Abstract
Release of lipid vesicle content induced by the amphipathic peptide δ-lysin was investigated as a function of lipid acyl chain length and degree of unsaturation for a series of phosphatidylcholines. Dye efflux and peptide binding were examined for three homologous lipid series: di-monounsaturated, di-polyunsaturated, and asymmetric phosphatidylcholines, with one saturated and one monounsaturated acyl chain. Except for the third series, peptide activity correlated with the first moment of the lateral pressure profile, which is a function of lipid acyl chain structure. In vesicles composed of asymmetric phosphatidylcholines, peptide binding and dye efflux are enhanced compared to symmetric, unsaturated lipids with similar pressure profiles. We attribute this to the entropically more favorable interaction of δ-lysin with partially saturated phospholipids. We find that lipid acyl chain structure has a major impact on the activity of δ-lysin and is likely to be an important factor contributing to the target specificity of amphipathic peptides.
INTRODUCTION
The cell membrane provides a tightly controlled barrier between cell interior and environment, and any form of communication between these two entities has to occur across this barrier. Binding of soluble agents to membrane-bound receptors triggers complex processes such as signaling cascades or receptor-mediated endocytosis. Conversely, protein export, quintessential for cell-cell communication in multicellular organisms, generally involves a secretory pathway from the endoplasmic reticulum, through the Golgi apparatus, to the extracellular matrix. A number of proteins and peptides, however, circumvent the regular import/export machinery and appear to cross the membrane directly (1). Movement of proteins and peptides across membranes thus seems to be a widespread and biologically important phenomenon. Studies of small peptides and their interaction with model membranes have been instrumental in the elucidation of the principles that govern the movement of peptides across hydrophobic barriers. A particularly well-studied class of peptides comprises cell-penetrating peptides that are able to transport cargo across membranes (2) and amphipathic, cytolytic peptides. Of the latter, antimicrobial peptides have received most attention due to their preferential lysis of prokaryotic cells and potential use as antibiotic agents (3).
Initially, antimicrobial peptides were thought to permanently insert into the membrane and aggregate as barrel staves to form ion-channels (4). Although alamethicin appears to form pores of this type, most other peptides have been found to form more transient structures, such as toroidal pores (5,6) or even relatively unstructured membrane defects (7–10). Alternatively, some peptides may not cross the cell membrane at all but instead lead to the micellization of the lipid bilayer through adsorption to the membrane surface, as has been proposed in the carpet model (11). The factors that determine peptide-membrane interactions have been the focus of research for much of the past decade. The majority of antimicrobial peptides are cationic and the distribution of charges within the peptide, the degree of amphipathicity, and the overall hydrophobicity clearly play an important role in peptide-membrane interactions. Model membrane systems that mimic the composition of bacterial membranes usually contain the anionic lipid diacylphosphatidylglycerol, which is the predominant phospholipid in many Gram-positive strains but also exists in the membranes of Gram-negative bacteria. Due to the cationic nature of most antimicrobial peptides, the charge of the lipid headgroup is considered paramount in determining peptide-lipid interactions, while the lipid acyl chain composition has received less attention by comparison. The current work focuses on the impact of lipid acyl chain composition, in particular hydrophobic thickness and the degree of unsaturation, on peptide-membrane interactions.
We used the cytolytic peptide δ-lysin, a 26-residue peptide that forms an amphipathic helix when bound to bilayer membranes, to probe the effect of lipid acyl chain structure on peptide activity. δ-Lysin is one of many toxins secreted by the Gram-positive bacterium Staphylococcus aureus and is extremely efficient in permeabilizing the cell membranes of a wide variety of organisms and artificial vesicles (12–15). In systems that exhibit lipid phase coexistence, it preferentially binds to liquid-disordered lipid phases and only poorly to solid and cholesterol-containing, liquid-ordered phases (16). Based primarily on the analysis of kinetic data, we recently introduced a model for the function of δ-lysin where a small aggregate of approximately three protomers is the state responsible for the release of content from lipid vesicles (7,16,17). The pores are formed by δ-lysin as the peptide equilibrates across the membrane. They are thus transient structures and the peptides do not remain permanently inserted in the membrane (7,17).
Membrane perturbation requires a distortion, sometimes transient, of the lipid matrix and often the formation of highly curved peptide-lipid structures. For instance, we proposed that defect formation by δ-lysin involves a short-lived intermediate in which the lipids are forced into a positively curved arrangement (7). Highly curved lipid structures also occur in the formation of toroidal pores and during micellization of the bilayer, as has been suggested in the carpet model. For a homologous series of membrane-forming lipids with the same headgroup, differences in the ability to form structures of high curvature are determined by the acyl chain structure—which also determines the bulk elastic properties of the bilayer.
Bilayer elastic properties have been shown to influence the activity of ion channels and of a number of small, membrane-active peptides such as melittin, GALA, alamethicin, and gramicidin (18–26). In previous studies, the elastic properties of the lipid matrix were often altered by the inclusion of lysolipids, which are prone to form structures of positive curvature; phosphatidylethanolamine, which tends to form inverted lipid structures of negative curvature due to its small headgroup (27); or cholesterol, which stiffens the membrane (28–30). Working with lipid mixtures, however, has, at least in this particular case, the disadvantage that the different lipid types may not mix well or change peptide binding to the membrane. In either case, it is not straightforward to correlate experimental observations with changes in the elastic properties of the lipid matrix. Therefore, we chose to use one-component lipid vesicles, in which the lipid headgroup was kept constant and only the acyl chain composition was varied. We found that the interaction of δ-lysin with lipid bilayers is strongly dependent on the elastic properties of the membrane and, in the case of many but not all membrane-forming lipids, seems to respond to the lateral pressure profile within the bilayer.
