucidate more refined SAR and optimize curcumin analogs to develop better anti-prostate cancer drugs.
Conclusion
Natural curcuminoids are compounds found in Curcuma species, which are used as a medicine of the upper class of traditional Chinese medicine herbs that are generally not toxic and are in rich content in natural foods and spices. Curcuminoids and other natural and synthetic curcuminoids possess various bioactivities including anti-inflammatory, anti-oxidant, anti-HIV, chemopreventive and anti-prostate cancer effects. In addition, curcumin was recently found to prevent experimental rheumatoid arthritis [87]. Recent studies on curcuminoids, particularly on curcumin, have discovered not only much on the therapeutic activities, but also on mechanisms of molecular biological action and major genomic effects. Our research group developed some anti-androgenic curcumin analogs as anti-prostate cancer agents.
Abbreviations
AIDS: acquired immune deficiency syndrome; ABTS: 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid); AR: androgen receptor; COX-2: cyclo-oxygenase-2; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ECECu: 4-ethoxycarbonyl curcumin; HIV: human immunodeficiency virus; IκBα: inhibitory protein κBα; IL-1β: interleukin-Iβ; LD50: median lethal dose; LDL: low density lipoprotein; MMP-9: matrix metalloproteinase-9; NF-κB: nuclear factor-κB; OA: osteoarthritis; PTP: permeability transition pore; QSAR: quantitative structure-activity relationships; SAR: structure-activity relationship; TNF-α: tumor necrosis factor-α
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KHL and HI conceived and drafted the paper. QS and TA provided technical assistance. SMN edited the manuscript.
Acknowledgments
Acknowledgements
We are grateful to the valuable contributions of Drs L Lin, H Ohtsu, and J Ishida of the NPRL. This work was financially supported by an NIH grant (No. CA-17625) awarded to KHL.
Contributor Information
Hideji Itokawa, Email: itokawah@nifty.com.
Qian Shi, Email: qshi1@email.unc.edu.
Toshiyuki Akiyama, Email: akiyama@email.unc.edu.
Susan L Morris-Natschke, Email: susan_natschke@unc.edu.
Kuo-Hsiung Lee, Email: khlee@unc.edu.
References
- Kuhn MA, Winston D. Herbal Therapy and Supplements: A Scientific & Traditional Approach. New York: Lippincott; 2001. pp. 330–335. [Google Scholar]
- Van Wyk BE, Wink M. Medicinal Plants of the World. Portland: Timber Press; 2004. p. 118. [Google Scholar]
- Lee KH. Antineoplastic agents and their analogues from Chinese traditional medicine. In: Kinghorn AD, Balandrin MF, editor. Human Medicinal Agents from Plants Symp Series 534. Washington DC: Amer Chem Soc; 1993. pp. 170–190. [Google Scholar]
- Itokawa H, Hirayama F, Funakoshi K, Takeya K. Studies on the antitumor bisabolane sesquiterpenoids isolated from Curcuma xanthorrhiza. Chem Pharm Bull. 1985;33:3488–3492. doi: 10.1248/cpb.33.3488. [DOI] [PubMed] [Google Scholar]
- Uehara S, Yasuda I, Akiyama K, Morita H, Takeya K, Itokawa H. Diarylheptanoids from the rhizome of Curcuma xanthorrhiza and Alpinia officinarum. Chem Pharm Bull. 1987;35:3298–3304. [Google Scholar]
- Uehara S, Yasuda I, Takeya K, Itokawa H. New bisaborane sesquiterpenoids from the rhizomes of Curcuma xanthorrhiza (Zingiberaceae) Chem Pharm Bull. 1989;37:237–240. [Google Scholar]
- Uehara S, Yasuda I, Takeya K, Itokawa H, Iitaka Y. New bisaborane sesquiterpenoids from the rhizomes of Curcuma xanthorrhiza (Zingiberaceae) II. Chem Pharm Bull. 1990;38:261–263. [Google Scholar]
- Uehara S, Yasuda I, Takeya K, Itokawa H. Terpenoids and curcuminoids of the rhizoma of Curcuma xanthorrhiza Roxb. Yakugaku Zasshi. 1992;112:817–823. doi: 10.1248/yakushi1947.112.11_817. [DOI] [PubMed] [Google Scholar]
