Abstract
Three major outer membrane proteins with apparent molecular masses of 43, 45, and 51 kDa were purified from Wolinella recta ATCC 33238, and their pore-forming abilities were determined by the black lipid bilayer method. The non-heat-modifiable 45-kDa protein (Omp 45) showed no pore-forming activity even at high KCl concentrations. The single-channel conductances in 1 M KCl of the heat-modifiable proteins with apparent molecular masses of 43 kDa (Omp 43) and 51 kDa (Omp 51) were 0.49 and 0.60 nS, respectively. The proteins formed nonselective channels and, as determined by experiments of ion selectivity and zero-current potential, were weakly anion selective.
Full text
PDF
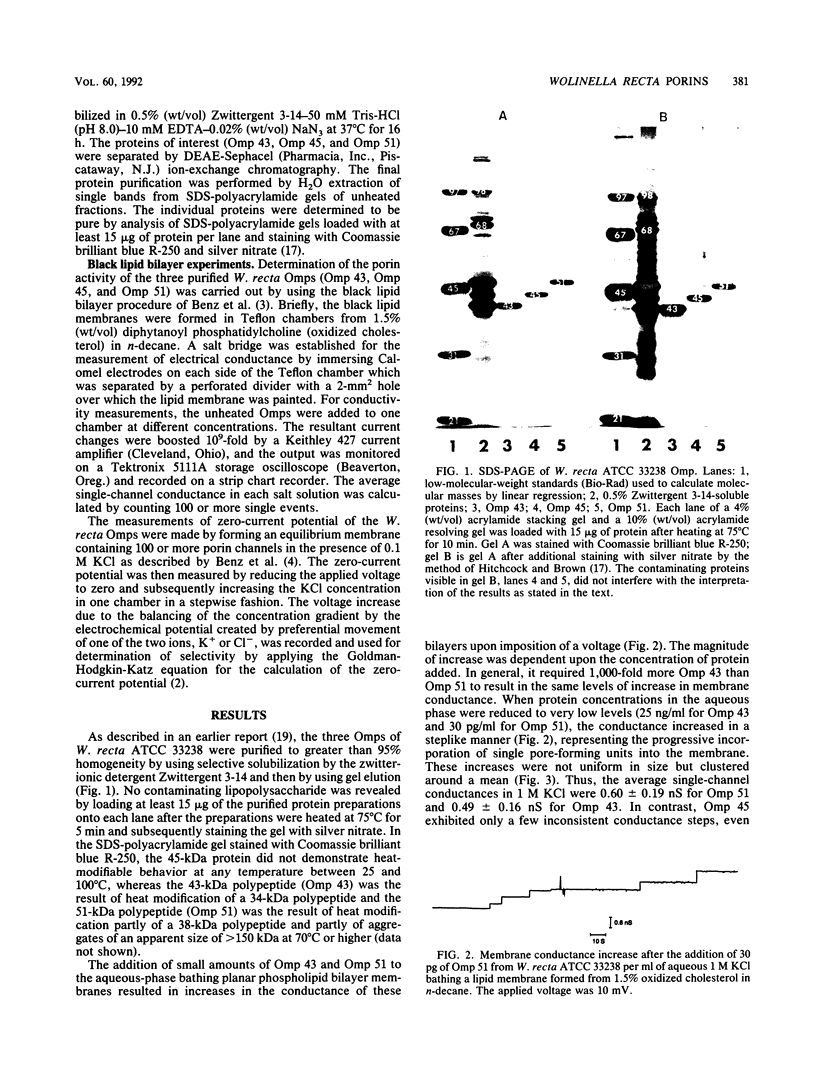
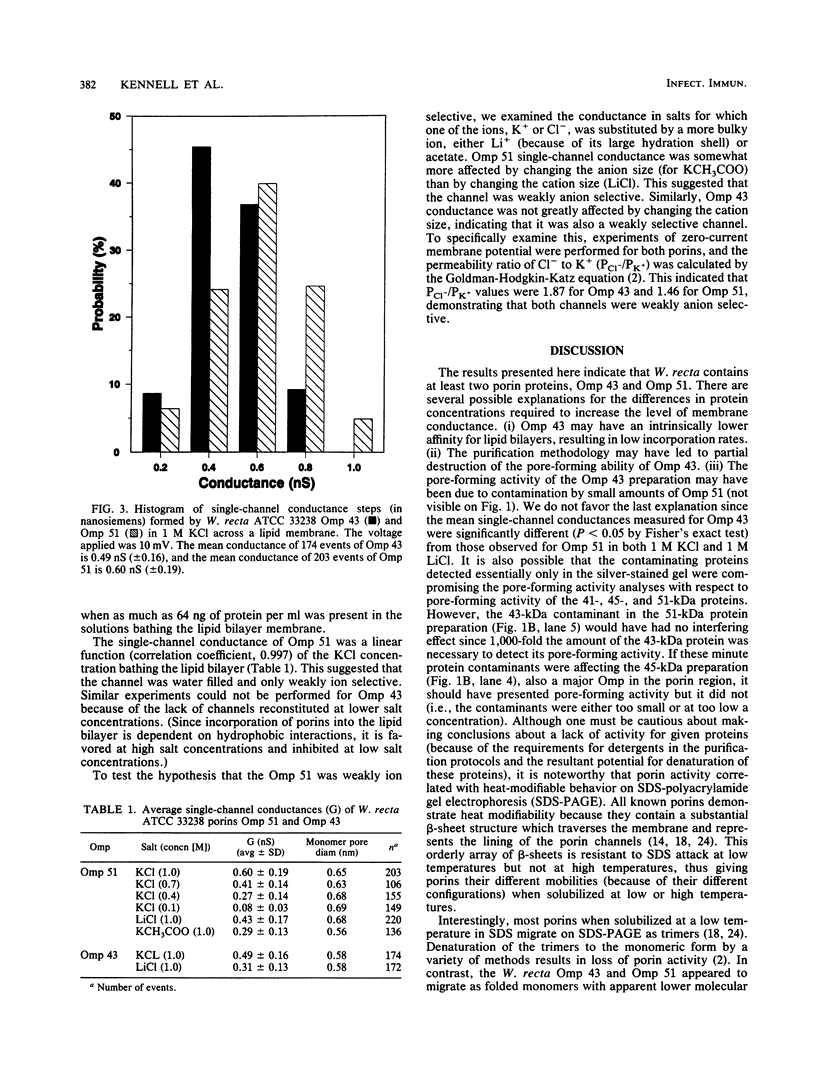


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. K., Parr T. R., Jr, Parker C. D., Hancock R. E. Bordetella pertussis major outer membrane porin protein forms small, anion-selective channels in lipid bilayer membranes. J Bacteriol. 1986 Apr;166(1):212–216. doi: 10.1128/jb.166.1.212-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Läuger P. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1979 Mar 8;551(2):238–247. doi: 10.1016/0005-2736(89)90002-3. [DOI] [PubMed] [Google Scholar]
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Donato A., Draetta G. F., Illiano G., Tufano M. A., Sommese L., Galdiero F. Do porins inhibit the macrophage phagocyting activity by stimulating the adenylate cyclase? J Cyclic Nucleotide Protein Phosphor Res. 1986;11(2):87–97. [PubMed] [Google Scholar]
- Dzink J. L., Tanner A. C., Haffajee A. D., Socransky S. S. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985 Sep;12(8):648–659. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Fauchère J. L., Kervella M., Rosenau A., Mohanna K., Véron M. Adhesion to HeLa cells of Campylobacter jejuni and C. coli outer membrane components. Res Microbiol. 1989 Jul-Aug;140(6):379–392. doi: 10.1016/0923-2508(89)90014-4. [DOI] [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Galdiero M., Masiello S., Di Rosa M. Inflammatory effects of Salmonella typhimurium porins. Infect Immun. 1990 Oct;58(10):3183–3186. doi: 10.1128/iai.58.10.3183-3186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Sommese L., Folgore A., Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984 Nov;46(2):559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J., Holt S. C. Growth studies of Wolinella recta, a gram-negative periodontopathogen. Oral Microbiol Immunol. 1987 Sep;2(3):105–111. doi: 10.1111/j.1399-302x.1987.