METHODS
Chemicals
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (SOPC), 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine (di14:1PC), 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine (di16:1PC), 1,2-dioleoyl-sn-glycero-3-phosphocholine (di18:1PC), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (di18:2PC), 1,2-dilinolenoyl-sn-glycero-3-phosphocholine (di18:3PC), 1,2-dieicosenoyl-sn-glycero-3-phosphocholine (di20:1PC), and 1,2-dierucoyl-sn-glycero-3-phosphocholine (di22:1PC) were purchased from Avanti Polar Lipids (Alabaster, AL). Carboxyfluorescein (99% pure, lot No. A015252901) was purchased from ACROS (Morris Plains, NJ).
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine-n-(7-methoxycoumarin) (7MC-POPE), POPE labeled with 7MC through an amide bond to the amino group of the ethanolamine headgroup, was synthesized as previously described (9). Organic solvents (high performance liquid chromatography/American Chemical Society grade) were purchased from Burdick & Jackson (Muskegon, MI). Lipids and probes were tested by thin layer chromatography and used without further purification.
δ-Lysin
δ-Lysin (formyl-NH-Met-Ala-Gln-Asp-Ile-Ile-Ser-Thr-Ile-Gly-Asp-Leu-Val-Lys-Trp-Ile-Ile-Asp-Thr-Val-Asn-Lys-Phe-Thr-Lys-Lys-COOH) was a gift from Dr. T. H. Birkbeck (University of Glasgow, Scotland). Its purification was described previously (17,31). For the dye efflux kinetics measurements, lyophilized δ-lysin was dissolved in distilled water acidified to pH ≃ 3, to a final concentration of 200 μM as described in Pokorny and Almeida (16). Just before the kinetic experiments δ-lysin was diluted into 0.10 M KCl, pH 3.0. The low pH imparts the peptide with a net positive charge, which minimizes its aggregation.
Preparation of large unilamellar vesicles
Large unilamellar vesicles (LUVs) were prepared by mixing the lipids in chloroform in a round-bottom flask. For vesicles containing 7MC-POPE, the probes were added to the lipid in chloroform solution at a final probe concentration of 1 mol %. The solvent was rapidly evaporated using a rotary evaporator (Büchi R-3000, Flawil, Switzerland) at 60°C. The lipid film was then placed under vacuum for 4 h and hydrated by the addition of buffer containing 20 mM MOPS, pH 7.5, 0.1 mM EGTA, 0.02% NaN3, and 100 mM KCl or appropriately modified as indicated below. The suspension of multilamellar vesicles was subjected to five freeze-thaw cycles. The suspension was then extruded 10 times through two stacked polycarbonate filters of 0.1-μm pore size (Nuclepore, Whatman, Florham, NJ), using a water-jacketed high pressure extruder (Lipex Biomembranes, Vancouver, Canada) at room temperature. Lipid concentrations were assayed by the Bartlett phosphate method (32), modified as previously described (17).
Kinetics of δ-lysin binding to and dissociation from lipid vesicles
The kinetics of association of δ-lysin with LUVs was recorded on an Applied Photophysics SX.18MV stopped-flow fluorimeter (Leatherhead, Surrey, UK). Fluorescence resonance energy transfer between the intrinsic Trp residue of δ-lysin and 7MC-POPE incorporated in the lipid membrane was used to monitor peptide binding and dissociation from LUVs. The Trp was excited at 280 nm and transferred energy to 7MC-POPE, which absorbs maximally at 348 nm. The emission of 7MC, with maximum at 396 nm, was measured using a GG-385 cutoff filter (Edmund Industrial Optics, Barrington, NJ). After mixing, the concentration of peptide was 0.5 μM.
Carboxyfluorescein efflux experiments
LUVs for carboxyfluorescein (CF) efflux kinetics measurements were prepared by hydrating the dried lipid film with CF-containing buffer (20 mM MOPS pH 7.5, 0.1 mM EGTA, and 0.02% NaN3, 50 mM CF) to give a final lipid concentration of 10 mM. After extrusion, CF-containing LUVs were passed through a Sephadex-G25 column (GE Healthcare Bio-Sciences, Piscataway, NJ) to separate the dye in the external buffer from the vesicles. The suspension was diluted in buffer to the desired lipid concentration and used for fluorescence measurements. The buffer used was 20 mM MOPS pH 7.5, containing 100 mM KCl, 0.1 mM EGTA, and 0.02% NaN3, which has the same osmolarity as the CF-containing buffer. The kinetics of carboxyfluorescein efflux were recorded in a model No. 8100 Spectrofluorimeter (SLM-Aminco, Foster City, CA), updated by ISS (ISS, Champaign, Il) and adapted with a RX2000 rapid kinetics spectrometer accessory (Applied Photophysics), equipped with a RX pneumatic drive accessory (Applied Photophysics). CF efflux was measured by the relief of self-quenching of fluorescence, measured by excitation at 470 nm and emission at 520 nm.
Calculation of average relaxation times
The curves of carboxyfluorescein release as a function of time were characterized by a mean relaxation time (τ), as described before (16). Briefly, the mean relaxation time is obtained from the integral (33,34),
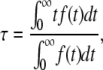 |
(1) |
where
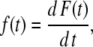 |
(2) |
and F(t) is the experimental curve of normalized fluorescence increase as a function of time. This curve increases as CF is released, until it essentially reaches a plateau (see Fig. 1). The time-derivative of F(t), f(t) behaves as the probability density function (33,34). For example, for a multiexponential decay τ is the weighted average of the relaxation times of each exponential function. Before numerical differentiation, the curves were smoothened as described before (16), to avoid errors due to experimental noise.
