Abstract
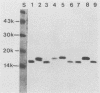
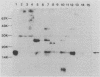
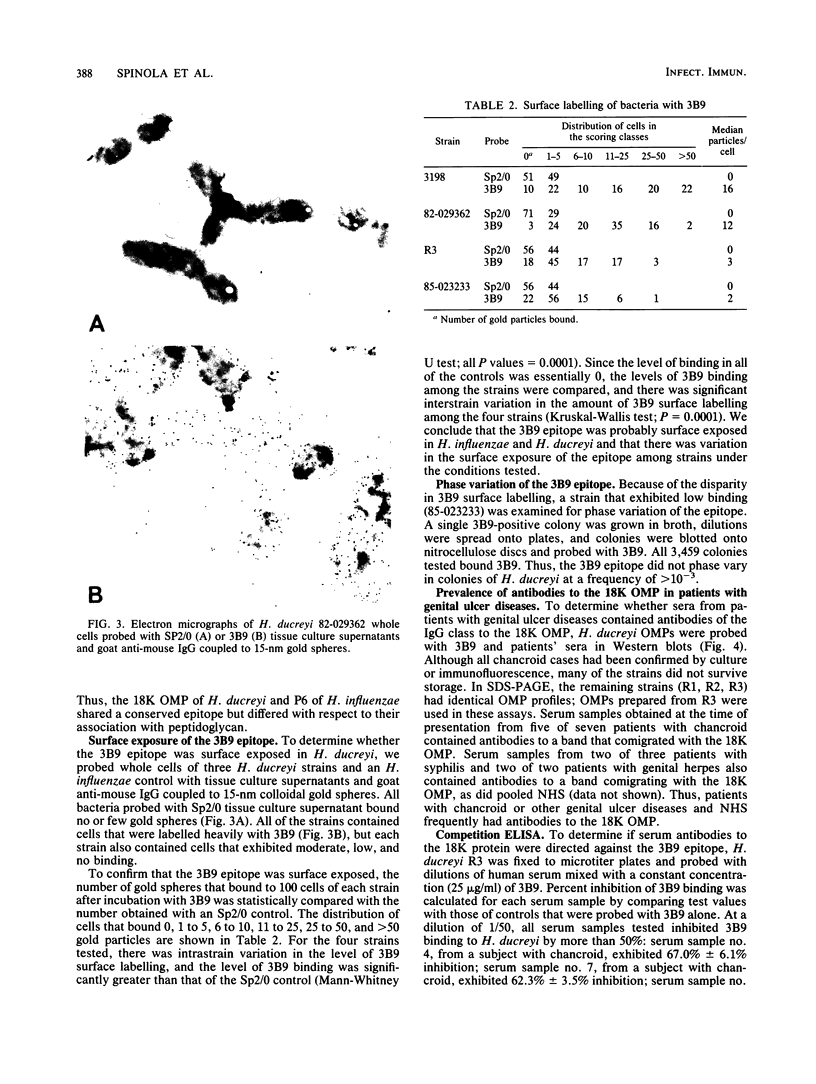
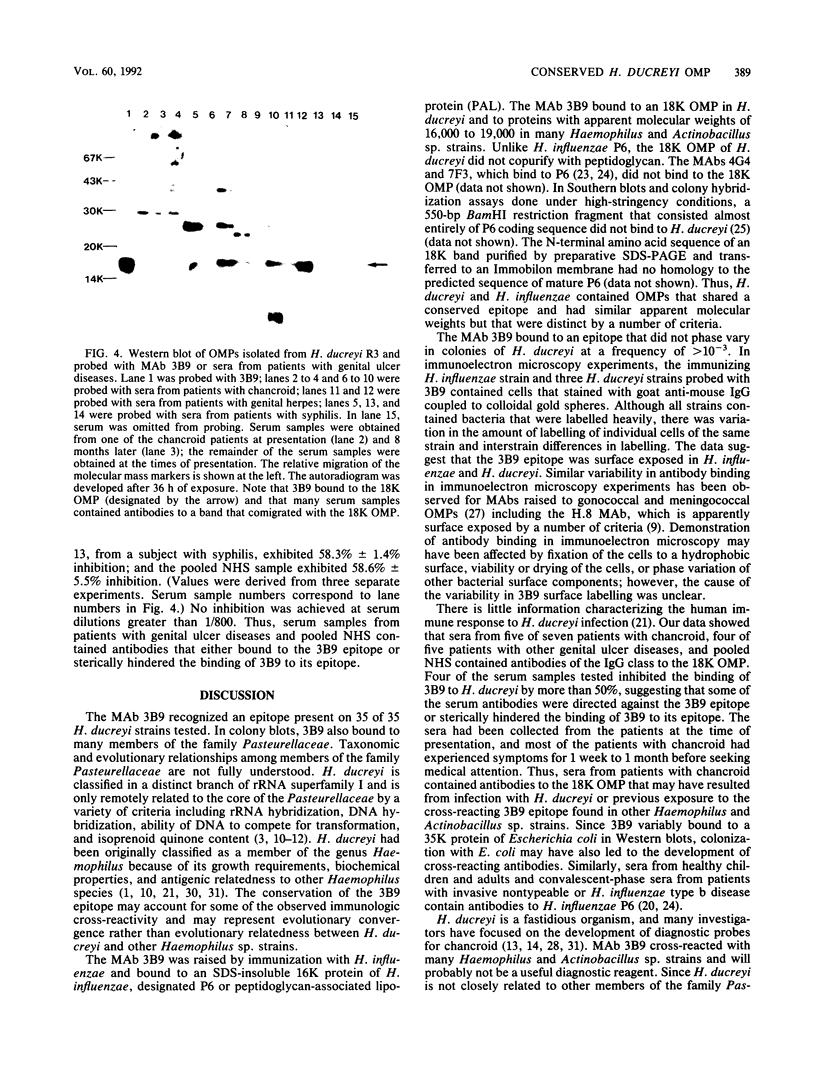
Identification of antigenically conserved surface components of Haemophilus ducreyi may facilitate the development of reagents to diagnose and prevent chancroid. A hybridoma derived from a mouse immunized with nontypeable Haemophilus influenzae produced a monoclonal antibody (MAb), designated 3B9, that bound to 35 of 35 H. ducreyi strains isolated from diverse geographic regions. The MAb 3B9 bound to a non-heat-modifiable H. ducreyi outer membrane protein (OMP) whose apparent molecular weight was 18,000 (the 18K OMP), and the 3B9 epitope did not phase vary at a rate of greater than 10(-3) in H. ducreyi. In immunoelectron microscopy, the 3B9 epitope was surface exposed, and there was intrastrain and interstrain variability in the amount of 3B9 labelling of whole cells. The MAb 3B9 cross-reacted with many species of the family Pasteurellaceae and bound to the 16.6K peptidoglycan-associated lipoprotein (P6 or PAL) of H. influenzae. Unlike P6, the 18K OMP did not copurify with peptidoglycan. In Western blots (immunoblots), five of seven serum samples obtained from patients with chancroid and four of five serum samples obtained from patients with other genital ulcer diseases at the time of presentation contained antibodies that bound to the 18K OMP. In a competition enzyme-linked immunosorbent assay, four of these serum samples inhibited the binding of 3B9 to H. ducreyi by more than 50%. We conclude that members of Pasteurellaceae expressed a conserved epitope on OMPs that sometimes had different physical characteristics. Patients with chancroid usually have antibodies to the 18K OMP and the 3B9 epitope that may have resulted from infection with H. ducreyi or previous exposure to other Haemophilus or Actinobacillus sp. strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Taylor-Robinson D. Antigenic analysis of Haemophilus ducreyi by Western blotting. Epidemiol Infect. 1988 Aug;101(1):151–157. doi: 10.1017/s0950268800029319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L. Biology of Haemophilus ducreyi. Microbiol Rev. 1989 Dec;53(4):377–389. doi: 10.1128/mr.53.4.377-389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Dudas K. C., Campagnari A., Rice P., Mylotte J. M., Murphy T. F. Antigenic heterogeneity of lipid A of Haemophilus influenzae. Infect Immun. 1985 Oct;50(1):9–14. doi: 10.1128/iai.50.1.9-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley S. F., Widy-Wirski R., Okware S. I., Downing R., Linnan M. J., White K. E., Sempala S. Risk factors associated with HIV infection in Uganda. J Infect Dis. 1989 Jul;160(1):22–30. doi: 10.1093/infdis/160.1.22. [DOI] [PubMed] [Google Scholar]
- Black J. R., Black W. J., Cannon J. G. Neisserial antigen H.8 is immunogenic in patients with disseminated gonococcal and meningococcal infections. J Infect Dis. 1985 Apr;151(4):650–657. doi: 10.1093/infdis/151.4.650. [DOI] [PubMed] [Google Scholar]
