Abstract
The purpose of this study was to examine whether dietary habits and physical activity patterns were independently associated with severity of sleep disordered breathing (SDB) in adults diagnosed with obstructive sleep apnea. Data collected from 320 adults participating in an ancillary study of The Apnea Positive Pressure Long-term Efficacy Study were analyzed as a cross-sectional assessment at study baseline. The respiratory disturbance index (RDI) was used as a measure of the severity of sleep disordered breathing. Separate linear regression models were fitted using RDI as the independent variable and various preselected components of dietary intake and physical activity as the dependent variables. The results indicated that even after adjusting for BMI, age, and daytime sleepiness, subjects with very severe and extremely severe SDB (RDI ≥ 50) consumed a diet that was higher in cholesterol, protein, total fat, and total saturated fatty acids. These findings were most evident among women. For all participants, those with RDI ≥ 50 in comparison to those < 50, on average consumed 88.16 more mg of cholesterol per day (95% CI: 44.45 to 131.86, p < 0.001). Among the women participants only, those with RDI ≥ 50 in comparison to those < 50, on average consumed 21.96 more grams of protein (95% CI: 2.64 to 41.29, p = 0.026), 27.75 more grams of total fat (95% CI: 3.38 to 52.11, p = 0.026), and 9.24 more grams of saturated fatty acids (95% CI: 0.67 to 17.80, p = 0.035). Furthermore, those with an RDI ≥ 50 had a 224.58 greater caloric expenditure than those with RDI < 50 from all activities including work and sleep (95% CI: 40.98 to 408.18, p = 0.017). Although significant results were seen in a reduction of physical activity from recreational activities, this finding was explained by the increase in BMI associated with higher levels of RDI.
Citation:
Vasquez MM; Goodwin JL; Drescher AA; Smith TW; Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the apnea positive pressure long-term efficacy study (APPLES). J Clin Sleep Med 2008;4(5):411–418.
Keywords: Sleep disordered breathing, sleep apnea, dietary intake, physical activity
Obstructive sleep apnea (OSA), characterized by repetitive episodes of complete or partial airway obstruction during sleep, is a serious health concern affecting an estimated 18 million Americans today.1 It is independently associated with cardiovascular disease, hypertension, and stroke.2,3 Symptoms of OSA include excessive daytime sleepiness as well as loud, disruptive snoring. These symptoms and poor sleep quality are associated with decreased cognitive functioning and motor vehicle accidents.4
OSA is commonly associated with obesity, with an estimated 70% of all OSA patients classified as being obese.3 The association between OSA is particularly strong with abdominal obesity, which is a key component of the metabolic syndrome (hypertension, abdominal obesity, hyperglycemia, insulin resistance, and hyperlipidemia).5,6 Thus, it is not surprising that recently OSA also has been independently linked to the presence of the metabolic syndrome.7
Factors important in promoting and maintaining obesity in OSA have not been clearly defined although several potential mechanisms have been identified. OSA is associated with alterations in leptin and ghrelin, which are important in appetite regulation.8,9 Furthermore, it has been demonstrated that sleep deprivation, a common occurrence among persons with OSA, is associated with increased body mass index (BMI) and increased cravings for carbohydrates, as well as decreased leptin and increased ghrelin.10,11 These hormone changes result in levels that may increase appetite and thus impact dietary intake, although the study did not include any appetite or dietary intake measures. Dietary patterns such as those with limited fruits and vegetables and excess sucrose and fat intake are currently being investigated in relation to promoting obesity.12,13 Although preference for fatty foods has been documented in early reports on sleep deprivation,14 there is a paucity of data on the dietary intake patterns specific to those with OSA. Therefore, it is unclear how dietary habits, sleep, OSA, and obesity interact.
Poor quality sleep, a consequence of OSA, is associated with fatigue and sleepiness. This may curtail physical activity and also result in a compensatory increase in caloric intake in an effort to boost energy levels,14 both of which promote weight gain. There is epidemiological evidence that increased physical activity is associated with a lower prevalence of sleep disorders, and some preliminary studies have explored the role of exercise in treating OSA.15–17 Unfortunately, these studies are confounded by small sample size,17 concurrent CPAP treatment,18 and lack of documentation of dietary change.15–18 Furthermore, most of the available studies used heterogeneous exercise assessment tools15 and did not ascertain detailed information concerning the types of activities performed.16 Thus, they had limited ability to identify the activities that might have been effective.
To investigate the relationships among dietary intake, physical activity, OSA, sleep, and obesity, this study uses data from a subset of the Apnea Positive Pressure Long-Term Efficacy Study (APPLES). We hypothesized that dietary habits and physical activity patterns were independently associated with severity of OSA.
MATERIALS AND METHODS
Study Population and Procedures
APPLES is a randomized, double-blinded, 2-arm, sham-controlled, multicenter, 6-month, intent-to-treat study of continuous positive airway pressure (CPAP) efficacy on neurocognitive function in OSA. A detailed description of the protocol has been published recently.4 Briefly, subjects were recruited into the study primarily from patients scheduled into a regular sleep clinic for evaluation of possible OSA and from local advertising. Symptoms indicative of OSA were used as prescreening questions. Initially, a clinical evaluation was conducted which included administering informed consent, screening questionnaires, history and physical examination, and a medical assessment by a study physician. Subjects subsequently returned 2–4 weeks later for a 24-h sleep laboratory visit, when a diagnostic polysomnogram (PSG) was performed to confirm the presence of OSA (vide infra) followed by a day of neurocognitive and maintenance of wakefulness testing. Approximately 10–14 days later, the central PSG scoring center provided the respiratory disturbance index (RDI) for each subject; only those subjects with a RDI ≥ 10 events per h were considered to have OSA, and were randomized to continue participation in the APPLES study.
