Abstract
Study Objectives:
Aerophagia is a complication of continuous positive airway pressure (CPAP) therapy for sleep disordered breathing (SDB), whereupon air is forced into the stomach and bowel. Associated discomfort can result in CPAP discontinuation. We hypothesize that aerophagia is associated with gastroesophageal reflux disease (GERD) via mechanisms involving GERD related lower esophageal sphincter (LES) compromise.
Methods:
Twenty-two subjects with aerophagia and 22 controls, matched for age, gender, and body mass index, who were being treated with CPAP for SDB were compared in regard to clinical aspects of GERD, GERD associated habits, SDB severity as measured by polysomnography, and mean CPAP pressure.
Results:
More subjects with aerophagia had symptoms of GERD (77.3% vs. 36.4%; p < 0.01) and were on GERD related medications (45.5% vs. 18.2%, p < 0.05) than controls. Regarding polysomnography, mean oxygen saturation percentages were lower in the aerophagia group than controls (95.0% vs. 96.5%, p < 0.05). No other differences were observed, including mean CPAP pressures. No one in the aerophagia group (vs. 27.3% of the control group) was a current tobacco user (p < 0.01). There was no difference in caffeine or alcohol use between the 2 groups.
Conclusions:
These results imply aerophagia is associated with GERD symptoms and GERD related medication use. This finding suggests a relationship between GERD related LES pathophysiology and the development of aerophagia in patients with SDB treated with CPAP.
Citation:
Watson NF; Mystkowski SK. Aerophagia and gastroesophageal reflux disease in patients using continuous positive airway pressure: a preliminary observation. J Clin Sleep Med 2008;4(5):434–438.
Keywords: Continuous positive airway pressure, aerophagia, gastroesophageal reflux disease, complications
Continuous positive airway pressure (CPAP), the preferred treatment for sleep disordered breathing (SDB), involves delivery of pressurized air into the upper airway via nasal or oronasal masks. CPAP provides a pneumatic splint, thereby resolving SDB by preventing pharyngeal collapse during sleep. Aerophagia is a complication of CPAP, in which air is inadvertently injected into the stomach and bowel resulting in painful abdominal bloating, belching, and flatulence. CPAP compliance frequently suffers, with patients often discontinuing treatment altogether due to these symptoms. The exact prevalence of CPAP related aerophagia in SDB is unknown, although one study found a prevalence of 13% in patients with chronic respiratory failure treated with noninvasive positive pressure ventilation.1
Gastroesophageal reflux disease (GERD), defined by at least weekly heartburn and/or acid regurgitation, is a common medical disorder affecting 10% to 20% of the population in the Western world.2 Nocturnal symptoms are common, with 2 nationwide telephone surveys reporting that 74% to 79% of adults with weekly GERD experience comorbid nighttime symptoms.3 Reflux events typically occur during transient relaxations of the lower esophageal sphincter (LES), the primary antireflux barrier.4 Patients with GERD develop pathophysiological changes at the gastroesophageal junction, which compromise the LES, predisposing to reflux during these intermittent episodes of sphincter relaxation.4,5 We hypothesize that GERD associated gastroesophageal pathology creates an environment susceptible to aerophagia in patients on CPAP for SDB. Therefore, the goal of this research is to establish an association between aerophagia and GERD.
METHODS
Ascertainment of Obstructive Sleep Apnea and Aerophagia
This case-control study involved patients treated at the University of Washington Sleep Disorders Center at Harborview between 2005 and 2006. Sleep disordered breathing was defined as an apnea-hypopnea index (AHI) > 10 on polysomnography (PSG) along with corresponding symptomology (e.g., snoring, excessive daytime sleepiness, morning headaches, witnessed apneas). Apneas were defined by ≥ 90% reduction in the pressure flow signal with corresponding respiratory effort; hypopneas were defined as 50% to 90% reduction in signal with corresponding respiratory effort. All study subjects were diagnosed with SDB and treated with CPAP.
