Abstract
Study Objectives:
To assess the ability of repeated daily oral ramelteon to facilitate re-entrainment of human circadian rhythms after an imposed phase advance of the sleep-wake cycle.
Methods:
A total of 75 healthy adult volunteers aged 18–45 years remained in a sleep laboratory for 6 days and 5 nights; a 5-h phase advance in their sleep-wake cycle was imposed under dim-light conditions. Oral ramelteon (1, 2, 4, or 8 mg once daily for 4 days) or placebo was administered 30 min before bedtime. The primary endpoint was the phase of the circadian rhythm as assessed by the time at which salivary melatonin concentrations declined below 3 pg/mL after morning awakening (dim-light melatonin offset [DLMoff]).
Results:
After 4 days of once-daily treatment, participants receiving 1, 2, or 4 mg ramelteon exhibited statistically significant phase shifts in DLMoff of −88.0 (16.6), −80.5 (14.8), and −90.5 (15.2) minutes respectively, versus −7.1 (18.6) minutes for placebo (least-squares mean(SEM), p = 0.002, p = 0.003, p = 0.001, respectively). Change in DLMoff for the 8 mg dose of ramelteon, −27.9 (16.4) minutes, was not significantly different than that for placebo (p = 0.392).
Conclusions:
Ramelteon (1, 2, or 4 mg per day) administered before bedtime significantly advanced the phase of the circadian rhythm after a 5-h phase advance in the sleep-wake cycle. These findings suggest that ramelteon has potential as a specific therapy for circadian rhythm sleep disorders.
Citation:
Richardson GS; Zee PC; Wang-Weigand S; Rodriguez L; Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med 2008;4(5):456–461.
Keywords: Melatonin receptor agonist, circadian rhythm, phase advance, dim-light melatonin offset
Circadian rhythm sleep disorders (CRSDs) include delayed sleep phase syndrome (DSPS), advanced sleep phase syndrome (ASPS), shift work sleep disorder (SWSD), irregular sleep-wake pattern, non–24-hour sleep-wake syndrome, and jet lag.1 These disorders are characterized most prominently by symptomatic disruption of sleep and wakefulness arising from an incompatible phase relationship between the endogenous circadian rhythm in sleep propensity and the desired sleep-wake schedule. In jet-lag and SWSD, circadian misalignment arises from the imposition of an incompatible sleep-wake schedule. In the case of other CRSDs, such as ASPS and DSPS, the exact mechanisms underlying the circadian misalignment are not entirely known. However, recent evidence indicates that functional polymorphisms or mutations in one or more of the circadian clock genes may be responsible for altered phase in some cases.2–5
It has been estimated that as many as 2% to 3% of the population experience sleep disruption as a consequence of shift work, and jet lag affects millions of people each year.1 Other CRSDs are less common overall, but often occur at higher frequencies in certain demographic groups. For example, delayed sleep phase syndrome has been estimated to occur in about 7% of adolescents,6 and non–24-hour sleep phase syndrome occurs in as many as 40% of blind persons.7
Current treatments for CRSDs include phototherapy, chronotherapy, and pharmacotherapy.8–10 Phototherapy involves using timed bright light exposure to reset the timing of circadian rhythms. This treatment has been shown to be effective for DSPS and ASPS, but may be difficult to implement clinically because patients must adhere to a strict schedule of light exposure, which may often conflict with work or social requirements.9,11,12 Chronotherapy is the progressive advancing or delaying (depending on the disorder) of the sleep-wake time until an optimal sleep schedule is attained. The length of treatment, as well as the strict sleep and wake schedules, have contributed to difficulties with adherence to chronotherapy for many patients.9,13
Pharmacotherapy has also been investigated as a potential treatment for CRSDs. Use of hypnotic medications (i.e., benzodiazepines and the newer benzodiazepine receptor agonists) can improve the insomnia symptoms associated with CRSDs, but these medications do not address the underlying circadian rhythm disorder.10,14 Melatonin has also been studied as a potential treatment for CRSDs with some success; however, the effects have been inconsistent.15–17 A more potent melatonin receptor agonist may provide an additional treatment option for inducing phase shifts, either alone or in combination with other therapies.