Two of the 5 sites in this multicenter trial participated in this ancillary study to assess if CPAP treatment would result in a change of diet or physical activity during the initial 4 months of therapy: the University of Arizona in Tucson, Arizona, and St. Mary Medical Center in Walla Walla, Washington. In addition to PSG and neurocognitive assessment, consenting subjects completed detailed dietary and physical activity questionnaires at initial diagnostic polysomnography and 4-month clinical APPLES examinations. Data used in this report are derived from the initial assessment of these participants before randomization to 6 months of CPAP or sham CPAP use. Therefore, we also included data from subjects with RDI < 10 who were not randomized into the study. The institutional review boards of both participating institutions approved this ancillary study.
Assessment of Dietary Intake
To maximize the quality and quantity of the dietary information, we selected a validated and reputable computerized food frequency questionnaire (FFQ) developed by the Fred Hutchinson Cancer Center (the paper version of the tool was used by The Women's Health Initiative, the largest research program to date designed to focus on diet and health).19 This computerized dietary questionnaire was completed the day after the baseline diagnostic polysomnogram. The questionnaire contained items that ascertained what was eaten over the past 3 months and included details such as the frequency and portion size of the food items consumed.
Assessment of Physical Activity
Assessment of physical activity was gathered from participants using the Arizona Activity Frequency Questionnaire (AAFQ). This instrument distinguishes between different types of physical activity, including recreational, household, and leisure activity. In addition to caloric expenditure, it provides the amount of activity in metabolic equivalents or the ratio of work metabolic rate to a standard resting metabolic rate (METs). One MET is roughly equivalent to the energy cost of sitting quietly or 0.0175 calories per minute per kilogram of body weight (kcal/minute/kg).20 This instrument has been validated using doubly labeled water and has been shown to be effective in predicting total energy expenditure and physical activity energy expenditure in epidemiological studies.21
Polysomnography
The PSG montage included monitoring of the electroencephalogram (EEG, C3-A2 or C4-A1, O2-A1 or O1-A2), electrooculogram (EOG, ROC-A1, LOC-A2), chin and anterior tibialis electromyograms (EMG), heart rate by 2-lead electrocardiogram, snoring intensity (anterior neck microphone), nasal pressure (nasal cannula), nasal/oral thermistor, thoracic and abdominal movement (inductance plethysmography bands), and oxygen saturation (pulse oximetry). All PSG records were electronically transmitted to the data coordinating and PSG reading center. Sleep and wakefulness were scored using Rechtschaffen and Kales criteria.22 Apneas and hypopneas were scored using American Academy of Sleep Medicine Task Force (1999) diagnostic criteria.23 Briefly, an apnea was defined by a clear decrease (> 90%) from baseline in the amplitude of the nasal pressure or thermistor signal lasting ≥ 10 sec. Hypopneas were identified if there was a clear decrease (> 50% but ≤ 90%) from baseline in the amplitude of the nasal pressure or thermistor signal, or if there was a clear amplitude reduction of the nasal pressure signal ≥ 10 sec that did not reach the above criterion but was associated with either an oxygen desaturation > 3% or an arousal. Obstructive events were scored if there was persistence of chest or abdominal respiratory effort. Central events were noted if no displacement occurred on either the chest or abdominal channels.
Data Analysis
To investigate potential associations among dietary intake, physical activity and OSA, RDI was stratified into 6 categories of increasing OSA severity: RDI < 10, 10 ≤ RDI < 15, 15 ≤ RDI < 30, 30 ≤ RDI < 50, 50 ≤ RDI < 75, and RDI ≥ 75. Analysis of variance was then performed to determine whether any differences in means existed among the RDI groups for demographic, sleep, dietary, and physical activity variables. As shown in Tables 1 and 2, it appeared that increasing severity of RDI was associated with changes in several variables with a threshold effect occurring at RDI ≥ 50. Consequently, we performed all subsequent analyses with to exam if there were differences between participants with RDI ≥ 50 and those with RDI < 50.
Table 1.
Descriptive Statistics by RDI
| <10 (NO/Little SDB) (N = 21) |
10–15 (Mild SDB) (N = 40) |
15–30 (Moderate SDB) (N = 84) |
30–50 (Severe SDB) (N = 72) |
50−75 (Very Severe SDB) (N = 58) |
≥75 (Extremely Severe SDB) (N = 45) |
ANOVA (F-test, p-value) |
|
|---|---|---|---|---|---|---|---|
| Gender (% Men) | 47.62 | 52.50 | 66.67 | 62.50 | 77.59 | 75.56 | |
| Ethnicity (% White) | 71.43 | 72.50 | 84.52 | 79.17 | 84.48 | 88.89 | |
| Marital status (% married) | 66.67 | 65.00 | 69.88 | 76.06 | 81.03 | 53.33 | |
| Body mass index (mean, SD) | 27.96 (4.78) | 30.10 (6.65) | 29.88 (5.57) | 31.96 (7.35) | 34.59 (8.51) | 39.64 (7.27) | 15.89, p = 0.00001 |
| Age (mean years, SD) | 41.99 (15.86) | 53.42 (13.28) | 53.71 (14.18) | 55.96 (12.27) | 53.37 (12.65) | 49.85 (11.27) | 4.26, p = 0.0009 |
| Years of education (mean, SD) | 15.45 (2.21) | 15.75 (2.96) | 15.12 (2.44) | 14.97 (2.35) | 14.98 (3.08) | 14.69 (2.84) | 0.82, p = 0.5375 |
| Epworth Sleepiness Scale | 8.95 (3.76) | 9.53 (4.15) | 9.00 (4.49) | 9.63 (4.23) | 10.14 (3.87) | 11.4 (4.34) | 2.19, p = 0.0556 |
| Total sleep time (mean minutes, SD) | 430.48 (68.21) | 402.90 (90.82) | 425.60 (79.30) | 413.89 (73.36) | 426.57 (74.79) | 423.27 (97.67) | 0.68, p = 0.6389 |
Table 2.