All patients with SDB treated with CPAP at our sleep center were eligible for inclusion in the study group. All patients were queried regarding CPAP complications at follow-up visits, which include issues such as mask and oral leak of air, nasal congestion, mask discomfort, feelings of claustrophobia, and aerophagia. Any history of painful abdominal bloating, eructation, or flatulence related to CPAP use was considered positive for aerophagia. Patients with a prior history of esophageal or gastric surgery were excluded as the effect of these procedures on CPAP related aerophagia is unknown. Age-, gender-, and body mass index (BMI)- matched controls were randomly selected from a clinical database representing patients seen at our sleep center between 2002 and 2006. During the initial clinic visit all subjects were assessed with a standardized questionnaire for clinical evidence of GERD (symptoms, history of physician diagnosis); current GERD related medication use (proton pump inhibitors, H2 blockers, and antacids); and GERD related habits (current tobacco, caffeine, and alcohol use). GERD symptoms included endorsement of heartburn on review of systems. Physician diagnosis of GERD was established from the past medical history, and GERD related medications were extracted from the patient's medication list at the initial clinic visit and again prior to the diagnostic polysomnogram. Habits related to GERD were ascertained from the social history. The combined endpoint of any GERD factor included the presence of any combination of the aforementioned GERD related items (symptoms, physician diagnosis, and medication use). This study was reviewed and approved by the UW Human Subjects Committee.
Polysomnography
EEG electrodes were positioned at 2 frontal (F7, F8), 2 central (C3, C4), and 2 occipital (O1, O2) locations (International 10–20 system of measurement) and were referenced to the contralateral mastoids. Chin electromyogram and right and left electro-oculogram electrodes were also applied. Airflow was measured using a nasal pressure cannula placed in the nose (Pro-Tech Services, Inc. Mukilteo, WA). Chest and abdominal respiratory effort were measured by piezo respiratory effort bands placed around the chest and abdomen (Pro-Tech Services, Inc. Mukilteo, WA). Oxygen saturation was measured from the left or right index finger via pulse oximetry (Nellcor, Pleasanton, CA). Snoring was assessed by a small microphone sensor (Pro-Tech Services, Inc. Mukilteo, WA) placed on the throat just lateral to the trachea. Electromyogram electrodes were placed on the anterior tibialis muscle of each leg to monitor leg movements. The electrocardiogram was measured via 2 leads placed according to the modified lead II configuration. Measured PSG characteristics included AHI, desaturation index, oxygen saturation nadir, oxygen saturation mean, arousal index, total sleep time, sleep efficiency, sleep latency, and prescribed CPAP pressure. All PSGs were scored in accordance with Rechtschaffen and Kales criteria.6 All PSGs were single-night diagnostic studies. All subjects underwent diagnostic polysomnography and were treated with CPAP within 30 days of their initial clinic visit.
Statistical Analysis
The nonparametric chi-square test was utilized for all statistical analyses involving binary exposure and outcome variables except for tobacco (Fischer exact test) due to sample size. Continuous outcome variables obtained from PSG were assessed with Student's t-test with unequal variances. Stata 9.0 statistical software package was used (StataCorp LP, College Station, TX).
RESULTS
As expected, the aerophagia and control groups were similar in age (49.3 vs. 48.9 y), gender (both groups 50% female), and BMI (31.1 vs. 31.0 kg/m2). The group with aerophagia demonstrated more GERD symptoms (77.3% vs. 36.4%, p < 0.01) and were taking more GERD-related medications (45.5% vs. 18.2%, p < 0.05) than the control group. In addition, the combined GERD endpoint of any symptoms, medication use, or physician diagnosis of GERD was more common in the aerophagia group than the controls (77.3% vs. 40.9%, p < 0.05). Interestingly, 27.3% of the control group endorsed current smoking; no one in the aerophagia group reported smoking (p < 0.01). Current caffeine and alcohol use was similar regardless of aerophagia status. Regarding PSG, the aerophagia group had a lower mean oxygen saturation level when compared to the control group (95.0% vs. 96.5%; p < 0.05). No other significant differences were observed. These results are summarized in Table 1.
Table 1.