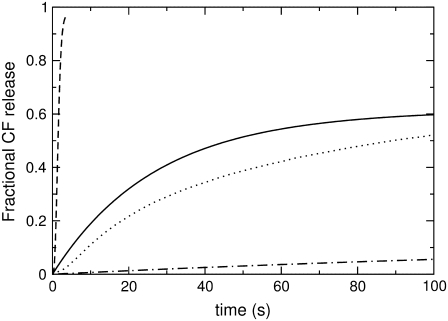
FIGURE 1.
CF efflux induced by δ-lysin (0.5 μM) for a series of di-monounsaturated PCs: di14:1PC (dashed line), di16:1PC (solid line), di18:1PC (dotted line), and di20:1PC (dashed-dotted line). The lipid concentration in all experiments was 50 μM.
RESULTS
δ-Lysin causes the rapid release of contents from unilamellar POPC vesicles (17). A convenient way to measure the kinetics of content release is via the increase of fluorescence (Fig. 1) that is observed when a high, self-quenching concentration of dye is diluted into the surrounding aqueous buffer after peptide-induced release from vesicles. We measured the average time required for the release of a fluorescent dye, carboxyfluorescein, as a function of the lipid chain length and unsaturation, at constant lipid and peptide concentrations.
The mean time constant, τ, of dye efflux (Eq. 1) provides an average, model-independent, and robust observable that can be directly related to changes in bulk bilayer properties. The analysis is based on the idea that lnτ should, in essence, be related to the Gibbs free energy of activation for dye efflux, ΔG‡. For a series of homologous phosphatidylcholines (PCs) to which peptide binding is similar, ΔG‡ then reflects the Gibbs free energy of activation associated with the rate-limiting step, which is dominated by the free energy required for the insertion of the peptide aggregate into the lipid bilayer, ΔGo (10). Thus, ΔGo for insertion and lnτ for dye efflux reflect the ease with which the membrane defect is formed in the lipid bilayer. Bulk elastic properties of the lipid bilayer are a direct consequence of lipid acyl chain structure. In the following, we will therefore relate lnτ of dye release to the elastic properties of the bilayer to assess the impact of lipid acyl chain structure on membrane perturbation by δ-lysin.
Degree of unsaturation
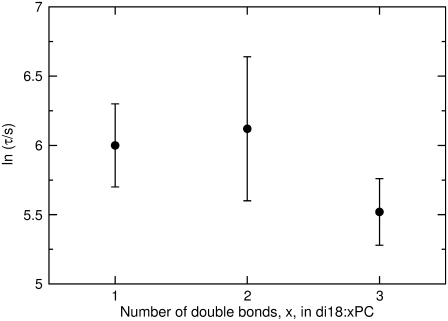
For a series of PCs that contain cis-unsaturated acyl chains in both the sn-1 and sn-2 positions, lnτ of dye release caused by δ-lysin is not a simple monotonic function of the number of double bonds (Fig. 2). Rather, lnτ appears to be the same for di18:1PC, di18:2PC, and di18:3PC, within experimental error.
FIGURE 2.
The average time constant (τ) for CF release induced by δ-lysin from lipid vesicles as a function of the number of double bonds in di18:xPC, where x represents the number of double bonds. Each error bar represents the standard deviation of a minimum of five individual traces. In the kinetic experiments, the peptide concentration was 0.5 μM and the lipid concentration was 50 μM.
Stretching modulus
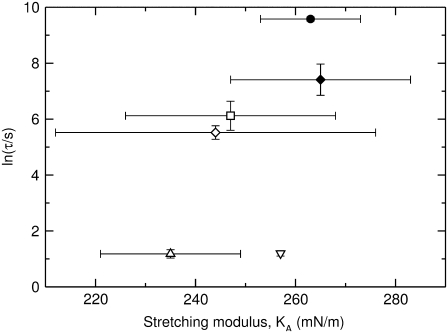
For a thin layer of material such as a bilayer membrane, the isothermal elastic stretching modulus, KA, reflects the area change in response to a uniform tension applied parallel to the surface (35,36). For the series of PCs considered here, KA remains essentially constant within the experimental error (37), but the τ of dye release varies over several orders of magnitude (Fig. 3), indicating a lack of correlation between membrane perturbation and the bilayer stretching modulus.
FIGURE 3.
The average time constant (τ) for CF release induced by δ-lysin as a function of the stretching modulus, KA, for POPC (∇), SOPC (Δ), di18:3PC (◊), di18:2PC (□), di18:1PC (♦), and di22:1PC (•). Each vertical error bar represents the standard deviation of a minimum of five individual kinetic traces. The peptide concentration in the dye release experiments was 0.5 μM and the lipid concentration was 50 μM. The values for KA and their standard deviations were taken from Rawicz et al. (39).
Bending modulus
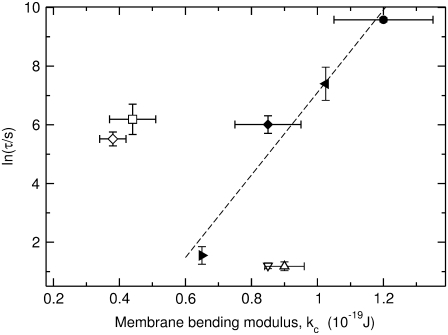
A change in bilayer curvature induced by a bending moment acting on the bilayer is described by the bending modulus, kc (35,36). In Fig. 4, lnτ is plotted as a function of kc. For the di-monounsaturated series, di14:1PC, di16:1PC, di18:1PC, di20:1PC, and di22:1PC, lnτ increases linearly with kc (Fig. 4, solid symbols). For the di-polyunsaturated lipids (Fig. 4, open square and diamond), lnτ is consistently larger than predicted from the straight line fit to the di-monounsaturated series. Surprisingly, dye release from vesicles composed of POPC and SOPC (Fig. 4, open triangles), both mixed-chain lipids with a saturated chain in the sn-1 position and a monounsaturated chain in the sn-2 position, occurs two orders-of-magnitude faster than from vesicles composed of a di-monounsaturated lipid with a comparable bending modulus.