Dietary Intake and Physical Sctivity by Categories of RDI
| Mean (SD) | <10 (NO/Little SDB) (N=20) |
10–15 (Mild SDB) (N=37) |
15–30 (Moderate SDB) (N=79) |
30–50 (Severe SDB) (N=71) |
50–75 (Very Severe SDB) (N=58) |
’75 (Extremely severe SDB) (N=40) |
ANOVA (F-test, p-value) |
|---|---|---|---|---|---|---|---|
| Calories (energy in kcal) | 1963.44 (771.81) | 2048.41 (1022.91) | 2106.18 (865.08) | 1997.29 (844.86) | 2304.61 (1101.31) | 2355.74 (984.03) | 1.33, p = 0.2495 |
| Total carbohydrates (g) | 237.94 (105.84) | 228.85 (115.85) | 240.51 (104.23) | 217.79 (97.74) | 243.80 (126.24) | 241.74 (124.57) | 0.50, p = 0.7752 |
| Cholesterol (mg) | 282.11 (127.95) | 268.65 (153.05) | 290.08 (144.44) | 289.25 (150.45) | 353.77 (184.68) | 428.46 (204.79) | 5.80, p = 0.00001 |
| Total fat (g) | 75.09 (32.60) | 88.38 (52.26) | 85.88 (48.50) | 86.21 (44.57) | 100.84 (54.38) | 106.67 (49.48) | 2.07, p = 0.0686 |
| Total dietary fiber (g) | 21.11 (10.94) | 19.50 (10.69) | 19.83 (8.45) | 18.73 (9.21) | 19.88 (10.51) | 17.87 (8.51) | 0.47, p = 0.8005 |
| Protein (g) | 85.63 (33.19) | 77.65 (30.76) | 86.62 (35.80) | 84.06 (35.65) | 95.61 (45.77) | 103.99 (38.54) | 2.65, p = 0.0230 |
| Total saturated fatty acids (g) | 25.54 (12.05) | 29.25 (19.38) | 29.41 (17.85) | 29.07 (16.17) | 34.02 (18.99) | 36.63 (17.82) | 1.92, p = 0.0909 |
| Sucrose (g) | 42.98 (24.27) | 40.98 (29.02) | 45.94 (35.52) | 39.46 (24.45) | 42.42 (26.54) | 34.81 (20.50) | 0.94, p = 0.4557 |
| Trans-fatty acids (g) | 3.59 (1.74) | 4.76 (4.22) | 4.12 (2.52) | 4.51 (2.63) | 5.30 (3.76) | 6.30 (3.84) | 3.44, p = 0.0049 |
| Daily servings of fruits | 1.99 (157) | 2.04 (2.02) | 2.02 (1.44) | 2.08 (1.82) | 1.78 (1.37) | 1.53 (1.37) | 0.83, p = 0.5322 |
| Daily servings of vegetables | 2.92 (2.48) | 2.53 (2.34) | 2.48 (1.55) | 2.49 (1.70) | 2.67 (1.62) | 2.10 (1.34) | 0.77, p = 0.5705 |
| (N=20) | (N=36) | (N=82) | (N=68) | (N=54) | (N=44) | ||
| Total adjusted energy expenditure per day (calories) for recreational activities | 676.56 (1062) | 221.74 (311.04) | 348.02 (451.46) | 232.02 (333.02) | 264.70 (341.28) | 221.88 (277.67) | 3.98, p = 0.0016 |
| Total adjusted energy expenditure per day (calories) for all activities (including work and sleep) | 3115.29 (1004.89) | 2750.39 (782.89) | 2768.12 (711.92) | 2721.14 (662.43) | 3050.76 (690.85) | 3285.48 (908.86) | 4.54, p = 0.0005 |
| % energy expenditure from recreational activities | 16.80 (20.03) | 7.52 (9.39) | 11.52 (12.47) | 8.44 (10.96) | 8.32 (10.29) | 6.45 (6.94) | 3.20, p = 0.0078 |
| (N=20) | (N=35) | (N=78) | (N=66) | (N=53) | (N=43) | ||
| Met minutes /week | 2972.58 (2786.22) | 1955.08 (1839.08) | 2419.99 (2303.29) | 1988.64 (2050.40) | 1785.00 (1622.20) | 1630.57 (1685.05) | 1.89, p = 0.0952 |
Separate unadjusted linear regression models were fit using RDI as the independent variable and various prespecified components of dietary intake and physical activity as the dependent variables. The dietary variables used were daily consumption of calories, protein, total fat, total carbohydrates, total saturated fatty acids, trans-fatty acids, sucrose, cholesterol, and total dietary fiber. Physical activity variables used were total adjusted energy expenditure/day for recreational activities, total adjusted energy expenditure/day for all activities (including work and sleep), percent of energy expenditure/day from recreational activities, and met minutes/week. For several outcome variables, gender interaction was significant; therefore, gender stratified analyses also were conducted.
To determine whether there was an independent association between consumption of various diet components and physical activity with RDI, we constructed multivariate models using RDI (< 50 vs. ≥ 50) as the independent variable. An initial examination of the data indicated that there were significant differences for several dietary and physical activity variables between sites. However, this finding was explained by differences in BMI between sites, with participants from Walla Walla being heavier (BMI: 31.7 vs. 36.1, p = 0.0013). Additional preliminary analyses demonstrated that a trend existed for increasing Epworth Sleepiness Scale scores as a function of RDI severity, but there was no association between total sleep time and RDI severity. Consequently, no additional adjustments were made for either study site or total sleep time in our modeling. Two multivariate models were fit by adjusting for (1) BMI and age and then for (2) BMI, age, and Epworth Sleep Scale with stratification by gender.
Data from 31 participants were excluded from the analysis. To account for potential false reporting of daily caloric consumption, 7 women reporting less than 700 calories and 7 men reporting less than 800 calories per day were considered outliers according to commonly used criteria.24 However, one criterion was adapted by increasing the upper limit for calorie exclusion from 5000 to 6000 to account for the level of obesity in our subjects with known sleep apnea. Using this modification, 1 man who reported consuming more than 6000 calories per day also was excluded. To account for potential false reporting of daily physical activity, we used the following criteria as given by Staten et al21 to exclude outliers: participants with a daily unadjusted time in all activities plus work and sleep < 10 h or > 32 h were excluded. Two participants reported < 10 h, and 14 reported > 32 h.