Clinical and Polysomnographic Variables in Patients with Sleep Disordered Breathing on CPAP with and without Aerophagia
| Aerophagia (n = 22) | Controls (n = 22) | p-value | |
|---|---|---|---|
| Clinical Variables (%) | |||
| Symptoms of GERD | 77.3 | 36.4 | <0.01 |
| Medications for GERD | 45.5 | 18.2 | <0.05 |
| Physician diagnosis of GERD | 50.0 | 27.3 | 0.12 |
| Any GERD* | 77.3 | 40.9 | <0.05 |
| Caffeine** | 72.7 | 68.2 | 0.74 |
| Tobacco** | 0.0 | 27.3 | <0.01 |
| Alcohol** | 50.0 | 68.2 | 0.22 |
| Polysomnographic Variables: Mean (95% CI) | |||
| AHI (events/hr) | 38.3 (31.0–45.6) | 43.9 (29.5–58.3) | 0.48 |
| Desaturation index† | 10.9 (6.2–15.6) | 11.0 (5.5–16.5) | 0.98 |
| Oxygen nadir (%) | 87.1 (84.4–89.9) | 88.7 (85.9–91.5) | 0.40 |
| Oxygen mean (%) | 95.0 (93.9–96.1) | 96.5 (95.8–97.2) | <0.05 |
| Arousal index (events/hour) | 39.6 (30.0–49.3) | 49.3 (39.7–58.9) | 0.15 |
| Total sleep time (TST; minutes) | 345.0 (309.3–380.7) | 332.5 (306.9–358.1) | 0.56 |
| Sleep efficiency (% TST) | 80.6 (75.3–85.8) | 76.6 (71.0–82.2) | 0.29 |
| Sleep latency (minutes) | 24.4 (13.9–34.9) | 23.4 (13.8–33.1) | 0.89 |
| CPAP (cm H2O) | 10.5 (9.3–11.8) | 9.6 (7.6–11.5) | 0.38 |
Any GERD = positive endorsement of GERD symptoms or GERD medication use or physician diagnosis of GERD.
Caffeine, tobacco, and alcohol were all related to current use.
Number of oxygen desaturations >3% from baseline per hour. One patient in the control group was missing CPAP data and one patient from the aerophagia group was missing desaturation index data. Chi-square test for all analyses except for tobacco (Fisher exact test).
GERD = gastroesophageal reflux disease, AHI = apnea-hypopnea index, TST = total sleep time, CPAP = continuous positive airway pressure.
DISCUSSION
This pilot study revealed a positive association between aerophagia and GERD symptoms and GERD-related medication use in patients using CPAP for SDB. The endpoint of the presence of any one of GERD symptoms, medication use, or physician diagnosis was more common in the aerophagia group. There are a number of potential physiological explanations for this association. Gastroesophageal reflux in normal individuals is closely related to transient LES relaxation episodes that occur during wakefulness and brief arousals from sleep.7,8 Patients with GERD are at increased risk of reflux events during these transient episodes of relaxation.4 These factors could provide an esophageal environment conducive to the development of aerophagia. Also, gastric distention is a potent stimulator of transient LES relaxation.8 Therefore, once initiated, aerophagia-associated increases in gastric pressure could create a positive feedback loop, leading to further air swallowing by the patient, with worsening aerophagia and increasing reflux.
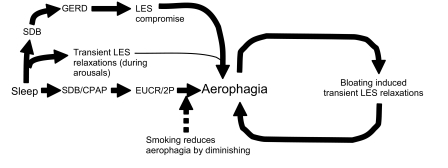
Interestingly, we found that controls were more likely to smoke than the aerophagia group. Nicotine transiently decreases LES tone, thereby contributing to GERD development.9,10 However, the effects of smoking on LES tone are self-limited, and would not extend into the sleep period.9,10 Smoking also diminishes the esophago-upper esophageal sphincter contractile reflex (EUCR) and secondary peristalsis (2P), with triggering volumes 2.6 times as high in smokers as nonsmokers.11 The EUCR/2P is a reflex contraction of the upper esophageal sphincter followed by non-deglutitive esophageal peristalsis. It has been shown that esophageal distention with air triggers a peristaltic response that traverses the entire esophagus.12 Animal models have demonstrated triggering of the EUCR/2P reflex by esophageal distension with air applied in a slow, continuous manner.13 In humans, stimulation of the EUCR/2P in NREM sleep can take ≥ 3.5 min from the application of the stimulus to triggering of the reflex, with multiple peristaltic events occurring per EUCR activation.14 Therefore, the inhibiting effect of smoking on the EUCR/2P might explain the reduced prevalence of aerophagia in smokers. Further speculation suggests that activation of the EUCR/2P reflex by CPAP could contribute to the development of aerophagia in patients with SDB. Additional research investigating the potential role of the EUCR/2P in aerophagia is necessary before these conclusions can be definitively drawn. Figure 1 is a theoretical model summarizing these physiological relationships and their potential contribution to the development of aerophagia.