FIGURE 4.
The average time constant for CF release induced by δ-lysin from lipid vesicles as a function of the bending modulus, kc. Solid symbols correspond to the di-monounsaturated PCs di16:1PC (▸), di18:1PC (♦), di20:1PC (◂), and di22:1PC (•). Open square symbols correspond to the di-polyunsaturated PCs di18:3PC (◊) and di18:2PC (□). Open triangular symbols correspond to POPC (∇) and SOPC (Δ). Each vertical error bar represents the standard deviation of a minimum of five individual kinetic traces. The dashed line is a linear fit to the data for the di-monounsaturated PCs (solid symbols). The peptide concentration in the dye release experiments was 0.5 μM and the lipid concentration was 50 μM. The values for kc and their standard deviations were taken from Rawicz et al. (39) and Kučerka et al. (52).
Bilayer thickness
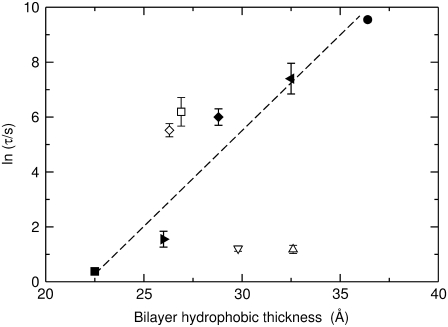
For thin sheets, kc scales with both KA and the square of the thickness of the material (38). KA remains essentially constant for the lipids considered here and thus we expect kc ∝ h2, where h is the thickness of the hydrophobic membrane core (39). Therefore, lnτ can be expected to scale as the square of hydrophobic bilayer thickness for di-monounsaturated PCs because lnτ scales linearly with kc. However, lnτ appears to be proportional to h rather than to h2 (Fig. 5, solid symbols). Again, similar to what we already observed for the dependence of lnτ on kc, dye efflux caused by δ-lysin from vesicles composed of di-polyunsaturated lipids (Fig. 5, open square and diamond) is systematically slower than expected from the behavior of vesicles composed of di-monounsaturated lipids of the same thickness. At the other extreme, dye efflux from PCs with asymmetric acyl chains (POPC, SOPC) occurs at least two orders-of-magnitude faster than from their di-monounsaturated counterparts and does not seem to depend strongly, if at all, on hydrophobic thickness (Fig. 5, open triangles).
FIGURE 5.
The average time constant for CF release induced by δ-lysin from lipid vesicles as a function of hydrophobic thickness, h. Solid symbols correspond to the di-monounsaturated PCs di14:1PC (▪), di16:1PC (▸), di18:1PC (♦), di20:1PC (◂), and di22:1PC (•). Open square symbols correspond to the di-polyunsaturated PCs di18:3PC (◊) and di18:2PC (□). Open triangular symbols correspond to POPC (∇) and SOPC (Δ). Each error bar represents the standard deviation of a minimum of five individual kinetic traces. The dashed line is a linear fit to the data for the di-monounsaturated PCs (solid circles). The peptide concentration in the dye release experiments was 0.5 μM and the lipid concentration was 50 μM. The values for h were taken from Hinderliter et al. (64).
Peptide binding
Binding of δ-lysin to vesicles composed of a series of lipids was measured by fluorescence resonance energy transfer from the tryptophan residue to the acceptor fluorophore 7MC-POPE embedded in the lipid matrix (9). If peptide binding occurs on a shorter timescale than subsequent membrane-perturbing events, the time course of peptide binding follows a simple exponential function. The apparent rate constant (kapp) of peptide binding is a function of the molecular on- and off-rate constants, kapp = kon[V] + koff, where [V] is the vesicle concentration. A plot of kapp versus [V] yields a straight line (not shown), which allows the determination of kon from the slope and koff from the y intercept (9,10). The ratio koff/kon is then a good estimate of the dissociation constant, KD. Equilibrium binding of δ-lysin to POPC, DOPC, di22:1PC, and di18:3PC vesicles was found to be similar. Binding to DOPC and di22:1PC is strongest (KD ≈ 30 μM), followed by POPC (KD ≈ 60 μM) and di18:3PC (KD ≈ 100 μM). Thus, binding does not determine the differences observed in lnτ.
DISCUSSION
Release of vesicle content by δ-lysin was investigated as a function of lipid acyl chain length and degree of unsaturation for a series of phosphatidylcholines. We had previously hypothesized that δ-lysin would introduce curvature strain upon binding to vesicles, which is relieved when the peptide crosses the bilayer in the form of small aggregates (7). In this model, graded dye release from vesicles occurs as the aggregates cross the membrane and cause a significant perturbation of the bilayer. If this hypothesis is correct, dye release should be very sensitive to bilayer elastic properties and lipid acyl chain composition, since the types of structures phospholipids can form are in large part determined by their acyl chain structures.
We found no correlation between the stretching modulus (KA) and lnτ of dye release (Fig. 3). The bending modulus (kc), on the other hand, influences dye release, but in a complex manner that depends on the lipid series under consideration. We believe that this complexity arises from the interplay of kc, the lateral pressure profile (π), and the spontaneous curvature of the lipids forming the bilayer (co), all of which are a direct consequence of lipid acyl chain structure. In the following, we will briefly review the connection between kc, π, and co, and then relate these parameters to the experimental results obtained with each lipid series.