To adjust for multiple comparisons within the 2 domains of dietary consumption and physical activity as well as statistical modeling based on preliminary data exploration, we applied a Bonferroni correction in determining the acceptable level of statistical significance. Consequently, p values < 0.026 and < 0.029 were considered statistically significant for dietary consumption and physical activity measures, respectively.
RESULTS
There were 320 participants—263 from Tucson and 57 from Walla Walla. More than half of the participants were obese (56.88%). According to the Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obese,25 the new recommendations of classifying obesity are as follows: (obesity class 1: 30 ≤ BMI < 35, obesity class 2: 35 ≤ BMI < 40, and extreme obesity class 3: BMI ≥ 40); 26.56% of our participants were classified as obesity class 1, 15.63% as obesity class 2, and 14.69% as extreme obesity class 3.
Table 1 shows descriptive characteristics of participants by RDI category. Age, BMI, and percentage of men were associated with increasing RDI severity. As noted previously, there was also trend towards higher Epworth Sleepiness Scale scores.
Table 2 shows the results from the analysis of variance, displaying mean values of dietary and physical activity variables stratified by all categories of RDI severity. With respect to dietary habits, increasing RDI severity was associated with greater consumption of cholesterol, protein, and trans-fatty acids. There also was a trend towards greater ingestion of total saturated fatty acids and total fat. When reviewing patterns in physical activity, there were statistically significant differences among means between groups for energy expenditure related to recreational activities per day, total energy expenditure for overall activities (including work and sleep) per day, as well as percent of energy expenditure from recreational activities per day.
In Table 3, we show results from both the unadjusted and adjusted linear regression models of the dietary variables as a function of RDI. In unadjusted models, we found that RDI ≥ 50 was significantly associated with consumption of greater amounts of total calories, protein, fat, total saturated fatty acids, trans-fatty acids and cholesterol, but not carbohydrates, sucrose or dietary fiber. We found that with the exception of cholesterol, these associations occurred primarily in women participants. After adjusting for BMI and age, significant associations, although slightly attenuated, were still seen overall for cholesterol and protein, and in women for cholesterol, protein, total fat, and total saturated fatty acids. Further adjustment for Epworth Sleepiness Scale score had a minimal effect on these findings. Thus, in the fully adjusted model, those with RDI ≥ 50 in comparison to those < 50, on average consumed 88.16 more mg of cholesterol per day (95% CI: 44.45 to 131.86, p < 0.001). Similarly, among the women participants only, those with RDI ≥ 50 in comparison to those < 50, on average consumed 21.96 more grams of protein (95% CI: 2.64 to 41.29, p = 0.026), 27.75 more grams of total fat (95% CI: 3.38 to 52.11, p = 0.026), and 9.24 more grams of saturated fatty acids (95% CI: 0.67 to 17.80, p = 0.035).
Table 3.
Associations Between Sleep Disordered Breathing and Dietary Intake†
| Unadjusted |
Adjusted For BMI and Age |
Adjusted For BMI, Age, and Epworth Sleep Scale |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | |
| Calories (energy in kcal) | 280.77 | 0.02 | (55.2, 506.4) | 237.71 | 0.05 | (−2.2, 477.6) | 217.3 | 0.08 | (−23.2, 457.9) |
| Men | 132.48 | 0.33 | (−133.2, 398.1) | 89.11 | 0.55 | (−204.8, 383.0) | 72.75 | 0.63 | (−221.2, 366.7) |
| Women | 551.22 | 0.02 | (108.9, 993.6) | 463.85 | 0.06 | (−11.7, 939.4) | 437.42 | 0.07 | (−42.0, 917.2) |
| Protein (g) | 14.99 | <0.01 | (6.0, 24.0) | 12.25 | 0.01 | (2.6, 21.9) | 11.73 | 0.02 | (2.0, 21.4) |
| Men | 10.37 | 0.06 | (−0.4, 21.1) | 5.43 | 0.37 | (−6.4, 17.3) | 5.11 | 0.40 | (−6.8, 17.0) |
| Women | 24.29 | <0.01 | (6.7, 41.9) | 22.76 | 0.02 | (3.66, 41.86) | 21.96 | 0.03 | (2.6, 41.3) |