- Uehara S, Yasuda I, Takeya K, Itokawa H. Comparison of the commercial turmeric and its cultivated plant by their constituents. Shouyakugaku Zasshi. 1992;46:55–61. [Google Scholar]
- Hwang JK, Sim JS, Baek NI, Pyun YR. Xanthorrhizol: a potential antibacterial agent from Curcuma xanthorrhiza against Streptococcus mutans. Planta Med. 2000;66:196–197. doi: 10.1055/s-2000-8569. [DOI] [PubMed] [Google Scholar]
- Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Nagabhushan M, Bhide SV. Curcumin as an inhibitor of cancer. J Amer Coll Nutr. 1992;11:192–198. [PubMed] [Google Scholar]
- Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, Tanaka y, Ohsawa T, Uraji T, Murakoshi M, Nishino H, Tsuda H. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis. 1998;19:81–85. doi: 10.1093/carcin/19.1.81. [DOI] [PubMed] [Google Scholar]
- Syu WJ, Shen CC, Don MJ, Ou JC, Lee KH, Sun CM. Cytotoxicity of curcuminoids and some novel compounds from Curcuma zedoaria. J Nat Prod. 1998;61:1531–1534. doi: 10.1021/np980269k. [DOI] [PubMed] [Google Scholar]
- Deters M, Siegers C, Muhl P, Hansel W. Choleretic effects of curcuminoids on an acute cyclosporin-induced cholestasis in the rat. Planta Med. 1999;65:610–613. doi: 10.1055/s-1999-14033. [DOI] [PubMed] [Google Scholar]
- Chun KS, Sohn Y, Kim HS, Kim OH, Park KK, Lee JM, Lee J, Lee JY, Moon A, Lee SS, Surh YJ. Anti-tumor promoting potential of naturally occurring diarylheptanoids structurally related to curcumin. Mutat Res. 1999;428:49–57. doi: 10.1016/s1383-5742(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Milobedzka J, Kostanecki SV, Lampe V. Curcumin. Chem Ber. 1910;43:2163–2170. [Google Scholar]
- Aggarwal BB, Kumar A, Bhartic AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- Leu TH, Maa MC. The molecular mechanisms for the antitumorigenic effect of curcumin. Curr Med Chem Anticancer Agents. 2002;2:357–370. doi: 10.2174/1568011024606370. [DOI] [PubMed] [Google Scholar]
- Kohli K, Ali J, Ansari MJ, Raheman Z. Curcumin: a natural antiinflammatory agent. Indian J Pharmacol. 2005;37:141–147. [Google Scholar]
- Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix matalloproteinase-9 in human articular chondrocytes; Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nita CW. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa) J Alternative Complementary Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Antitumor and oxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-J. [DOI] [PubMed] [Google Scholar]
- Rao TS, Basu N, Siddiqui HH. Anti-inflammatory activity of curcumin analogues. Indian J Med Res. 1982;75:574–578. [PubMed] [Google Scholar]
- Mukhopadhyay A, Basu N, Ghatak N, Gujiral PK. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12:508–515. doi: 10.1007/BF01965935. [DOI] [PubMed] [Google Scholar]
- Ali M, Bagati A, Gupta J. Comparison of anti-inflammatory activity of curcumin analogs. Indian Drugs. 1995;32:502–505. [Google Scholar]
- Ali M, Bagati A, Gupta J. Synthesis and anti-inflammatory activity of some curcumin analogs. Indian J Chem, Org Chem. 1995;34B:884–888. [Google Scholar]
- Nurfina AN, Reksohadiprodjo MS, Timmerman H, Jenie UA, Sugiyanto D, Goot H Van Der. Synthesis of some symmetrical curcumin derivatives and their antiinflammatory activity. Europ J Med Chem. 1997;32:321–328. doi: 10.1016/S0223-5234(97)89084-8. [DOI] [Google Scholar]
- Nurrochmad A, Supardjan AM, Sardjiman Inhibition of cyclooxygenase by cyclovalone and its three analogue compounds. Majalah Farmasi Indonesia. 1998;9:180–185. [Google Scholar]
- Opletalova V. Constituents of the plants of the Zingiberaceae family and their synthetic analogs as potential anti-inflammatory agents. Ceska a Slovenska Farmacie. 1995;44:305–307. [Google Scholar]