By definition, a symmetrical bilayer has no intrinsic curvature, but the individual monolayers may still experience packing stress resulting from forcing unsaturated lipid acyl chains into a planar configuration (36,40,41). The resulting distribution of lateral stresses in the membrane is given by the profile of the lateral pressure, π(z), along the membrane normal, z (42). The first moment (P1) of the lateral pressure profile of each monolayer is physically equivalent to a torque stress acting on the membrane, the magnitude of which is determined by the shape of the pressure profile, 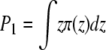 (40,43). This integral is equal to the product of kc and the spontaneous curvature of each monolayer, which is P1 = cokc (36,42–45).
(40,43). This integral is equal to the product of kc and the spontaneous curvature of each monolayer, which is P1 = cokc (36,42–45).
Let us first consider peptide-induced efflux from vesicles composed of the homologous series of di-polyunsaturated PCs, di18:1PC (DOPC), di18:2PC, and di18:3PC. We found that the average τ of dye efflux is not very sensitive to the number of double bonds and, in fact, appears to be the same within experimental error (Fig. 2). However, with the introduction of two or more unsaturations, the bending modulus decreases substantially (37,39). Acyl chain packing stress, on the other hand, will increase with unsaturation such that co is likely to increase in absolute value for this series, as has been observed for other lipids (46–48). We therefore posit that the decrease in kc will, to some extent, be compensated for by an increase in co, which will lead to a P1 that is roughly constant for the lipids of this series. Indeed, calculations using a statistical thermodynamic lattice model for the membrane predict that P1 for these three lipids should be essentially the same (44,45). We thus conclude that δ-lysin does not respond directly to either co or kc of the target membrane but primarily to P1. If this is correct, lnτ of dye efflux should scale with kc only if the spontaneous curvature remains constant for a particular lipid series.
Next, we investigated the homologous series of di-monounsaturated PCs, di14:1PC, di16:1PC, di18:1PC, di20:1PC, and di22:1PC. In this case we found a clear dependence of lnτ on kc and chain length (Figs. 4 and 5), which indicates that formation of a membrane defect and insertion of the δ-lysin aggregate becomes progressively harder as the membrane becomes thicker and less flexible. In principle, kc scales with the square of the thickness of the hydrophobic core, or acyl chain length, rather than linearly (39). Thus, lnτ should scale with h2, since lnτ appears to depend linearly on kc (Fig. 4). But for the thicker bilayers of this series, efflux occurs faster than expected (Fig. 5). This could be due to the limited range of bilayer chain lengths studied here, but this explanation appears unlikely to us and we will revisit this issue after the discussion of the asymmetric lipids.
There are no calculations regarding the pressure profiles as a function of chain length for di-monounsaturated PCs, but the trend in P1 for saturated chains is clear: the pressure profiles broaden as a function of chain length, thus increasing the magnitude of P1 (45). It is reasonable to assume that P1 follows the same trend in di-monounsaturated PCs and, if so, we see that lnτ of efflux again scales with P1. There are no data on the dependence of co on chain length for di-monounsaturated PCs, but co increases in magnitude with chain length for phosphoethanolamines (PEs) (36,46–48). Due to the larger PC headgroup, it is very likely that co increases only slightly, if at all, with chain length for this series. In that case, P1 is a function of chain length only, and lnτ of efflux can be expected to correlate linearly with kc, which appears to be the case experimentally (Fig. 4).
Last, we compared dye efflux from vesicles composed of the asymmetric, monounsaturated lipids POPC and SOPC with the corresponding di-monounsaturated lipid DOPC. In this case, the correlation of lnτ with P1 breaks down quite dramatically, with dye release occurring two orders-of-magnitude faster from POPC or SOPC vesicles than from DOPC vesicles (Fig. 4). This behavior does not appear to be unique to δ-lysin. For instance, melittin, one of the most studied amphipathic peptides, is known to be more active toward POPC than DOPC bilayers, as is GALA, a synthetic peptide (21,49). The effect is surprising because POPC and SOPC have bending moduli very similar to DOPC (Fig. 4) and P1 has been predicted to be also very similar, or even somewhat larger, for POPC and SOPC compared to DOPC (44,45). Hence, if both kc and P1 are so similar for POPC, SOPC, and DOPC, the same must be true for co, and we would expect lnτ of dye efflux to be similar as well, which it clearly is not. It is curious to note in this context that nature hugely favors the use of asymmetric membrane lipids over symmetric ones in eukaryotic cell membranes.
Theoretical calculations of pressure profiles assume that asymmetric lipids behave like a 1:1 mixture of saturated and unsaturated chains and that the profile is dominated by the effect of the unsaturated chain (44,45). This may be an oversimplification and the effect of an asymmetric acyl chain composition on peptide activity may be due to interactions on the molecular level that are currently not captured by the calculations. Other calculations, including molecular dynamics simulations, have proposed a reduced bending modulus for lipids with asymmetric acyl chains compared to their symmetric counterparts as a reason for their difference (50,51). However, the values for kc used here have been determined experimentally and do not vary much among POPC, SOPC, and DOPC (39,52). Clearly, bulk properties of the lipid bilayer such as the bending modulus and the resulting lateral pressure profile are not sufficient to describe the significantly different behavior of δ-lysin and other amphipathic peptides toward bilayers composed of asymmetric phospholipids.