| Total Fat (g) | 17.82 | <0.01 | (6.2, 29.5) | 13.40 | 0.04 | (1.0, 25.9) | 12.40 | 0.05 | (−0.1, 24.9) |
| Men | 9.56 | 0.18 | (−4.4, 23.5) | 5.34 | 0.50 | (−10.1, 20.8) | 4.49 | 0.57 | (−11.0, 20.0) |
| Women | 36.01 | <0.01 | (13.6, 58.4) | 28.88 | 0.02 | (4.8, 53.0) | 27.75 | 0.03 | (3.4, 52.1) |
| Total Carbohydrates (g) | 12.59 | 0.36 | (−14.2, 39.4) | 9.49 | 0.51 | (−18.8, 37.8) | 6.18 | 0.67 | (−22.1, 34.4) |
| Men | 3.90 | 0.81 | (−27.6, 35.4) | 3.19 | 0.86 | (−31.3, 37.7) | 0.49 | 0.98 | (−33.8, 34.8) |
| Women | 25.59 | 0.35 | (−28.4, 79.6) | 16.09 | 0.58 | (−41.9, 74.1) | 12.17 | 0.68 | (−46.1, 70.4) |
| Total Saturated Fatty Acids (g) | 6.19 | <0.01 | (2.0, 10.4) | 4.78 | 0.04 | (0.3, 9.3) | 4.29 | 0.06 | (−0.2, 8.8) |
| Men | 3.42 | 0.19 | (−1.7, 8.5) | 1.78 | 0.53 | (−3.8, 7.4) | 1.36 | 0.63 | (−4.2, 7.0) |
| Women | 11.82 | <0.01 | (3.9, 19.8) | 9.75 | 0.03 | (1.3, 18.3) | 9.24 | 0.04 | (0.7, 17.8) |
| Trans-Fatty Acids (g) | 1.39 | <0.01 | (0.6, 2.2) | 0.80 | 0.05 | (0.0, 1.6) | 0.72 | 0.08 | (−0.1, 1.5) |
| Men | 0.95 | 0.04 | (0.1, 1.8) | 0.59 | 0.24 | (−0.4, 1.6) | 0.52 | 0.30 | (−0.5, 1.5) |
| Women | 2.52 | <0.01 | (0.9, 4.12) | 1.36 | 0.11 | (−0.3, 3.0) | 1.26 | 0.14 | (−0.4, 2.9) |
| Sucrose (g) | −3.23 | 0.35 | (−10.0, 3.6) | −2.66 | 0.48 | (−10.0, 4.7) | −3.62 | 0.33 | (−10.9, 3.7) |
| Men | −4.65 | 0.28 | (−13.0, 3.7) | −3.52 | 0.46 | (−12.9, 5.9) | −4.20 | 0.34 | (−13.6, 5.2) |
| Women | −1.89 | 0.76 | (−14.3, 10.5) | −2.17 | 0.75 | (−15.7, 11.4) | −3.58 | 0.60 | (−17.0, 9.8) |
| Cholesterol (mg) | 98.84 | <0.01 | (58.8, 138.9) | 87.56 | <0.01 | (44.3, 130.9) | 88.16 | <0.01 | (44.5, 131.9) |
| Men | 92.56 | <0.01 | (43.0, 142.1) | 59.84 | 0.03 | (5.1, 114.6) | 60.64 | 0.03 | (5.6, 115.7) |
| Women | 85.34 | 0.02 | (15.8, 154.9) | 82.52 | 0.04 | (5.2, 159.9) | 82.90 | 0.04 | (4.1, 161.7) |
| Total Dietary Fiber (g) | −0.46 | 0.70 | (−2.7, 1.8) | −0.95 | 0.45 | (−3.4, 1.5) | −1.18 | 0.35 | (−3.7, 1.3) |
| Men | −0.70 | 0.56 | (−3.3, 1.9) | −0.49 | 0.74 | (−3.4, 2.4) | −0.69 | 0.64 | (−3.6, 2.2) |
| Women | 1.24 | 0.61 | (−3.6, 6.1) | 0.18 | 0.95 | (−5.1, 5.5) | −0.06 | 0.98 | (−5.4, 5.3) |
Comparing participants with RDI ≥ 50 to participants with RDI < 50.
Example interpretation for cholesterol (unadjusted): On average, participants with RDI ≥ 50 consume 98.84 more mg of cholesterol a day than participants with RDI < 50.
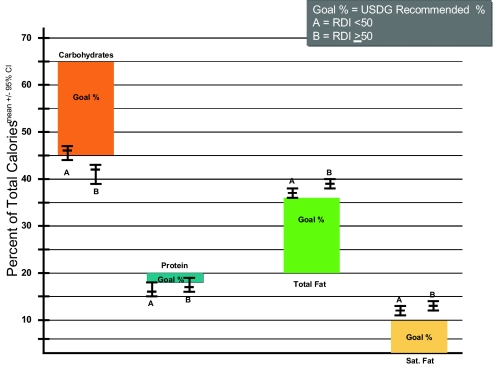
Figure 1 shows a comparison of the macronutrient and saturated fat intake by RDI with the 2005 U.S. Dietary Guidelines.26–28 The distribution of calories reflects that both groups ate less than the recommended proportion of calories from carbohydrate and more than recommended from total and saturated fat. The protein allotment, while in correct proportion, is high when considered in absolute terms, partly as a consequence of high overall caloric intake.
Figure 1.
Comparison of Major Dietary Components to Recommended Daily Allowances. Data are grouped according RDI and expressed as a % of total caloric intake.
Table 4 shows the results from both unadjusted and adjusted linear regression models with physical activity variables as a function of RDI. When examining the unadjusted models, we found RDI ≥ 50 was associated with greater total adjusted energy expenditure per day for overall activities (including work and sleep) and a trend towards a decreased percent of energy expenditure from recreational activities. For the latter finding, this was explained primarily by a decrease in recreational activity in men. In a model adjusted for BMI and age, the increase in total energy expenditure was attenuated, but remained significant. Further adjustment for Epworth Sleepiness Scale had little effect. Thus, those with a RDI ≥ 50 expended 224.58 more calories than those with RDI < 50 from all activities. In contrast, the decrease in percent recreational activity was eliminated after BMI and age adjustment. Further analysis indicated that this finding was explained entirely by the effect of increasing BMI, and not by differences in age (data not shown).
Table 4.