Ramelteon is a specific MT1 and MT2 melatonin receptor agonist that has been shown to have chronobiotic properties in preclinical studies.18 In a previous clinical study, a single dose of ramelteon administered in the evening produced a significant phase advance of the melatonin rhythm in humans.19 The current study was designed to examine the efficacy of ramelteon to facilitate resynchronization following an acute 5-h phase advance of the sleep-wake cycle, and to determine the optimal dose of ramelteon.
METHODS
Study Participants
Participants in the study were healthy males or nonpregnant, nonlacting females between the ages of 18 and 45 years, and with a body mass index of 18 to 30 kg/m2. Eligible participants had a habitual bedtime between 22:00 and 01:00, a median subjective sleep latency of less than 30 min, and a median subjective total sleep time between 6.5 and 9 h as determined by a sleep diary maintained during a 7-day screening period (completed within the 21 days before randomization). Exclusionary criteria included previous exposure to ramelteon or to another investigational drug (within the preceding 30 days or 5 half-lives), recent sleep schedule changes (such as shift work or air travel across ≥ 3 time zones), history of a primary sleep disorder or other condition affecting sleep (including alcohol or drug abuse and psychiatric disorders), caffeine consumption > 500 mg per day, smoking > 3 cigarettes per day or during nighttime awakenings, significant medical or psychiatric disease, or use of any medications known to affect sleep/wake functions. One day before randomization, potential study participants were screened using polysomnography (PSG) in the sleep laboratory beginning at habitual sleep time (calculated from pre-study sleep diaries). Single-blind placebo was administered before the screening/baseline recording. Baseline DLMoff was determined by a saliva sample collected immediately upon awakening after 8 h of PSG-monitored sleep. Subjects were excluded from further participation if they exhibited an apnea-hypopnea index (calculated as the number of apnea-hypopnea events per hour of sleep) > 10 or periodic limb movement associated with an arousal index (calculated as the number of periodic movements with arousal per hour) > 10.
Study procedures and informed consent forms were approved by a central Institutional Review Board (Coast Independent Review Board, San Clemente, CA), which was responsible for overseeing compliance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practices, federal regulations (21 Code of Federal Regulations), and state and local regulations. Each subject who chose to participate in the study signed the Informed Consent Form before any study-related procedures were performed.
Study Design
This study was a randomized, double-blind, placebo-controlled trial of 4 fixed doses of ramelteon administered once daily for 4 consecutive days. Investigators at 16 sites in the United States participated in the study (12 sites randomized participants) (Appendix 1). Participants remained in a sleep laboratory for 6 days and 5 nights. Dim lighting conditions (<20 lux) were maintained throughout the treatment period to avoid photic interference with endogenous melatonin secretion. On treatment day 1, a 5-hour phase advance of the sleep cycle was induced and maintained for the duration of the study. PSG recordings of sleep were performed for each sleep period.
The primary objective of the study was to examine whether oral ramelteon (1, 2, 4, or 8 mg once daily), in comparison to placebo, significantly improved the re-entrainment of circadian rhythms in healthy adults subjected to a 5-h phase advance in their sleep cycle. Circadian phase was measured using dim-light melatonin offset time (DLMoff), which is defined as the time at which falling-phase salivary melatonin concentrations declined below 3.0 pg/mL while participants were maintained in dim light.20 The selection of melatonin offset (rather than the more commonly used onset of melatonin secretion, DLMon) was necessary in this study because salivary measurements would have occurred during the sleep phase (as a consequence of the induced phase advance) and would have caused sleep disruptions that would have interfered with the assessment of sleep parameters. Drug effect was measured as change in DLMoff from Baseline to Day 4, relative to the placebo group. The impact on sleep was quantified as PSG-derived latency to persistent sleep (LPS), total sleep time, sleep efficiency, wake time after sleep onset, number of awakenings after persistent sleep, percent of sleep time in different sleep stages, and latency to REM sleep. Subjective measures of sleep were measured by a post-sleep questionnaire.
Study Procedures
Subjects completed a 2-phase screening procedure (described above) to determine their eligibility for randomization. Eligible participants were admitted to the sleep laboratory 8 h before habitual bedtime and received a physical examination and assessments of vital signs, clinical laboratory parameters, pregnancy status (women of childbearing potential), and screens for alcohol and drug use. Subjects were randomized to receive placebo or one of 4 doses of ramelteon (1, 2, 4, or 8 mg) and remained in a sleep clinic for the remaining 5 days of the study under dim-light conditions (< 20 lux).