- Chun KS, Park KK, Lee J, Kang M, Surh YJ. Inhibition of mouse skin tumor promotion by anti-inflammatory diarylheptanoids derived from Alpinia oxyphylla Miquel (Zingiberaceae) Oncology Res. 2002;13:37–45. doi: 10.1159/000047945. [DOI] [PubMed] [Google Scholar]
- Itokawa H, Aiyama R, Ikuta A. A pungent principle from Alpinia oxyphylla. Phytochem. 1982;21:241–243. doi: 10.1016/0031-9422(82)80061-7. [DOI] [Google Scholar]
- Itokawa H, Aiyama R, Ikuta A. Synthesis of diarylheptanoids and assessment of their pungency. Chem Pharm Bull. 1983;31:2491–2496. [Google Scholar]
- Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidant activity of tetrahydrocurcuminoids. Biosci Biotechnol Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- Masuda T, Maekawa T, Hidaka K, Bando H, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcumin: Analysis of oxidative coupling products from curcumin and linoleate. J Agric Food Chem. 2001;49:2539–2547. doi: 10.1021/jf001442x. [DOI] [PubMed] [Google Scholar]
- Kunchandy E, Rao M. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–240. doi: 10.1016/0378-5173(90)90201-E. [DOI] [Google Scholar]
- Toennesen HH. Studies on curcumin and curcuminoids. XIII. Catalytic effect of curcumin on the peroxidation of linoleic acid by 15-lipoxygenase. Int J Pharm. 1989;50:67–69. doi: 10.1016/0378-5173(89)90183-X. [DOI] [Google Scholar]
- Ahsan H, Parveen N, Khan NU, Hadi SM. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–175. doi: 10.1016/S0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan P, Rao MNA. Structure-activity relationships for the inhibition of lipid peroxidation and the scavenging of free radicals by synthetic symmetrical curcumin analogues. J Pharm Pharmacol. 2000;52:1123–1128. doi: 10.1211/0022357001774886. [DOI] [PubMed] [Google Scholar]
- Barclay LRC, Vinqvist MR, Mukai K, Goto H, Tokunaga A, Uno H. On the antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Organic Lett. 2000;2:2841–2843. doi: 10.1021/ol000173t. [DOI] [PubMed] [Google Scholar]
- Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG, Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Rad Biol Med. 2003;35:475–484. doi: 10.1016/S0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- Gorman AA, Hamblett I, Srinivasan VS, Wood PD. Curcumin-derived transients: a pulsed laser and pulse radiolysis study. Photochem Photobiol. 1994;59:389–398. doi: 10.1111/j.1751-1097.1994.tb05053.x. [DOI] [PubMed] [Google Scholar]
- Priyadarsini KI. Free radical reactions of curcumin in membrane models. Free Rad Biol Med. 1997;23:838–843. doi: 10.1016/S0891-5849(97)00026-9. [DOI] [PubMed] [Google Scholar]
- Khopde SM, Priyadarsini KI, Venkatesan P, Rao MNA. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys Chem. 1999;80:85–91. doi: 10.1016/S0301-4622(99)00070-8. [DOI] [PubMed] [Google Scholar]
- Kapoor S, Priyadarsini KI. Protection of radiation-induced protein damage by curcumin. Biophys Chem. 2001;92:119–126. doi: 10.1016/S0301-4622(01)00188-0. [DOI] [PubMed] [Google Scholar]
- Jovanovic SV, Steenken S, Boone CW, Simic G. How curcumin works preferentially with water soluble antioxidants. J Am Chem Soc. 1999;121:9677–9681. doi: 10.1021/ja991446m. [DOI] [PubMed] [Google Scholar]
- Patro BS, Rele S, Chhintalwar GJ, Chattopadhyay S, Adhikari S, Mukerjee T. Protective activities of some phenolic 1,3-diketones against lipid peroxidation: possible involvement of the 1,3-diketone moiety. Chembiochem. 2002;3:364–370. doi: 10.1002/1439-7633(20020402)3:4<364::AID-CBIC364>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ligeret H, Barthelemy S, Zini R, Tillement JP, Labidalle S, Morin D. Effects of curcumin and curcumin derivatives on mitochondrial permeability transition pore. Free Radic Biol Med. 2004;36:919–929. doi: 10.1016/j.freeradbiomed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Toennesen HH, Greenhill JV. Studies on curcumin and curcuminoids. XXII: Curcumin as a reducing agent and as a radical scavenger. Int J Pharm. 1992;87:79–87. doi: 10.1016/0378-5173(92)90230-Y. [DOI] [Google Scholar]