Associations Between Sleep Disordered Breathing and Physical Activity (Comparing Participants with RDI ≥50 to Participants with RDI <50)
| Unadjusted |
Adjusted For BMI and Age |
Adjusted For BMI, Age, and Epworth Sleep Scale |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | Coefficient | p-value | 95% CI | |
| Total adjusted energy expenditure per day (calories) for recreational activities * | −74.08 | 0.18 | (−183.29, 35.13) | −15.72 | 0.79 | (−133.51, 102.06) | −9.84 | 0.87 | (128.72, 109.04) |
| Men | −159.58 | 0.04 | (−309.39, −9.77) | −110.78 | 0.19 | (−277.96, 56.41) | −106.49 | 0.21 | (−274.66, 61.68) |
| Women | 24.07 | 0.63 | (−75.08, 123.23) | 77.92 | 0.15 | (−27.96, 183.80) | 83.27 | 0.12 | (−23.13, 189.67) |
| Total adjusted energy expenditure per day (calories) for all activities (including work and sleep)* | 372.92 | <0.01 | (188.70, 557.15) | 221.15 | 0.02 | (39.40, 402.90) | 224.58 | 0.02 | (40.98, 408.18) |
| Men | 239.31 | 0.03 | (25.89, 452.73) | 41.99 | 0.70 | (−171.28, 255.25) | 41.64 | 0.70 | (−173.06, 256.34) |
| Women | 280.52 | 0.02 | (37.83, 523.21) | −41.57 | 0.66 | (−229.36, 146.22) | −32.12 | 0.74 | (−222.21, 157.97) |
| % energy expenditure per day (calories) from recreational activities* | −2.84 | 0.05 | (−5.64, −0.04) | −0.63 | 0.67 | (−3.58, 2.31) | −0.48 | 0.75 | (−3.45, 2.49) |
| Men | −4.70 | 0.01 | (−8.35, −1.05) | −2.81 | 0.16 | (−6.77, 1.15) | −2.75 | 0.18 | (−6.74, 1.24) |
| Women | −0.52 | 0.80 | (−4.49, 3.45) | 2.20 | 0.30 | (−1.94, 6.34) | 2.46 | 0.24 | (−1.68, 6.61) |
| Mets/min† | −534.87 | 0.04 | (−1034.16, −35.89) | −254.10 | 0.35 | (−792.14, 283.94) | −234.76 | 0.40 | (−777.14, 307.62) |
| Men | −829.82 | 0.01 | (−1464.77, −194.87) | −663.05 | 0.07 | (−1382.48, 56.38) | −658.43 | 0.08 | (−1384.23, 67.37) |
| Women | −253.31 | 0.52 | (−1032.83, 526.21) | 116.41 | 0.78 | (−707.37, 940.19) | 149.68 | 0.72 | (−673.97, 973.3) |
N = 304 (men = 200, women = 104);
N = 295 (men = 194, women = 101)
DISCUSSION
In this study we found that subjects with very severe and extremely severe SDB consumed a diet higher in cholesterol, protein, total fat, and total saturated fatty acids than those with less severe SDB and those without significant SDB. Furthermore, these observations persisted even after adjusting for several potential confounders, including BMI and daytime sleepiness, and were most evident in women. With respect to physical activity, we found that the component of caloric expenditure related to recreational physical activity was reduced in those with very severe or extremely severe SDB. However, this finding was explained by the increase in BMI associated with higher levels of RDI. These findings suggest that unrelated to obesity, those with the most severe SDB consume a more unhealthy diet, and that this may be a factor contributing to greater cardiovascular morbidity and mortality.2 In contrast, reductions in physical activity in severe SDB are primarily related to decreased activity associated with obesity.
We have demonstrated that persons with very severe and extremely severe SDB consume a diet that would be considered atherogenic with high amounts of total fat, cholesterol, and saturated fatty acids.29 Figure 1 demonstrates how their intake differs from the 2005 U.S. Dietary Guidelines.26–28 Additionally, they consumed more cholesterol than recommended by the American Heart Association.26 It is well known that severity of SDB is correlated with BMI and that obese persons are more likely to consume diets high in fat and cholesterol.13,30,31 Thus, our dietary findings in those with RDI ≥ 50 may in part be explained by the greater prevalence of obesity in these subjects. Although these analyses are cross-sectional, the persistence of these results even after controlling for BMI suggests that a high RDI has an independent effect on dietary intake. To our knowledge, no previous study has explored the dietary habits of persons with SDB. Consequently, there are few clues to explain our observation. It has been suggested that fatigue and sleepiness, as well as sleep deprivation, influence dietary intake via alterations in neuroendocrine control of feeding behavior. In individuals with short sleep duration, the gastric-derived peptide hormone ghrelin, which is orexigenic is increased and conversely the anorexigenic adipocyte-derived hormone leptin is decreased.10 However, controlling for Epworth Sleepiness Scale score did little to affect our results; and in bivariate analysis, total sleep time was not associated with alterations in dietary intake. Thus, it is unlikely that sleep deprivation explains our observations. Nevertheless, SDB independent of changes in sleep duration may affect levels of appetite regulating hormones. In support of this possibility, levels of neuropeptide Y, a potent appetite stimulating peptide produced in the hypothalamus, were recently observed to be elevated in obstructive sleep apnea patients independent of obesity.32 It also is interesting to speculate that sleep disruption from SDB by some mechanism increases craving for fatty foods. However, we know of no studies that directly address this issue.
In our study, differences in consumption of protein, cholesterol, fat, and saturated fatty acids were primarily observed in women. The prevalence of obesity in the United States is higher in women,33 but our results remained significant even after controlling for BMI. Nevertheless, previous studies have demonstrated that the dietary habits of women differ from those of men. In one study using data from the NHANES surveys, the energy density of snacks was higher in women than for men.34 In another study, predictive associations of total energy, fat, protein, and fiber intake with obesity were higher among women than men.35 Therefore, it appears that the mechanism producing the higher dietary consumption of unhealthy nutrients in persons with extremely severe SDB is exaggerating behaviors already observed among obese women.
We found that caloric expenditure related to recreational activities was decreased in men with RDI ≥ 50, supporting generally accepted empiric observations that such persons are sedentary. However, it appears that this finding is explained by coexisting obesity, as the decrease in caloric expenditure was eliminated after controlling for BMI. Previous studies have shown that obese persons are less likely to engage in physical activity and are more likely to watch television.36,37 However, it is unclear why our observation was limited to only men. It is possible that men with either milder degrees of SDB or no SDB engage in greater amounts of high caloric recreational activity in comparison to women and are thus more affected as BMI increases.