Bedtime for the double-blind portion of the study was established as 5 h before habitual bedtime for each participant in order to induce a 5-h advance in the sleep-wake cycle. Study drug was administered 30 min (± 5 min) before bedtime. Ramelteon was administered during the phase-advance portion of the melatonin phase-response curve. Previous studies have determined that this is the optimal time to induce a phase advance in humans using exogenous melatonin.21,22 No food was to be given within 2 h of bedtime. Participants were put to bed for 8 h of PSG-monitored sleep. Each morning, a saliva sample was collected within 5 min of awakening, and subjects completed a post-sleep questionnaire as well as assessments of vital signs, adverse events, and concomitant medications. Additional saliva samples were obtained every 60 min (± 5min) during all waking hours.
Participants were fed meals in the sleep laboratory and had access to snacks, but were required to refrain from strenuous exercise and were not permitted to nap. On the final morning of the study, after awakening and the collection of study data, participants were again assessed by physical examination, measurement of vital signs, and clinical laboratory tests before being discharged.
Data Analysis
DLMoff was calculated by linear interpolation using the times and salivary melatonin concentrations bracketing the time when salivary melatonin concentration declined below 3 pg/mL for at least 2 consecutive determinations. Saliva samples were analyzed for melatonin concentration by Bioanalytical Systems, Inc (West Lafayette, IN). Melatonin was extracted from the saliva by a 96-well automated solid phase extraction, using a LC/MC/MS system with a SIELC Primesep 200 reverse phase ion-exchange column in series with a Synergi Hydro-RP MercuryMS LC/MS Cartridge and a Synergi Max-RP C-12 column with an acetonitrile/water/glacial acetic acid mobile phase. The calibration range for melatonin was 1.00 pg/mL–100 pg/mL. Calibration curves for melatonin were obtained using a 1/concentration weighted linear squares regression of peak height vs concentration. Objective and subjective measures of sleep were obtained from PSG or a post-sleep questionnaire of subjective sleep quality, respectively. PSG data were scored from digital recordings at a central site by qualified scorers blind to study condition. Standard safety variables were assessed using clinical laboratory tests, vital signs, physical examination, ECG recordings, and incidence of adverse events.
Statistical analyses were performed on an intent-to-treat basis using t-tests, least-square (LS) means, and standard errors obtained from an analysis of covariance (ANCOVA) model containing terms for study center and treatment group, and using the baseline value as a covariate. All statistical tests were 2-sided and performed at the 0.05 significance level.
RESULTS
In total, 113 volunteers entered the screening phase; 75 satisfied the entry criteria and were randomized to receive study drug (38 male, 37 female). One participant (8 mg ramelteon arm) voluntarily withdrew consent during the study but is included in the intent-to-treat analysis. No participants were lost to follow up or withdrew because of adverse events. Demographic and baseline characteristics of randomized participants are shown in Table 1. There were no significant differences among the 5 arms in any demographic or baseline characteristics.
Table 1.