Figure 1.
Theoretical model of the relationship between gastroesophageal reflux disease (GERD) and aerophagia following continuous positive airway pressure (CPAP) therapy for sleep disordered breathing (SDB). LES = lower esophageal sphincter; EUCR/2P = esophago-upper esophageal sphincter contractile reflex and secondary peristalsis. Solid lines indicate relationships predisposing to aerophagia. Dotted lines indicate relationships reducing aerophagia.
GERD is common in patients with SDB, occurring in 54% to 76% of patients.15,16 Interestingly, CPAP reduces GERD symptoms in SDB patients predominantly through increased intraesophageal pressure, with higher CPAP pressures causing greater symptom relief.17–19 Therefore it appears the same air pressure that relieves GERD symptoms by forcing refluxate back into the stomach also has the potential to cause aerophagia in susceptible individuals. CPAP also reflexively increases LES tone. As previously discussed, exposure of the gastroesophageal junction to refluxate compromises the LES over time.4 Therefore, CPAP and chronic reflux have opposite effects on LES tone. Overall, the balance of this relationship appears to favor compromised LES tone, since CPAP improves, but does not completely resolve, GERD symptoms,19 leading to the association we observe between GERD and aerophagia in the current study.
We found a slight reduction in mean SpO2 in the aerophagia group, suggesting more severe SDB in these subjects. However, the lack of differences in other SDB related PSG variables between the 2 groups, particularly CPAP pressures, argues against this notion. Regarding other habits, our finding of no relationship between alcohol and caffeine use and aerophagia are consistent with the literature showing no clear association between specific types of food and GERD.4
We observed a trend toward increased physician-diagnosed GERD in subjects with aerophagia, supporting our findings of an association between aerophagia, GERD symptoms, and medication use. The lack of a significant association here likely reflects frequent self-medication with over-the-counter remedies by patients with GERD symptoms.20 This is not unusual considering that proton pump inhibitors and H2 blockers are both highly effective and available without a prescription. Therefore a formal diagnosis by a physician may be considered unnecessary and not sought by the patient.
Current treatments for aerophagia, such as avoiding bedtime meals, propping the head of the bed, and reducing mean airway pressure with autotitrating PAP, are marginally effective. The apparent role of the LES in aerophagia presents the possibility of novel treatments. The antimuscarinic medication atropine reduces secondary peristalsis following the introduction of air into the esophagus.12 Perhaps other antimuscarinics, such as scopolamine, would prevent aerophagia. Similarly, certain SDB cases may benefit from laparoscopic or open gastric fundoplication around the lower esophagus to mitigate aerophagia and allow ongoing CPAP treatment. This option has potential importance in severely affected patients at high cardiovascular risk. Clearly, further research is needed into the prevention and treatment of aerophagia before any of these interventions can be recommended.
What can aerophagia teach us about GERD? Could this be a clinical sign of more severe GERD? Should these patients be evaluated for a hiatal hernia? Are patients with aerophagia without GERD symptoms prone to future GERD development? These issues deserve further investigation. In the meantime, clinicians may wish to refer aerophagia patients to gastroenterologists for detailed esophageal evaluations.
Our study has limitations, as we lack objective measures of GERD and aerophagia. Also, we did not gather CPAP compliance data or assess all patients at risk. As a result, we cannot comment on the prevalence of aerophagia or its effect on CPAP usage. Also, we did not ascertain time of initial GERD diagnosis or initial exposure to anti-GERD medications in relation to the development of aerophagia. Nevertheless, we feel that ongoing treatment with anti-GERD medications and concurrent symptomology are strong indicators that these patients were exhibiting GERD pathophysiology at the time they developed aerophagia. Overall, the findings of this pilot study suggest that future studies with objective GERD and aerophagia measures are warranted.