- Campagnari A. A., Spinola S. M., Lesse A. J., Kwaik Y. A., Mandrell R. E., Apicella M. A. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog. 1990 May;8(5):353–362. doi: 10.1016/0882-4010(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Cannon J. G. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S1–S4. doi: 10.1128/cmr.2.suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Mannheim W., Mutters R., Piechulla K., Tytgat R., Segers P., Bisgaard M., Frederiksen W., Hinz K. H., Vanhoucke M. Inter- and intrafamilial similarities of rRNA cistrons of the Pasteurellaceae. Int J Syst Bacteriol. 1990 Apr;40(2):126–137. doi: 10.1099/00207713-40-2-126. [DOI] [PubMed] [Google Scholar]
- Finn G. Y., Karim Q. N., Easmon C. S. The production and characterisation of rabbit antiserum and murine monoclonal antibodies to Haemophilus ducreyi. J Med Microbiol. 1990 Mar;31(3):219–224. doi: 10.1099/00222615-31-3-219. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Loftus T. A. Monoclonal antibodies reactive with all strains of Haemophilus ducreyi. Infect Immun. 1984 Apr;44(1):196–198. doi: 10.1128/iai.44.1.196-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg S. D., Horsburgh C. R., Jr, Ward J. W., Jaffe H. W. Biologic factors in the sexual transmission of human immunodeficiency virus. J Infect Dis. 1989 Jul;160(1):116–125. doi: 10.1093/infdis/160.1.116. [DOI] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Coombs R., Plummer F., Holmes K. K., Nikora B., Cameron W., Ngugi E., Ndinya Achola J. O., Corey L. Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J Infect Dis. 1989 Sep;160(3):380–384. doi: 10.1093/infdis/160.3.380. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Koech D., Plummer F. A., Holmes K. K., Lightfoote M., Piot P., Ronald A. R., Ndinya-Achola J. O., D'Costa L. J., Roberts P. AIDS virus infection in Nairobi prostitutes. Spread of the epidemic to East Africa. N Engl J Med. 1986 Feb 13;314(7):414–418. doi: 10.1056/NEJM198602133140704. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Human antibody response to individual outer membrane proteins of Haemophilus influenzae type b. Infect Immun. 1982 Sep;37(3):1032–1036. doi: 10.1128/iai.37.3.1032-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Campagnari A. A., Nelson M. B., Apicella M. A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986 Dec;54(3):774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C., Rice P. A., Nelson M. B., Dudas K. C., Apicella M. A. Identification of a 16,600-dalton outer membrane protein on nontypeable Haemophilus influenzae as a target for human serum bactericidal antibody. J Clin Invest. 1986 Oct;78(4):1020–1027. doi: 10.1172/JCI112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. B., Apicella M. A., Murphy T. F., Vankeulen H., Spotila L. D., Rekosh D. Cloning and sequencing of Haemophilus influenzae outer membrane protein P6. Infect Immun. 1988 Jan;56(1):128–134. doi: 10.1128/iai.56.1.128-134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Relationship between lipopolysaccharide composition and virulence of Haemophilus ducreyi. J Med Microbiol. 1987 Mar;23(2):155–162. doi: 10.1099/00222615-23-2-155. [DOI] [PubMed] [Google Scholar]