Demographic and Baseline Characteristics of Subjects in All Groups
| Characteristic | Placebo (n = 15) | Ramelteon |
|||
|---|---|---|---|---|---|
| 1 mg (n = 14) | 2 mg (n = 16) | 4 mg (n = 15) | 8 mg (n = 15) | ||
| Age (years), Mean (SD) | 26.9 (8.0) | 25.9 (6.3) | 29.6 (7.6) | 26.2 (7.0) | 26.1 (5.7) |
| Sex (N), M/F | 5/10 | 7/7 | 8/8 | 9/6 | 9/6 |
| Race (N) | |||||
| White | 12 | 12 | 13 | 9 | 8 |
| Black | 3 | 1 | 2 | 6 | 7 |
| Asian or Multiracial | 0 | 1 | 1 | 0 | 0 |
| Height (cm), Mean (SD) | 170.9 (10.3) | 170.4 (11.0) | 168.9 (11.4) | 171.1 (11.9) | 173.9 (11.2) |
| Weight (kg), Mean (SD) | 71.8 (11.7) | 74.1 (14.5) | 71.3 (12.1) | 72.1 (15.2) | 77.4 (15.1) |
| Caffeine Consumption | |||||
| None | 1 | 3 | 2 | 5 | 0 |
| ≤ 500 mg/day | 14 | 11 | 14 | 10 | 15 |
| Baseline DLMoff (time), Mean (SD) | 8:50 (1:22) | 9:29 (1:13) | 9:08 (1:12) | 9:28 (1:06) | 8:50 (1:21) |
| Baseline LPS (minutes), Mean (SD) | 19.9 (19.1) | 18.4 (22.0) | 20.5 (17.8) | 14.3 (10.2) | 17.9 (18.2) |
| Baseline TST (minutes), Mean (SD) | 412.2 (58.9) | 421.8 (53.6) | 408.8 (47.3) | 429.6 (26.9) | 423.6 (22.9) |
LPS = latency to persistent sleep; TST = total sleep time; SD = standard deviation
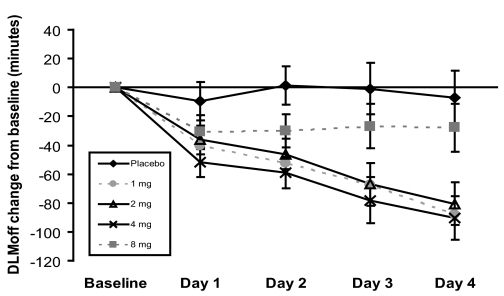
Change in Dim-Light Melatonin Secretion Offset Time (DLMoff)
The changes from baseline in salivary dim-light melatonin secretion offset time (DLMoff) for Days 1–4 are shown in Figure 1. Four days after a 5-h advance in their sleep-wake cycle, participants receiving placebo exhibited a nonsignificant LS mean (SE) shift in DLMoff of −7.1 (18.63) min (i.e., 7.1 min earlier than baseline). In contrast, those receiving 1, 2, 4, or 8 mg ramelteon exhibited respective LS mean (SE) shifts in their DLMoff of −88.0 (16.6), −80.5 (14.8), −90.5 (15.2) and −27.9 (16.4) min. The 95% confidence intervals for changes at Day 4 from Baseline were (−130.8, −30.9), (−120.0, −26.7), (−130.8, −35.9), and (−69.3, 27.7) for 1 mg, 2 mg, 4 mg, and 8 mg ramelteon, respectively. Compared to the placebo group, DLMoff changes from baseline were significantly different in the 1 mg (p = 0.002), 2 mg (p = 0.003), and 4 mg (p = 0.001) ramelteon groups, but not in the 8 mg group (p = 0.392).
Figure 1.
Changes from baseline in DLMoff during each of the 4 days of double-blind administration of study drug for placebo, ramelteon 8 mg, ramelteon 4 mg, ramelteon 2 mg, and ramelteon 1 mg. Values shown are the least-square means (SE) with standard error bars obtained from ANCOVA. The number of subjects with evaluable DLMoff data for each time point was: Day 1–placebo (9), 8 mg (10), 4 mg (13), 2 mg (14), 1 mg (11); Day 2–placebo (10), 8 mg (11), 4 mg (13), 2 mg (14), 1 mg (11); Day 3–placebo (10), 8 mg (11), 4 mg (11), 2 mg (13), 1 mg (11); Day 4–placebo (10), 8 mg (11), 4 mg (13), 2 mg (14), 1 mg (11).
During the course of the double-blind treatment period, LS mean DLMoff for participants in the 4 mg ramelteon group showed significantly greater change from baseline as compared to the placebo group as early as Day 1 (−51.6 minutes versus −9.6 minutes, respectively; p = 0.015). The 1 mg and 2 mg ramelteon groups were significantly different from placebo on Day 2 (p = 0.004 and 0.006, respectively). The LS mean differences from placebo continued to increase on subsequent days for participants in these three groups, suggesting a cumulative phase-shifting effect of ramelteon (1, 2, and 4 mg) administered during successive days. At no time did participants in the 8 mg group exhibit a DLMoff that was statistically significantly different than participants in the placebo group.
Polysomnography
No significant differences were observed with regard to any of the PSG-measured parameters in comparisons of the ramelteon groups with the placebo group from Baseline to Day 4 (Table 2). In addition, ramelteon was not associated with any significant differences in sleep architecture, as determined by the percentage of sleep time spent in any sleep stage (stages 1, 2, 3/4, REM sleep), or in latency to REM sleep.