In conclusion, we demonstrate an association between GERD symptoms and medication use and CPAP-related aerophagia. We propose a mechanism involving transient LES relaxation in GERD-related LES compromise. Once initiated, gastric bloating may serve to further increase transient LES relaxation, creating a feedback loop worsening the aerophagia. Triggering of the EUCR/2P by CPAP pressure in the proximal esophagus may also contribute to aerophagia development, as supported by the finding that smoking, which inhibits the EUCR/2P, was protective against aerophagia in our sample. Future studies should focus on objective measures of GERD, aerophagia, and CPAP compliance, and the impact of GERD treatment, both medical and surgical, on aerophagia.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Rebecca Larson, Simon Zirians, and Monte Carlow for their assistance in assembling the data for this study.
This work was performed at the University of Washington and Harborview Medical Center.
REFERENCES
- 1.Criner GJ, Brennan K, Travaline JM, et al. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999;116:667–75. doi: 10.1378/chest.116.3.667. [DOI] [PubMed] [Google Scholar]
- 2.Dent J, El-Serag HB, Wallander MA, et al. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–17. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaker R, Castell DO, Schoenfeld PS, et al. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98:1487–93. doi: 10.1111/j.1572-0241.2003.07531.x. [DOI] [PubMed] [Google Scholar]
- 4.Fox M, Forgacs I. Gastro-oesophageal reflux disease. BMJ. 2006;332:88–93. doi: 10.1136/bmj.332.7533.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute, UCLA; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 7.Dodds WJ, Dent J, Hogan WJ, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547–52. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- 8.Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–10. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 9.Stanciu C, Bennett JR. Smoking and gastro-oesophageal reflux. Br Med J. 1972;3:793–5. doi: 10.1136/bmj.3.5830.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennish GW, Castell DO. Inhibitory effect of smoking on the lower esophageal sphincter. N Engl J Med. 1971;284:1136–7. doi: 10.1056/NEJM197105202842007. [DOI] [PubMed] [Google Scholar]
- 11.Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut. 1998;43:537–41. doi: 10.1136/gut.43.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoeman MN, Holloway RH. Stimulation and characteristics of secondary oesophageal peristalsis in normal subjects. Gut. 1994;35:152–8. doi: 10.1136/gut.35.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang IM, Medda BK, Shaker R. Mechanisms of reflexes induced by esophageal distension. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1246–63. doi: 10.1152/ajpgi.2001.281.5.G1246. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj JS, Bajaj S, Dua KS, et al. Influence of sleep stages on esophago-upper esophageal sphincter contractile reflex and secondary esophageal peristalsis. Gastroenterology. 2006;130:17–25. doi: 10.1053/j.gastro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Graf KI, Karaus M, Heinemann S, et al. Gastroesophageal reflux in patients with sleep apnea syndrome. Z Gastroenterol. 1995;33:689–93. [PubMed] [Google Scholar]
- 16.Teramoto S, Ohga E, Matsui H, et al. Obstructive sleep apnea syndrome may be a significant cause of gastroesophageal reflux disease in older people. J Am Geriatr Soc. 1999;47:1273–4. doi: 10.1111/j.1532-5415.1999.tb05216.x. [DOI] [PubMed] [Google Scholar]
- 17.Dent J, Dodds WJ, Friedman RH, et al. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980;65:256–67. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr P, Shoenut JP, Steens RD, et al. Nasal continuous positive airway pressure. A new treatment for nocturnal gastroesophageal reflux? J Clin Gastroenterol. 1993;17:276–80. doi: 10.1097/00004836-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Green BT, Broughton WA, O'Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med. 2003;163:41–5. doi: 10.1001/archinte.163.1.41. [DOI] [PubMed] [Google Scholar]
- 20.Pettit M. Treatment of gastroesophageal reflux disease. Pharm World Sci. 2005;27:432–5. doi: 10.1007/s11096-005-4798-7. [DOI] [PubMed] [Google Scholar]