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Benz R. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim Biophys Acta. 1986 Sep 11;860(3):699–707. doi: 10.1016/0005-2736(86)90569-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Siehnel R., Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990 Jul;4(7):1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Kennell W. L., Holt S. C. Extraction, purification, and characterization of major outer membrane proteins from Wolinella recta ATCC 33238. Infect Immun. 1991 Oct;59(10):3740–3749. doi: 10.1128/iai.59.10.3740-3749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A., Beesley J. E. Bordetella adenylate cyclase: a genus specific protective antigen and virulence factor. Dev Biol Stand. 1985;61:27–41. [PubMed] [Google Scholar]
- Takada H., Ogawa T., Yoshimura F., Otsuka K., Kokeguchi S., Kato K., Umemoto T., Kotani S. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect Immun. 1988 Apr;56(4):855–863. doi: 10.1128/iai.56.4.855-863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M. A., Berlingieri M. T., Sommese L., Galdiero F. Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica. 1984 Oct;7(4):353–366. [PubMed] [Google Scholar]
- Tufano M. A., Ianniello R., Galdiero M., De Martino L., Galdiero F. Effect of Salmonella typhimurium porins on biological activities of human polymorphonuclear leukocytes. Microb Pathog. 1989 Nov;7(5):337–346. doi: 10.1016/0882-4010(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Tufano M. A., Sommese L., Capasso C., Folgore A., Scafa F., Galdiero E. Comparative study of some biological activities of porins extracted from various microorganisms. Microbiologica. 1986 Oct;9(4):431–442. [PubMed] [Google Scholar]
- Van Dyke T. E., Dowell V. R., Jr, Offenbacher S., Snyder W., Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun. 1986 Sep;53(3):671–677. doi: 10.1128/iai.53.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Zalman L. S., Nikaido H. Porin from Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jul;159(1):199–205. doi: 10.1128/jb.159.1.199-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Hancock R. E. Pseudomonas aeruginosa outer membrane protein F: structural role and relationship to the Escherichia coli OmpA protein. J Bacteriol. 1989 Jun;171(6):3304–3309. doi: 10.1128/jb.171.6.3304-3309.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Zalman L. S., Nikaido H. Purification and properties of Pseudomonas aeruginosa porin. J Biol Chem. 1983 Feb 25;258(4):2308–2314. [PubMed] [Google Scholar]