Our analyses demonstrated that overall caloric consumption increased as a function of increasing RDI. Although it was attenuated after controlling for obesity, there still was a trend for persons with RDI ≥ 50 to have a greater caloric intake than those with less severe SDB. This finding was more apparent in our analysis of physical activity data, which demonstrated greater caloric expenditures in those with RDI ≥ 50 even after controlling for obesity and sleepiness. Energy expenditures in those with significant sleep apnea are increased during sleep presumably as a result of a greater energy cost of repetitive breathing efforts at night against an obstructed upper airway.38 Although this explanation is consistent with our dietary consumption data, it does not adequately account for the caloric expenditures calculated from the physical activity data because the collection instrument does not capture caloric expenditure related to increased respiratory effort. Thus, our findings with respect to increased caloric consumption and expenditures are not totally explained.
There are limitations to our study. First, our data is based on completion of questionnaire data. Food frequency questionnaires are a well-established, user-friendly method of ranking and aggregating the diets of large groups in a cost-effective manner, but they have some well-documented limitations. The main errors associated with FFQs are the restrictions imposed by a fixed list of foods, perception of portion sizes, and the cognitive challenge of assessing the frequency of consuming foods over a broad time range.39 There are also several types of reporting bias that may be relevant. These include social desirability,39 the tendency of some persons to respond to questionnaires with what is perceived to be socially appropriate (rather than an objective response), and the impact of characteristics such as BMI and age. In contrast, stringent energy balance dietary studies, while more precise, do not emulate real life eating patterns of free-living subjects. The complex nature of physical activity is known to be difficult to accurately measure with physical activity questionnaires (PAQ). The criterion field measure of determining individual energy expenditure (EE) is doubly labeled water, which gives a direct measure of EE but is very expensive and labor intensive to analyze. Despite a large number of available PAQs, few have been validated by comparison to doubly labeled water. The PAQ we selected (AAFQ) was validated in a group of sedentary women and found to overestimate total EE by only 7%, compared to measured doubly labeled water.21 Nevertheless, factors attributed to accuracy of the PAQ method include social desirability theory as well as errors associated with increased age and higher BMI. Consequently, obese subjects have been found to overestimate their duration of moderate and high intensity activities.40 Second, our study population is not a random sample of either the general population or a population of persons with SDB. Rather, they are a selected group of previously untreated persons who were recruited into an intervention study of CPAP therapy for SDB. Thus, our observations may not be generalizable to all other persons with SDB. Third, we performed a cross-sectional analysis, and thus our finding that an unhealthy diet and increased caloric consumption is related to very severe and extremely severe SDB while suggestive, cannot be considered causal, and the reverse hypothesis could be true. Fourth, our analytic technique involved some exploratory analyses before construction of our statistical models resulting in some falsely low p values. However, we adjusted for this concern by utilizing a more stringent standard for statistical significance.
SDB has been associated with the development of cardiovascular disease and metabolic syndrome independent of obesity.2 This study provides important new information on the dietary habits of people with OSA, which may be another mechanism through which SDB leads to these diseases. Based on these findings, unhealthful dietary and activity patterns warrant further study of their role in cardiovascular and metabolic syndrome development in patients with OSA.
DISCLOSURE STATEMENT
This study was supported by National Heart, Lung, and Blood Institute; CPAP equipment was provided by Respironics. Dr. Quan has received research support from Respironics and has participated in speaking engagements for Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute 5 UO1 HL068060.
Participating APPLES Institutions, Investigators and Staff
Administrative Core
Clete A. Kushida, M.D., Ph.D., Deborah A. Nichols, M.S., Eileen B. Leary, B.A., RPSGT, Pamela R. Hyde, M.A., Tyson H. Holmes, Ph.D., Daniel A. Bloch, Ph.D., William C. Dement, M.D., Ph.D.
Data Coordinating Center
Daniel A. Bloch, Ph.D., Tyson H. Holmes, Ph.D., Deborah A. Nichols, M.S., Rik Jadrnicek, Microflow, Ric Miller, Microflow, Usman Aijaz, M.S., Aamir Farooq, M.S., Darryl Thomander, Ph.D., Chia-Yu Cardell, RPSGT, Emily Kees, Michael E. Sorel, M.P.H., Oscar Carrillo, Ray Balise, Ph.D., Tracy Kuo, Ph.D.
Clinical Coordinating Center
Clete A. Kushida, M.D., Ph.D., William C. Dement, M.D., Ph.D., Pamela R. Hyde, M.A., Rhonda M. Wong, B.A., Pete Silva, Max Hirshkowitz, Ph.D., Alan Gevins, D.Sc., Gary Kay, Ph.D., Linda K. McEvoy, Ph.D., Cynthia S. Chan, B.S., Sylvan Green, M.D.
Clinical Centers
Stanford University
Christian Guilleminault, M.D., Eileen B. Leary, B.A., RPSGT, David Claman, M.D., Stephen Brooks, M.D., Julianne Blythe, P.A.-C, RPSGT, Jennifer Blair, B.A., Pam Simi, Ronelle Broussard, B.A., Emily Greenberg, M.P.H., Bethany Franklin, M.S., Amirah Khouzam, M.A., Sanjana Behari Black, B.S., RPSGT, Viola Arias, RPSGT, Romelyn Delos Santos, B.S., Tara Tanaka, Ph.D.
University of Arizona
Stuart F. Quan, M.D., James L. Goodwin, Ph.D., Wei Shen, M.D., Phillip Eichling, M.D., Rohit Budhiraja, M.D., Charles Wynstra, M.B.A., Cathy Ward, Colleen Dunn, B.S., Terry Smith, B.S., Dane Holderman, B.S., Michael Robinson, B.S., Osmara Molina, B.A., B.S., Aaron Ostrovsky, B.S., Jesus Wences, Sean Priefert, Julia Rogers, B.S., Megan Ruiter, B.S., Leslie Crosby, B.S., R.N.