- Parsons L. M., Shayegani M., Waring A. L., Bopp L. H. DNA probes for the identification of Haemophilus ducreyi. J Clin Microbiol. 1989 Jul;27(7):1441–1445. doi: 10.1128/jcm.27.7.1441-1445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâques M., Teppema J. S., Beuvery E. C., Abdillahi H., Poolman J. T., Verkleij A. J. Accessibility of gonococcal and meningococcal surface antigens: immunogold labeling for quantitative electron microscopy. Infect Immun. 1989 Feb;57(2):582–589. doi: 10.1128/iai.57.2.582-589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Glasser D., Cannon R. O., Matuszak D. L., Dunning R. W., Kline R. L., Campbell C. H., Israel E., Fauci A. S., Hook E. W., 3rd Human immunodeficiency virus infection among patients attending clinics for sexually transmitted diseases. N Engl J Med. 1988 Jan 28;318(4):197–203. doi: 10.1056/NEJM198801283180401. [DOI] [PubMed] [Google Scholar]
- Saunders J. M., Folds J. D. Immunoblot analysis of antigens associated with Haemophilus ducreyi using serum from immunised rabbits. Genitourin Med. 1986 Oct;62(5):321–328. doi: 10.1136/sti.62.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalla W. O., Sanders L. L., Schmid G. P., Tam M. R., Morse S. A. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986 May;153(5):879–887. doi: 10.1093/infdis/153.5.879. [DOI] [PubMed] [Google Scholar]
- Schmid G. P., Sanders L. L., Jr, Blount J. H., Alexander E. R. Chancroid in the United States. Reestablishment of an old disease. JAMA. 1987 Dec 11;258(22):3265–3268. [PubMed] [Google Scholar]
- Simonsen J. N., Cameron D. W., Gakinya M. N., Ndinya-Achola J. O., D'Costa L. J., Karasira P., Cheang M., Ronald A. R., Piot P., Plummer F. A. Human immunodeficiency virus infection among men with sexually transmitted diseases. Experience from a center in Africa. N Engl J Med. 1988 Aug 4;319(5):274–278. doi: 10.1056/NEJM198808043190504. [DOI] [PubMed] [Google Scholar]
- Spinola S. M., Kwaik Y. A., Lesse A. J., Campagnari A. A., Apicella M. A. Cloning and expression in Escherichia coli of a Haemophilus influenzae type b lipooligosaccharide synthesis gene(s) that encodes a 2-keto-3-deoxyoctulosonic acid epitope. Infect Immun. 1990 Jun;58(6):1558–1564. doi: 10.1128/iai.58.6.1558-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Peacock J., Denny F. W., Smith D. L., Cannon J. G. Epidemiology of colonization by nontypable Haemophilus influenzae in children: a longitudinal study. J Infect Dis. 1986 Jul;154(1):100–109. doi: 10.1093/infdis/154.1.100. [DOI] [PubMed] [Google Scholar]
- Stamm W. E., Handsfield H. H., Rompalo A. M., Ashley R. L., Roberts P. L., Corey L. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA. 1988 Sep 9;260(10):1429–1433. [PubMed] [Google Scholar]