To determine whether different degrees of peptide binding to lipid vesicles were the cause for the observed dependence of peptide activity on lipid composition, we also measured δ-lysin binding as a function of lipid composition. We found that the interaction of δ-lysin with di18:1PC (DOPC), POPC, di22:1PC, and di18:3PC is characterized by similar dissociation constants, which differ at most by a factor of 3. Binding occurs with the highest affinity to the di-monounsaturated PCs DOPC and di22:1PC (KD ≈ 30 μM), followed by POPC (KD ≈ 60 μM), and di18:3PC (KD ≈ 100 μM). Clearly, the striking difference in the rate of dye efflux from vesicles composed of the asymmetric lipids POPC and SOPC on one hand and the di-monounsaturated lipid DOPC on the other hand cannot be accounted for by weaker binding to DOPC. Rather, it appears that peptide-induced membrane perturbation is reduced in proportion to the degree of lipid unsaturation. We suggest that the interaction of δ-lysin with membrane lipids will “freeze” acyl chain motion, which is entropically unfavorable, particularly for membranes composed only of unsaturated acyl chains. However, this entropic penalty should depend on acyl chain length and be smaller for longer chains, because, at the same temperature, they are more ordered than shorter ones. We believe that this explanation can also account for the higher-than-expected rate of dye efflux from vesicles composed of the long-chain members of the di-monounsaturated series. In this case, the linear dependence of lnτ on kc is probably only apparent but not exact. Comparing fluid bilayers of similar thicknesses and bending moduli, δ-lysin should interact more strongly with bilayers composed of saturated lipids than with unsaturated ones, if this interpretation is correct. In fact, δ-lysin has been shown to interact very favorably with bilayers composed of the saturated phospholipid 1,2-dimyristoyl-sn-glycero-3-phosphocholine (15).
Finally, co is clearly not the only factor that determines peptide-bilayer interactions. A number of membrane-active peptides of both natural and synthetic origin induce positive curvature stress in the bilayer upon binding (8,20,53–59). Thus, the spontaneous curvature of the target lipid membrane has been thought to be critical in determining peptide-lipid interactions. As a result, the formation of a bilayer-perturbing structure should be inhibited in bilayers composed of lipids with negative spontaneous curvature. This question is often addressed experimentally by including diacylphosphatidylethanolamine (PE) in the bilayer along with other bilayer-forming lipids. The tendency to form an inverted hexagonal phase of negative intrinsic curvature rather than a lamellar phase correlates directly with lipid acyl chain length and degree of unsaturation, and inversely with headgroup size (36,46–48). Phosphatidylethanolamines, especially when unsaturated, are prone to form inverted hexagonal phases due to their small headgroup (27), and peptide activity is frequently reduced in proportion to the PE content. However, an unambiguous interpretation of these results in terms of the impact of co on peptide activity is hampered by complications arising from multiple lipid-peptide interactions and nonideal lipid mixing. Also, introduction of more highly unsaturated lipids is likely to change peptide binding, co, and kc, making it difficult to attribute experimental observations to variations in co only.
The effect of cholesterol in eukaryotic membranes on peptide-membrane interactions can also be partially understood in terms of its effect on P1. Generally, adding cholesterol to a PC matrix causes an increase in hydrophobic membrane thickness (60,61) and kc (29,62). Peptide partitioning into the bilayer core can thus be predicted to be reduced, which is true for δ-lysin (16,63). However, the case is further complicated by poor binding of δ-lysin to liquid-ordered bilayers that exist at a cholesterol content >35%. The behavior of δ-lysin in systems that exhibit lipid phase separation between a cholesterol-rich, liquid-ordered and a cholesterol-poor, liquid-disordered phase at cholesterol concentrations characteristic for eukaryotic cell membranes, therefore becomes quite complex (16,63).
In summary, we have shown that the capacity to promote or inhibit peptide-induced membrane perturbation is intimately related to the fatty acid structure of the lipids that constitute the bilayer. This finding emphasizes the point that lipid acyl chain structure is an important determinant in peptide-lipid interactions and that even subtle differences in structure such as the presence of one extra unsaturation can have a dramatic impact on peptide activity. In this sense, DOPC is a poor—but commonly used—mimic of POPC in studies that seek to elucidate peptide-membrane interactions. This study further emphasizes that the specificity of antimicrobial peptides may only in part be determined by the headgroup composition of the target membrane.
Acknowledgments
We thank Melissa Cherry and Sarah Pagentine for their assistance in the determination of some of the dissociation constants.
This work was supported by National Institutes of Health grant No. GM072507.
Editor: Paul H. Axelsen.
References
- 1.Nickel, W. 2003. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 270:2109–2119. [DOI] [PubMed] [Google Scholar]
- 2.Handbook of Cell-Penetrating Peptides, 2nd Ed. 2006. U. Langel, editor. CRC, Boca Raton, FL.