Table 2.
Sleep Measurement Changes from Baseline to Day 4 for All Groups
| Sleep Measurement | Change from Baseline to Day 4 |
||||
|---|---|---|---|---|---|
| Placebo n = 15 | Ramelteon 1 mg n = 14 | Ramelteon 2 mg n = 16 | Ramelteon 4 mg n = 15 | Ramelteon 8 mg n = 15 | |
| LPS (min) | |||||
| LS Mean (SE) | −6.99 | −8.60 | −6.11 | 4.18 | −5.39 |
| p value* | 0.829 | 0.901 | 0.132 | 0.829 | |
| TST (min) | |||||
| LS Mean (SE) | 8.82 | 24.28 | 12.08 | −13.11 | 2.49 |
| p value* | 0.369 | 0.842 | 0.198 | 0.710 | |
| Sleep Efficiency (%) | |||||
| LS Mean (SE) | 1.84 | 5.06 | 2.51 | −2.75 | 0.52 |
| p value* | 0.368 | 0.843 | 0.197 | 0.711 | |
| WASO (min) | |||||
| LS Mean (SE) | −5.85 | −19.43 | −6.68 | 8.03 | 0.96 |
| p value* | 0.404 | 0.957 | 0.387 | 0.673 | |
| NWAK (count) | |||||
| LS Mean (SE) | −1.8 | −1.2 | −1.1 | −1.1 | −0.8 |
| p value* | 0.624 | 0.517 | 0.542 | 0.396 | |
p value compared with placebo; LPS = latency to persistent sleep; TST = total sleep time; WASO = wake after sleep onset; NWAK = number of awakenings; SE = standard error
Subjective Sleep Assessment
Several subjective measures of sleep were assessed using a post-sleep questionnaire administered each morning after awakening. By the fourth day of treatment with study drug, participants receiving ramelteon reported a decrease in the average subjective sleep latency (LS mean change from baseline: −6.7, −10.3, −3.3, −4.9 minutes for the 1 mg, 2 mg, 4 mg, and 8 mg groups, respectively, versus −1.6 minutes for placebo) although the differences were not statistically significant. Ramelteon 1 mg and 2 mg recipients also reported an increase in subjective total sleep time in comparison to placebo, but again the difference was not statistically significant. Likewise, there were no significant differences across the treatment groups with respect to the subjective assessments of the number of awakenings, the ease of falling asleep, sleep quality, or the restorative nature of sleep.
Safety and Adverse Events
A total of 24 subjects (32.0%) experienced at least 1 adverse event (AE) during the study. The incidence of AEs was similar between the placebo (4) and the ramelteon 1 mg (5), 2 mg (6), 4 mg (4), and 8 mg (5) groups. The majority of AEs were mild or moderate in intensity with headache and nausea most commonly reported. There were no serious adverse events reported. The overall safety profile of ramelteon 1 mg, 2 mg, 4 mg, and 8 mg was similar to placebo.
DISCUSSION
The results of this study indicate that ramelteon 1, 2, or 4 mg, taken nightly, can facilitate the re-entrainment of circadian rhythms after a 5-h phase advance in healthy adults. Such a phase advance is similar to that experienced during eastward jet travel across 5 time zones. Subjects who took low doses of ramelteon, 30 min before bedtime, exhibited phase advances in DLMoff that were significantly greater than placebo. Furthermore, there was continued improvement in adaptation to the new sleep-wake cycle during successive days for subjects receiving ramelteon. In contrast, those receiving placebo exhibited little or no adaptation of their circadian rhythm, with no evidence of an accumulating phase advance during the 4-day study period.