- Wright JS. Predicting the antioxidant activity of curcumin and curcuminoids. Theochem. 2002;591:207–217. doi: 10.1016/S0166-1280(02)00242-7. [DOI] [Google Scholar]
- Cohly HHP, Asad S, Das SK, Angel MF, Rao M. Effect of antioxidant (turmeric, turmerin and curcumin) on human immunodeficiency virus. Int J Mol Sci. 2003;4:22–33. [Google Scholar]
- Mazumder A, Raghavan K, Weinstein J, Kohn KW, Pomier Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995;49:1165–1170. doi: 10.1016/0006-2952(95)98514-A. [DOI] [PubMed] [Google Scholar]
- Artico M, Di Santo R, Costi R, Novellino G, Greco G, Massa S, Tramontano E, Marongiu ME, De Montis A, La Colla P. Geometrically and conformationally restrained cinnamoyl compounds as inhibitors of HIV-1 integrase: synthesis, biological evaluation, and molecular modeling. J Med Chem. 1998;41:3948–3960. doi: 10.1021/jm9707232. [DOI] [PubMed] [Google Scholar]
- Li CJ, Zhang LJ, Denzube BJ, Crumpacker CS, Pardee AB. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci USA. 1993;90:1839–1842. doi: 10.1073/pnas.90.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A, Neamati N, Sunder S, Schulz J, Pertz H, Erich E, Pommier Y. Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J Med Chem. 1997;40:3057–3063. doi: 10.1021/jm970190x. [DOI] [PubMed] [Google Scholar]
- Mazumder A, Neamati N, Pilon A, Sunder S, Pommier Y. Chemical trapping of ternary complexes of human immunodeficiency virus type 1 integrase, divalent metal, and DNA substrates containing an a basic site. Implications for the role of lysine 136 in DNA binding. J Biol Chem. 1996;271:27330–27338. doi: 10.1074/jbc.271.44.27330. [DOI] [PubMed] [Google Scholar]
- Mazumder A, Wang S, Neamati N, Nicklaus S, Pilon A, Sunder S, Chen J, Milne WA, Rice WG, Burk TRJ, Pommier Y. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J Med Chem. 1996;39:2472–2481. doi: 10.1021/jm960074e. [DOI] [PubMed] [Google Scholar]
- Yuan H, Parrill AL. QSAR studies of HIV-1 integrase inhibition. Bioorg Med Chem. 2002;10:4169–4183. doi: 10.1016/S0968-0896(02)00332-2. [DOI] [PubMed] [Google Scholar]
- Swan DK, Ford B. Chemoprevention of cancer: review of the literature. Oncol Nurs Forum. 1997;24:719–727. [PubMed] [Google Scholar]
- Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Susan M, Rao MNA. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittel-Forschung. 1992;42:962–964. [PubMed] [Google Scholar]
- Arbiser JL, Klauber N, Rohan R, Van Leeuwen R, Huang MT, Fisher C, Flynn E, Byers HR. Curcumin is an in vivo inhibitor of angiogenesis. Mol Med. 1998;4:376–383. [PMC free article] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Wang TTI, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. doi: 10.1016/S0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Lubet R, Steele VE, Kelloff , Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- Landis SH, Muray T, Bolden S, Wingo PA. Cancer statistics 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- Ross RK, Pike MC, Coetzee GA, Reichard MC, Yu MC, Feigelson H, Stanczyk FZ, Kolonel LN, Henderson BE. Androgen metabolism and prostate cancer. Establishing a model of genetic susceptibility. Cancer Res. 1998;58:4497–4504. [PubMed] [Google Scholar]
- Goldenberg SL, Bruchovsky N. Use of cyproterone acetate in prostate cancer. Urol Clin North Am. 1991;18:111–112. [PubMed] [Google Scholar]
- de Voogt HJ. The position of cyproterone acetate (CPA), a steroid anti-androgen, in the treatment of prostate cancer. Prostate. 1992:91–95. doi: 10.1002/pros.2990210514. [DOI] [PubMed] [Google Scholar]