- 3.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenstein, G., and H. Lecar. 1977. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 10:1–34. [DOI] [PubMed] [Google Scholar]
- 5.Ludtke, S. J., K. He, W. T. Heller, T. A. Harroun, L. Yang, and H. W. Huang. 1996. Membrane pores induced by magainin. Biochemistry. 35:13723–13728. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki, K., O. Murase, N. Fujii, and K. Miyajima. 1996. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 35:11361–11368. [DOI] [PubMed] [Google Scholar]
- 7.Pokorny, A., and P. F. F. Almeida. 2004. Kinetics of dye efflux and lipid flip-flop induced by δ-lysin in phosphatidylcholine vesicles and the mechanism of graded release by amphipathic, α-helical peptides. Biochemistry. 43:8846–8857. [DOI] [PubMed] [Google Scholar]
- 8.Leontiadou, H., A. E. Mark, and S. J. Marrink. 2006. Antimicrobial peptides in action. J. Am. Chem. Soc. 128:12156–12161. [DOI] [PubMed] [Google Scholar]
- 9.Gregory, S. M., A. C. Cavenaugh, V. Journigan, A. Pokorny, and P. F. F. Almeida. 2008. A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide Cecropin A. Biophys. J. 94:1667–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yandek, L. E., A. Pokorny, and P. F. F. Almeida. 2008. Small changes in the primary structure of Transportan 10 alter the thermodynamics and kinetics of its interaction with phospholipid vesicles. Biochemistry. 47:3051–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shai, Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers. 66:236–248. [DOI] [PubMed] [Google Scholar]
- 12.Kreger, A. S., K.-S. Kim, F. Zaboretzky, and A. W. Bernheimer. 1971. Purification and properties of staphylococcal δ-hemolysin. Infect. Immun. 3:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, K. H., J. E. Fitton, and K. Wüthrich. 1987. Nuclear magnetic resonance investigation of the conformation of δ-hemolysin bound to dodecylphosphocholine micelles. Biochim. Biophys. Acta. 911:144–153. [DOI] [PubMed] [Google Scholar]
- 14.Thiaudière, E., O. Siffert, J. C. Talbot, J. Bolard, J. E. Alouf, and J. Dufourcq. 1991. The amphiphilic α-helix concept. Consequences on the structure of staphylococcal δ-toxin in solution and bound to lipids. Eur. J. Biochem. 195:203–213. [DOI] [PubMed] [Google Scholar]
- 15.Lohner, K., E. Staudegger, E. J. Prenner, R. N. A. H. Lewis, M. Kriechbaum, G. Degovics, and R. N. McElhaney. 1999. Effect of staphylococcal δ-lysin on the thermotropic phase behavior and vesicle morphology of dimyristoylphosphatidylcholine lipid bilayer model membranes. Differential scanning calorimetry, 31P nuclear magnetic resonance, Fourier transform infrared spectroscopy, and x-ray diffraction studies. Biochemistry. 38:16514–16528. [DOI] [PubMed] [Google Scholar]
- 16.Pokorny, A., and P. F. F. Almeida. 2005. Permeabilization of raft-containing lipid vesicles by δ-lysin: a mechanism for cell sensitivity to cytotoxic peptides. Biochemistry. 44:9538–9544. [DOI] [PubMed] [Google Scholar]
- 17.Pokorny, A., T. H. Birkbeck, and P. F. F. Almeida. 2002. Mechanism and kinetics of δ-lysin interaction with phospholipid vesicles. Biochemistry. 41:11044–11056. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H. W. 1986. Deformation free energy of bilayer membrane and its effect on gramicidin channel lifetime. Biophys. J. 50:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faucon, J. F., J. M. Bonmatin, J. Dufourcq, and E. J. Dufourc. 1995. Acyl chain length dependence in the stability of melittin-phosphatidylcholine complexes. A light scattering and 31P-NMR study. Biochim. Biophys. Acta. 1234:235–243. [DOI] [PubMed] [Google Scholar]
- 20.Monette, M., and M. Lafleur. 1996. Influence of lipid chain unsaturation on melittin-induced micellization. Biophys. J. 70:2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicol, F., S. Nir, and F. C. Szoka. 2000. Effect of phospholipid composition on an amphipathic peptide-mediated pore formation in bilayers vesicles. Biophys. J. 78:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, F. Y., M. T. Lee, and H. W. Huang. 2002. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study on alamethicin. Biophys. J. 82:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen, F. Y., M. T. Lee, and H. W. Huang. 2003. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys. J. 84:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghuraman, H., and A. Chattopadhyay. 2004. Influence of lipid chain unsaturation on membrane-bound melittin: a fluorescence approach. Biochim. Biophys. Acta. 1665:29–39. [DOI] [PubMed] [Google Scholar]
- 25.Allende, D., S. A. Simon, and T. J. McIntosh. 2005. Melittin-induced bilayer leakage depends on lipid material properties: evidence for toroidal pores. Biophys. J. 88:1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIntosh, T. J., and S. A. Simon. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35:177–198. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh, T. J. 1996. Hydration properties of lamellar and non-lamellar phases of phosphatidylcholine and phosphatidylethanolamine. Chem. Phys. Lipids. 81:117–131. [DOI] [PubMed] [Google Scholar]
- 28.Evans, E., and D. Needham. 1987. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. J. Phys. Chem. 91:4219–4228. [Google Scholar]
- 29.Evans, E., and W. Rawicz. 1990. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 64:2094–2097. [DOI] [PubMed] [Google Scholar]
- 30.Rawicz, W., B. A. Smith, T. J. McIntosh, S. A. Simon, and E. Evans. 2008. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys. J. 94:4725–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birkbeck, T. H., and J. H. Freer. 1988. Purification and assay of staphylococcal δ-lysin. Methods Enzymol. 165:16–22. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. [PubMed] [Google Scholar]
- 33.Colquhoun, D. 1971. Lectures on Biostatistics. Clarendon Press, Oxford, UK.
- 34.Colquhoun, D., and A. G. Hawkes. 1987. The interpretation of single channel recordings. In Microelectrode Techniques. The Plymouth Workshop Handbook, 2nd Ed. D. Ogden, editor. The Company of Biologists Ltd., Cambridge, UK.