Unlike subjects receiving lower doses of ramelteon, those receiving 8 mg per day did not exhibit a statistically significant phase advance in DLMoff compared with placebo. The mechanism underlying this complex dose-response relationship is unclear. One possible explanation, drawing on similar results with melatonin,23 is that higher doses of ramelteon may cause blood levels of the drug to remain elevated into the morning hours, producing an antagonistic phase delay. The mean half-life of ramelteon is 0.83 h with an elimination half-life ranging from 0.83 to 1.9 h.24 However, the main metabolite of ramelteon, M-II, has a mean half-life of 2.27 h and an elimination half-life ranging from 2.27 to 3.39 h. M-II has much lower affinity for MT1 and MT2 melatonin receptors than ramelteon (one-fifth to one-tenth) and is 17- to 25-fold less potent in in vitro functional assays,24 but it may play a role in inducing phase shifts. Exogenous melatonin has been shown to produce phase delays when administered in the morning,22,25,26 suggesting that the human phase response curve to melatonin receptor agonists includes the capacity for phase delays.
In addition, results from a previous smaller study of ramelteon 4 mg and 16 mg showed a significant phase advance of DLMO after a single evening administration when compared with placebo (39.4 min advance with 4 mg, p = 0.009; 32.2 min advance with 16 mg, p = 0.031).19 It is possible that the different determination of circadian phase (DLMO as opposed to DLMoff) may account for the apparent incongruous results of the two studies. Due to the sleep requirements in the study design, DLMoff was the only marker of phase measured in the current study. While previous studies have shown DLMoff to be a reliable phase marker,27 other measurements of melatonin, including DLMO, may have yielded a more complete picture.
In the current study, none of the doses of ramelteon produced significant improvements in objective or subjective sleep parameters relative to the placebo group. This appears to reflect the relatively normal sleep in the placebo group, despite the imposition of a 5-h phase advance in scheduled sleep. The reason for the absence of significant sleep disturbances is unclear. Initial sleep assessments at baseline suggest high variability among subjects in all treatment groups, suggesting possible difficulty adapting to the laboratory environment or inaccuracies in the estimation of biological sleep phase using habitual sleep time from subject diaries. This may have limited the ability to detect any disruptive effects of the phase shift as well as any corrective effects of medication.
Some limitations of the current study were the small number of subjects in each group, the high variability in sleep parameters at baseline, and the use of DLMoff as the sole marker of phase. In some subjects, melatonin concentrations after awakening were below quantifiable levels or did not fall below the identified threshold (3 pg/mL) on various days, thereby reducing the number of data points available for analysis in an already small number of subjects. Because of these limitations, additional studies using a greater number of subjects and multiple phase markers may be needed to fully evaluate the effect of ramelteon on both phase and sleep in this setting.
DLMoff measures of circadian phase at baseline did show substantially smaller intersubject variance than did the sleep measures, and a significant effect of ramelteon on circadian phase was demonstrated. These results confirm that ramelteon does have the potential to phase-advance the melatonin circadian cycle. However, further studies are needed to determine the optimal dose of ramelteon required to phase-advance the melatonin circadian cycle. Additionally, the ability of ramelteon to precipitate phase delays has not been evaluated. Because there is evidence that endogenous melatonin may lead to phase delays when given in the morning, this is another potential area for further research with ramelteon.22,28,29
Conclusion
In persons subjected to a 5-h phase advance in the sleep-wake cycle, such as experienced by eastward jet travel across 5 time zones, ramelteon (1, 2, or 4 mg per day) advanced the phase of the endogenous circadian rhythm significantly better than placebo. The higher dose of ramelteon (8 mg) did not result in a significant phase shift compared with placebo. These results suggest that low-dose ramelteon may be effective for the treatment of CRSDs, although further study is required to determine its efficacy for the treatment of specific clinical conditions.
ABBREVIATIONS
- AE
adverse event
- ANCOVA
analysis of covariance
- ASPS
advanced sleep phase syndrome
- CRSD
circadian rhythm sleep disorder
- DLMoff
dim-light melatonin offset
- DLMO
dim-light melatonin onset
- DSPS
delayed sleep phase syndrome
- PSG
polysomnography
- REM
rapid eye movement
- SWSD
shift work sleep disorder
DISCLOSURE STATEMENT
This study was supported by the Takeda Pharmaceutical Company, Ltd. Dr. Richardson has received research support from industry and has consulted to and been a speaker for Takeda. Dr. Zee has received research support from GlaxoSmithKline and has participated in speaking engagements for Takeda and Sanofi-Aventis. Dr. Wang-Weigand, Dr. Peng, and Ms. Rodriguez are employees of Takeda.
ACKNOWLEDGMENTS
Assistance with manuscript preparation and writing was provided by Sara Sarkey, PhD, an employee of Takeda Pharmaceuticals North America.