- de Voogt HJ, Smith PH, Pavone-Macaluso M, de Pauw M, Sucin S. Cardiovascular side effects of diethylstilbestrol, cyproterone acetate, methoxyprogesterone acetate and estraumustine phosphateused for the treatment of advanced prostate cancer. Results from European Organization for research on treatment of cancer trials 3076 and 30762. J Urol. 1986;135:303–307. doi: 10.1016/s0022-5347(17)45620-5. [DOI] [PubMed] [Google Scholar]
- Kelly WK, Scher HI. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J Urol. 1993;149:607–609. doi: 10.1016/s0022-5347(17)36163-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Akakura K, Komiya A, Aida S, Akimoto S, Shimazaki J. Codon 877 mutation in the androgen receptor gene in advanced prostate cancer: relation to androgen withdrawal syndrome. Prostate. 1996;29:153–158. doi: 10.1002/1097-0045(199609)29:3<153::AID-PROS2990290303>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicaltamide in prostate cancer. Proc Natl Acad Sci USA. 2005;102:6201–6206. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(SICI)1097-0215(20000101)85:1<60::AID-IJC11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lin L, Shi Q, Nyarko AK, Bastow KF, Wu CC, Su CY, Shih CY, Lee KH. Antitumor agents 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J Med Chem. 2006;49:3963–3972. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu H, Itokawa H, Xiao Z, Su CY, Shi CC, Chiang T, Chang E, Lee Y, Chiu SY, Lee KH. Antitumor agents 222. Synthesis and anti-androgen activity of new diarylheptanoids. Bioorg Med Chem. 2003;11:5083–5090. doi: 10.1016/j.bmc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H, Su CY, Shih c, Chiang T, Chang E, Lee Y, Tsai MY, Chang C, Lee KH. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45:5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- Ishida J, Kozuka M, Wang HK, Konoshima T, Tokuda H, Okuda M, Mou XY, Nishino H, Sakurai N, Lee KH. Antitumor-promoting effects of diarylheptanoids on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett. 2000;159:135–140. doi: 10.1016/S0304-3835(00)00538-3. [DOI] [PubMed] [Google Scholar]
- Lin L, Shi Q, Su CY, Shih CCY, Lee KH. Antitumor agents 247. New 4-ethoxycarbonylethyl curcumin analogs as potential antiandrogenic agents. Bioorg Med Chem. 2006;14:2527–2534. doi: 10.1016/j.bmc.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Lee KH, Ishida J, Ohtsu H, Wang HK, Itokawa H, Chang C, Shih CCY. Curcumin analogs for the treatment of cancers and androgen-related diseases. PCT Int Appl, USA. 2003. p. 36.
- Lee DK, Chang CS. Endocrine mechanisms of disease. Expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metabol. 2003;88:4043–4054. doi: 10.1210/jc.2003-030261. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang CS. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- Masuda T, Matsumura H, Oyama Y, Takeda Y, Jitoe A, Kida A, Hidak K. Synthesis of (±)-cassuminins A and B, new curcuminoid antioxidants having protective activity of the living cell against oxidative damage. J Nat Prod. 1998;61:609–613. doi: 10.1021/np970555g. [DOI] [PubMed] [Google Scholar]
- Pederson U, Rasmussen PB, Lawesson SO. Synthesis of naturally occurring curcuminoids and related compounds. Liebigs Ann Chem. 1985:1557–1569. doi: 10.1002/jlac.198519850805. [DOI] [Google Scholar]
- Baanowsky A, Schmit B, Fowler DJ, Schneider B. Synthesis of new biosynthetically important diarylheptanoids and their oxa- and fluoro-analogues by three different strategies. Synth Commun. 2003;33:1019–1045. doi: 10.1081/SCC-120016367. [DOI] [Google Scholar]
- Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solom AM, Timmerman BN. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]