- 35.Evans, E., and R. Skalak. 1979. Mechanics and thermodynamics of biomembranes: part 1. CRC Crit. Rev. Bioeng. 3:181–330. [PubMed] [Google Scholar]
- 36.Marsh, D. 2006. Elastic curvature constants of lipid monolayers and bilayers. Chem. Phys. Lipids. 144:146–159. [DOI] [PubMed] [Google Scholar]
- 37.Olbrich, K., W. Rawicz, D. Needham, and E. Evans. 2000. Water permeability and mechanical strength of polyunsaturated lipid bilayers. Biophys. J. 79:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans, E. 1974. Bending resistance and chemically induced moments in membrane bilayers. Biophys. J. 14:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rawicz, W., K. C. Olbrich, T. McIntosh, D. Needham, and E. Evans. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 28c:693–703. [DOI] [PubMed] [Google Scholar]
- 41.Gruner, S. M. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA. 82:3665–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seddon, J. M., and R. H. Templer. 1995. Polymorphism of lipid-water systems. In Structure and Dynamics of Membranes, Vol. 1. R. Lipowski and E. Sackmann, editors. Elsevier Science, Amsterdam, The Netherlands.
- 43.Marsh, D. 1996. Lateral pressure in membranes. 1996. Biochim. Biophys. Acta. 1286:183–223. [DOI] [PubMed] [Google Scholar]
- 44.Cantor, R. S. 1999. Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76:2625–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantor, R. S. 1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 101:45–56. [DOI] [PubMed] [Google Scholar]
- 46.Dekker, C. J., W. S. Geurts van Kessel, J. P. Klomp, J. Pieters, and B. De Kruijff. 1983. Synthesis and polymorphic phase behavior of polyunsaturated phosphatidylcholines and phosphatidylethanolamines. Chem. Phys. Lipids. 33:93–106. [DOI] [PubMed] [Google Scholar]
- 47.Rand, R. P., N. L. Fuller, S. M. Gruner, and V. A. Parsegian. 1990. Membrane curvature, lipid segregation, and structural transitions for phospholipids under dual-solvent stress. Biochemistry. 29:76–87. [DOI] [PubMed] [Google Scholar]
- 48.Szule, J. A., N. L. Fuller, and R. P. Rand. 2002. The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholines on membrane monolayer curvature. Biophys. J. 83:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rex, S. 1996. Pore formation induced by the peptide melittin in different lipid vesicle membranes. Biophys. Chem. 58:75–85. [DOI] [PubMed] [Google Scholar]
- 50.Illya, G., R. Lipowsky, and J. C. Shillcock. 2005. Effect of chain length and asymmetry on material properties of bilayer membranes. J. Chem. Phys. 122:244901. [DOI] [PubMed] [Google Scholar]
- 51.Dan, N. 2007. Lipid tail chain asymmetry and the strength of membrane-induced interactions between membrane proteins. Biochim. Biophys. Acta. 1768:2393–2399. [DOI] [PubMed] [Google Scholar]
- 52.Kučerka, N., S. Tristram-Nagle, and J. F. Nagle. 2005. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. 208:193–202. [DOI] [PubMed] [Google Scholar]
- 53.Prenner, E. J., R. N. Lewis, K. C. Neuman, S. M. Gruner, L. H. Kondejewski, R. S. Hodges, and R. N. McElhaney. 1997. Nonlamellar phases induced by the interaction of gramicidin S with lipid bilayers. A possible relationship to membrane-disrupting activity. Biochemistry. 36:7906–7916. [DOI] [PubMed] [Google Scholar]
- 54.Matsuzaki, K., K. Sugishita, N. Ishibe, M. Ueha, S. Nakata, K. Miyajima, and R. M. Epand. 1998. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry. 37:11856–11863. [DOI] [PubMed] [Google Scholar]
- 55.Epand, R. F., R. M. Epand, V. Monaco, S. Stoia, F. Formaggio, M. Crisma, and C. Toniolo. 1999. The antimicrobial peptide trichogin and its interaction with phospholipid membranes. Eur. J. Biochem. 266:1021–1028. [DOI] [PubMed] [Google Scholar]
- 56.Liu, F., R. N. Lewis, R. S. Hodges, and R. N. McElhaney. 2001. A differential scanning calorimetric and 31P NMR spectroscopic study of the effect of transmembrane α-helical peptides on the lamellar-reversed hexagonal phase transition of phosphatidylethanolamine model membranes. Biochemistry. 40:760–768. [DOI] [PubMed] [Google Scholar]
- 57.Epand, R. F., N. Umezawa, E. A. Porter, S. H. Gellman, and R. M. Epand. 2003. Interactions of the antimicrobial β-peptide β-17 with phospholipid vesicles differ from membrane interactions of magainins. Eur. J. Biochem. 270:1240–1248. [DOI] [PubMed] [Google Scholar]
- 58.Lee, M. T., W. C. Hung, F. Y. Chen, and H. W. Huang. 2005. Many-body effect of antimicrobial peptides: on the correlation between lipid's spontaneous curvature and pore formation. Biophys. J. 89:4006–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramamoorthy, A., S. Thennarasu, D. K. Lee, A. Tan, and L. Maloy. 2006. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys. J. 91:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sankaram, M. B., and T. E. Thompson. 1990. Modulation of phospholipid acyl chain order by cholesterol. A solid-state 2H nuclear magnetic resonance study. Biochemistry. 29:10676–10684. [DOI] [PubMed] [Google Scholar]
- 61.Nezil, F. A., and M. Bloom. 1992. Combined influence of cholesterol and synthetic amphiphilic peptides upon bilayer thickness in model membranes. Biophys. J. 61:1176–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen, Z., and R. P. Rand. 1997. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pokorny, A., L. E. Yandek, A. I. Elegbede, A. Hinderliter, and P. F. Almeida. 2006. Temperature and composition dependence of the interaction of δ-lysin with ternary mixtures of sphingomyelin/cholesterol/POPC. Biophys. J. 91:2184–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinderliter, A., R. L. Biltonen, and P. F. Almeida. 2004. Lipid modulation of protein-induced membrane domains as a mechanism for controlling signal transduction. Biochemistry. 43:7102–7110. [DOI] [PubMed] [Google Scholar]