REFERENCES
- 1.Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs. 2001;15:311–28. doi: 10.2165/00023210-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–3. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 3.Satoh K, Mishima K, Inoue Y, Ebisawa T, Shimizu T. Two pedigrees of familial advanced sleep phase syndrome in Japan. Sleep. 2003;26:416–7. doi: 10.1093/sleep/26.4.416. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKI[delta] mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–4. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 5.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–5. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 6.Partinen M. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 2nd ed. Philadelphia: WB Saunders; 1994. pp. 437–52. [Google Scholar]
- 7.Tabandeh H, Lockley SW, Buttery R, et al. Disturbance of sleep in blindness. Am J Ophthalmol. 1998;126:707–12. doi: 10.1016/s0002-9394(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 8.Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–77. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lack LC, Wright HR. Clinical management of delayed sleep phase disorder. Behav Sleep Med. 2007;5:57–76. doi: 10.1207/s15402010bsm0501_4. [DOI] [PubMed] [Google Scholar]
- 10.Lemmer B. The sleep-wake cycle and sleeping pills. Physiol Behav. 2007;90:285–93. doi: 10.1016/j.physbeh.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–77. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesson AL, Jr, Anderson WM, Littner M, et al. Practice parameters for the nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep. 1999;22:1128–33. doi: 10.1093/sleep/22.8.1128. [DOI] [PubMed] [Google Scholar]
- 13.Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8:566–77. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamieson AO, Zammit GK, Rosenberg RS, Davis JR, Walsh JK. Zolpidem reduces the sleep disturbance of jet lag. Sleep Med. 2001;2:423–30. doi: 10.1016/s1389-9457(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey KM, Eastman CI. Melatonin phase shifts human circadian rhythms in a placebo-controlled simulated night-work study. Am J Physiol Regul Integr Comp Physiol. 2002;282:R454–R463. doi: 10.1152/ajpregu.00135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hack LM, Lockley SW, Arendt J, Skene DJ. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J Biol Rhythms. 2003;18:420–9. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 17.Edwards BJ, Atkinson G, Waterhouse J, Reilly T, Godfrey R, Budgett R. Use of melatonin in recovery from jet-lag following an eastward flight across 10 time-zones. Ergonomics. 2000;43:1501–13. doi: 10.1080/001401300750003934. [DOI] [PubMed] [Google Scholar]
- 18.Hirai K, Kita M, Ohta H, et al. Ramelteon (TAK-375) accelerates reentrainment of circadian rhythm after a phase advance of the light-dark cycle in rats. J Biol Rhythms. 2005;20:27–37. doi: 10.1177/0748730404269890. [DOI] [PubMed] [Google Scholar]
- 19.Richardson G, Zammit G, Rodriguez L, Zhang J. Evaluation of circadian phase-shifting effects of ramelteon in healthy subjects. Chronobiol Int. 2005;22:1271–2. [Google Scholar]
- 20.Danilenko KV, Wirz-Justice A, Krauchi K, Weber JM, Terman M. The human circadian pacemaker can see by the dawn's early light. J Biol Rhythms. 2000;15:437–46. doi: 10.1177/074873000129001521. [DOI] [PubMed] [Google Scholar]
- 21.Lewy AJ, Bauer VK, Ahmed S, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 22.Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 23.Lewy A, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int. 2006;23:403–12. doi: 10.1080/07420520500545862. [DOI] [PubMed] [Google Scholar]
- 24.Karim A, Tolbert D, Cao C. Disposition kinetics and tolerance of escalating single doses of ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist indicated for treatment of insomnia. J Clin Pharmacol. 2006;46:140–8. doi: 10.1177/0091270005283461. [DOI] [PubMed] [Google Scholar]
- 25.Lewy AJ, Sack RL. Exogenous melatonin's phase-shifting effects on the endogenous melatonin profile in sighted humans: a brief review and critique of the literature. J Biol Rhythms. 1997;12:588–94. doi: 10.1177/074873049701200614. [DOI] [PubMed] [Google Scholar]
- 26.Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586:639–47. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 28.Wirz-Justice A, Werth E, Renz C, Muller S, Krauchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J Pineal Res. 2002;32:1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- 29.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]