St. Mary Medical Center
Richard D. Simon, Jr., M.D., Kevin Hurlburt, RPSGT, Michael Bernstein, M.D., Timothy Davidson, M.D., Jeannine Orock-Takele, RPSGT, Shelly Rubin, M.A., Phillip Smith, RPSGT, Erica Roth, RPSGT, Julie Flaa, RPSGT, Jennifer Blair, B.A., Jennifer Schwartz, B.A., Anna Simon, B.A., Amber Randall, B.A.
St. Luke's Hospital
James K. Walsh, Ph.D., Paula K. Schweitzer, Ph.D., Anup Katyal, M.D., Rhody Eisenstein, M.D., Stephen Feren, M.D., Nancy Cline, Dena Robertson, R.N., Sheri Compton, R.N., Susan Greene, Kara Griffin, M.S., Janine Hall, M.S.
Brigham and Women's Hospital*
Daniel J. Gottlieb, M.D., M.P.H., David P. White, M.D., Denise Clarke, B.Sc., RPSGT, Kevin Moore, B.A., Grace Brown, B.A., Paige Hardy, M.S., Kerry Eudy, Ph.D., Lawrence Epstein, M.D., Sanjay Patel, M.D.
Industry Support
Respironics, Inc.
Footnotes
Gratitude is expressed to Sleep Health Centers for the use of their clinical facilities to conduct this research
REFERENCES
- 1.Tishler PV, Larkin EK, Schluchter MD, Redline S. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. 2003;289:2230–7. doi: 10.1001/jama.289.17.2230. [DOI] [PubMed] [Google Scholar]
- 2.Budhiraja R, Quan SF. Sleep-disordered breathing and cardiovascular health. Curr Opin Pulm Med. 2005;11:501–6. doi: 10.1097/01.mcp.0000183058.52924.70. [DOI] [PubMed] [Google Scholar]
- 3.Wolk R, Somers VK. Obesity-related cardiovascular disease: implications of obstructive sleep apnea. Diabetes Obes Metab. 2006;8:250–60. doi: 10.1111/j.1463-1326.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 4.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]
- 5.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 6.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 7.Lam JC, Ip MS. An update on obstructive sleep apnea and the metabolic syndrome. Curr Opin Pulm Med. 2007;13:484–9. doi: 10.1097/MCP.0b013e3282efae9c. [DOI] [PubMed] [Google Scholar]
- 8.Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology. 2007;132:2116–30. doi: 10.1053/j.gastro.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671–80. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 10.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 12.Millen BE, Quatromoni PA, Pencina M, et al. Unique dietary patterns and chronic disease risk profiles of adult men: the Framingham nutrition studies. J Am Diet Assoc. 2005;105:1723–34. doi: 10.1016/j.jada.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Quatromoni PA, Copenhafer DL, D'Agostino RB, Millen BE. Dietary patterns predict the development of overweight in women: the Framingham Nutrition Studies. J Am Diet Assoc. 2002;102:1239–46. doi: 10.1016/s0002-8223(02)90275-0. [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Carter EA, Aloia M, Phillips B. Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: results from the third national health and nutrition examination survey. J Clin Sleep Med. 2006;2:163–9. [PubMed] [Google Scholar]
- 15.Quan SF, O'Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–57. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 16.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–4. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 17.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 18.Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea-an open trial. Sleep Breath. 2000;4:173–6. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 19.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 21.Staten LK, Taren DL, Howell WH, et al. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33:1959–67. doi: 10.1097/00005768-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. Bethesda, MD: U.S. Dept. of Health, Education, and Welfare; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 23.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Rock CL, Thornquist MD, Kristal AR, et al. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra Post-Marketing Surveillance Study. J Nutr. 1999;129:855–64. doi: 10.1093/jn/129.4.855. [DOI] [PubMed] [Google Scholar]
- 25.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 26.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 27.United States Department of Health and Human Services, United States Department Agriculture. Dietary Guidelines Advisory Committee. Dietary guidelines for Americans. 2005 http://www.health.gov/dietaryguidelines/dga2005/report/
- 28.Institute of Medicine Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington DC: National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 29.Weintraub WS. Is atherosclerotic vascular disease related to a high-fat diet? J Clin Epidemiol. 2002;55:1064–72. doi: 10.1016/s0895-4356(02)00541-3. discussion 73-4. [DOI] [PubMed] [Google Scholar]
- 30.Duvigneaud N, Wijndaele K, Matton L, et al. Dietary factors associated with obesity indicators and level of sports participation in Flemish adults: a cross-sectional study. Nutr J. 2007;6:26. doi: 10.1186/1475-2891-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murtaugh MA, Herrick JS, Sweeney C, et al. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J Am Diet Assoc. 2007;107:1311–21. doi: 10.1016/j.jada.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Barcelo A, Barbe F, Llompart E, et al. Neuropeptide Y and leptin in patients with obstructive sleep apnea syndrome: role of obesity. Am J Respir Crit Care Med. 2005;171:183–7. doi: 10.1164/rccm.200405-579OC. [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 34.Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84:1215–23. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maskarinec G, Takata Y, Pagano I, et al. Trends and dietary determinants of overweight and obesity in a multiethnic population. Obesity (Silver Spring) 2006;14:717–26. doi: 10.1038/oby.2006.82. [DOI] [PubMed] [Google Scholar]
- 36.Cournot M, Ruidavets JB, Marquie JC, Esquirol Y, Baracat B, Ferrieres J. Environmental factors associated with body mass index in a population of Southern France. Eur J Cardiovasc Prev Rehabil. 2004;11:291–7. doi: 10.1097/01.hjr.0000129738.22970.62. [DOI] [PubMed] [Google Scholar]
- 37.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000;24:1683–8. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 38.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271:E1036–43. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 39.Willett W. Nutritional epidemiology. New York: Oxford University Press; 1990. [Google Scholar]
- 40.Buchowski MS, Townsend KM, Chen KY, Acra SA, Sun M. Energy expenditure determined by self-reported physical activity is related to body fatness. Obes Res. 1999;7:23–33. doi: 10.1002/j.1550-8528.1999.tb00387.x. [DOI] [PubMed] [Google Scholar]